Abstract
Aims
To test a neurobehavioral model of adolescent substance use disorder (SUD) resulting from an imbalance between a hyperactive reward motivation system and a hypoactive executive control system. Specifically, we tested (1) if early weakness in working memory (WM) and associated imbalance indicators of acting-without-thinking (AWT) and delay discounting (DD) predict SUD in late adolescence, and (2) if early drug use progression mediates this relation.
Design
Five waves of longitudinal data collected annually from 2005–2010, with a final follow-up in 2012.
Setting & Participants
Sample of 387 community adolescents (baseline ages 11–13) recruited from the Philadelphia, Pennsylvania, USA area.
Measurements
WM was assessed at baseline using 4 different computerized tasks. AWT and DD were assessed at baseline using self-reports. Early drug use patterns were modeled using annual self-reports of recent drug use across the first four waves. Final outcome of SUD was assessed at last wave using self-reports matched to the DSM-5 criteria for three commonly used substances: alcohol, marijuana, and tobacco.
Findings
Weakness in WM at baseline, associated with neurobehavioral imbalance indicators of AWT, B (SE) = −0.06(0.02), p<0.01, and DD, B (SE) = −7.30(1.93), p<0.01, was a significant predictor of SUD at final follow-up. WM predicted SUD both independent of early drug use, B (SE) = −0.36(0.12), p<0.01, and as mediated by early drug use progression, B (SE) = −0.06(0.02), p<0.01.
Conclusions
Adolescents with weak working memory have less control over impulsive urges, placing them at risk for later substance use disorder with some of the effects mediated by early drug use progression.
Keywords: Working memory ability, impulsivity, early drug use, substance use disorders, adolescence
Introduction
Most substance use onset occurs during adolescence [1] with a strong link between early onset and later dependence [2,3]. Longitudinal evidence linking early onset to dependence has relied heavily on retrospective reports of drug use assessed with high-risk samples [4–6]. Further, this research has overlooked the significant heterogeneity in early drug use patterns [7,8], leaving unaddressed the question whether any early onset or specific forms of early drug use are predictive of later substance use disorders (SUD). Considerable research also finds that an underlying liability for disinhibition predisposes to externalizing behaviors during adolescence, including both early drug use and SUD [9,10]. However, less is known about the neuropsychological mechanisms underlying this liability. Our study tests the role of weak executive control in relation to reward-seeking tendencies as a predisposing mechanism for early progression in drug use and subsequent SUD in adolescence.
In our prior longitudinal research with a cohort of community adolescents (N=387; baseline ages 10–12), we tested and found support for a neurobehavioral imbalance model [11,12] which proposes that an imbalance between a hyperactive reward system and a hypoactive executive control system predisposes adolescents to engage in progressive drug use after initial experimentation. Two forms of impulsivity that index this imbalance, namely acting without thinking (AWT) and delay discounting (DD), predicted progressive drug use trajectories from early to mid-adolescence [7]. AWT is a form of impulsive action that represents the tendency for rapid and unplanned responses to behavioral urges [13,14]. DD is a form of impulsive choice reflecting a preference for smaller immediate rewards over larger delayed rewards [15]. These forms of impulsivity reflect a lack of top-down control over rewarding or impulsive urges, and are negatively associated with working memory ability (WM) [16,17] – an important component of the executive control system that enables one to ignore distracting stimuli and to access information in memory that would discourage drug use during planning and problem solving [18,19]. Individuals with better WM are therefore hypothesized to be more able to resist strong impulses, such as those associated with drug reward, and maintain focus on less urgent or more distant negative consequences [18–20].
According to our proposed model, the imbalance resulting from strong attraction to rewarding experience (e.g., drug use) in combination with weak executive control leads to progressive drug use, which eventuates in SUD. Although sensation seeking (a tendency to seek exciting or novel experiences) has often been linked to early drug use [21], the imbalance model distinguishes between any early use and early progressive use that is associated with weak executive control. Although predictions of this model regarding early progression of drug use in human subjects have been confirmed [7], its utility in predicting substance dependence has so far only been tested in animals [11,12]. Here we extend our prior findings by testing whether the neurobehavioral imbalance model predicts SUD in human adolescents by re-interviewing the same cohort for SUD symptoms in late adolescence (ages 18–20).
