Abstract
Purpose Simulation training offers a useful opportunity to appreciate vascular anatomy and develop the technical expertise required to clip intracranial aneurysms of the posterior circulation.
Materials and Methods In cadavers, a comparison was made between the endoscopic transclival approach (ETA) alone and a combined multiportal approach using the ETA and a transorbital precaruncular approach (TOPA) to evaluate degrees of freedom, angles of visualization, and ergonomics of aneurysm clip application to the posterior circulation depending on basilar apex position relative to the posterior clinoids.
Results ETA alone provided improved access to the posterior circulation when the basilar apex was high riding compared with the posterior clinoids. ETA + TOPA provided a significantly improved functional working area for instruments and visualization of the posterior circulation for a midlevel basilar apex. A single-shaft clip applier provided improved visualization and space for instruments. Proximal and distal vascular control and feasibility of aneurysmal clipping were demonstrated.
Conclusions TOPA is a medial orbital approach to the central skull base; a transorbital neuroendoscopic surgery approach. This anatomical simulation provides surgical teams an alternative to the ETA approach alone to address posterior circulation aneurysms, and a means to preoperatively prepare for intraoperative anatomical and surgical instrumentation challenges.
Keywords: posterior circulation aneurysms, endoscopic approach, endonasal transclival approach, transorbital neuroendoscopic surgery, simulation training
Introduction
The success of any technique used to treat cerebral aneurysms is significantly influenced by aneurysm location. Posterior circulation aneurysms are the most technically challenging to treat through open skull base approaches. Posterior circulation aneurysms also present a greater rate of treatment complications. 1 At most centers, endovascular techniques have become the mainstay of treatment for posterior circulation aneurysms, for example, the Barrow Ruptured Aneurysm Trial. 1 Basilar tip and posterior cerebral artery (PCA) aneurysms are the most common aneurysms of the posterior circulation 2 and are most amenable to endovascular treatment in comparison to superior cerebellar artery (SCA) or anterior inferior cerebellar artery (AICA) aneurysms. SCA and AICA aneurysms, however, are particularly challenging to treat through open skull base or endovascular techniques. Endovascular treatment for SCA and AICA aneurysms have been reported to have a 12.5% dog-ear remnant postprocedure. 3 Clipping of these aneurysms is more commonly curative, and microsurgical approaches without temporal lobe retraction have been used. 4 Clipping has been successfully employed when coiling has failed or when coiling is not feasible. 5 An endoscopic transclival approach (ETA) provides direct access to the posterior circulation without brain retraction. It may also provide adequate decompression in the setting of mass effect secondary to hemorrhage. Surgical outcomes for the posterior circulation have been improved by adequate decompression. 6 Endoscopic endonasal clippings of aneurysms have been increasingly performed successfully. 7 8
In this anatomical and simulation study, ETA alone was compared with ETA and a transorbital neuroendoscopic surgery (TONES). 9 10 11 An ETA was previously shown to increase visualization to the basilar artery (BA) and posterior circulation, allowing enhanced feasibility of clip placement. 12 To improve the technical skill sets required for these endoscopic approaches, feasible simulation experiences are necessary. Narayanan et al have shown direct improvement of endoscopic endonasal skull based procedures due to participation in simulated anatomical studies. 13
Labib and Dehdashti have demonstrated the clinical feasibility of ETA clip placement for posterior circulation aneurysms at the hands of experienced surgeons. 14 To increase widespread clinical applicability, it will be important to improve training experiences for residents and junior faculty. Simulated anatomical experiences offer a unique advantage in that approaches and visualization can be rehearsed numerous times prior to entering the operative arena. The extended transsphenoidal endoscopic view in comparison to microsurgery allows better visualization of the blind corners associated with posterior circulation aneurysms, offering clear advantages. 15 The evolution toward endoscopic clipping of aneurysms can be seen by the increased use of endoscopic assisted/endonasal expanded surgeries. 16 In a recent feasibility case series, the endoscopic endonasal technique was used to clip 11 intracranial aneurysms including several vertebrobasilar aneurysms. 17 Although there is inherent risk of cerebrospinal fluid leak with clip placement, posterior circulation aneurysms can be adequately treated by clipping with good success rates. 18 The ETA is now being investigated as an alternative for aneurysm clipping of the posterior circulation arteries, although experienced surgeons suggest that a steep learning curve exists. 2 There is a significant learning curve to endoscopic skull base surgery and more so as it relates to the more complex approaches. 19 Vascular endoscopic surgery falls within this realm. As the diameter from the face of the sphenoid progressively “cones down” through the clival and dural opening to the posterior circulation, the advantages of removing a rod lens instrument from the endonasal cavity becomes apparent to optimize the four-handed microsurgical technique. An experienced team with dynamic and multiangled endoscopes can be used and has been previously published to be successful. 20 We present an approach to be considered as an alternative, or in the case where the ETA approach is used and found be too limiting intraoperatively, the addition of the TONES can be beneficial. The endoscopic endonasal approach (EEA) was originally used for treating pituitary adenomas, acromegaly, Cushing's disease, and clival chordomas, but its potential suitability for other treatments has expanded over the years. 21 In this cadaveric simulation and anatomical study, we highlight the benefit, feasibility, and cost-effectiveness of cadaveric models for presurgical training prior to attempting endoscopic approaches in the surgical arena.
Methods
Preparation
Three adult cadaveric heads were used for this anatomical study: one with a high-riding basilar apex, one with a midlevel basilar apex, and one with a low-riding basilar apex. The heads were acquired as part of the Oregon Health & Science University (OHSU) Body Donation Program and were handled according to the Code of Ethics approved by the OHSU Institutional Review Board and VirtuOHSU laboratories. Heads were frozen and thawed once prior to use. A 1:100 solution of anticoagulant citrate dextrose (Pierce Laboratories, New Haven, Connecticut, United States) mixed with warm water was prepared. The great vessels in the neck (jugular veins, carotid arteries, and vertebral arteries) were washed out for 15 minutes and the head was allowed to sit overnight in a cold room at 5°C. The following day, the heads were embalmed and stored in formalin fixative solution (10%).
