Abstract
Aims
To test whether binge drinking, the density of familial alcoholism (FHD), and their interaction are associated with an altered developmental trajectory of impulsive choice across adolescence, and whether more lifetime drinks is associated with a greater change in impulsive choice across age.
Design
Alcohol-naïve adolescents, with varying degrees of FHD, were recruited as part of an ongoing longitudinal study on adolescent development, and were grouped based on whether they remained non-drinkers (n = 83) or initiated binge drinking (n = 33) during follow-up. During all visits, adolescents completed a monetary delay discounting task to measure impulsive choice. The effects of binge-drinking status, FHD, and their interaction on impulsive choice across adolescence were tested.
Setting
Developmental Brain Imaging Lab, Oregon Health & Science University, Portland, Oregon, USA.
Participants
116 healthy male and female adolescents (ages 10–19) completed 2–4 visits between July 2008 and May 2016.
Measurements
Discounting rates were obtained based on adolescents’ preference for immediate or delayed rewards. FHD was based on parent-reported prevalence of alcohol use disorder in the participant’s first and second degree relatives. Binge-drinking status was determined based on the number of recent binge-drinking episodes.
Findings
There was a significant interaction effect of binge-drinking status and FHD on impulsive choice across age (b = 1.090, p < 0.05, β = 0.298). In adolescents who remained alcohol-naïve, greater FHD was associated with a steeper decrease in discounting rates across adolescence (b = −0.633, p < 0.05, β = −0.173); however, this effect was not present in binge-drinkers. Furthermore, total lifetime drinks predicted escalated impulsive choice (b = 0.002, p < 0.05, β = 0.295) in binge-drinking adolescents.
Conclusions
A greater degree of familial alcoholism is associated with a steeper decline in impulsive choice across adolescence, but only in those who remain alcohol-naïve. Meanwhile, more lifetime drinks during adolescence is associated with increases in impulsive choice across age.
Keywords: alcohol, impulsivity, decision making, adolescent, genetic, longitudinal
Introduction
Using delay discounting paradigms, alcohol-dependent individuals discount (or devalue) delayed rewards to a greater degree than non-dependent individuals (1–3). That is, when forced to choose, alcohol-dependent individuals are more likely to select smaller immediate rewards over larger delayed rewards, thus making what is often considered an impulsive choice. However, the temporal nature of this relationship between alcohol use and impulsive choice (i.e. discounting rates) remains unclear. While some speculate that greater impulsive choice leads to the initiation of alcohol use, others argue that alcohol use itself alters underlying neural mechanisms responsible for increases in impulsive choice. It is also possible that these two behaviors are both products of some underlying risk phenotype, and share a common genetic component (4). Adolescence is a critical period during which many first initiate alcohol use, and a time during which impulsive choice develops, as evidenced by both human and rodent studies. For example, both cross-sectional and longitudinal work in human adolescents has shown that impulsive choice decreases across adolescence and into young adulthood (5–8). Meanwhile, cross-sectional pre-clinical models have found that adolescent rodents exhibit more impulsive responding for food rewards than adults (9–11). Thus, adolescence is an important period for investigating the development of impulsive choice.
Previous cross-sectional work in humans and rodents has established that both alcohol use and a familial history of addiction (FH+) are associated with altered impulsive choice. Compared to light drinkers, heavy-drinking human adolescents show greater impulsive choice for monetary and alcohol rewards (12, 13). Meanwhile, alcohol exposure has a greater impact on impulsive choice in adolescent rodents than adults (14). Further, studies in drug- and alcohol-naïve human adolescents and young adults found that those with FH+ (drugs and alcohol) made more impulsive choices (15, 16) and had significantly slower reaction times (alcohol only), than adolescents without a FH+ (17). Similarly, alcohol-naïve rodents bred to consume high levels of alcohol demonstrate more impulsive responding for sucrose rewards than those bred for low levels of alcohol consumption (18–20). In combination, these findings suggest that both alcohol use and a FH+ may predispose adolescents to be more impulsive.
Despite evidence supporting the influence of both alcohol use and FH+ on impulsive choice, few studies have investigated their effects concurrently. In a cross-sectional study, impulsive choice correlated with age in light-drinking adults, but not heavy drinkers, and in light drinkers, those with a FH+ (alcohol only) showed greater impulsive choice (21). Further, another study in adults found that higher rates of impulsive choice partially mediated the relationship between greater parental substance use and greater alcohol consumption (22). However, these studies were both in adult populations, and thus were unable assess the combined associations of alcohol use and FH+ with the development trajectory of impulsive choice. Understanding the association of this combined effect with development is crucial, as binge drinking and FH+ (alcohol only) have been shown to interact and are associated with impaired neuropsychological functioning during adolescence (23).