The aims of this study were (1) to test if early weakness in working memory (WM) and associated imbalance indicators of acting-without-thinking (AWT) and delay discounting (DD) predict SUD in late adolescence, and (2) to evaluate if early drug use progression mediates this relation. We hypothesized that weakness in WM and associated neurobehavioral imbalance would be a prospective risk factor for SUD, as mediated by early drug use progression. In the absence of targeted intervention, individual differences in WM were likely to persist [20,22], leaving the adolescent vulnerable to SUD apart from early drug use progression. Weak WM could therefore continue to pose a risk for later SUD through poor impulse control or limited capacity to attend to the harms of drug use during planning and decision-making. These potential pathways to SUD are illustrated in Figure 1.
Figure 1. Neurobehavioral imbalance model predicting SUD during adolescence.
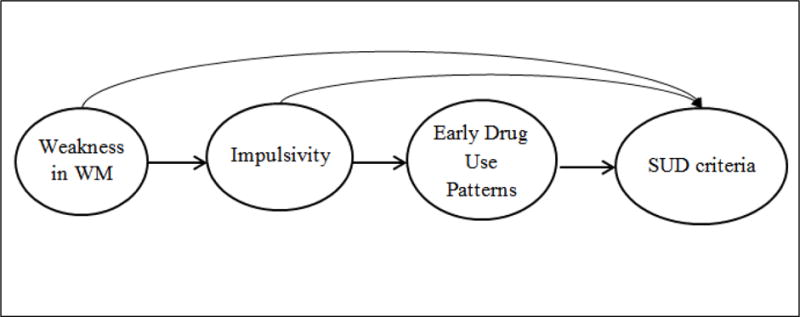
Note. SUD = Substance use disorder; WM = Working memory. We hypothesized that the imbalance captured by weakness in WM and associated impulsivity dimensions will predict later SUD both directly and as mediated by early drug use experiences.
We chose to focus our analysis on alcohol, marijuana, and tobacco, which are the three most commonly used substances by adolescents [1], including in our sample. There is a high degree of covariance in the trajectories of use of these three drugs [23,24] as well as a high degree of comorbidity in early onset of SUD for all three drugs [9]. We used a latent SUD factor that captured dependence symptoms for these three substances as our main outcome variable.
Method
Study Design
Longitudinal data were obtained from 387 adolescents who participated in the Philadelphia Trajectory study (PTS) –a six wave study of a community cohort of adolescents recruited at ages 10–12 from the Philadelphia area. Participants were assessed annually for the first five years (waves 1–5) from 2004–2010, with a final follow-up two years later (wave 6). All self-reports were obtained using audio computer assisted interviewing. Sample descriptives can be found in Table 1. Further details about sample recruitment and characteristics can be found elsewhere [25]. We analyzed data from the last five waves (waves 2–6) due to the very low rates of drug use (<4%) during the first wave. Baseline (wave 2) assessments of WM and associated impulsivity dimensions were used as predictors of early drug use progression (from waves 2–5) and SUD (at wave 6). The study was approved by the Institutional Review Board of the Children’s Hospital of Philadelphia. A certificate of confidentiality was obtained for the last wave when participants had reached the age of majority.
Table 1.
Sample demographics (N = 387).
| Variable Name | Mean (sd)/Frequencies |
|---|---|
| Age in years (wave 2) | 12.61 (0.89) |
| Sex | 52% |
| Race-Ethnicity | |
| Non-Hispanic White | 56% |
| Non-Hispanic Black | 26% |
| Hispanic | 9% |
| Others (including Asians, Native Americans) | 9% |
| Socioeconomic status (Hollingshead two-factor index; reverse scored) | 47.0 (15.8) |
Measures
Working Memory (WM)
Participant WM was assessed based on performance on the following tasks that were largely nonverbal and thus not dependent on differences in reading comprehension: (1) Digit span backwards (2) Corsi-block tapping (3) Letter-two-back, and (4) a spatial WM task. WM assessments from wave 2 of the study were included in present analyses. All four WM tasks (described below) have been linked to activation in executive control brain regions [25] and loaded significantly on a single latent factor, with loadings ranging from 0.40–0.60.
Digit Span
This task tests the auditory-verbal WM of participants by having them repeat back in reverse order, sequences of digits to the experimenter. The test was administered in standard format according to the procedures listed in the Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV) manual [26].
Corsi-block tapping
This task is a non-verbal variant of the digit span task [27]. Participants view a set of identical blocks that are spatially dispersed on the screen, and are individually lit up in a random sequence. Participants are asked to tap each box in the reverse order of the sequence of lit boxes. This task also assesses spatial WM as the visual sequence must be maintained and reversed in WM in order to guide the response.