Microfil Injections
Red microfil (Flow Tech, Carver, Massachusetts, United States) was prepared according to the manufacturer's instructions for use on three heads at a time (50-μL diluent, 40-μL red microfil dye, and 4.5-μL curative agent). A total of 20 μL of microfil solution was injected into the right internal carotid artery through a clamped catheter. The external carotid artery was clamped to focus microfil flow into the intracerebral vasculature. The injection was stopped once the red microfil filled the common carotid artery on the opposite side. Both common carotid arteries were then clamped. A total of 10 μL of microfil was injected into the right vertebral artery until flow was noted out of the vertebral artery on the opposite side. Both vertebral arteries were clamped and the head was allowed to sit at room temperature for 30 minutes before the clamps were removed. All three heads were injected in a similar fashion.
Dissection and Clip Placement
The heads were placed in a slightly extended position with good visibility of the nasal passages. Zero- and thirty-degree endoscopes of 4-mm diameter and 18-cm length were used for the study (Karl Storz, Tuttlingen, Germany). The endoscope was connected to a fiber optic camera and light source. For the ETA, exposure of the sphenoid sinus was performed as previously described. 22 Briefly, the middle turbinates were lateralized and a wide bilateral sphenoidotomy was performed. The incision was made at the articulation of the rostrum and vomer. A periosteal elevator was used to clear the mucoperiosteum, and the rostral bone was removed bilaterally with a Kerrison 2 (Symmetry Surgical) and drilled flush to the floor of the sphenoid sinus. Identification of the bone impressions of the clivus, sella, cavernous carotid arteries, and opticocarotid recesses was made. The bone was removed overlying these critical structures. The pituitary gland was retracted and the clivus was drilled out with a 5-mm coarse diamond burr drill. A Kerrison was used to clear remaining bone fragments until the clival dura was exposed. The posterior clinoids were removed to optimize pituitary gland mobilization. The dura over the pituitary gland was preserved and its dural attachments were dissected free laterally. The clival dura was excised, and the BA, AICAs, SCAs, and PCAs were exposed.
TONES was performed as previously described. 22 Briefly, an incision was made medial to the caruncle into the avascular plane. The incision was widened superiorly and inferiorly using iris scissors. The frontoethmoidal suture was identified along the medial orbital wall. This suture and the anterior and posterior ethmoidal arteries are used as key anatomical landmarks. The transorbital port was created in the lamina papyracea below the level of the frontoethmoidal suture and through the ethmoid air cells more medially. The cavernous carotid, clivus, and sella were visualized. The binares endonasal approach was used as the instrumentation port in all specimens. The comparisons of functional working area, ergonomics, and visualization were made between using the ETA approach alone, which included the endoscope, and removing the endoscope from the endonasal port and placing it through the transorbital port while all other instrumentation and aneurysm clip appliers remained in the endonasal port. The clip applicator was used to show feasibility of clip placement at sites of proximal and distal control and potential aneurysm origination within all regions posterior circulation: BA (proximal to its apex), PCA, SCA, and AICA.
Results
Anatomy
The endoscopic dissection with the ETA revealed detailed bilateral carotid anatomy as the arteries coursed through the cavernous sinus. The opticocarotid recess, optic chiasm, and clival recess were visualized. The microfil-injected arteries of the posterior circulation were clearly visualized through the bilateral sphenoidotomies with the 0-degree endoscope. The BA coursed atop the pons, with pontine branches readily seen. A clear delineation of the basilar tip was observed ( Fig. 1 ). The occulomotor nerves were visualized between the SCA and PCA. Adequate working space was obtained with this approach for placement of the endoscope as well as surgical instrumentation through the bilateral nasal passages.
Fig. 1.
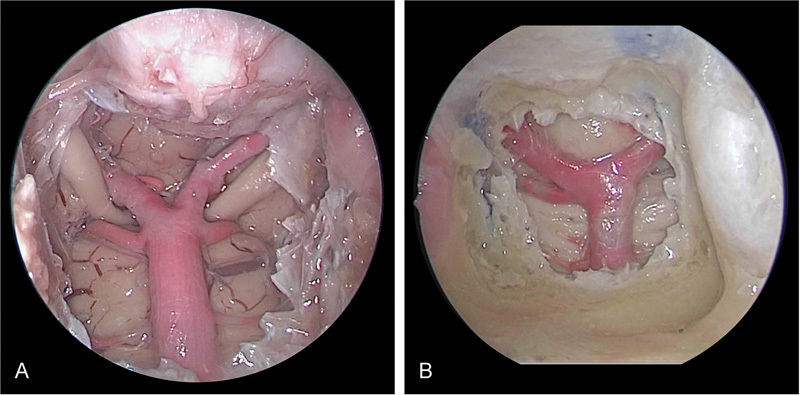
Endoscopic endonasal transclival approach to the posterior circulation for ( A ) high-riding basilar apex and ( B ) midlevel basilar apex.
For the high-riding basilar apex, above the level of the posterior clinoids, the TONES required more significant mobilization of the pituitary gland to view the BA, PCA, and SCA ( Fig. 2 ). Whereas, less manipulation to view these segments was needed using the ETA alone ( Fig. 3 ). When the BA was at the level of the posterior clinoids (mid) or below the level of the posterior clinoids (low riding), there was a significant advantage to using a biportal technique. The ETA combined with the TONES ( Fig. 4 ) improved the degrees of freedom. Using the biportal technique, the fixed rod lens of the endoscope was removed from the endonasal port allowing for approximately 25% more working room given that the four-handed technique of an EEA includes the endoscope. By placing the endoscope through the transorbital port, more instruments can be used through the binares endonasal approach. The area of working room dramatically decreases from the sphenoid face to the region of the clivus ( Fig. 5 ). Restriction occurred bilaterally by the cavernous carotid arteries, inferiorly by the floor of the sphenoid sinus, and superiorly by the pituitary gland or the sella turcica if the gland is not mobilized. Mobilization of the pituitary gland with instrumentation has been associated with pituitary dysfunction, 8 but extra or intradural transposition has been performed successfully. 23 Thus, improving the working area and degrees of freedom to perform the necessary microsurgical techniques to dissect the aneurysm from the brain stem and parent vessel, to obtain proximal and distal vascular control, and to perform the clipping (which may require placement and readjustment of the clip) and possible intraoperative aneurysm rupture is potentially beneficial ( Video 1 ). As the dimensions from the sphenoid opening to the clivus dramatically narrow and cone down, the degrees of freedom and visualization are challenged ( Fig. 6 ). In this restricted region, gaining more degrees of freedom for instruments and improved visualization is critical ( Fig. 7 ). This can be especially critical if surgeons are faced with a highly vascular tumor or brisk arterial bleeding that requires more suction and instrumentation to assist with vascular control. In addition, instruments to provide mobilization of the pituitary gland for improved access to the posterior circulation are needed.
