Abstract
Background
Hypotension and bradycardia are known side effects of spinal anesthesia in pregnant women undergoing cesarean section and adults undergoing other surgical procedures. Whether children experience similar hemodynamic changes is unclear.
Aims
The purpose of this study is to evaluate hemodynamic effects of spinal anesthesia compared to general anesthesia in a cohort of healthy infants.
Methods
The University of Vermont Medical Center almost exclusively used spinal anesthesia for infant pyloromyotomy surgery between 2008–2013, while Columbia University Medical Center relied on general anesthesia. The primary outcome assessed was the percentage change in intraoperative heart rate and blood pressure (systolic [SBP] and mean [MAP] blood pressure) from baseline. Analysis was performed using t-tests for continuous variables, followed by linear regression to account for differences in demographic and clinical covariates.
Results
The study sample consisted of 51 infants with spinal anesthesia at the University of Vermont and 52 infants with general anesthesia at Columbia University. The decrease from baseline for mean intraoperative SBP was −8.2 ± 16.8% for SA and −24.2 ± 17.2% for GA (difference between means: 16.2% [95% confidence interval (CI), 9.5–22.9]), while the decrease from baseline for mean intraoperative MAP was −16.3 ± 19.9% for SA and −24.6 ± 19.3% for GA (difference between means: 8.4% [95% CI, 0.8–16]). SA patients also had smaller drops in minimum intraoperative MAP and SBP. These blood pressure differences persisted even after adjusting for covariates. No differences in heart rate were seen between spinal and general anesthesia.
Discussion
Our findings show that spinal anesthesia performed in healthy infants undergoing pyloromyotomy results in reduced intraoperative blood pressure changes from baseline, significantly higher blood pressure readings, and no increased bradycardia compared to general anesthesia. Further research is needed to assess whether any clinical impact of these hemodynamic differences between spinal and general anesthesia exists.
Keywords: General Anesthesia, Local Anesthetics, Infant, Hemodynamics, Blood Pressure, Heart Rate
Introduction
The use of regional anesthesia in children may be beneficial in a variety of clinical scenarios.(1) These situations include a desire for improved intraoperative pain management, the avoidance of airway manipulation and respiratory depression, or the concern for potential neurotoxic effects of inhaled or intravenous anesthetics.(2–4) Spinal anesthesia (SA) specifically can be used in infants for certain intra-abdominal and lower extremity procedures with success rates ranging from 93 to 95%.(5, 6) While SA can potentially allow for the avoidance of intubation and exposure to intravenous (IV) and inhaled anesthetics, hypotension and bradycardia are potential side effects. Hemodynamic changes associated with spinal anesthesia are commonly seen in adults with hypotension occurring in 33% and bradycardia in 13% of patients.(7) Decreases in blood pressure are particularly common in pregnant women receiving SA for cesarean section, where 50–60% are found have hypotension.(8) While hemodynamic changes after SA in adults are well known, few studies have been performed in children. The purpose of this study is to evaluate the hemodynamic effects of SA compared to general anesthesia (GA) in a cohort of healthy infants.
Methods
The University of Vermont Medical Center (UVM) uses regional anesthesia (either spinal or epidural anesthesia) as its preferred anesthetic technique for all infant surgery below the level of the chest, and almost exclusively uses spinal anesthesia for pyloromyotomy surgery, while Columbia University Medical Center (CUMC) almost exclusively uses general anesthesia. Infants receiving a pyloromyotomy procedure at CUMC or UVM had been previously identified, and the present study involves those patients who had available electronic anesthetic record and baseline hemodynamic data, and were American Society of Anesthesiologists (ASA) physical status of 1 or 2 at the time of surgery.(9) Children undergoing pyloromyotomy procedures were chosen because they are often otherwise healthy full-term infants. Infants at UVM where successful placement of a spinal block could not be achieved were excluded because they did not actually receive a spinal anesthetic injection.
