Abstract
Aims
Decreased arylesterase (ArylE) activity of paraoxonase-1, a HDL-associated protein with anti-inflammatory and antioxidant properties, has been associated with increased risk of cardiac events in patients with ischemic heart failure (HF). We aim to investigate the prognostic significance of changes in serum ArylE activity over time.
Methods and results
We examined the association between baseline and follow up serum ArylE activity and HF outcomes (death, cardiac transplantation or ventricular assist device implantation) in 299 patients with HF enrolled in a prospective cohort study from 1/2008 to 7/2009, with 145 patients have available follow up levels at 1 year. A significant drop in ArylE activity on follow up was defined as a drop of ≥25% vs. baseline levels. Mean baseline and follow up ArylE activity levels were 110.5±29.8µmol/min/ml and 106.2±29.9µmol/min/ml, respectively. After mean follow up of 2.8±1.1 years, low baseline ArylE activity was associated with increased risk of adverse HF events [HR (lowest vs highest tertile)=2.6(95% CI 1.3–5.5), p=0.01] and HF related hospitalization [incidence rate ratio (lowest vs highest tertile)=2.1(95%CI 1.2–4.1), p=0.016], which remained significant after adjustment for age, male, systolic blood pressure, diabetes, creatinine clearance, coronary artery disease, and HDL Cholesterol levels. Patients who had a significant drop in ArylE activity on follow up (≥25% of baseline levels, n=18), had significantly increased risk of HF events [HR=4.9(95%CI 1.6–14.6), p=0.005], even after adjustment for baseline levels of ArylE activity.
Conclusions
Reduced baseline ArylE activity and decreased levels on follow up are associated with adverse outcomes in stable outpatients with HF.
Keywords: Paraoxonase, Heart failure, High density lipoprotein, Prognosis
INTRODUCTION
Oxidative stress has been implicated in the pathophysiology of heart failure (HF) as well as other disease states.(1, 2) While there are many contributing factors leading to heightened oxidative stress, the downstream consequences may lead to disease progression and adverse outcomes.(1) As one of the most abundant circulating anti-oxidants in the body, high-density lipoprotein (HDL) serves as an important regulator of oxidative stress but the major focus has been its role in reverse cholesterol transport as well as its effects on countering the pathogenesis of atherosclerosis.(3) The clinical relevance of HDL cholesterol in HF has only been recently appreciated with data from epidemiological studies recognizing low HDL cholesterol as an important predictor of incident HF.(4, 5)
Circulating paraoxonase-1 (PON-1) has long been associated with anti-oxidant functions of HDL, being the major “detoxification” protein that is bound to apolipoprotein A1 in the HDL particle.(6) PON-1 catalytic activity within crude serum mixtures has traditionally been measured by quantifying enzymatic hydrolysis rates of 2 known in vitro substrates, with functional activities named paraoxonase (hydrolyzes organophosphates, like paraoxon) and arylesterase (hydrolyzes aromatic esters, like phenyl acetate) activities.(3, 7) We have recently demonstrated that in a cohort of stable cardiac patients with HF undergoing cardiac catheterization, diminished serum arylesterase (ArylE) activity, a functional measure of PON-1 activity corresponds to higher rates of long-term major adverse cardiac events including death, myocardial infarction, and stroke. (8) Herein, we examined the impact of HDL anti-oxidant function on disease progression of chronic HF by measuring serum ArylE and paraoxonase activities levels in ambulatory patients with chronic HF.
METHODS
Study Population
A total of 299 subjects with HF were enrolled from the Atlanta Cardiomyopathy Consortium, a prospective cohort study conducted between 2007–13 enrolling outpatients with HF from 3 university-affiliated hospitals in the greater metropolitan Atlanta area. The inclusion criteria included age older than 18 years, ability to understand and sign written informed consent to participate, and a diagnosis of HF with either reduced or preserved ejection fraction (EF). The diagnosis of HF with preserved EF required, in addition to the clinical diagnosis of HF, elevated B-type natriuretic peptide level >200 pg /dL and / or echocardiographic evidence of diastolic dysfunction. Exclusion criteria included congenital heart disease, previous heart transplant, known cardiac infiltrative disease (e.g., amyloidosis), previous other solid organ transplantation, and end-stage HF requiring outpatient continuous inotrope infusion. Creatinine clearance was calculated using Cockcroft-Gault formula. Patients were followed up prospectively for HF events [death, transplant and left ventricular assist device implantation (LVAD)] and HF related hospitalizations. The Emory University Institutional Review Board approved the research protocol, and all participants provided written informed consent.