Fig. 2.
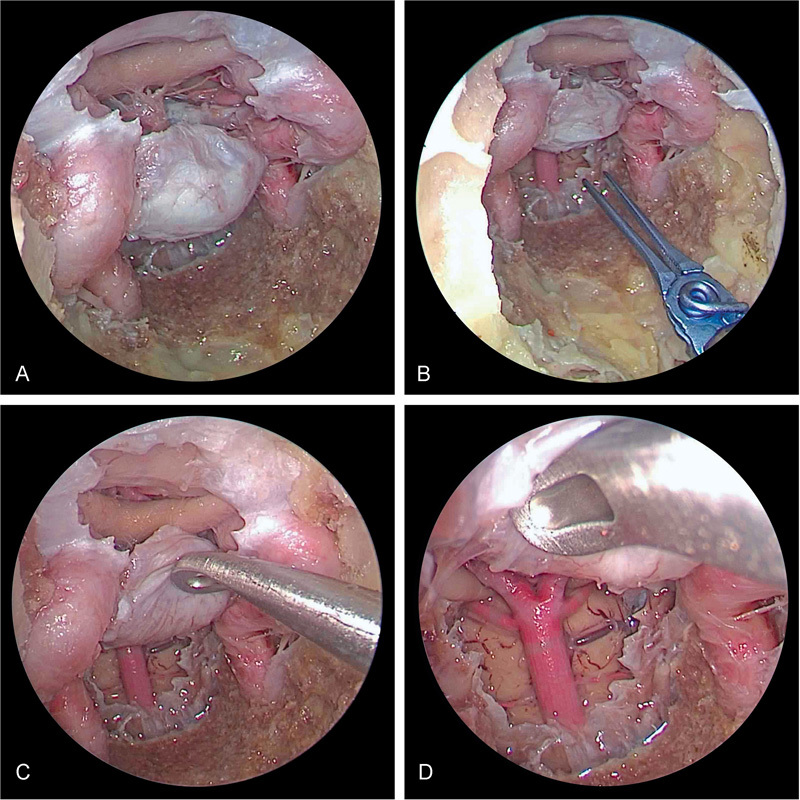
Right transorbital view of combined endoscopic multiportal transorbital and endonasal view and approach for posterior circulation clipping with a high-riding basilar apex. The dual approach is limited by transposition of the pituitary gland to achieve visualization of the posterior circulation.
Fig. 3.
Endoscopic endonasal transclival approach demonstrating successful clip application for ( A ) posterior cerebral artery, ( B ) basilar artery, ( C ) anterior inferior cerebellar artery, and ( D ) superior cerebellar artery when there is a high-riding basilar apex.
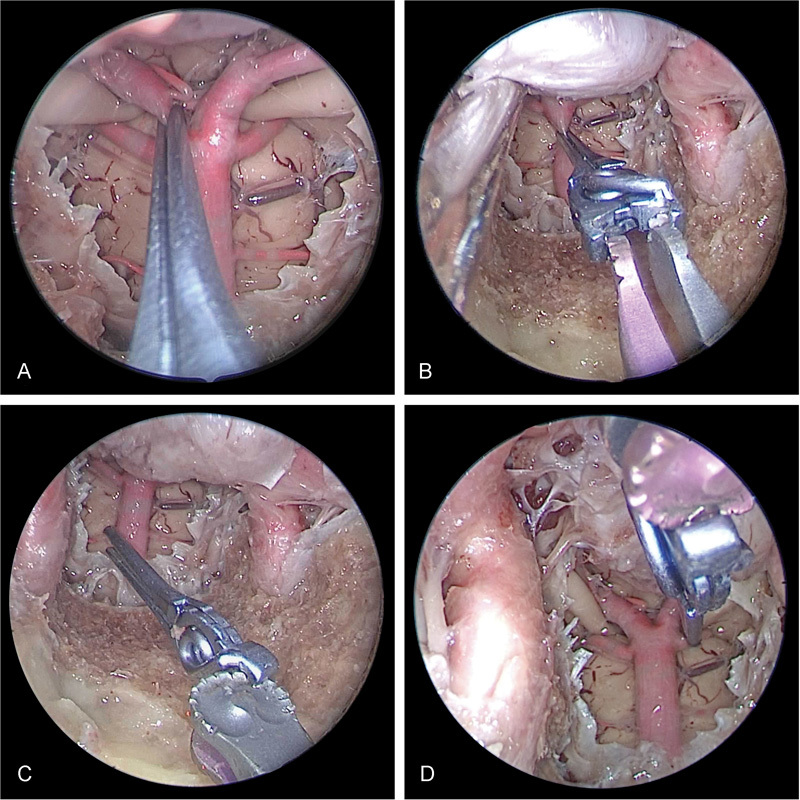
Fig. 4.
Right transorbital view of combined endoscopic multiportal transorbital and endonasal view and approach for posterior circulation clipping with a midlevel basilar apex. Sufficient clipping of ( A ) posterior cerebral artery, ( B ) Basilar, and ( C ) superior cerebellar artery is obtained with good visualization.