Letter two-back
This task involves monitoring a series of letters for a repeat “two-back.” Letters are presented for 500 milliseconds each, separated by a 1 second interval. Participants must continually update their WM in order to compare the current letter to the letter shape presented two trials back. This task was adapted for children by Casey et al. [28].
Spatial Working Memory
This self-directed computerized task requires the participant to search for hidden tokens one at a time within sets of four to eight randomly positioned boxes. WM skills are tapped as the participant while searching must hold in WM the locations already checked and as tokens are found, must remember and update information about the locations of those tokens [29]. Between-search errors are made if the participant returns to a box where a token had already been found during a previous search sequence, and was used as a measure of WM performance.
Impulsivity dimensions
We assessed two dimensions of impulsivity: Acting-without-thinking (AWT), a measure of impulsive action, and delay discounting (DD), a measure of impulsive choice. Both measures have been associated with hyperactivation in the reward motivational system [30,31].
Acting Without Thinking
AWT was assessed using a 9-item self-report measure adapted from the Junior Eysenck Impulsivity Scale [32] that assesses the predisposition towards rapid, unplanned reactions to impulsive urges without thinking though the consequences (e.g., do you usually do or say things without thinking?) with binary (Y/N) response options. We used AWT assessed at wave 2 corresponding to WM and early drug use assessments.
Delay Discounting
DD was assessed using a hypothetical monetary choice task [33] where the participant is asked in the context of payment for a job to select an amount between $10 and $90 that if received immediately would be equivalent to receiving $100 6 months later. Respondents are initially asked if they would accept an immediate payment of $50. Using an iterative procedure, those who accept/reject this offer are asked if they would accept an amount lower/higher than $50 in $10 decrements. Scores on this variable ranged from 10 to 100, which were reverse-scored such that higher scores were indicative of greater DD. Similar procedures have been shown to be valid with this age-group [34] and to be valid indicators of the ability to delay gratification [35]. Research comparing hypothetical with real rewards and delays indicates that this procedure produces comparable estimates of individual differences [36]. We used DD at wave 3, when it was first assessed in the study.
Additionally, we also included sensation seeking assessed at wave 2. Previously, we have found that sensation seeking, which is positively correlated with AWT [30], does not predict progression in drug use [7,20] controlling for the effect of AWT. Given that sensation seeking does not reflect an underlying weakness in executive control, we did not expect it to be a significant predictor of SUD. Nonetheless, we tested its effect in our model.
Early Drug Use Patterns
Based on our previous study [7], we used a nominal variable with three categories (abstainers, experimenters, progressors) as a measure of early drug use trajectory classes. These classes were generated using latent growth class analysis to examine heterogeneity in early drug use patterns in PTS waves 2–5. Specifically, we used self-reported recent (past 30 days) use of alcohol, marijuana, and tobacco on a cumulative scale coded 0 (no use), 1 (used only one drug), 2 (used two of the three drugs), and 3 (used all three drugs) in the past 30 days. This score from waves 2–5 was used to generate the latent class solution which suggested a two-class solution: abstainers/experimenters (69.8%) and progressors (30.2%). The progressor class was defined by rapid escalation and persistent use over the four years, while experimenters had intermittent or low use at all waves. Given the high entropy score (0.80) [37], classification criteria (>91% classification probabilities), and model fit indices (LRT and bootstrap LRT results), we assigned participants to the latent classes they were most likely to belong based on highest posterior probabilities. We separated the experimenters from the abstainers, and used the nominal variable with three categories (progressors, experimenters, abstainers), with abstainers serving as the reference group.
Substance Use Disorder (SUD)
At wave 6, if participants reported use of a specific drug in the past year, they were asked questions pertaining to substance abuse and dependence with an interview used by the National Survey on Drug Use and Health to identify SUD in the United States [38]. The DSM-5 has replaced the substance abuse and dependence classifications with “substance use disorder” which includes a new craving item. We matched our survey items to the DSM-5 criteria for each of the three substances –alcohol, marijuana, and tobacco. Craving was only asked for marijuana and tobacco. DSM-5 defines a mild drug use disorder as meeting 2–3 criteria. We used a continuous score which indicated number of criteria met, representing a continuum of severity of disorder. In case of tobacco, only 14% of our sample met one or more criteria for SUD, of which 39% endorsed only one criterion. Therefore, we collapsed the tobacco use disorder severity scores to a binary variable. The scores for the three drugs were significantly correlated (r = 0.22–0.44). Further, given the co-morbidity in early-onset SUD [39] and research suggesting a single underlying factor for consumption, dependence, and abuse [40], we used a latent factor with the criterion scores of alcohol, marijuana, and tobacco as our outcome variable. Confirmatory factor analyses revealed that the criterion scores loaded on a single latent factor, with loadings ranging from 0.31–0.65. There was a significant residual correlation between alcohol and marijuana criterion scores (r = 0.30, p<0.001), suggesting additional comorbidity in these two drugs. This covariance pathway was retained in the final model.