Serum ArylE activity and paraoxonase activity measurements
Blood samples were collected on enrollment for all patients and in 145 patients who had clinic visit after one year. Serum ArylE activity and paraoxonase activity assays were measured by spectrometry in an open channel on Architect ci8200 platform, and in a 96-well plate format (Spectramax 384 Plus, Molecular Devices, Sunnyvale, California), respectively, as previously described.(3, 8) Briefly, for serum ArylE activity measurement, initial hydrolysis rates were determined at 270 nm in 50-fold diluted serum (final) in reactions mixtures composed of 3.4mM phenyl acetate (Sigma-Aldrich, St Louis, Missouri), 9mM Tris hydrochloride, pH 8, and 0.9mM calcium chloride at 24°C. An extinction coefficient (at 270 nm) of 1310M−1•cm−1 was used for calculating units of ArylE activity, which are expressed as micromoles of phenyl acetate hydrolyzed per minute per milliliter of serum. The intra-assay and inter-assay coefficients of variance for ArylE activity assay were 3.4% and 3.9%, respectively, on 20 replicates performed on 10 different days. For serum paraoxonase activity, the rate of generation of para-nitrophenol was determined at 405 nm in 40-fold diluted serum (final) in reaction mixtures compose of 1.5mM paraoxon (Sigma-Aldrich, St Louis, Missouri), 10mM Tris hydrochloride, pH 8, 1M sodium chloride, and 2mM calcium chloride at 24°C. An extinction coefficient (at 405 nm) of 17 000 M−1•cm−1 was used for calculating units of paraoxonase activity, which is expressed as nanomoles of para-nitrophenol produced per minute per milliliter of serum. The intra-assay and inter-assay coefficients of variance for the high throughput paraoxonase activity assay were 1.9% and 3.3%, respectively, on 30 replicates performed on 15 different days.
Statistical analysis
The Student t test or Analysis of variant (ANOVA) test, for continuous variables, and Chi-square test for categorical variables were used to examine differences between the groups. Linear regression was used to study factors correlation with ArylE activity. Survival and event rates were described with the Kaplan Meier method. Cox proportional hazards regression was used to determine hazard ratios (HR) and 95% confidence intervals (CI) for events. In multivariable models, we adjusted for age, gender, diabetes mellitus, systolic blood pressure, calculated creatinine clearance, coronary artery disease and HDL-cholesterol levels. The negative binomial regression model was used to obtain an estimate of the effect of ArylE activity on the rate of HF hospitalizations giving the skewed distribution in the frequency of hospitalizations.(9, 10) The negative binomial naturally accommodates the different probabilities for events across members of the population. General linear models with repeated measures were used to study the change of ArylE overtime. A Significant drop in ArylE activity over time was defined as a drop of ≥25% of the baseline activity.
RESULTS
Table 1 describes the baseline characteristics of the study population. Baseline ArylE activity was normally distributed with a mean of 110.6±29.9 µmol/min/mL. No significant difference in baseline characteristics was observed between groups with different ArylE activity. No difference was observed in ArylE activity between patients with preserved or reduced EF.
Table 1.
Baseline Characteristics
| ArylE activity, µmol/L/min/mL |
Total | Tertile 1 <97 |
Tertile 2 97–120 |
Tertile 3 ≥120 |
p value |
|---|---|---|---|---|---|
| Number | 299 | 100 | 99 | 100 | |
| Age, years | 57 ± 12 | 56 ± 13 | 57 ± 13 | 57 ± 12 | 0.93 |
| Male, % | 64 | 71 | 64 | 57 | 0.15 |
| Black, % | 44 | 48 | 34 | 50 | 0.04 |
| Body mass index, kg/m2 |
31 ± 7 | 31 ± 7 | 32 ± 7 | 31 ± 7 | 0.69 |
| Systolic blood pressure, mmHg |
113 ± 19 | 112 ± 18 | 112 ± 18 | 115 ± 22 | 0.42 |
| Diabetes, % | 33 | 35 | 31 | 32 | 0.81 |
| Smoking, % | 31 | 33 | 34 | 25 | 0.36 |
| Coronary artery disease, % |
40 | 40 | 46 | 35 | 0.29 |
| Creatinine clearance, mL/min |
93 ± 43 | 92 ± 46 | 94 ± 42 | 93 ± 40 | 0.92 |
| Ejection Fraction, % |
30 ± 15 | 29 ± 15 | 31 ± 16 | 31 ± 14 | 0.51 |
| Ejection Fraction ≥ 40% |
29 | 27 | 29 | 29 | 0.95 |
| B-type Natriuretic Peptide, pg/mL |
163 (57 – 564) |
233 (75 – 684) |
147 (57 – 543) |
110 (39 – 301) |
0.003 |
| ACE inhibitors or ARBs, % |
78 | 73 | 75 | 87 | 0.03 |
| Beta blockers, % |
91 | 90 | 93 | 90 | 0.72 |
| Hydralazine, % | 19 | 16 | 18 | 24 | 0.34 |
| Nitrate, % | 23 | 16 | 29 | 25 | 0.1 |
| Statins, % | 54 | 50 | 62 | 51 | 0.16 |
| High density lipoprotein cholesterol, mg/dL |
41 ± 13 | 38 ± 11 | 40 ± 13 | 46 ± 15 | <0.001 |
| Low density lipoprotein cholesterol, mg/dL |
97 ± 36 | 93 ± 38 | 96 ± 30 | 101 ± 38 | 0.33 |
Abbreviations: ArylE: Arylesterase. ACE: Angiotensin converting enzyme. ARB: Angiotensin II receptor blockers.
Serum ArylE activity and clinical variables
On univariate linear regression analysis, increased HDL-cholesterol levels (β=0.61, p<0.001), female gender (β=8.2, p=0.024) and ACEi/ARBs use (β=10.8, p=0.01) were associated with increased activity of baseline ArylE activity, while log transformed BNP (β=−3.1, p=0.01) was inversely correlating to ArylE activity. However, on multivariate linear regression including basic demographics, medication use and EF, only HDL-cholesterol (β=0.63, p<0.001) and ACEi/ARBs use (β=13.3, p=0.01) were independently associated with increased baseline ArylE activity.