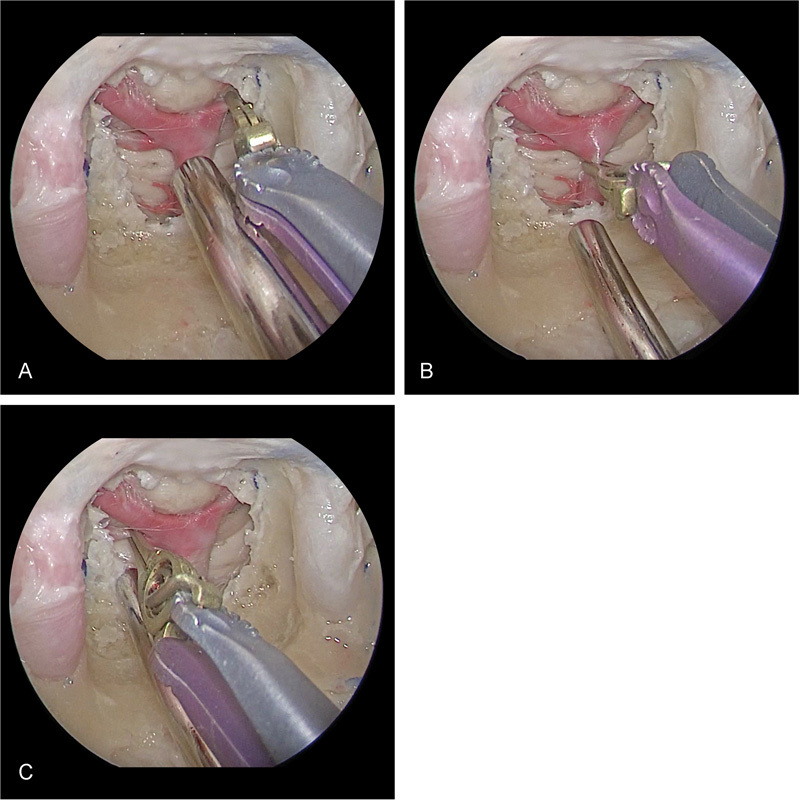
Fig. 5.
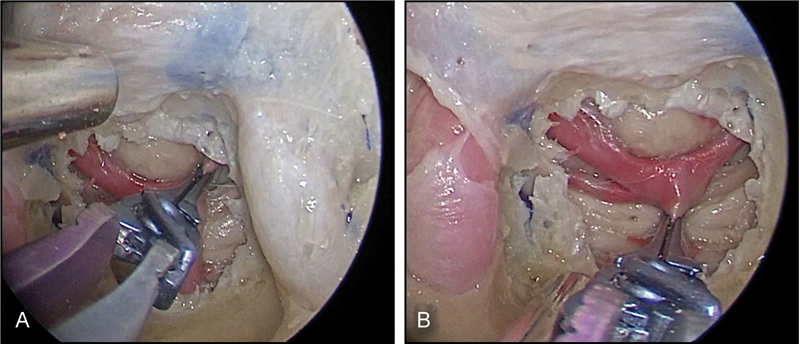
Endoscopic endonasal transclival approach alone is limited by instrument “sword fighting” for posterior circulation clipping with a midlevel basilar apex.
Fig. 6.
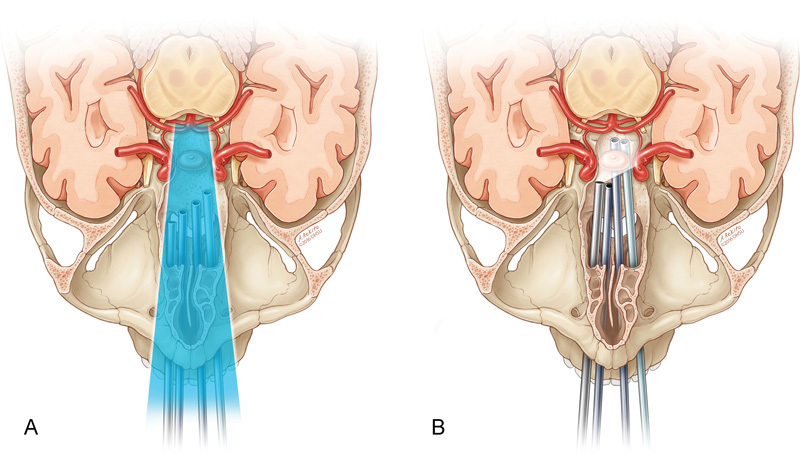
Schematics showing that the endoscopic endonasal transclival approach alone is limited by ( A ) coning down effect and ( B ) instrument sword fighting.
Fig. 7.
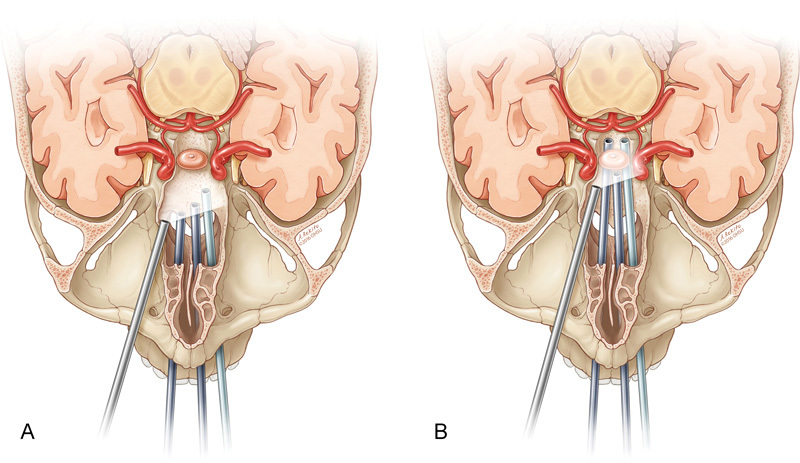
Schematics showing that the right transorbital view of the combined endoscopic multiportal transorbital and endonasal approaches increases ( A ) visualization and ( B ) working area for instrumentation.