Urine Drug Screens
Although we relied on self-reports to assess SUD criteria, we also acquired a urine sample to determine the presence of the following drugs: amphetamine, barbiturates, benzodiazepines, cocaine, cotinine, opiates, phencyclidine (PCP), and tetrahydrocannabinol (THC; the active ingredient in cannabis). Enzyme immunoassay was used for the initial screen and positive screens were confirmed by gas chromatography-mass spectrometry [41].
Analytic Plan
Five waves of PTS data (waves 2–6) were analyzed using structural equation modeling (SEM) procedures in Mplus v7 using robust estimation procedures [42]. There was a 25% loss to follow-up over the six waves of the PTS, with only 13% attrition across the first five waves. Missingness was unrelated to participant demographics or key study variables, and was handled using Full information maximum likelihood which provides reliable estimates when data are missing at random [43]. Confidence intervals for mediated effects were obtained using the bias-corrected bootstrap re-sampling method [44]. Age and gender were included as covariates. Model fit was evaluated using multiple indices of global fit and an examination of residual diagnostics. The criteria for good model fit included a low chi-square test statistic, a root mean square error of approximation (RMSEA) value less than 0.05 and values of the comparative fit index (CFI) and the Tucker–Lewis Index (TLI) greater than 0.95.
Results
At the final follow-up (ages 18–20 years), 13.4% (n = 39) of the sample had developed alcohol use disorder (≥ 2 criteria), 14.5% (n = 42) had developed marijuana use disorder (≥ 2 criteria), while 14.1% (n = 41) showed at least one criterion for tobacco dependence (see Table 2). In total, about a quarter of our sample (25.2%) reported sufficient criteria for a mild SUD – slightly higher than national estimates of 16% in 18–25 year olds [45]. Alcohol and marijuana criteria scores were correlated at 0.44. The correlation of tobacco with alcohol and marijuana scores was lower at 0.22 and 0.27, respectively.
Table 2.
Percentage of sample who met criteria for alcohol, marijuana, and tobacco disorder at wave 6 (N=290).
| Number of criteria met | Alcohol | Marijuana | Tobacco |
|---|---|---|---|
| 0 | 71.7% (n=208) | 76.2% (n=221) | 85.9% (n=249) |
| 1 | 14.8% (n=43) | 9.3% (n=27) | 5.5% (n=16) |
| 2 | 8.3% (n=24) | 5.2% (n=15) | 1.4% (n=4) |
| 3+ | 5.2% (n=15) | 9.3% (n=27) | 7.2% (n=21) |
Note. DSM-5 describes presence of 2–3 criteria as a mild case of SUD.
As expected, more recent drug use was reported at the final follow-up (57.6% reported recent alcohol use, 34.8% cannabis use, and 22.3% cigarette use) than during earlier waves when only 30.2% of the sample reported consistent drug use. Drug screening revealed high agreement with final wave self-reports for both cannabis (92%) and nicotine (77%). We did not have a reliable drug test for alcohol. Bivariate associations between key variables (see Table 3) revealed that the adolescents who developed SUD were more likely to have weaker WM performance and higher AWT and DD scores at baseline. No other differences were observed in terms of age, gender, race-ethnicity, or socioeconomic background.
Table 3.