Baseline ArylE activity and HF adverse outcomes
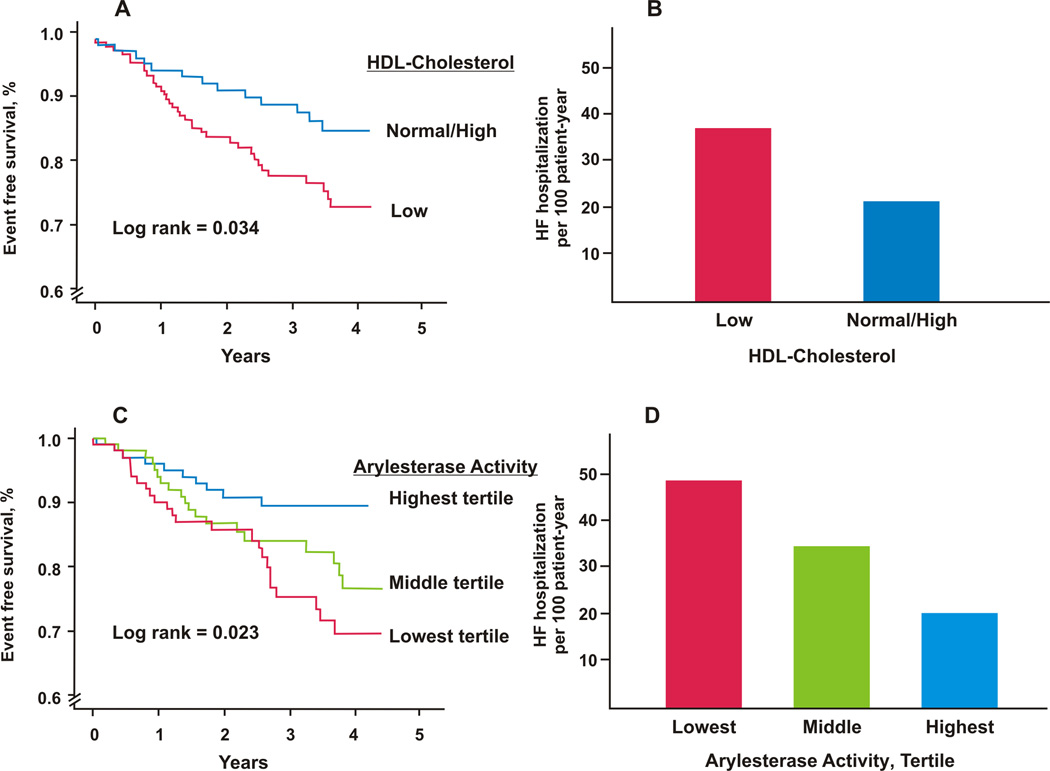
After mean follow up of 2.8 ± 1.1 years, 53 patients had HF events (42 deaths, 7 transplants, 4 ventricular assist devices), while 95 patients experienced a total of 283 HF related hospitalizations. Distribution of HF related hospitalizations is shown in Online Figure 1. Patients with low HDL-cholesterol (<40 mg/dL for men and <50 mg/dL for women) had higher risk of HF events (log rank = 0.034) but not HF related hospitalizations (p=0.298), (Figure 1A&B).
Figure 1.
Baseline HDL-Cholesterol levels association with: A) HF events (Death, transplant, or LVAD), and B) HF related hospitalizations. Low HDL-Cholesterol was defined as HDL-Cholesterol <40 mg/dL for men or <50 mg/dL for women. Baseline ArylE activity association with: C) HF events, and D) HF related hospitalizations.
Patients, who had HF events, had significantly lower ArylE activity (102.4±27.8 vs 112.3±30.2 µmol/ml/min, p=0.028). Patients with low ArylE activity had a higher incidence of HF events (23.5% vs 9.9%, for lowest vs highest tertile, p=0.031) and HF related hospitalization (49 vs 20.2 per 100-patient-years for lowest vs highest tertile, respectively). Patients with lower levels of ArylE activity, have a significantly increased risk of HF events [HR(95%CI) for lowest vs highest tertile of 2.6 (1.3–5.5), p=0.01] and HF related hospitalization [incidence rate ratio (95%CI) for lower vs higher tertile of 2.1 (1.2–4.1), p=0.016] (Figure 1 C & D, respectively) (Table 2). ArylE activity remained significant even after adjustment for age, gender, diabetes, systolic blood pressure, creatinine clearance and CAD (Table 2). In a subset of patients with available baseline HDL-cholesterol levels (n=253), low ArylE activity was independently associated with adverse HF events and hospitalization, even after adjustment for HDL-cholesterol levels.
Table 2.