Video 1 Right transorbital view of the combined endoscopic multiportal transorbital and endonasal approaches allows trainees to practice clipping a bleeding superior cerebellar artery with good visualization of the anterior inferior cerebellar artery, posterior cerebral artery, brain stem perforating arteries, and cranial nerve III. Online content including video sequences viewable at: www.thiemeconnect.com/products/ejournals/html/10.1055/s-0036-1597278 .
The transcranial open skull base microsurgical approaches to clip posterior circulation aneurysms are typically determined by the relationship of the BA to the posterior clinoids. A high-riding BA apex aneurysm is above the level of the posterior clinoids. The most common surgical approach is a cranial orbitozygomatic or pterional craniotomy with a wide Sylvian fissure dissection. Whereas, BA aneurysms below the posterior clinoids are most commonly approached with open microsurgical skull base techniques through either a subtemporal, transpetrosal, or anterior petrous apicectomy/kawase approach. 24 At most centers, endovascular treatment of posterior circulation aneurysms has become the most common approach for treatment. 1 However, SCA and AICA aneurysms remain the most challenging to treat endovascularly as well as through open skull base microsurgical techniques. EEAs provide the most direct surgical route obviating the need for brain retraction to approach the BA, especially the middle to lower third of the vessels originating from the BA that can harbor aneurysms SCA and AICA. When there was a midposition basilar apex compared with the clivus, the combination of the TONES and ETA provided the improved visualization of the posterior circulation ( Fig. 4 ) when compared with the ETA alone. The TONES was used as a visualization port for positioning the endoscope. The ETA was used for placing the surgical instruments, performing the fourhanded microsurgical dissection, and clip application. The dual approach and four-handed technique provided improved working area, degrees of freedom, and accurate clip placement ( Video 1 ). When there was a low-riding basilar apex relative to the clivus, the TONES alone provided the best visualization and working angle to reach all of the arteries of the posterior circulation from the basilar apex, PCA, SCA, to AICA.
Visualization of the basilar apex, PCA, SCA, and AICA was accomplished with both ETA and TONES approaches. Visualization through both the approaches required more mobilization of the pituitary gland with a high-riding basilar apex relative to the posterior clinoids and less so for the SCA and AICA. The ETA had the advantage in visualization and clip application of the basilar apex region versus the addition of the TONES when the BA was high riding. This was less so with the approach to the SCA region. There was no significant difference in terms of visualization of the AICA with either approach. TONES combined with ETA did yield a significantly improved difference in visualization, improved functional degrees of freedom, and accurate clip application of the BA, SCA, and AICA regions compared with the ETA alone in middle- and low-positioned BA relative to the clivus.
The TONES provided an oblique visual angle of the clip tines with the 0-degree endoscope. This optimized visualization, improved optimal clip application, and decreased the incidence of pass pointing the clip tines beyond the vessel being clipped. In addition, the addition of the TONES minimized the risk of incorporating the brain stem tissue in the clip tines. Whereas, the ETA approach alone is a more inline view of the clip tines, decreasing the perspective of the clip tines to the vessel being clipped and the brain stem. Pass pointing was more common using this approach. The use of angled endoscopes (30 and 45 degrees) improved the perspective of the clip tines, yet the working area was significantly more limited. Angled endoscopes were not required for optimal visualization of the clip tines with the ETA and TONES. The addition of TONES to the ETA improved the degrees of working freedom, given the dynamic nature of the procedure in all specimens as well as the visualization of the SCA and AICA in all specimens.
Clip Placement
A Lazic clip with vessel clip system was used for this study (Peter Lazic Microsurgical Innovations, Tuttlingen, Germany). The clip was applied to the major arteries of the posterior circulation. The clip applicator offers flexibility for clip removal and placement ( Video 1 ). The different angles of visualization through the ETA and TONES can be appreciated in regard to clip placement ( Fig. 4 ). The ease of use can be appreciated ( Fig. 7 ).
Discussion
Cadaveric workshops for postgraduate training have been performed for the past decade but underused despite evidence that these workshops improve operative performance 25 Aboud et al have developed successful cadaveric simulations to teach microsurgical approaches. 26 However, one of the most challenging teaching principles is the management of vascular pathologies. 27 Microfil-injected arteries provide good visualization, allowing the neurosurgeon and otolaryngologist to better appreciate the intricate intraoperative anatomy. Virtual reality training and simulation models can be useful for initial training and decreasing the learning curve 28 29 ; however; developing expertise with endoscopic procedures require hands-on experience, and appreciation for anatomy is most realistic with cadavers. 28 30 Nowhere is this more tactically evident than for visualizing vascular anatomy in preparation for endoscopic clipping of aneurysms. Di Somma et al have shown that cadaveric models are ideal for learning the complex anatomy adjacent to the posterior circulation arteries. 15 The use of instrumentation in a confined space with a limited field of view requires both planning and practice. 31 In the operative environment, both neurosurgeons and otolaryngologists must work together to approach the problem.
In this feasibility study, we successfully demonstrate how to place an aneurysm clip through an ETA, and ETA and TONES, depending on the location of the basilar apex. Treating posterior circulation aneurysms, especially aneurysms of the SCA and AICA, is a technical challenge due to the close proximity to the cranial nerves as well as the brain stem. 9 Using cadaveric models for surgical practice allows for visualization of the relevant anatomy prior to performing the procedure in the operating arena. It also helps visualize the necessity of different approaches with subtle differences in the basilar apex location. We demonstrate the feasibility of clip placement at relevant vascular sites on multiple cadaveric heads. The oculomotor nerve was appreciated as it emerged between the SCA and PCA, highlighting the importance of careful instrumentation placement to avoid nerve damage. Recent advancement in modeling simulation allows for the incorporation of vascular complications into the training scenario as well. 32 Future studies involving a simulated aneurysm with clip placement are warranted.