Data Source
Electronic anesthetic record keeping was implemented in January 2008 at UVM and August 2008 at CUMC, and as result all pyloromyotomies performed before those dates were excluded. Following IRB approval at UVM and CUMC, eligible patients were identified between the date of implementation of electronic anesthetic records at each center and January 31st 2013. We included only those infants with available electronic anesthetic records, as automatically collected intraoperative vital signs are likely to be more reliable than those recorded in paper records.(10) Hemodynamic data were recorded using CompuRecord at CUMC (CompuRecord, Philips Medical, Andover, MA) and Optum Anesthesia Manager at UVM (Optum Anesthesia Manager, Optum, Eden Prairie, MN). The hemodynamic parameters evaluated included intraoperative systolic (SBP), mean blood pressure (MAP), as well as intraoperative heart rate (HR). All blood pressure (BP) readings were performed using appropriately sized non-invasive BP cuffs and heart rates were obtained from the intraoperative electrocardiogram. CUMC used General Electric Solar 8000i (GE Medical Systems, Milwaukee, WI) patient monitors while UVM used Philips Intellivue (Philips Healthcare, Andover, MA) monitors. Both monitors are oscillometric BP measurement machines. Baseline BP and HR readings were measured prior to the patient entering the operating room. Since pre-operative records only recorded baseline SBP and DBP, baseline MAP was calculated using the following formula: (2*DBP + SBP)/3. Patients without baseline hemodynamic data recorded were excluded. Perioperative data including anesthetic medications were abstracted from anesthetic and medical records.
Statistical analysis
Intraoperative BP was assessed approximately every 5 minutes at UVM and every 3 minutes at CUMC. Since BP is commonly rechecked when a particularly low or high BP value is seen, this can potentially generate a higher number of recorded outlier BP values. In order to account for potential differences in the timing of the recorded BP values, each BP value was considered to be representative of the time period (in seconds) between when it was recorded and when the following BP value was recorded. The mean intraoperative SBP and MAP values for each patient were calculated by summing up all BP values for each second of the case and dividing it by the total duration of the case in seconds. The mean percentage change from the baseline hemodynamic values for each patient was then calculated by comparing the minimum, maximum, and mean intraoperative BP and HR values with the respective baseline BP and HR values. The mean percentage change from baseline for SBP, MAP, and HR were the primary outcomes of interest. Differences in baseline hemodynamic values and mean percentage changes in hemodynamic values from baseline between SA and GA patients were evaluated with t-tests. The a priori P-value was set to P<0.05 with 95% confidence intervals (CI) calculated. As a sensitivity analysis, linear regression was used to evaluate the association between absolute differences in hemodynamic parameters and anesthetic technique after adjusting for categorical variables: sex, preterm birth (defined as <37 weeks gestational age), and continuous variables: baseline hemodynamic value (BP or HR in each model), age in days at the time of procedure, and duration in minutes of the procedure. Further sensitivity analyses including assessing the impact of open vs. laparoscopic surgery and location of the BP cuff were also performed by evaluating mean percentage changes in hemodynamic values from baseline in subgroups of SA vs. GA patients. Absolute differences in intraoperative hemodynamic values between the children at UVM included in the primary analysis and those excluded due to lack of baseline hemodynamic data were also evaluated. T-tests were used for these sensitivity analyses with the a priori P-value also set to P<0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Spinal Anesthesia Technique
Since this is an observational study, no standardized anesthetic protocols were followed. At UVM lumbar puncture is performed in the sitting or lateral decubitus position with a midline approach at the L3/L4 or L4/L5 level. Cerebrospinal fluid (CSF) is obtained using a 22g or 25g Quincke needle, and local anesthetic with epinephrine in a 1-mL tuberculin syringe injected to produce a subarachnoid block with adequacy of block tested as previously described.(9) If a sufficient sensory level cannot be obtained, the block is considered a failure and the child intubated and maintained under general anesthesia for the procedure and excluded from analysis in this study. Peripheral IV access is typically established in the lower extremity after performing the spinal block.