ArylE activity and adverse HF outcomes.
| ArylE activity | Continuous variable, per 10 µmol/L/min/mL |
Tertile 1 <97 |
Tertile 2 97–120 |
Tertile 3 ≥120 |
|---|---|---|---|---|
| Baseline levels (n=299) | ||||
| Events, % | 23.5 | 18.8 | 9.9 | |
| Unadjusted model, HR(95%CI) |
β = −0.12* | 2.6 (1.3 – 5.5)* |
2 (0.9 – 4.2) | Reference |
| Adjusted model1, HR(95%CI) |
β = −0.11* | 2.2 (1.04 – 4.7)* |
1.7 (0.8 – 3.8) |
Reference |
| Adjusted model 2, HR(95%CI) |
β = −0.11* | 2.4 (1.03 – 5.4)* |
1.8 (0.7–4.2) | Reference |
| HF admission | ||||
| Per 100-patient-year | 49 | 31.4 | 20.2 | |
| Unadjusted model, Incidence rate ratio (95CI%) |
β = −0.1* | 2.2 (1.2– 4.1)* |
1.5 (0.8–1.5) | Reference |
| Adjusted model 1, Incidence rate (95CI%) |
β =−0.1* | 1.9 (1.004– 3.6)* |
1.4 (0.7 – 2.8) |
Reference |
| Adjusted model 2, Incidence rate (95CI%) |
β = − 0.1* | 3.4 (1.7– 6.7)* |
1.9 (0.9–3.9) | Reference |
| Follow up levels (n=145) | ||||
| Events, % | 14.9 | 10.2 | 4.1 | |
| Unadjusted model, HR(95%CI) |
β =−0.22* | 4.2 (0.9– 20.3) |
2.9 (0.6– 15.1) |
Reference |
| Adjusted model for baseline levels, HR(95%CI) |
β = −0.29* | 6 (1.04– 34.7)* |
3.6 (0.7– 20.1) |
Reference |
| HF admission | ||||
| Per 100-patient-years | 30.1 | 33.8 | 23.2 | |
| Unadjusted model, Incidence rate (95CI%) |
β = −0.02 | 1.2 (0.4–3.3) | 1.3 (0.48– 3.7) |
Reference |
| Adjusted for baseline levels, Incidence rate (95CI%) |
β =−0.01 | 1.1 (0.35– 3.2) |
1.2 (0.43– 3.6) |
Reference |
Model 1: Age, male, systolic blood pressure, diabetes, creatinine clearance and coronary artery disease.
Model 2: Model 1 + HDL-cholesterol levels (only 253 patients had available HDL-cholesterol).
p<0.05
Abbreviations: ArylE: Arylesterase. HR: hazard ratio. CI: confidence interval.
Follow up ArylE activity and HF outcomes
Total of 145 patients had 1 year follow up clinic visit and ArylE activity was reassessed. No significant difference was found in patients with follow up levels and those without in regard to basic demographics and HF severity (EF, BNP)(Online Table 1). However, HF events were lower (9.7% vs 25.3%, p=0.001) in the group with available follow up samples for ArylE activity measurement.
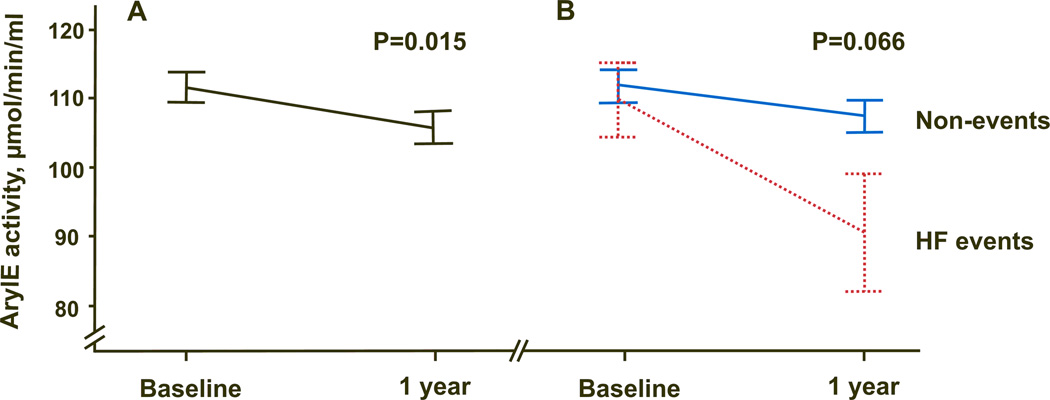
Significant decrease in ArylE activity was observed on follow up (−5.9 ± 28.6 µmoles/min/mL, p=0.015)(Figure 2A). No significant correlation was found between basic demographics, medications, BNP or EF and change in arylE activity overtime. When the change in ArylE activity overtime was stratified by HF events, those who developed HF events had a trend of higher drop in ArylE activity overtime (mean of −19.2±37.3 vs −4.4±27.3 µmol/min/mL, for events and non-events, respectively, p=0.066) (Figure 2B).
Figure 2.
ArylE activity decreased over time in patients with available follow up blood samples (n=145) in A) overall population, B) stratified by event status.
After adjustment for baseline level, low follow up ArylE activity was also independently associated with increased HF events [lowest tertile vs highest tertile HR(95%CI) of 6 (1.04–34.7), adjusted p=0.046](Table 2). Drop in ArylE activity was associated with increased risk of HF events [40% (95%CI of 8–80%) increased risk for every 10% drop; or HR(95%CI) of 4.9 (1.6–14.6), for a drop ≥25%, adjusted p value for baseline levels of 0.01 and 0.005, respectively] (Figure 3). No correlation between follow up ArylE activity and HF related hospitalization was observed (Table 2).