Balloon angiography with a controlled perfusion pump may provide a viable technique for creating simulated aneurysms in a cadaveric model. Ideally, the models would be incorporated into a two-stage training workshop. For instance, in the first part of a workshop, participants would familiarize themselves with the endoscope and instrumentation through different surgical approaches. Use of instrumentation in a confined space could be practiced in a controlled environment. Microfil-injected cadaver heads would be used to appreciate vascular intraoperative anatomy. In the second part, the participant would manage clipping a simulated aneurysm or handling a vascular complication in a timed scenario. The purpose would be to develop the skill sets necessary to perform with excellence using endoscopic procedures in a real operative environment.
A current challenge with clipping aneurysms is acute morbidity and mortality due to surgical complexity and invasiveness ( Table 1 ). 9 12 14 15 33 34 35 36 37 Endoscopic approaches provide a novel way to reach and clip posterior circulation aneurysms. 17 The ETA was superior to the TONES as demonstrated in this study when there is a high-riding basilar apex above the posterior clinoid. Obstruction of visibility by the pituitary gland prevented feasibility of TONES and would require pituitary mobilization, resection of the posterior clinoids, and removal of a portion of the clivus. The ETA and TONES can be used to successfully clip AICA aneurysms by lateralizing the pituitary gland, but clipping posterior circulation aneurysms with pituitary mobilization is limited. 38 When the basilar apex is within the middle to lower third of the clivus, the TONES provides excellent visualization of the posterior circulation arteries. The advances in clip applicator design have also made intraoperative clip readjustment plausible. The use of the endoscope and improved clip applicators may be a suitable approach for large aneurysms. 39 Endonasal endoscopic procedures may be associated with fewer complications than traditional approaches. 40 With the advent of the malleable endoscope, visualization will continue to improve, further reducing complications. 41 It will be possible to reach the three portions of the AICA feasibly, and enhanced visualization will decrease potential damage to the BA. 42 Endoscopic approaches for clipping aneurysms are a recent development, and prospective studies are needed to determine long-term effectiveness.
Table 1. Literature summary: endoscopic posterior circulation approaches.
| Study | Approach | Clipping | Limitations |
|---|---|---|---|
| Di Somma et al (2014) | Extended endoscopic endonasal | Feasibility study for anterior and posterior circulation aneurysms | Difficult to maintain posterior vascular control, major arterial bleeding, and cerebrospinal fluid leak |
| Enseñat et al (2011) | Endoscopic endonasal | Vertebral-posterior inferior cerebellar artery aneurysm | Deep location and intimate relation with the medulla and cranial nerves |
| Galzio et al (2013) | Endoscope-assisted microneurosurgery | Anterior and posterior circulation aneurysms | Wider working area required for posterior circulation and large anterior circulation aneurysms |
| Lai et al (2013) | Endonasal transclival approach | Proximal and distal basilar artery aneurysms | Steep learning curve for surgical skills |
| Perneczky and Boecher-Schwarz (1998) | Endoscope-assisted microsurgery | Giant aneurysms of posterior circulation | Potential for premature rupture with accompanying hemiparesis and neurologic impairment |
| Rodríguez-Hernández et al (2013) | Endoscopic transsphenoidal approach | Superior cerebellar artery-posterior cerebral artery bypass | Pseudoaneurysm formation and ischemia |
| Somanna et al (2015) | Endoscopic endonasal transclival approach | Basilar apex, basilar trunk, and poster cerebral artery aneurysms | Steep learning curve with high morbidity |
| Szentirmai et al (2016) | Endoscopic endonasal transclival approach | Basilar trunk and bifurcation | Difficult to visualize perforating arteries, making it hard to handle large hemorrhages |
| Zhao et al (2006) | Neuroendoscope-assisted microsurgery | Anterior and posterior circulation aneurysms | Complications: hemiplegia, pseudomembranous enteritis, and optic blur |
BA and PCA aneurysms are more commonly being treated through endovascular techniques with lower morbidity and mortality than microsurgical techniques at most centers. 1 However, the challenges remain regarding SCA and AICA aneurysms. Typically, these aneurysms are too small, or at a branch, point at the BA, SCA, or AICA origin that would be too great a risk of parent vessel occlusion, stenosis, thrombosis, or source of embolism with endovascular techniques. The other limiting factor to endovascular treatment of a ruptured SCA or AICA aneurysm is the increase risk of complications related to stenting and need for therapeutic antiplatelet therapy in the setting of an acute subarachnoid hemorrhage. Challenges still remain regarding the treatment of SCA and AICA aneurysms from both the endovascular and microsurgical open skull base approaches. EEAs also have their challenges. In an effort to improve EEAs to posterior circulation aneurysms (in particular the SCA and AICA aneurysms) or highly vascular neoplasms, the ETA combined with the TONES was used and demonstrated significant improvement in the visualization, ergonomics, and working degrees of freedom.
This feasibility study shows an adequate operative space for clip placement and application. The use of cadaveric models provides a unique opportunity to develop and perfect skill sets associated with new approaches prior to use in an operative arena. When used in conjunction with virtual reality and simulation models, the neurosurgeon in training can effectively tackle the steep learning curve.
Conclusion
We show a feasible, replicable simulation and anatomical study that can be used to demonstrate the combined multiportal endoscopic endonasal and transorbital approaches, ETA combined with the TONES, and provide enhanced visualization and degrees of working freedom when addressing pathology involving the posterior circulation. This dual approach is perhaps most applicable to SCA and AICA aneurysms. Endoscopic approaches are emerging as a potential alternative to clipping noncoilable aneurysms. With advances in technology and simulated cadaveric training, EEAs may become a more widely used strategy for aneurysm treatment of the posterior circulation. The addition of the TONES may provide improved safety and outcomes in these patients. There is potential of using the endoscope and instruments though the endonasal and TONES approach interchangeably as needed. Further studies are required as the field progresses to determine long-term outcomes with these approaches.
Acknowledgments
The authors would like to thank Oregon Health & Science University for use of facilities. Brandon Lucke-Wold received a predoctoral grant from the American Foundation of Pharmaceutical Education, an American Medical Association Seed Grant, and a Neurosurgery Research and Education Foundation Medical Student Summer Research Fellowship.