General Anesthesia Technique
At CUMC, infants are brought into the operating room and awake oral gastric suctioning performed to empty the stomach. Patients are then placed in the supine position and intubated awake or using a rapid sequence intubation (RSI) technique. The decision to perform either an awake intubation or RSI was based on attending preference. At the end of the procedure, all patients in this study were extubated awake.
Results
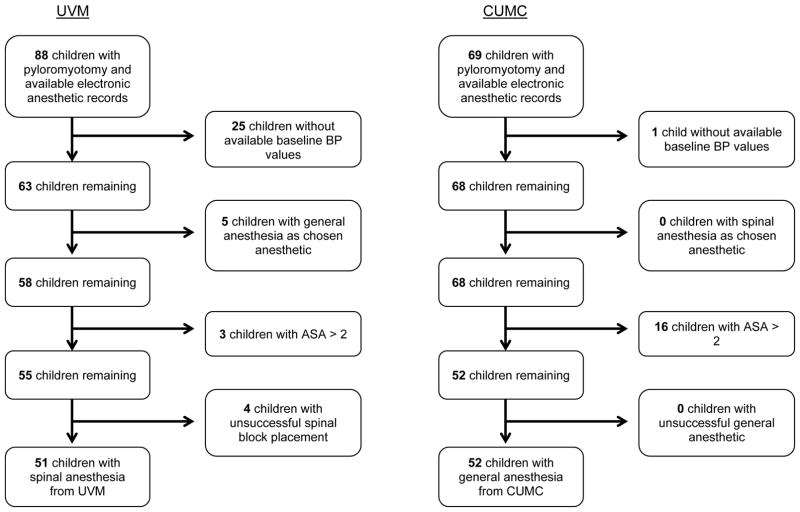
Records were obtained for 51 eligible infants from UVM and 52 eligible infants from CUMC. (Figure 1) The infants from the two groups were demographically similar, with the SA group composed of 84.3% boys and GA group 80.8% boys. The weights (4.0 ± 0.7 in SA and 4.1 ± 0.8 kg in GA) and ages at the time of surgery (39.6 ± 18.5 in SA vs. 38.9 ± 17.2 days in GA) were also similar. (Table 1) Of the SA group, 11.8% were preterm with a gestation age less than 37 weeks, while in the GA group 9.6% were preterm. Preoperative levels of sodium (138.3 ± 2.7 in SA vs. 137.9 ± 2.6 mmol/L in GA), chloride (102.7 ± 6.3 in SA vs. 105.2 ± 4.1 mmol/L in GA), and potassium (4.7 ± 0.8 in SA and 4.6 ± 0.5 mmol/L in GA) were also found to be similar. All of the patients who had SA had an open pyloromyotomy, while in the patients with GA, 36.5% (n=19) had an open procedure and the remainder had a laparoscopic procedure. The mean duration of anesthesia was longer for the GA patients than the SA patients (90.3 ± 21.2 vs. 72.6 ± 14.1 minutes respectively).
Figure 1.
Children with pyloromyotomy at the University of Vermont Medical Center and Columbia University Medical Center
Table 1.
Demographic and Clinical Data
| Spinal Anesthesia (n=51) | General Anesthesia (n=52) | |
|---|---|---|
| mean ± SD | mean ± SD | |
| Mean Age (days) | 39.6 ± 18.5 | 38.9 ± 17.2 |
| Weight (kg) | 4.0 ± 0.7 | 4.1 ± 0.8 |
| n (%) | n (%) | |
| Sex | ||
| Female | 8 (15.7) | 10 (19.2) |
| Male | 43 (84.3) | 42 (80.8) |
| Gestational Age | ||
| ≥37 weeks | 45 (88.2) | 47 (90.4) |
| <37 weeks | 6 (11.8) | 5 (9.6) |
| Surgical Technique | ||
| Open | 51 (100) | 19 (36.5) |
| Laparoscopic | 0 (0) | 33 (63.5) |
Spinal Anesthesia for Pyloromyotomy
Of the 51 infants where an adequate level of surgical anesthesia was obtained after SA, 2 infants were also exposed to inhalational anesthesia due to a block duration that was inadequate for completion of the procedure. These patients remained in the cohort. Tetracaine was the local anesthetic of choice for 47 of the 51 SA patients, with a mean dose of 0.77 ± 0.1 mg/kg. Of the 51 infants who had SA, 16 (31.4%) required a supplemental IV or inhaled anesthetic agent. Of these 16 infants, 6 received propofol, 2 received fentanyl, 11 received IV midazolam, and 2 received an inhalational anesthetic after an initially adequate spinal anesthetic level. Of the 2 patients who needed an inhalational anesthetic after an initial adequate spinal anesthetic level, one was exposed to sevoflurane while the other was exposed to nitrous oxide.