Figure 3.
Correlation between ArylE activity change between baseline and one year follow up samples, and long term HF events (Death, transplant or LVAD).
Serum paraoxonase activity and HF outcomes
We also assessed paraoxonase activity (hydrolyzes organophosphates, like paraoxon) of PON-1 on baseline and on follow up. Paraoxonase activity was positively skewed with a median (interquartile range; IQR) baseline of 829 (350 – 1233) nmol/min/mL, which increased significantly on follow up to 883 (351 – 1414)nmol/min/mL, p=0.015. In contrast to ArylE activity, patients who had HF events, did not have lower paraoxonase activity on baseline [median (IQR) of 754 (291–1023) vs 883 (368–1270), p=0.123) or follow up [474 (286 – 1044) vs 915 (405–1424) nmol/min/mL, p=0.301). No significant correlation was found between either baseline or follow up paraoxonase activity and HF events or HF related hospitalization.
Results validation and association with Apolipoprotein A1
We validated our results using a longer follow up time (5 years) of our previously published cohort of 830 patients with HF undergoing elective coronary angiography enrolled from the Cleveland Clinic Genbank database (Online supplement). The mean age was 67±11 years, 60% were males and 76% had coronary artery disease (Online table 2). Mean ArylE activity was 94.2 ± 26.3, and mean apolipoprotein A1 was 113.6 ± 25.2 mg/dL. There was a positive correlation between apolipoprotein A1 and ArylE activity (β=0.42, R2=0.16, p<0.001). In this cohort, both ArylE activity and apolipoprotein A1 were associated with 5-year all-cause mortality. However, when both included in one model, only ArylE activity was significantly associated with all-cause mortality (Online table 3).
DISCUSSION
We demonstrate that in an outpatient population of chronic HF, impaired anti-oxidant function of HDL as measured by serum ArylE activity was associated with adverse HF events and HF related hospitalizations. We also observed a significant decrease in ArylE activity overtime, mainly in patients who developed adverse HF events. We didn’t see any correlation between HF events or hospitalizations and paraoxonase activity. Furthermore, using our previously published cohort we found ArylE activity to be independent of apolipoprotein A1 levels in predicting adverse outcomes. These findings are supportive of our initial observations, and confer HDL as an important mediator that may serve to prevent disease progression in HF.
Patients with HF have increased oxidative stress in comparison to healthy population.(11, 12) Furthermore, HF patients with high oxidative stress, have higher disease severity, (11–13) hospitalization and adverse outcomes.(11) Myocardial ischemia, toxins, and infection, are main factors suggested to increase oxidative stress in HF patients.(1) Increased oxidative stress in cardiomyocytes can cause exaggerated autophagy, apoptosis or necrosis, which subsequently cause progressive loss of cardiomyocytes and left ventricular remodeling.(1, 2, 14–16) On the other hand, progression of HF with increased volume status and myocardial stretching, increases myocardial oxidative stress and mitochondrial dysfunction.(13, 17) Thus, anti-oxidant activity may play a major role in modifying oxidative stress and subsequently, may prevent progression of HF.(17)
Circulating PON-1 is the major “detoxification” protein that is bound to apolipoprotein A1 in the HDL particle.(3) PON-1 has been shown to be involved in inhibiting LDL oxidation, cholesterol efflux, monocyte binding and transmigration, plaque inflammation, as well as destroying oxidized phospholipids and thus decrease atherosclerosis and plaque formation.(18, 19) Furthermore, by hydrolyzing homocysteine thiolactones, PON-1 may prevent endothelial dysfunction and vascular damage.(7, 20, 21) The clinical significance of PON-1 activity is still controversial. Concordant with the athero-protective role of ArylE activity, several studies have shown low activity to be associated with increased CAD risk, CAD severity, endothelial dysfunction,(22) worse collateral flow and adverse cardiovascular outcomes.(7, 19, 20, 23–25) However, in contrary to this, few other studies showed high activity to be associated with increased CAD severity, coronary calcification and increased risk of myocardial infarction,(19, 26, 27) while other studies have not shown any such associations.(28, 29)
Only few studies assessed serum ArylE activity in the setting of HF. In our previous report, we found patients with HF to have diminished ArylE activity in comparison to healthy control group.(8) We also showed that diminished ArylE activity is associated with all-cause mortality, myocardial infarction, and stroke in patients with ischemic cardiomyopathy.(3) We have largely related this to the anti-atherogenic role of PON-1 since the study population included mainly patients with ischemic HF. However, in this group of patients with mixed ischemic and non-ischemic HF, we also showed a significant association with adverse HF events (death, cardiac transplantation, LVAD). Furthermore, using long term data from our previously cohort, (3) we found ArylE activity to carry more prognostic information above and beyond the levels of apolipoprotein A1. Taken together, the association of diminished serum ArylE activity and adverse outcomes is likely independent of the underlying etiology of HF, and more reflective of anti-oxidative properties of HDL than the levels of apolipoprotein A1. It’s possible that PON-1, in addition to its anti-atherosclerotic effect, plays a direct protective role on cardiac myocyte by decreasing oxidative injury and thus alters the progression of HF.