The current study focused on the developmental trajectory of impulsive choice across adolescence. The longitudinal design allowed us to test whether binge drinking and degree of familial alcoholism are associated with an altered trajectory of impulsive choice during this critical period. Further, this study aimed to test whether an increase in lifetime drinks is associated with increases in impulsive choice across age, in binge-drinking adolescents. While many studies utilize family history status (based on alcoholism in at least one parent), for this study, a continuous family history density (FHD) score was calculated based on the number and degree of relatives with an alcohol use disorder, to improve effect sizes, power, and measurement reliability (24). Based on previous literature, we hypothesized that alcohol-naïve adolescents would show a decrease in impulsive choice across age, and that this relationship would be diminished in adolescents who ultimately engaged in binge drinking. Furthermore, we hypothesized that greater FHD would be associated with greater impulsive choice prior to alcohol consumption, and that FHD would interact with binge drinking status across age. For this interaction effect, we predicted non-drinking adolescents with low FHD would show the greatest age-dependent decrease in impulsive choice compared to binge drinkers and those with higher FHD, similar to the behavioral pattern found in previous neuropsychological work (23). Additionally, we hypothesized that among binge-drinking adolescents there would be a positive association between lifetime drinks and impulsive choice across age.
Methods
Design
This study is part of an ongoing longitudinal investigation of the emergence and effects of alcohol use during development, and includes all participants that completed at least two (and up to four) in-person study visits between July 2008 and May 2016. Following the baseline visit, quarterly telephone interviews were conducted to assess alcohol/substance use, and participants were brought in for in-person re-assessment once they reported reaching criteria for binge drinking (see below). For every binge-drinking adolescent that was re-assessed, a time-since-baseline and developmentally (based on sex, age, and pubertal stage) matched non-drinking control was also brought in for in-person re-assessment. Additional non-drinking controls were also brought in for re-assessment, approximately 1 year after recruitment, as part of an ongoing investigation of adolescent development. The majority of controls reported no lifetime drinking during follow-up visits, with a small subsample (n = 9) reporting minimal drinking experience (< 15 lifetime drinks, with no binge episodes). This design resulted in a total of 272 visits among 33 binge-drinking adolescents and 83 largely drug- and alcohol-naïve controls (Fig. 1). This study was approved by the Oregon Health & Science University (OHSU) Institutional Review Board.
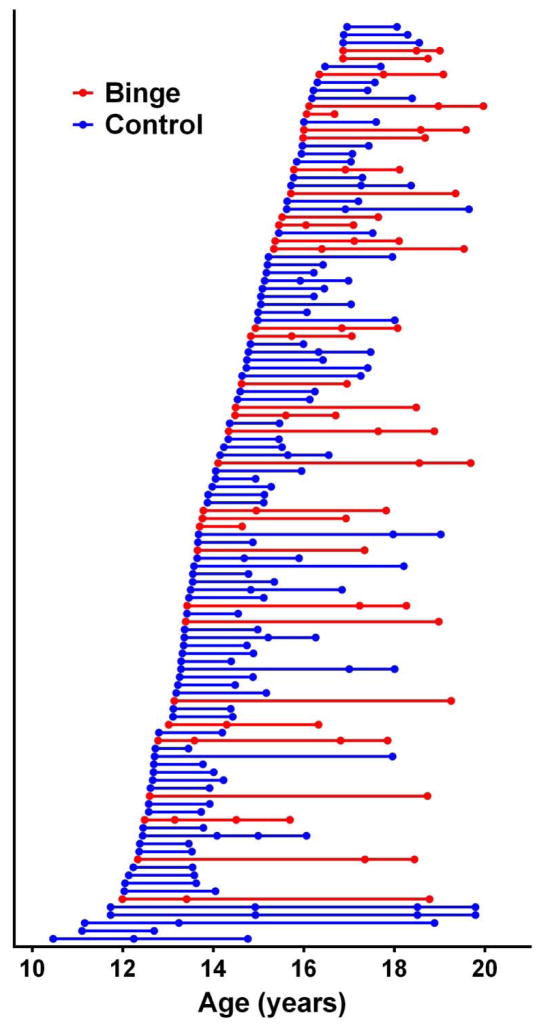
Figure 1.
Ages for all scans depicted within subject and separated by binge-drinking status. There were a total of 272 total visits, divided amongst 33 binge drinking adolescents (88 visits) and 83 controls (184 visits). There was a median of 1.35 years between visits (range = 0.51–6.14 years) and a median of 1.74 years between first and last visit (range = 0.62–8.06 years).