Correlation matrix of key study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. WM factor score (wave 2) | 1 | |||||||||
| 2. AWT (wave 2) | −0.22 | 1 | ||||||||
| 3. DD (wave 3) | −0.16 | 0.11 | 1 | |||||||
| 4. Early drug use experimentation (waves 2–5) | −0.07 | −0.03 | −0.04 | 1 | ||||||
| 5. Early drug use progression (waves 2–5) | −0.05 | 0.35 | 0.14 | −0.21* | 1 | |||||
| 6. Alcohol dependence symptoms (wave 6) | −0.04 | 0.11† | 0.07 | 0.04 | 0.11† | 1 | ||||
| 7. Marijuana dependence symptoms (wave 6) | −0.08 | 0.09 | 0.10 | −0.03 | 0.25 | 0.44 | 1 | |||
| 8. Tobacco dependence symptoms (wave 6) | −0.23 | 0.09 | 0.12 | −0.08 | 0.27 | 0.23 | 0.30 | 1 | ||
| 9. Female | −0.05 | −0.10 | −0.04 | −0.05 | −0.04 | −0.09 | −0.16 | −0.05 | 1 | |
| 10. Age (wave 2) | 0.09 | 0.09 | 0.06 | −0.11 | 0.25 | 0.06 | 0.07 | −0.02 | −0.08 | 1 |
|
| ||||||||||
| Mean (SD); Range | 0.39 (0.28); 0 – 1 |
50.58 (29.09); 10–100 |
0.10 (0.30); 0–1 |
0.28 (0.45); 0– 1 |
0.47 (0.85); 0–3 |
0.44 (0.89); 0–3 |
0.14 (0.35); 0 – 1 |
0.51 (0.51); 0 – 1 |
18.41 (0.63); 18–20 |
|
Note. Bold values signify correlations significant at p < 0.05;
denotes significance at p < 0.08. WM=Working Memory, AWT=Acting Without Thinking, DD=Delay Discounting. WM factor scores were used for the correlation matrix even though a latent WM factor with 4 indicators was used in the final SEM model.
Early drug use progression and experimentation are negatively correlated by virtue of the group assignment, i.e., those in the progressors group were not included in the experimenter group.
In a direct effects model (with no mediational pathways of influence), the direct effect of weak WM on SUD remained significant, even after accounting for early drug use patterns, B(SE) = −0.44(0.17), p<0.01. Early progression in drug use was also an independent predictor of SUD, B(SE) =1.41(0.47), p<0.01, controlling for the effects of WM and impulsivity dimensions. Mediational analyses revealed that some of the effects of weak WM on SUD were channeled through early drug use patterns, and this model (see Figure 2) provided the best fit to the data, Chi sq. (df=57) = 69.24, p=0.13; RMSEA= 0.02 (0.00, 0.04), CFI=0.97, TLI=0.96.
Figure 2. Final SEM model showing effects of underlying weakness in executive control on SUD that is both direct and mediated by early drug use progression.
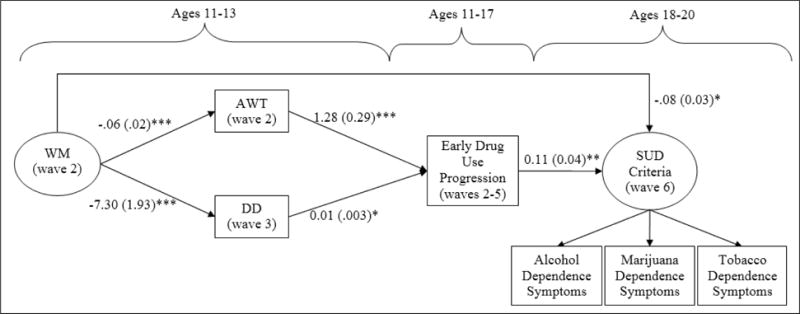
Note. *p<.05, **p<.01, ***p<.001. WM=Working Memory, AWT=Acting Without Thinking, DD=Delay Discounting. “Early drug use experiences” was a nominal variable including abstainers, experimenters, and progressors; abstainers was omitted as the reference group. Only the “progressors” group was a significant predictor of SUD risk, and is shown in the model above. There was a significant residual correlation between alcohol and marijuana criterion scores. Model Fit: Chi Sq. (df=57) =69.24, p=0.13; RMSEA=0.02 (0.00, 0.04), CFI= 0.97, TLI= 0.96. The effect of age and gender was controlled for. The model explained 33% of variance in the latent SUD risk factor.
Adolescents with weak WM and associated impulsivity dimensions of AWT and DD were more likely to engage in early and progressive drug use, which was an important mediated pathway of influence for developing SUD, B(SE) = −0.06(0.02), p<0.01. Additionally, weakness in WM remained a liability for SUD apart from early progression in drug use. Separate analysis revealed that this left-over effect of WM on SUD was direct and not mediated by impulsivity. We also tested the effect of sensation seeking, but it did not emerge as a significant predictor of SUD, B(SE) =0.10(0.10), p=0.33, and was not retained in the final model. Our model explained 33% of variance in the latent SUD symptoms factor.