Disclosures The authors have no conflicts of interests to disclose.
Denotes equal contribution for first author.
References
- 1.Spetzler R F, McDougall C G, Zabramski J M et al. The Barrow Ruptured Aneurysm Trial: 6-year results. J Neurosurg. 2015;123(03):609–617. doi: 10.3171/2014.9.JNS141749. [DOI] [PubMed] [Google Scholar]
- 2.Lee C H, Chen S M, Lui T N. Posterior cerebral artery pseudoaneurysm, a rare complication of pituitary tumor transsphenoidal surgery: case report and literature review. World Neurosurg. 2015;84(05):14930–1.493E6. doi: 10.1016/j.wneu.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 3.Velioglu M, Selcuk H, Kizilkilic O, Basekim C, Kocer N, Islak C. Endovascular management of superior cerebellar artery aneurysms: mid and long-term results. Turk Neurosurg. 2015;25(04):526–531. doi: 10.5137/1019-5149.JTN.8611-13.0. [DOI] [PubMed] [Google Scholar]
- 4.Nair P, Panikar D, Nair A P, Sundar S, Ayiramuthu P, Thomas A. Microsurgical management of aneurysms of the superior cerebellar artery - lessons learnt: an experience of 14 consecutive cases and review of the literature. Asian J Neurosurg. 2015;10(01):47. doi: 10.4103/1793-5482.151513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petr O, Brinjikji W, Thomé C, Lanzino G. Safety and efficacy of microsurgical treatment of previously coiled aneurysms: a systematic review and meta-analysis. Acta Neurochir (Wien) 2015;157(10):1623–1632. doi: 10.1007/s00701-015-2500-y. [DOI] [PubMed] [Google Scholar]
- 6.Hwang U S, Shin H S, Lee S H, Koh J S. Decompressive surgery in patients with poor-grade aneurysmal subarachnoid hemorrhage: clipping with simultaneous decompression versus coil embolization followed by decompression. J Cerebrovasc Endovasc Neurosurg. 2014;16(03):254–261. doi: 10.7461/jcen.2014.16.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Germanwala A V, Zanation A M.Endoscopic endonasal approach for clipping of ruptured and unruptured paraclinoid cerebral aneurysms: case report Neurosurgery 201168(1, Suppl Operative):234–239., discussion 240 [DOI] [PubMed] [Google Scholar]
- 8.Kassam A B, Gardner P A, Snyderman C H, Carrau R L, Mintz A H, Prevedello D M. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg. 2008;108(04):715–728. doi: 10.3171/JNS/2008/108/4/0715. [DOI] [PubMed] [Google Scholar]
- 9.Enseñat J, Alobid I, de Notaris Met al. Endoscopic endonasal clipping of a ruptured vertebral-posterior inferior cerebellar artery aneurysm: technical case report Neurosurgery 201169(1, Suppl Operative):E121–E127., discussion E127–E128 [DOI] [PubMed] [Google Scholar]
- 10.Moe K S, Bergeron C M, Ellenbogen R G.Transorbital neuroendoscopic surgery Neurosurgery 201067(3, Suppl Operative):ons16–ons28. [DOI] [PubMed] [Google Scholar]
- 11.Ramakrishna R, Kim L J, Bly R A, Moe K, Ferreira M., Jr Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J Clin Neurosci. 2016;24:99–104. doi: 10.1016/j.jocn.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai L T, Morgan M K, Chin D C et al. A cadaveric study of the endoscopic endonasal transclival approach to the basilar artery. J Clin Neurosci. 2013;20(04):587–592. doi: 10.1016/j.jocn.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Narayanan V, Narayanan P, Rajagopalan R et al. Endoscopic skull base training using 3D printed models with pre-existing pathology. Eur Arch Otorhinolaryngol. 2015;272(03):753–757. doi: 10.1007/s00405-014-3300-3. [DOI] [PubMed] [Google Scholar]
- 14.Somanna S, Babu R A, Srinivas D, Narasinga Rao K V, Vazhayil V. Extended endoscopic endonasal transclival clipping of posterior circulation aneurysms--an alternative to the transcranial approach. Acta Neurochir (Wien) 2015;157(12):2077–2085. doi: 10.1007/s00701-015-2610-6. [DOI] [PubMed] [Google Scholar]
- 15.Di Somma A, de Notaris M, Stagno V et al. Extended endoscopic endonasal approaches for cerebral aneurysms: anatomical, virtual reality and morphometric study. BioMed Res Int. 2014;2014:703792. doi: 10.1155/2014/703792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drazin D, Zhuang L, Schievink W I, Mamelak A N. Expanded endonasal approach for the clipping of a ruptured basilar aneurysm and feeding artery to a cerebellar arteriovenous malformation. J Clin Neurosci. 2012;19(01):144–148. doi: 10.1016/j.jocn.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Gardner P A, Vaz-Guimaraes F, Jankowitz B et al. Endoscopic endonasal clipping of intracranial aneurysms: surgical technique and results. World Neurosurg. 2015;84(05):1380–1393. doi: 10.1016/j.wneu.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Bambakidis N C, Manjila S, Dashti S, Tarr R, Megerian C A. Management of anterior inferior cerebellar artery aneurysms: an illustrative case and review of literature. Neurosurg Focus. 2009;26(05):E6. doi: 10.3171/2009.1.FOCUS0915. [DOI] [PubMed] [Google Scholar]
- 19.Starke R M, Jane J A, Jr, Asthagiri A R, Jane J A., Sr International rotations and resident education. J Neurosurg. 2015;122(02):237–239. doi: 10.3171/2014.9.JNS142171. [DOI] [PubMed] [Google Scholar]
- 20.Mamelak A N, Carmichael J, Bonert V H, Cooper O, Melmed S. Single-surgeon fully endoscopic endonasal transsphenoidal surgery: outcomes in three-hundred consecutive cases. Pituitary. 2013;16(03):393–401. doi: 10.1007/s11102-012-0437-1. [DOI] [PubMed] [Google Scholar]