Participants
Healthy adolescent participants, ages 10 to 17 at baseline (n = 116), were recruited from the local community (Portland, OR and surrounding suburbs). Following a telephone prescreen to determine initial eligibility, adolescents and their parents provided written consent and assent, respectively, followed by separate comprehensive screening interviews. As the goals of the ongoing longitudinal study are to investigate the emergence of mental illness and psychopathology during development, baseline exclusionary criteria included a likely diagnosis of a DSM-IV psychiatric disorder [Diagnostic Interview Schedule for Children Predictive Scales (25)], inability to obtain family history information, serious medical problems (including head trauma), mental retardation or learning disability, psychotic illness in a biological parent, and known prenatal drug/alcohol exposure. Additionally, at the time of recruitment, adolescents were excluded for prior drug or alcohol use that exceeded >10 lifetime alcohol drinks, >2 drinks on any one occasion, >5 uses of marijuana, >4 cigarettes per day, or any other drug use [Brief Lifetime version of the Customary Drinking and Drug Use Record (CDDR) (26)].
Measures
Socioeconomic status
To assess socioeconomic status (SES) at baseline, parents completed the Hollingshead Index of Social Position, a measure based on the educational attainment and occupation of each parent (27).
IQ
To estimate overall intellectual functioning at baseline, adolescents were administered the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (28).
Pubertal development
Self-assessment of puberty was obtained at all visits using a modified line drawing version of the Tanner’s Sexual Maturation Scale (29), with drawings ranging from stage 1 (pre-adolescent) through stage 5 (adult-like maturation).
Family history density
To evaluate family history of alcohol use disorders (AUD), a family history density (FHD) score was calculated at baseline using the Family History Assessment Module (30). FHD was based on the number of adolescents’ relative(s) with an AUD; parents contributed 0.5, grandparents 0.25, and aunts and uncles a weighted ratio of 0.25 divided by the number of their siblings, with higher scores indicating greater prevalence of familial AUDs.
Binge-drinking status
For this study, binge-drinking criterion was defined as ≥3 occasions of binge drinking (≥5 drinks for males or ≥4 drinks for females, in one occasion) within the last 90 days, and was assessed using the CDDR (26) and 90-day Timeline Followback (31). This criterion is in accordance with NIAAA guidelines of binge drinking (32) and has been utilized previously by our lab (33, 34).
Delay discounting rates
A computerized, and self-paced, version of the delay discounting paradigm, described previously (17, 35), was administered to adolescents during all in-person visits. Briefly, the task presented adolescents with the choice between a variable monetary reward ($0 to $10.50) available immediately, or a set monetary reward ($10) available after a delay (0, 7, 30, 90, 180, or 365 days). Choice pairs, consisting of one immediate variable reward and one delayed set reward, were presented in random order to make up a total of 138 questions. Participants were asked to choose the option they preferred from each choice pair. To enhance the saliency of the task, participants were informed that one of their choices would be randomly selected, following the task, and money would be awarded based on their choice during the task.
Indifference points, the point at which a person switched from choosing the immediate reward to choosing the delayed reward, were calculated for each delay length. Using these indifference points, the rate of discounting (k) was calculated by fitting a hyperbolic discounting function: V = A/(1 +kD). In this equation, V represents the value of the $10 reward (the indifference point) at a given delay length (D), and A represents the amount of the set delayed reward ($10). Using this equation, greater k values represent lower indifference points, or a greater preference for more immediate rewards.
Statistical analysis
All statistical analyses were carried out using R (v 3.2.3). Baseline demographic variables were examined for outliers (> 2.5 SD from the mean) and normal distribution and were compared between binge-drinking adolescents and controls, using independent-samples t-tests, or Mann-Whitney and chi-square tests where appropriate. Prior to multilevel modeling, k values were log transformed due to non-normal distribution; this also reframed our outcome measure, such that the estimated impacts of the predictor variables represent percent change in impulsive choice. Age was re-centered at the average baseline age (14.2 years) to aid in interpretation of the results.
To address the first aim of this study, a series of multilevel models were used to test the effects of binge-drinking status and FHD on the association between age and discounting rate using full maximum likelihood. This approach is similar to a mixed repeated-measures ANOVA design, modeling within- and between-subjects factors simultaneously, and helps account for individual level growth or change by accounting for the nested nature of longitudinal data. First, we tested an unconditional means model (Model A), analogous to a traditional one-way ANOVA, to determine how much of the observed variation in the outcome could be attributed to between-subjects differencesa. Next, a linear slope was added to create an unconditional growth model (Model B), which accounted for both within individual changes in discounting rates over time, as well as between individual differences in change over time, and estimated a unique baseline and slope over time, for each participant. This model is a necessary step to determine whether adolescents’ discounting rates vary across age, and additionally, provides an estimate of between individual variability, which represents a second level of differences from those estimated over time within individuals. The variance estimates from this model, both among individual starting points, or intercepts, and among individual trajectories of change, or slopes, served as a baseline model for testing the effects of level-2 predictors. Subsequent models included the addition of level-2 predictors to account for the estimated differences from Model B; these included binge-drinking status (Model C), and FHD (Model D), separately, and in combination (Model E).