Discussion
Our purpose here was to test the utility of a neurobehavioral imbalance model [11] for predicting SUD risk in human adolescents. Inspired by research in animals, this model posits that an imbalance resulting from a hyperactive reward system and a hypoactive executive control system increases risk for progressive drug use that eventually leads to SUD. Previously, we documented the utility of this model in predicting early and progressive drug use trajectories starting at ages 11 to 13 in a cohort of community adolescents. The present study re-assessed the same cohort at ages 18–20 to determine if early progression in drug use predicted greater drug dependence during later years as compared to mere experimentation with drugs or non-use during younger years. We also tested the hypothesis that the same liabilities (i.e., underlying weakness in WM along with indicators of impulsivity) that predicted early progression of drug use would also continue to predict later dependence, regardless of early drug use experiences. The results supported our hypotheses.
Specifically, we found that weak WM and associated impulsivity dimensions of AWT and DD, indexing the imbalance between the two neurobehavioral systems, predicted early drug use progression and subsequent SUD. Weakness in executive control continued to act as a liability for later drug use and SUD apart from early progression and impulsivity. We interpret this liability as a weakness in the capacity to screen out impulses associated with immediate drug rewards and to access information in memory that discourages drug use. Other research testing this model has found similar vulnerabilities associated with sexual urges [17,46].
Weak WM, even if not associated with an underlying imbalance as indexed by AWT and DD, could still put adolescents at risk for later SUD. It is possible that other indicators of neurobehavioral imbalance, besides the two we assessed, could mediate this left-over direct effect. Weakness in WM is also associated with a compromised ability to process complex information, leading to poor decision-making. Regardless, it is clear that early weakness in executive control can pose risk for later SUD even apart from early progression in drug use.
Our findings have implications for theories that focus on early drug use initiation as a primary risk factor for later SUD [2,3]. Although we found early progression in drug use to be an important predictor for SUD, it is also clear that the underlying liability of poor executive control continues to pose a risk for SUD during later years. This suggests that focusing exclusively on preventing early adolescent drug use will miss a significant amount of later risk for SUD unless the underlying liability is changed. Thus, interventions to strengthen executive control over behavioral impulses early in adolescence may be a strategy that can reduce the risk of SUD whether drug use is initiated early or later.
Our results are consistent with other models of drug dependence and addictive behaviors that focus on a general liability for disinibition [9] as well as dual systems models that focus on individual differences in self-control [47,48]. Broadly, these models suggest that a general disinhibitory tendency is predictive of a wide range of externalizing behaviors. Our findings identify weakness in executive control as a critical component of this liability and provide greater specification of the cognitive and motivational mechanisms that underlie this tendency. Popular theories of adolescent risk behaviors (e.g., developmental imbalance models) attribute the rise in risk-taking during adolescence to an imbalance between the same processes proposed by our model [49,50]. Nevertheless, we suggest that these imbalance models overgeneralize tendencies that are especially characteristic of a subset of youth with weakness in executive control relative to reward seeking urges. Although there is a surge in reward seeking during adolescence, as captured by higher levels of sensation seeking [51,52], it may not pose significant risk unless there is an accompanying imbalance created by weakness in executive control. This imbalance is likely present only in a subset of adolescents who exhibit early indications of low self-control [9,10].
Interventions targeting self-regulation have shown promising results in reducing drug use and other externalizing behaviors. In particular, family-focused interventions, such the Family Check-Up [53,54] and Iowa Strengthening Families program [55], that use positive parenting strategies to prevent youth disinhibitory behaviors appear to be effective in reducing drug use. Interventions to prevent the escalation in drug use and later SUD could profit by specifically targeting weakness in executive control functions such as WM and inhibitory control. So far these cognitive training interventions have focused mainly on academic skills [56]. Our findings suggest that improvements in WM could have protective effects on impulsivity. There is some evidence that WM training in adults with SUD can reduce impulsive responding on DD tasks [57]. However, whether this can be successfully implemented in adolescents remains a fertile area for further research.
Although we studied a large sample of community adolescents, we are limited in terms of the generalizations we can make in comparison to more nationally representative samples. Further, our reports of drug use and SUD symptoms were based on self-reports, which even though are not as reliable, were corroborated by biological assessments. We were also unable to include the DSM-5 craving item in our assessment of alcohol use disorder. Finally, although our model predicted SUD risk specifically for the three commonly used substances during adolescence, the use of other drugs like cocaine, methamphetamine, or heroin was negligible in our sample (0% −3%). This lends greater confidence in the model’s ability to predict dominant SUD risk in this population.
Acknowledgments
The project was supported by grants R01DA018913 and R01DA033996 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Kristin Arena, Nancy Brodsky, and Joan Giannetta for their invaluable contributions to the execution of the study.