It is still unclear what the factors that influence diminished serum ArylE activity are. In our population, only ACEi/ARBs use and baseline HDL-cholesterol levels were independent predictors of high baseline ArylE activity. However, no factor was associated with increased ArylE activity on follow up. Several other studies have shown an increase in ArylE activity after statin treatment, (30–32) however, only one was a randomized control study.(30) Since diminished PON-1 activity is linked to adverse outcomes in patients with HF, targeting those with diminished PON-1 activity may provide a protective role in HF. Although previous trials that used anti-oxidants or statins, failed to show mortality benefit, (33–38) these trials were testing the effect of the intervention regardless of the oxidative status of the patient. Thus, the beneficial effect could be masked by those with low oxidative status, who may not benefit from the intervention. Whether identifying the heightened risk patients, like those with diminished PON-1 activity, could affect HF outcomes need to be further investigated.
Interestingly, we observed a discordance between the two PON-1 activities in predicting adverse HF outcomes. These findings are consistent with our previous observations of no association between paraoxonase activity and subclinical myocardial necrosis and adverse cardiovascular outcomes in patients with CAD undergoing coronary angiogram.(3) The unique relationship between the 2 PON-1 activity measurements is largely the result of a very strong association between serum paraoxonase activity and its underlying genetic determinants, which may also explain why serum ArylE activity is normally distributed whereas serum paraoxonase activity was not.
In clinical practice, clinicians tend to treat HF patients based on their ejection fraction, without considering the underlying mechanism. Our findings are important in the context that, among different HF patients, there is a variable involvement of different mechanisms, like inflammation,(39–41) oxidative stress, (11, 12) microvascular rarefaction,(42) pressure overload,(43) endothelial dysfunction,(1)…etc. Thus, profiling HF patients by a more mechanistic driven way, may help identifying heightened risk patients and provide a more individualized HF therapy. Whether treating patients with low PON-1 activity with statins, or other medications have an impact on outcome need to be further investigated.(44)
Study strengths and limitations
Our study is the first study to assess serial PON-1 activity in outpatients with HF and correlate that with their prospective outcomes. However, the small sample size and low number of patients with follow up samples are the major limitations of our study. Also, it’s worth noting that, serum paraoxonase and ArylE activities used substrates that are not the endogenous substrates for PON-1, but are used because they are not readily influenced by other esterases/lactonases in serum, and are presumed to reflect the underlying catalytic activity of PON-1.(8) We did not assess genotyping or circulating levels of PON-1 that may also impact the prognostic value of ArylE activity in this population.
CONCLUSION
Diminished serum PON-1 activity, measured by ArylE activity, is associated with adverse outcomes in stable outpatients with HF.
Supplementary Material
Acknowledgments
FUNDING
This research was supported by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL103931, R01HL103866, P20HL113452, R01DK106000, P01HL076491, P01HL098055).
Dr. Kalogeropoulos reports research support from the NIH and AHA. Dr.Georgiopoulou reports research support from NIH. Dr. Hazen is named as co-inventor on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Hazen reports having been paid as a consultant for the following companies: Esperion, and Procter &Gamble. Dr. Hazen reports receiving research funds from Procter &Gamble, Pfizer Inc., Roche Diagnostics and Takeda. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Cleveland Heart Lab., Siemens, Esperion, Frantz Biomarkers, LLC, and P&G. Dr. Butler reports research support from the NIH, and the European Union; and is a consultant to Amgen, Bayer, Cardiocell, Celladon, Novartis, Ono Pharma, Takeda, StealthPeptide, Trevena, and Zensun.
ABBREVIATIONS
- ArylE
Arylesterase
- PON-1
paraoxonase-1
- HF
heart failure
- EF
ejection fraction
- CAD
Coronary artery disease
Footnotes
DISCLOSURE
All other authors have no relationships to disclose.