To further examine dose-related associations between alcohol use and impulsive choice, and to address the second aim of this study, a separate series of linear models were fit in the binge-drinking adolescents only. These models allowed for a more thorough examination of the influence of other important predictors that were unique to this group of adolescence (i.e. lifetime drinks), and followed a similar modeling progression as the earlier analysis. After fitting the unconditional means (Model F) and unconditional growth (Model G) models, individuals’ number of lifetime drinks was added to the model as a time-varying covariate (Model H) to estimate the dose-related relationship between drinking and impulsive choice.
A chi-square test comparing deviance statistics was used to assess the goodness-of-fit of each model when appropriate. Furthermore, effect sizes for all predictors in the final model of each aim (Model E and H) were reported as either Cohen’s d (for categorical predictors) or standardized regression estimates (for continuous variables). To obtain standardized regression estimates, all continuous variables were first z-transformed, and then the final model was rerun using these z-transformed variables.
Results
Participant baseline demographics are presented in Table 1.
Table 1.
Baseline Demographics
| Bingers (n = 33) M (SD) |
Controls (n = 83) M (SD) |
Significance test | |
|---|---|---|---|
| Sex (male/female) | 19/14 | 43/40 | X2 = 0.32 |
| Age | 14.52 (1.39) | 13.97 (1.47) | t114 = 1.85 |
| Pubertal stage | 4.00 (1.04)a | 3.75 (1.07)b | U103 = 952.0, Z = −1.13 |
| IQ | 112.82 (9.01) | 110.70 (10.38)c | t113 = 1.03 |
| SES | 27.12 (12.62) | 31.63 (13.81) | t114 = 1.62 |
| FHD | 0.39 (0.32) | 0.39 (0.33) | U103 = 1354.5, Z = −0.09 |
n = 29 due to missing data;
n = 76 due to missing data;
n = 82 due to outliers. There were no statistically significant differences between groups on any variables
To ensure valid and consistent discounting behavior, indifference points were examined to determine nonsystematic discounting behavior outliers, as previously described (36). Thus, 11 data points were excluded for nonsystematic discounting behavior, and 19 were excluded due to missing data. The remaining 242 data points (across 33 binge-drinking adolescents and 81 non-drinking controls) were included in multilevel modeling.
Results from the multilevel models investigating the effects of binge-drinking status and FHD on the development of discounting rates, addressing the first aim of this study, are presented in Table 2. Model A demonstrated that roughly half the variation in discounting rates was between subjects (ρ = 0.532), supporting the need for the addition of both within- (age) and between-subjects (drinking status and FHD) predictors. Next, Model B demonstrated a significant decrease in discounting rates across age. The addition of age to the model decreased the amount of within subject variance by 22%, and was an improved model compared to Model A [χ2(3) = 13.71, p < 0.05].
Table 2.
Multilevel model parameter estimates of discounting rates in all subjects
| Model A | Model B | Model C | Model D | Model E | Effect Size | ||
|---|---|---|---|---|---|---|---|
| Fixed Effects | |||||||
| Initial Status | Intercept | −4.981*** (0.198) | −4.628*** (0.218) | −4.573*** (0.248) | −5.199*** (0.339) | −5.210*** (0.383) | −0.100 |
| BINGE | −0.205 (0.475) | 0.069 (0.764) | 0.029 | ||||
| FHD | 1.401* (0.645) | 1.530* (0.725) | 0.090 | ||||
| BINGE* FHD | −0.699 (1.452) | 0.103 | |||||
| Rate of Change | Intercept | −0.242** (0.077) | −0.420*** (0.098) | −0.142 (0.120) | −0.177 (0.148) | −0.358 | |
| BINGE | 0.394** (0.147) | 0.020 (0.225) | 0.373 | ||||
| FHD | −0.245 (0.256) | −0.633* (0.309) | −0.173 | ||||
| BINGE* FHD | 1.090* (0.495) | 0.298 | |||||
| Variance Components | |||||||
| Level 1 | Within-person | 2.707 | 2.122 | 2.237 | 2.136 | 2.256 | |
| Level 2 | Initial status | 3.072 | 3.089 | 2.804 | 2.838 | 2.573 | |
| Rate of change | 0.142 | 0.078 | 0.137 | 0.047 | |||
| Covariance | −0.247 | −0.127 | −0.209 | −0.037 | |||
| Goodness-of-fit | |||||||
| Deviance | 1064.7 | 1051.0 | 1043.5 | 1046.4 | 1033.7 | ||
| AIC | 1070.7 | 1063.0 | 1059.5 | 1062.4 | 1057.7 | ||
| BIC | 1081.2 | 1084.0 | 1087.4 | 1090.3 | 1099.6 | ||
p < 0.05;
p < 0.01;
p < 0.001; standard errors are in parentheses
Model A is an unconditional means model; Model B is an unconditional growth model (including AGE); Model C includes the effects of binge drinking status (BINGE) on both initial status and rate of change (BINGE*AGE); Model D includes the effects of family history density of alcoholism (FHD) on both initial status and rate of change (FHD*AGE); Model E includes the effects of BINGE and FHD, as well as the effects of the interaction of the two predictors (BINGE*FHD) on both initial status and rate of change (BINGE*AGE, FHD*AGE, BINGE*FHD*AGE). Effect sizes for the final model (Model E) are presented as either standardized regression estimates, for effects including continuous predictors (FHD and BINGE*FHD), or as Cohen’s d for effects including only categorical variables (BINGE).