Footnotes
Declaration of Competing Interests: None.
References
- 1.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg J. Overview, key findings on adolescent drug use. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2016. Monitoring the Future national survey results on drug use, 1975–2015. [Google Scholar]
- 2.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. J Subst Abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 3.Odgers CL, Caspi A, Nagin DS, Piquero AR, Slutske WS, Milne BJ, et al. Is it important to prevent early exposure to drugs and alcohol among adolescents? Psychol Sci. 2008 Oct 1;19(10):1037–44. doi: 10.1111/j.1467-9280.2008.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colder CR, Campbell RT, Ruel E, Richardson JL, Flay BR. A finite mixture model of growth trajectories of adolescent alcohol use: Predictors and consequences. J Consult Clin Psychol. 2002;70(4):976–85. doi: 10.1037//0022-006x.70.4.976. [DOI] [PubMed] [Google Scholar]
- 5.Richmond-Rakerd LS, Fleming KA, Slutske WS. Investigating progression in substance use initiation using a discrete-time multiple event process survival mixture (mepsum) approach. Clin Psychol Sci. 2015 Jul 24; doi: 10.1177/2167702615587457. 2167702615587457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chassin L, Flora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. J Abnorm Psychol. 2004;113(4):483–98. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- 7.Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Experimentation versus progression in adolescent drug use: A test of an emerging neurobehavioral imbalance model. Dev Psychopathol. 2015;27(3):901–13. doi: 10.1017/S0954579414000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li F, Duncan TE, Hops H. Examining developmental trajectories in adolescent alcohol use using piecewise growth mixture modeling analysis. J Stud Alcohol Drugs. 2001 Mar 1;62(2):199–210. doi: 10.15288/jsa.2001.62.199. [DOI] [PubMed] [Google Scholar]
- 9.Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annu Rev Clin Psychol. 2008;4(1):325–48. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- 10.Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci. 2011;108(7):2693–8. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine taking. Science. 2008 Jun 6;320(5881):1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010 Aug 1;34(8):1306–18. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patton JH, Stanford MS, Barratt ES. Factor structure of the barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Eysenck SBG, Eysenck HJ. Impulsiveness and venturesomeness in children. Personal Individ Differ. 1980;1(1):73–8. [Google Scholar]
- 15.Wesley MJ, Bickel WK. Remember the Future II: Meta-analyses and Functional Overlap of Working Memory and Delay Discounting. Biol Psychiatry. 2014 Mar 15;75(6):435–48. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamosh NA, Deyoung CG, Green AE, Reis DL, Johnson MR, Conway AR, et al. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008 Sep;19(9):904–11. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 17.Khurana A, Romer D, Hurt H, Betancourt L, Brodsky NL, Giannetta JM. Early adolescent sexual debut: The mediating role of working memory ability, sensation seeking and impulsivity. Dev Psychol. 2012;48(5):1416–28. doi: 10.1037/a0027491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 19.Kane MJ, Conway ARA, Hanbrick DZ, Engle RW. Variation in working memory capacity as variation in executive attention and control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 21–48. [Google Scholar]
- 20.Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsivity. Addiction. 2013;108(3):506–15. doi: 10.1111/add.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk‐taking and the adolescent brain: who is at risk? Dev Sci. 2007;10(2):8–14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 22.Hackman DA, Gallop R, Evans GW, Farah MJ. Socioeconomic status and executive function: Developmental trajectories and mediation. Dev Sci. 2015 Sep 1;18(5):686–702. doi: 10.1111/desc.12246. [DOI] [PubMed] [Google Scholar]
- 23.Jackson KM, Sher KJ, Schulenberg JE. Conjoint developmental trajectories of young adult substance use. Alcohol Clin Exp Res. 2008;32(5):723–737. doi: 10.1111/j.1530-0277.2008.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson KM, Sher KJ, Schulenberg JE. Conjoint developmental trajectories of young adult alcohol and tobacco use. J Abnorm Psychol. 2005;114(4):612–26. doi: 10.1037/0021-843X.114.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009 Nov;47(13):2916–26. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Intelligence Scale for Children® — Fourth Edition (WISC®-IV) San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 27.Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27(3):272–7. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 28.Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. NeuroImage. 1995;2(3):221–9. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- 29.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28(10):1021–34. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- 30.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010 Jul 30;329(5991):532–532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pattij T, Vanderschuren LJMJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29(4):192–9. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Eysenck SBG, Easting G, Pearson PR. Age norms for impulsiveness, venturesomeness and empathy in children. Personal Individ Differ. 1984;5(3):315–21. [Google Scholar]