REFERENCES
- 1.Paulus WJ, Tschöpe C. A Novel Paradigm for Heart Failure With Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. Journal of the American College of Cardiology. 2013 Jul 23;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 2.Hare JM. Oxidative stress and apoptosis in heart failure progression. Circulation research. 2001 Aug 3;89(3):198–200. [PubMed] [Google Scholar]
- 3.Tang WHW, Hartiala J, Fan Y, Wu Y, Stewart AFR, Erdmann J, Kathiresan S, The CC, Roberts R, McPherson R, Allayee H, Hazen SL. Clinical and Genetic Association of Serum Paraoxonase and Arylesterase Activities with Cardiovascular Risk. Arteriosclerosis, thrombosis, and vascular biology. 2012 Sep 13;32(11):2803–2812. doi: 10.1161/ATVBAHA.112.253930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis C, Williams OD, Hulley SB. Racial Differences in Incident Heart Failure among Young Adults. The New England journal of medicine. 2009;360(12):1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velagaleti RS, Massaro J, Vasan RS, Robins SJ, Kannel WB, Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation. 2009 Dec 8;120(23):2345–2351. doi: 10.1161/CIRCULATIONAHA.109.830984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Wu Z, Riwanto M, Gao S, Levison BS, Gu X, Fu X, Wagner MA, Besler C, Gerstenecker G, Zhang R, Li XM, DiDonato AJ, Gogonea V, Tang WH, Smith JD, Plow EF, Fox PL, Shih DM, Lusis AJ, Fisher EA, DiDonato JA, Landmesser U, Hazen SL. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. The Journal of clinical investigation. 2013 Sep;123(9):3815–3828. doi: 10.1172/JCI67478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarandol A, Kirli S, Akkaya C, Ocak N, Eroz E, Sarandol E. Coronary artery disease risk factors in patients with schizophrenia: effects of short term antipsychotic treatment. J Psychopharmacol. 2007 Nov;21(8):857–863. doi: 10.1177/0269881107077609. [DOI] [PubMed] [Google Scholar]
- 8.Wilson Tang WH, Wu Y, Mann S, Pepoy M, Shrestha K, Borowski AG, Hazen SL. Diminished Anti-Oxidant Activity of High-Density Lipoprotein-Associated Proteins in Systolic Heart Failure. Circulation Heart failure. 2011 Nov 09;4(1):59–64. doi: 10.1161/CIRCHEARTFAILURE.110.958348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers JK, McMurray JJ, Pocock SJ, Zannad F, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms: analysis of repeat hospitalizations. Circulation. 2012 Nov 6;126(19):2317–2323. doi: 10.1161/CIRCULATIONAHA.112.110536. [DOI] [PubMed] [Google Scholar]
- 10.Jahn-Eimermacher A. Comparison of the Andersen-Gill model with poisson and negative binomial regression on recurrent event data. Comput Stat Data Anal. 2008;52(11):4989–4997. [Google Scholar]
- 11.Hirata Y, Yamamoto E, Tokitsu T, Kusaka H, Fujisue K, Kurokawa H, Sugamura K, Maeda H, Tsujita K, Yamamuro M, Kaikita K, Hokimoto S, Sugiyama S, Ogawa H. Reactive oxidative metabolites are associated with the severity of heart failure and predict future cardiovascular events in heart failure with preserved left ventricular ejection fraction. International Journal of Cardiology. 2015 Jan 20;179:305–308. doi: 10.1016/j.ijcard.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (SOLVD) Journal of the American College of Cardiology. 1996 Apr;27(5):1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 13.Lommi J, Pulkki K, Koskinen P, Naveri H, Leinonen H, Harkonen M, Kupari M. Haemodynamic, neuroendocrine and metabolic correlates of circulating cytokine concentrations in congestive heart failure. European heart journal. 1997 Oct;18(10):1620–1625. doi: 10.1093/oxfordjournals.eurheartj.a015142. [DOI] [PubMed] [Google Scholar]
- 14.Gurusamy N, Das DK. Autophagy, redox signaling, and ventricular remodeling. Antioxidants & redox signaling. 2009 Aug;11(8):1975–1988. doi: 10.1089/ars.2009.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penn MS. The role of leukocyte-generated oxidants in left ventricular remodeling. The American journal of cardiology. 2008 May 22;101(10a):30d–33d. doi: 10.1016/j.amjcard.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Tang WH, Tong W, Shrestha K, Wang Z, Levison BS, Delfraino B, Hu B, Troughton RW, Klein AL, Hazen SL. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. European heart journal. 2008 Oct;29(20):2506–2513. doi: 10.1093/eurheartj/ehn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yancey DM, Guichard JL, Ahmed MI, Zhou L, Murphy MP, Johnson MS, Benavides GA, Collawn J, Darley-Usmar V, Dell'Italia LJ. Cardiomyocyte mitochondrial oxidative stress and cytoskeletal breakdown in the heart with a primary volume overload. American journal of physiology Heart and circulatory physiology. 2015 Mar 15;308(6):H651–H663. doi: 10.1152/ajpheart.00638.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackness B, Davies GK, Turkie W, Lee E, Roberts DH, Hill E, Roberts C, Durrington PN, Mackness MI. Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol. 2001 Sep;21(9):1451–1457. doi: 10.1161/hq0901.094247. [DOI] [PubMed] [Google Scholar]
- 19.Kresanov P, Vasankari T, Ahotupa M, Kaikkonen J, Hutri-Kahonen N, Juonala M, Kahonen M, Lehtimaki T, Viikari J, Raitakari OT. Paraoxonase-1 and oxidized lipoprotein lipids. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2015 Jun 6;241(2):502–506. doi: 10.1016/j.atherosclerosis.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Yildiz A, Sezen Y, Gur M, Yilmaz R, Demirbag R, Erel O. Association of paraoxonase activity and coronary collateral flow. Coron Artery Dis. 2008 Nov;19(7):441–447. doi: 10.1097/MCA.0b013e328309b9f0. [DOI] [PubMed] [Google Scholar]
- 21.Perla-Kajan J, Jakubowski H. Paraoxonase 1 protects against protein N-homocysteinylation in humans. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010 Mar;24(3):931–936. doi: 10.1096/fj.09-144410. [DOI] [PubMed] [Google Scholar]