Next, Model C (including binge-drinking status) revealed a significant association between binge-drinking status and change in discounting rate across age. Discounting rates decreased significantly across age in control adolescents (b = −0.420, p < .05); however, this slope differed significantly in binge-drinking adolescents, with an estimated greater rate of change across age (b = 0.394, p < .05), compared to controls. The combined estimates resulted in a slight (but non-significant) decrease in discounting rates across age (b = −0.026) estimated for binge-drinking adolescents. Further, Model D (including FHD), revealed a significant association between FHD and baseline discounting rates (at age 14.2); greater FHD was associated with greater baseline discounting rates (b = 1.401, p < .05). Only Model C was a significantly improved model, compared to model B [χ2(2) = 7.56, p < 0.05]; however, due to the significance of FHD as a predictor of adolescents’ discounting rates, and the extent of literature suggesting an association between FH+ and discounting rates (16–18, 22), it was retained for Model E.
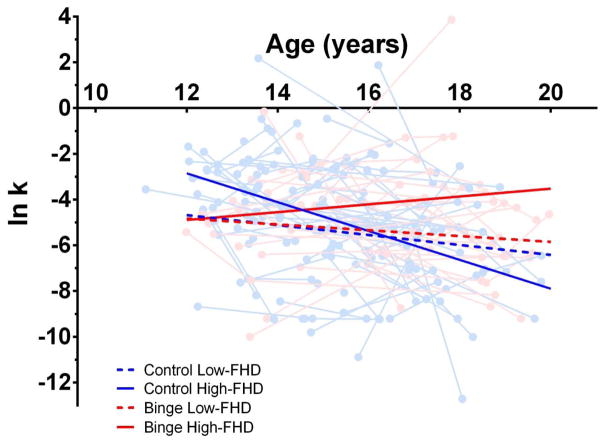
Model E, our final model (including binge-drinking status, FHD, and their interaction), revealed the interaction of FHD and binge-drinking status was significantly associated with discounting rates across age (b = 1.090, p < 0.05, β = 0.298). For control adolescents, increased FHD resulted in a significantly steeper decrease in discounting rates across age (b = −0.633, p < 0.05, β = −0.173). This relationship was different in binge-drinking adolescents, such that higher FHD was associated with a slight increase in the slope of discounting rates across age (b = 0.457). It is important to note that when binge-drinking status was reverse-coded, FHD had no effect on the rate of change of discounting rates in binge-drinking adolescents, suggesting that the binge drinking-by-FHD interaction was driven by an effect of FHD on discounting rates in control adolescents, but not binge-drinking adolescents. Additionally, greater FHD was also associated with higher discounting rates at baseline (b = 1.530, p < 0.05, β = 0.090), an effect that did not differ based on ultimate binge-drinking status. To aid in the interpretation of these findings, Figure 2 depicts prototypical trajectories for an individual falling 0.5 SDs above/below the mean FHD. Comparing models, we found that Model E, explained significantly more variation in discounting rates than Model B [χ2(6) = 17.34, p < 0.05], Model C [χ2(4) = 9.78, p < 0.05], and Model D [χ2(4) = 12.67, p < 0.05].
Figure 2.
Prototypical trajectories of discounting rates (ln k) across age are plotted for binge-drinking adolescents and controls with a high (+0.5 SDs from the mean) and low FHD (−0.5 SDs from the mean). Binge-drinking (light red) and control (light blue) adolescents’ individual discounting rates (ln k) across age are plotted in the background. For reference, the prototypical trajectory of high-FHD individuals is one with a FHD of 0.55, or roughly the equivalent of having at least one parent with an AUD, similar to the definition of a FH+ in prior research (15–17). Meanwhile, the prototypical trajectory of a low-FHD individual is one with a FHD of 0.25, or an individual with AUD only in a second-degree relative.
To address our second aim, and examine the dose-related association between binge drinking and discounting rates, we created a separate set of models in only the binge-drinking adolescents, depicted in Table 3. Results from Model F (unconditional means model) and Model G (unconditional growth model) are in line with the previous models, suggesting that binge-drinking adolescents show a non-significant change in discounting rates across age. Model H (including lifetime drinks) revealed that adolescents who had a greater number of drinks at baseline had lower baseline discounting rates (b = −0.012, p < 0.05, β = −0.469), and those who showed a greater escalation of drinking also had a significantly greater increase in discounting rates across age (b = 0.002, p < 0.05, β = 0.295). Also, the addition of lifetime drinks improved upon Model G, albeit at a trend level [χ2(2) = 5.37, p = 0.07].