- 21.Gondim J, Schops M, Tella O I., JrTransnasal endoscopic surgery of the sellar region: study of the first 100 cases [in Portuguese] Arq Neuropsiquiatr 200361(3B):836–841. [DOI] [PubMed] [Google Scholar]
- 22.Ciporen J N, Moe K S, Ramanathan D et al. Multiportal endoscopic approaches to the central skull base: a cadaveric study. World Neurosurg. 2010;73(06):705–712. doi: 10.1016/j.wneu.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Miranda J C, Gardner P A, Rastelli M M, Jr et al. Endoscopic endonasal transcavernous posterior clinoidectomy with interdural pituitary transposition. J Neurosurg. 2014;121(01):91–99. doi: 10.3171/2014.3.JNS131865. [DOI] [PubMed] [Google Scholar]
- 24.Sekhar L, Mantovani A, Mortazavi M, Schwartz T H, Couldwell W T. Open vs endoscopic: when to use which. Neurosurgery. 2014;61 01:84–92. doi: 10.1227/NEU.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 25.Gilbody J, Prasthofer A W, Ho K, Costa M L. The use and effectiveness of cadaveric workshops in higher surgical training: a systematic review. Ann R Coll Surg Engl. 2011;93(05):347–352. doi: 10.1308/147870811X582954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aboud E, Al-Mefty O, Yaşargil M G. New laboratory model for neurosurgical training that simulates live surgery. J Neurosurg. 2002;97(06):1367–1372. doi: 10.3171/jns.2002.97.6.1367. [DOI] [PubMed] [Google Scholar]
- 27.Olabe J, Olabe J, Sancho V. Human cadaver brain infusion model for neurosurgical training. Surg Neurol. 2009;72(06):700–702. doi: 10.1016/j.surneu.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Winkler-Schwartz A, Bajunaid K, Mullah M A et al. Bimanual psychomotor performance in neurosurgical resident applicants assessed using NeuroTouch, a virtual reality simulator. J Surg Educ. 2016:S1931-7204(16)30026-5. doi: 10.1016/j.jsurg.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Rooney D M, Tai B L, Sagher O, Shih A J, Wilkinson D A, Savastano L E. Simulator and 2 tools: validation of performance measures from a novel neurosurgery simulation model using the current Standards framework. Surgery. 2016;160(03):571–579. doi: 10.1016/j.surg.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Alaraj A, Lemole M G, Finkle J H et al. Virtual reality training in neurosurgery: review of current status and future applications. Surg Neurol Int. 2011;2:52. doi: 10.4103/2152-7806.80117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cappabianca P, Alfieri A, Thermes S, Buonamassa S, de Divitiis E.Instruments for endoscopic endonasal transsphenoidal surgery Neurosurgery 19994502392–395., discussion 395–396 [DOI] [PubMed] [Google Scholar]
- 32.Pham M, Kale A, Marquez Y et al. A perfusion-based human cadaveric model for management of carotid artery injury during endoscopic endonasal skull base surgery. J Neurol Surg B Skull Base. 2014;75(05):309–313. doi: 10.1055/s-0034-1372470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galzio R J, Di Cola F, Raysi Dehcordi S, Ricci A, De Paulis D. Endoscope-assisted microneurosurgery for intracranial aneurysms. Front Neurol. 2013;4:201. doi: 10.3389/fneur.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perneczky A, Boecher-Schwarz H G.Endoscope-assisted microsurgery for cerebral aneurysms Neurol Med Chir (Tokyo) 199838(Suppl):33–34. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Hernández A, Huang C, Lawton M T. Superior cerebellar artery-posterior cerebral artery bypass: in situ bypass for posterior cerebral artery revascularization. J Neurosurg. 2013;118(05):1053–1057. doi: 10.3171/2013.2.JNS122250. [DOI] [PubMed] [Google Scholar]
- 36.Szentirmai O, Hong Y, Mascarenhas L et al. Endoscopic endonasal clip ligation of cerebral aneurysms: an anatomical feasibility study and future directions. J Neurosurg. 2016;124(02):463–468. doi: 10.3171/2015.1.JNS142650. [DOI] [PubMed] [Google Scholar]
- 37.Zhao J, Wang Y, Zhao Y, Wang S. Neuroendoscope-assisted minimally invasive microsurgery for clipping intracranial aneurysms. Minim Invasive Neurosurg. 2006;49(06):335–341. doi: 10.1055/s-2006-958729. [DOI] [PubMed] [Google Scholar]
- 38.Yildirim A E, Divanlioglu D, Karaoglu D, Cetinalp N E, Belen A D. Pure endoscopic endonasal clipping of an incidental anterior communicating artery aneurysm. J Craniofac Surg. 2015;26(04):1378–1381. doi: 10.1097/SCS.0000000000001760. [DOI] [PubMed] [Google Scholar]
- 39.Yoshioka H, Kinouchi H. The roles of endoscope in aneurysmal surgery. Neurol Med Chir (Tokyo) 2015;55(06):469–478. doi: 10.2176/nmc.ra.2014-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lobo B, Heng A, Barkhoudarian G, Griffiths C F, Kelly D F. The expanding role of the endonasal endoscopic approach in pituitary and skull base surgery: a 2014 perspective. Surg Neurol Int. 2015;6:82. doi: 10.4103/2152-7806.157442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elhadi A M, Zaidi H A, Hardesty D Aet al. Malleable endoscope increases surgical freedom compared with a rigid endoscope in endoscopic endonasal approaches to the parasellar region Neurosurgery 20141003393–399., discussion 399 [DOI] [PubMed] [Google Scholar]
- 42.Sugrue P A, Hage Z A, Surdell D L, Foroohar M, Liu J, Bendok B R. Basilar artery occlusion following C1 lateral mass fracture managed by mechanical and pharmacological thrombolysis. Neurocrit Care. 2009;11(02):255–260. doi: 10.1007/s12028-008-9159-7. [DOI] [PubMed] [Google Scholar]