- 22.Pasqualini L, Cortese C, Marchesi S, Siepi D, Pirro M, Vaudo G, Liberatoscioli L, Gnasso A, Schillaci G, Mannarino E. Paraoxonase-1 activity modulates endothelial function in patients with peripheral arterial disease. Atherosclerosis. 2005 Dec;183(2):349–354. doi: 10.1016/j.atherosclerosis.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, Watt M, Mackness M. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation. 2003 Jun 10;107(22):2775–2779. doi: 10.1161/01.CIR.0000070954.00271.13. [DOI] [PubMed] [Google Scholar]
- 24.Graner M, James RW, Kahri J, Nieminen MS, Syvanne M, Taskinen MR. Association of paraoxonase-1 activity and concentration with angiographic severity and extent of coronary artery disease. J Am Coll Cardiol. 2006 Jun 20;47(12):2429–2435. doi: 10.1016/j.jacc.2006.01.074. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya T, Nicholls SJ, Topol EJ, et al. RElationship of paraoxonase 1 (pon1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299(11):1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birjmohun RS, Vergeer M, Stroes ES, Sandhu MS, Ricketts SL, Tanck MW, Wareham NJ, Jukema JW, Kastelein JJ, Khaw KT, Boekholdt SM. Both paraoxonase-1 genotype and activity do not predict the risk of future coronary artery disease; the EPIC-Norfolk Prospective Population Study. PLoS One. 2009;4(8):e6809. doi: 10.1371/journal.pone.0006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burdon KP, Langefeld CD, Beck SR, Wagenknecht LE, Carr JJ, Freedman BI, Herrington D, Bowden DW. Association of genes of lipid metabolism with measures of subclinical cardiovascular disease in the Diabetes Heart Study. Journal of medical genetics. 2005 Sep;42(9):720–724. doi: 10.1136/jmg.2004.029850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackness B, Marsillach J, Elkeles RS, Godsland IF, Feher MD, Rubens MB, Flather MD, Humphries SE, Cooper J, Mackness M. Paraoxonase-1 is not associated with coronary artery calcification in type 2 diabetes: results from the PREDICT study. Disease markers. 2012;33(2):101–112. doi: 10.3233/DMA-2012-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thyagarajan B, Jacobs DR, Jr, Carr JJ, Alozie O, Steffes MW, Kailash P, Hayes JH, Gross MD. Factors associated with paraoxonase genotypes and activity in a diverse, young, healthy population: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Clinical chemistry. 2008 Apr;54(4):738–746. doi: 10.1373/clinchem.2007.099044. [DOI] [PubMed] [Google Scholar]
- 30.Akalin Ciftci G, Ertorun I, Akalin A, Alatas IO, Musmul A. The effects of atorvastatin on antioxidant/antiinflammatory properties of HDLs in hypercholesterolemics. Turk J Med Sci. 2015;45(2):345–351. doi: 10.3906/sag-1311-91. [DOI] [PubMed] [Google Scholar]
- 31.Mirdamadi HZ, Sztanek F, Derdak Z, Seres I, Harangi M, Paragh G. The human paraoxonase-1 phenotype modifies the effect of statins on paraoxonase activity and lipid parameters. British journal of clinical pharmacology. 2008 Sep;66(3):366–374. doi: 10.1111/j.1365-2125.2008.03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomas M, Senti M, Garcia-Faria F, Vila J, Torrents A, Covas M, Marrugat J. Effect of simvastatin therapy on paraoxonase activity and related lipoproteins in familial hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2000 Sep;20(9):2113–2119. doi: 10.1161/01.atv.20.9.2113. [DOI] [PubMed] [Google Scholar]
- 33.Thomson MJ, Puntmann V, Kaski JC. Atherosclerosis and oxidant stress: the end of the road for antioxidant vitamin treatment? Cardiovasc Drugs Ther. 2007 Jun;21(3):195–210. doi: 10.1007/s10557-007-6027-1. [DOI] [PubMed] [Google Scholar]
- 34.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002 Jul 6;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 35.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005 Mar 16;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 36.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J., Jr Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006 Apr 13;354(15):1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 37.Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Janosi A, Kamensky G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007 Nov 29;357(22):2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 38.investigators G-H. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. The Lancet. 372(9645):1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 39.Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JD, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. The Journal of experimental medicine. 2003 Mar 3;197(5):615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang WH, Brennan ML, Philip K, Tong W, Mann S, Van Lente F, Hazen SL. Plasma myeloperoxidase levels in patients with chronic heart failure. The American journal of cardiology. 2006 Sep 15;98(6):796–799. doi: 10.1016/j.amjcard.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Hammadah M, Fan Y, Wu Y, Hazen SL, Tang WH. Prognostic value of elevated serum ceruloplasmin levels in patients with heart failure. Journal of cardiac failure. 2014 Dec;20(12):946–952. doi: 10.1016/j.cardfail.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammadah M, Georgiopoulou VV, Kalogeropoulos AP, Weber M, Wang X, Samara MA, Wu Y, Butler J, Tang WH. Elevated Soluble Fms-Like Tyrosine Kinase-1 and Placental-Like Growth Factor Levels Are Associated With Development and Mortality Risk in Heart Failure. Circ Heart Fail. 2016 Jan;9(1):e002115. doi: 10.1161/CIRCHEARTFAILURE.115.002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson KL. Pressure overload hypertrophy and congestive heart failure*Where is the “Achilles’ heel”? Journal of the American College of Cardiology. 2002;39(4):672–675. doi: 10.1016/s0735-1097(01)01790-9. [DOI] [PubMed] [Google Scholar]
- 44.Katsiki N, Doumas M, Mikhailidis DP. Lipids, Statins and Heart Failure: An update. Current pharmaceutical design. 2016 Jun 30; doi: 10.2174/1381612822666160701073452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.