Table 3.
Multilevel model parameter estimates of discounting rates in binge drinkers
| Model F | Model G | Model H | Effect Size | ||
|---|---|---|---|---|---|
| Fixed Effects | |||||
| Initial Status | Intercept | −4.866*** (0.301) | −4.635*** (0.374) | −4.663*** (0.380) | −0.125 |
| DRINKS | −0.012* (0.005) | −0.469 | |||
| Rate of Change | Intercept | −0.094 (0.156) | −0.046 (0.161) | 0.204 | |
| DRINKS | 0.002* (0.001) | 0.295 | |||
| Variance Components | |||||
| Level 1 | Within-person | 2.752 | 0.833 | 0.744 | |
| Level 2 | Initial status | 1.845 | 3.101 | 3.145 | |
| Rate of change | 0.587 | 0.490 | |||
| Covariance | −0.539 | −0.468 | |||
| Goodness-of-fit | |||||
| Deviance | 360.0 | 348.3 | 342.9 | ||
| AIC | 366.0 | 360.3 | 358.9 | ||
| BIC | 373.3 | 375.0 | 378.5 | ||
p < 0.05;
p < 0.01;
p < 0.001; standard errors are in parentheses
Model F is an unconditional means model; Model G is an unconditional growth model (including AGE); Model H includes the effects of lifetime drinks (DRINKS) on both initial status and rate of change (DRINKS*AGE). Effect sizes for the final model are presented as standardized regression estimates.
Discussion
The first aim of this study was to investigate the effects of binge-drinking status and FHD on the development of impulsive choice across adolescence. To our knowledge, this is the first study to use a prospective longitudinal design with data both before and after initiation of binge drinking. Our results suggest that as hypothesized, the interaction between FHD and binge drinking is associated with an altered developmental trajectory of impulsive choice across adolescence. More specifically, higher FHD was associated with a greater decline in impulsive choice across age in non-drinkers, but not in adolescents who went on to binge drink. This suggests that if adolescents refrain from binge drinking, greater FHD may be developmentally protective, at least with respect to reducing impulsive decision making.
Our study is not the first to suggest that FHD may be protective in those that do not drink. Studies of children of alcoholics suggest that many individual and social factors may contribute to an adolescents’ resilience against binge drinking (for review, see 37). Additionally, there could be a biological explanation behind this resilience. For example, Volkow and colleagues (38) found that individuals with alcoholic families, but who themselves were not alcoholics, had greater D2 receptor availability in the caudate and ventral striatum than non-alcoholics without a FH+, suggesting that greater D2 receptor levels could protect against alcoholism by regulating brain regions involved in behavioral inhibition and impulsivity (for review, see 39). Another possible explanation is that not all heritable predispositions toward high-alcohol drinking are associated with impulsive choice. That is, there is some degree of phenotypical variability in adolescents with a FH+ that causes some to engage in more impulsive choice and others less. For example, when comparing a high alcohol-consuming and alcohol-seeking strain of mice to a high-consuming but moderate-seeking strain, high alcohol-seeking animals had greater discounting rates, suggesting that impulsive choice may be more closely associated with a propensity to drug seek than to consume (40). In humans, this is supported by findings that novelty seeking is a significant predictor of alcohol dependence in FH+ individuals, but not those without FH+ (41). Additional work is necessary to determine the mechanism behind the association between the interaction of binge drinking and FHD, and impulsive choice.
Another benefit of this longitudinal study was that the multilevel modeling analytic strategy allowed us to investigate the association between our predictors and impulsive choice at the intercept (placed at the average age at baseline), when all adolescents were alcohol naïve. As hypothesized, our results showed that higher FHD was associated with more impulsive choices at baseline, prior to alcohol consumption. This is in line with previous studies in both humans (15–17) and rodents (18–20). Additionally, our findings suggest that despite those with greater FHD initially demonstrating greater impulsive choice, this effect becomes negligible, and may in fact reverse, across development in those that remain alcohol-naïve. This is consistent with longitudinal work showing that the association between FH+ and greater impulsive choice diminished across early adolescents in alcohol-naïve individuals (42). Further, our results showed that ultimate binge-drinking status on its own, or in interaction with FHD, was not associated with baseline impulsive choice. This suggests that adolescents who later go on to drink have comparable levels of impulsive choice to controls prior to alcohol initiation and supports the notion that alcohol use may alter underlying neural mechanisms involved in impulsive choice. To further strengthen this notion, the second aim of this study investigated the dose-related association between alcohol use and impulsive choice. We found that an escalation of drinking was associated with a greater increase in impulsive choice across adolescence, suggesting that the greater rates of impulsive choice previously observed in drinking adolescents (12, 13) may be the result of alcohol use, as opposed to a premorbid risk phenotype; however, additional studies are necessary to confirm this finding.