- 33.Green L, Fry AF, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychol Sci. 1994;5(1):33–6. [Google Scholar]
- 34.Duckworth AL, Seligman MEP. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol Sci. 2005 Dec 1;16(12):939–44. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds B, Schiffbauer R. Delay of gratification and delay discounting: A unifying feedback model of delay-related impulsive behavior. Psychol Rec. 2005;55(3):439. [Google Scholar]
- 36.Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77(2):129–46. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. J Classif. 1996 Sep;13(2):195–212. [Google Scholar]
- 38.Substance Abuse and Mental Health Services Administration. 2011 National Survey on Drug Use and Health. Rockville, Maryland: 2010. Retrieved from https://www.samhsa.gov/data/sites/default/files/NSDUH2011MRB/NSDUH2011MRB/2k11Q.pdf. [PubMed] [Google Scholar]
- 39.Jackson KM, Sher KJ, Wood PK. Trajectories of Concurrent Substance Use Disorders: A Developmental, Typological Approach to Comorbidity. Alcohol Clin Exp Res. 2000 Jun 1;24(6):902–13. [PubMed] [Google Scholar]
- 40.Jackson KM, Bucholz KK, Wood PK, Steinley D, Grant JD, Sher KJ. Towards the characterization and validation of alcohol use disorder subtypes: integrating consumption and symptom data. Psychol Med. 2014 Jan;44(1):143–159. doi: 10.1017/S0033291713000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Institute on Drug Abuse Research. Urine Testing for Drugs of Abuse. Monograph. 1986;73 [Google Scholar]
- 42.Muthen LK, Muthen BO. Mplus user’s guide. Los Angeles, CA: Muthen & Muthen; 2006. [Google Scholar]
- 43.Enders CK. The Performance of the Full Information Maximum Likelihood Estimator in Multiple Regression Models with Missing Data. Educ Psychol Meas. 2001 Oct 1;61(5):713–40. [Google Scholar]
- 44.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivar Behav Res. 2004 Jan 1;39(1):99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Center for Behavioral Health Statistics and Quality. (Report No.: HHS Publication No. SMA 15-4927, NSDUH Series H-50).Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health [Internet] 2015 Available from: http://www.samhsa.gov/data/
- 46.Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Stronger working memory reduces sexual risk taking in adolescents, even after controlling for parental influences. Child Dev. 2015 Jul 1;86(4):1125–41. doi: 10.1111/cdev.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClure SM, Bickel WK. A dual-systems perspective on addiction: contributions from neuroimaging and cognitive training: Dual-systems models of addiction. Ann N Y Acad Sci. 2014 Oct;1327(1):62–78. doi: 10.1111/nyas.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 49.Somerville LH, Casey B. Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol. 2010 Apr;20(2):236–41. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- 51.Romer D, Hennessy M. A biosocial-affect model of adolescent sensation seeking: the role of affect evaluation and peer-group influence in adolescent drug use. Prev Sci. 2007 Jun;8(2):89–101. doi: 10.1007/s11121-007-0064-7. [DOI] [PubMed] [Google Scholar]
- 52.Romer D. Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Dev Psychobiol. 2010;52(3):263–276. doi: 10.1002/dev.20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Connell AM, Dishion TJ, Yasui M, Kavanagh K. An adaptive approach to family intervention: Linking engagement in family-centered intervention to reductions in adolescent problem behavior. J Consult Clin Psychol. 2007;75(4):568–79. doi: 10.1037/0022-006X.75.4.568. [DOI] [PubMed] [Google Scholar]
- 54.Van Ryzin MJ, Stormshak EA, Dishion TJ. Engaging parents in the family check-up in middle school: Longitudinal effects on family conflict and problem behavior through the high school transition. J Adolesc Health. 2012;50(6):627–33. doi: 10.1016/j.jadohealth.2011.10.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spoth RL, Redmond C, Shin C. Randomized trial of brief family interventions for general populations: Adolescent substance use outcomes 4 years following baseline. J Consult Clin Psychol. 2001;69(4):627–42. doi: 10.1037//0022-006x.69.4.627. [DOI] [PubMed] [Google Scholar]
- 56.Holmes J, Gathercole SE, Dunning DL. Adaptive training leads to sustained enhancement of poor working memory in children. Dev Sci. 2009;12(4):F9–F15. doi: 10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 57.Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69(3):260–5. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