This study is not without limitation. First, as mentioned, there is a possibility that the association between greater FHD and more impulsive choice over time could be driven by a third variable (e.g. sensation seeking) (43). While investigation into this is beyond the scope of this manuscript, it should be explored in future experiments. Second, this study did not investigate sex differences. While a meta-analysis suggests that there are no sex differences in delay discounting behavior (44), whether this changes in the context of alcohol use is unclear. Unfortunately, with only 14 binge-drinking females, this study lacks power to detect potential three-way interactions between predictor variables; however, this is also an important future direction. Third, in light of the effect of binge drinking on impulsive choice, it is unclear if abstinence returns binge-drinking adolescents to a trajectory similar to that of non-drinking adolescents, given that abstinence has been shown to reduce some of the behavioral consequences of alcohol use in adolescents (45). Finally, while this study utilized a longitudinal dataset, the analyses were primarily correlational in nature and thus causality cannot be inferred. That said, while the lack of group differences at baseline and the dose-dependent association between alcohol use and impulsive choice would suggest that alcohol may be altering the development of impulsive choice, additional longitudinal studies will be necessary to sufficiently support this claim.
In conclusion, we showed that FHD interacts with binge drinking during adolescence and is associated with an altered developmental trajectory of impulsive choice. While greater FHD may be protective in adolescents who remain alcohol naïve, this effect is not present in adolescents who go on to binge drink. Furthermore, in binge-drinking adolescents, escalated drinking was associated with a greater increase in impulsive choice across adolescence. Understanding how alcohol use is associated with the development of impulsive choice may inform intervention strategies, such as episodic future thinking (46), in an effort to reduce rates of both impulsive choice and alcohol consumption. Knowledge of the interaction between FHD and binge drinking in relation to impulsive choice may help identify which individuals will benefit the most from behavioral intervention. Future work is important to understand what mechanism(s) may be responsible for this association between alcohol use and FHD and the development of impulsive choice across adolescence.
Acknowledgments
Past and current members of the Developmental Brain Imaging Lab are thanked for assisting in participant scheduling and data collection. This research was supported by the National Institute on Alcohol Abuse and Alcoholism [T32 AA007468 (Ryabinin); R01 AA017664 (Nagel)].
Footnotes
A common metric computed from this model is the intra-class correlation coefficient (ICC), which represents the percentage of variance in the outcome explained by inter-individual differences.
References
- 1.Mitchell JM, Fields HL, D’Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcoholism: Clinical and Experimental Research. 2005;29(12):2158–69. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- 2.Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154(3):243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- 3.Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and clinical psychopharmacology. 1998;6(3):292. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell SH. The genetic basis of delay discounting and its genetic relationship to alcohol dependence. Behavioural processes. 2011;87(1):10–7. doi: 10.1016/j.beproc.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achterberg M, Peper JS, van Duijvenvoorde AC, Mandl RC, Crone EA. Frontostriatal White Matter Integrity Predicts Development of Delay of Gratification: A Longitudinal Study. The Journal of Neuroscience. 2016;36(6):1954–61. doi: 10.1523/JNEUROSCI.3459-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson EA, Hooper CJ, Collins P, Luciana M. Adolescents’ performance on delay and probability discounting tasks: Contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Personality and Individual Differences. 2007;43(7):1886–97. doi: 10.1016/j.paid.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child development. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- 8.Water E, Cillessen AH, Scheres A. Distinct Age-Related Differences in Temporal Discounting and Risk Taking in Adolescents and Young Adults. Child development. 2014;85(5):1881–97. doi: 10.1111/cdev.12245. [DOI] [PubMed] [Google Scholar]
- 9.Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behavioral neuroscience. 2003;117(4):695. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- 10.Doremus-Fitzwater TL, Barreto M, Spear LP. Age-related differences in impulsivity among adolescent and adult Sprague-Dawley rats. Behavioral neuroscience. 2012;126(5):735. doi: 10.1037/a0029697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinkston JW, Lamb R. Delay discounting in C57BL/6J and DBA/2J mice: Adolescent-limited and life-persistent patterns of impulsivity. Behavioral neuroscience. 2011;125(2):194. doi: 10.1037/a0022919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102(4):579–86. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan EV, Brumback T, Tapert SF, Fama R, Prouty D, Brown SA, et al. Cognitive, emotion control, and motor performance of adolescents in the NCANDA study: Contributions from alcohol consumption, age, sex, ethnicity, and family history of addiction. Neuropsychology. 2016;30(4):449. doi: 10.1037/neu0000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mejia-Toiber J, Boutros N, Markou A, Semenova S. Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behavioural brain research. 2014;266:19–28. doi: 10.1016/j.bbr.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the Oklahoma family health patterns project. Alcoholism: Clinical and Experimental Research. 2011;35(9):1607–13. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty DM, Charles NE, Mathias CW, Ryan SR, Olvera RL, Liang Y, et al. Delay discounting differentiates pre-adolescents at high and low risk for substance use disorders based on family history. Drug and alcohol dependence. 2014;143:105–11. doi: 10.1016/j.drugalcdep.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcoholism, clinical and experimental research. 2010;34(9):1590–602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcoholism: Clinical and Experimental Research. 2009;33(7):1294–303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkel JK, Bentzley BS, Andrzejewski ME, Martinetti MP. Delay discounting for sucrose in alcohol-preferring and nonpreferring rats using a sipper tube within-sessions task. Alcoholism: Clinical and Experimental Research. 2015;39(2):232–8. doi: 10.1111/acer.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes, Brain and Behavior. 2008;7(7):705–13. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith CT, Steel EA, Parrish MH, Kelm MK, Boettiger CA. Intertemporal choice behavior in emerging adults and adults: effects of age interact with alcohol use and family history status. Frontiers in human neuroscience. 2015:9. doi: 10.3389/fnhum.2015.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanderBroek L, Acker J, Palmer AA, de Wit H, MacKillop J. Interrelationships among parental family history of substance misuse, delay discounting, and personal substance use. Psychopharmacology. 2016;233(1):39–48. doi: 10.1007/s00213-015-4074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95(7):1043–53. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- 24.MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological methods. 2002;7(1):19. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(4):443–9. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of studies on alcohol. 1998;59(4):427–38. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 27.Hollingshead AB, Redlich FC. Social class and mental illness: Community study. 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; 1999. [Google Scholar]
- 29.Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatric and perinatal epidemiology. 2001;15(1):88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 30.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism, clinical and experimental research. 1995;19(4):1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 31.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug and alcohol dependence. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- 32.NIAAA NIoAAaA. NIAAA council approves definition of binge drinking. NIAAA newsletter. 2004;3(3) [Google Scholar]
- 33.Cservenka A, Jones SA, Nagel BJ. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Developmental cognitive neuroscience. 2015;16:110–20. doi: 10.1016/j.dcn.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones SA, Cservenka A, Nagel BJ. Binge drinking impacts dorsal striatal response during decision making in adolescents. NeuroImage. 2016;129:378–88. doi: 10.1016/j.neuroimage.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146(4):455–64. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 36.Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay-discounting data. Exp Clin Psychopharmacol. 2008;16(3):264–74. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park S, Schepp KG. A systematic review of research on children of alcoholics: Their inherent resilience and vulnerability. Journal of Child and Family Studies. 2015;24(5):1222–31. [Google Scholar]
- 38.Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Archives of general psychiatry. 2006;63(9):999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 39.Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2014;76(Pt B):498–509. doi: 10.1016/j.neuropharm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beckwith SW, Czachowski CL. Increased delay discounting tracks with a high ethanol-seeking phenotype and subsequent ethanol seeking but not consumption. Alcoholism, clinical and experimental research. 2014;38(10):2607–14. doi: 10.1111/acer.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grucza RA, Robert Cloninger C, Bucholz KK, Constantino JN, Schuckit MI, Dick DM, et al. Novelty seeking as a moderator of familial risk for alcohol dependence. Alcoholism: Clinical and Experimental Research. 2006;30(7):1176–83. doi: 10.1111/j.1530-0277.2006.00133.x. [DOI] [PubMed] [Google Scholar]
- 42.Dougherty DM, Lake SL, Mathias CW, Ryan SR, Bray BC, Charles NE, et al. Behavioral Impulsivity and Risk-Taking Trajectories Across Early Adolescence in Youths With and Without Family Histories of Alcohol and Other Drug Use Disorders. Alcoholism, clinical and experimental research. 2015;39(8):1501–9. doi: 10.1111/acer.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiland BJ, Welsh RC, Yau WY, Zucker RA, Zubieta JK, Heitzeg MM. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug and alcohol dependence. 2013;128(1–2):130–9. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: a meta-analysis. Psychological bulletin. 2011;137(1):97. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- 45.Winward JL, Hanson KL, Bekman NM, Tapert SF, Brown SA. Adolescent heavy episodic drinking: neurocognitive functioning during early abstinence. Journal of the International Neuropsychological Society : JINS. 2014;20(2):218–29. doi: 10.1017/S1355617713001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snider SE, LaConte SM, Bickel WK. Episodic Future Thinking: Expansion of the Temporal Window in Individuals with Alcohol Dependence. Alcoholism, clinical and experimental research. 2016;40(7):1558–66. doi: 10.1111/acer.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]