General Anesthesia for Pyloromyotomy
In the GA group, awake ETT placement was initially attempted in 25 of 52 infants (48.1%) and IV induction and RSI used in the remaining infants. All 52 infants who had GA were exposed to sevoflurane, for a mean duration of 67 ± 17.2 minutes, 1 was also exposed to desflurane for 78 minutes and 2 infants were also exposed to nitrous oxide for 10 minutes each. The mean intraoperative exhaled sevoflurane concentration for all GA infants was 1.43%, which was calculated using all exhaled sevoflurane values for each infant and then calculating the overall mean for all infants. The exhaled sevoflurane values were recorded every 15 seconds by the CompuRecord electronic anesthetic record keeper at CUMC. Of the 52 GA infants, 48 (92.3%) received propofol, 23 (44.2%) received fentanyl, 4 (7.8%) received remifentanil, 33 (63.5%) received rectal acetaminophen, and no child received IV midazolam. The mean propofol dose was 3.4 ± 1.2mg/kg and the mean fentanyl dose was 1.7 ± 1.0mcg/kg. Of the four children who received remifentanil, two received a 10mcg bolus for intubation while the other two received infusions of 0.15 and 0.2mcg/kg/min.
The volume of IV crystalloid administered in the GA group was 21.4 ± 9.3 mL/kg, and in the SA group was 13.2 ± 8.5 mL/kg (difference between means: 8.2 mL/kg [95% CI, 4.7–11.7]). No blood, blood products, or any vasoactive medications were administered to any GA or SA infants. Adverse events based on our prior criteria were seen in 3 patients, 2 GA patients who required more than 3 direct laryngoscopy attempts, and 1 SA patient with inadequate spinal block duration and was converted to GA.(9)
Intraoperative Hemodynamics
No baseline hemodynamic differences between the SA and GA groups were found. (Table 2) There were however several differences in intraoperative BP values, particularly the mean and minimum SBP and MAP values between SA and GA patients. For example, the mean intraoperative SBP was 18.8 mmHg higher in the SA patients compared to the GA patients (89.3 ± 13.5 in SA vs. 70.5 ± 11.1 mmHg in GA, difference between means: 18.8 mmHg [95% CI, 14–23.6]). The results in this table however did not take into account baseline HR and BP values. In subsequently evaluating the primary outcome of interest for the percentage change from baseline of the intraoperative HR and BP values, there were no differences in SA and GA with regard to changes from baseline HR. (Table 3) Patients undergoing GA however experienced significantly lower intraoperative BP values than SA patients. In the SA group, the average percent decrease from baseline in mean intraoperative MAP was −16.3 ± 19.9% while in the GA group it was −24.6 ± 19.3% (difference between means: 8.4% [95% CI, 0.8–16]). The average decrease from baseline for the minimum recorded intraoperative MAP was −36.1 ± 16.7% in the SA group and −54.5 ± 18.3% in the GA group (difference between means: 18.4% [95% CI, 11.5–25.2]). Differences in SBP were also seen with decreases in mean SBP (−8.2 ± 16.8% in SA and −24.2 ± 17.2% in GA; difference between means: 16.2% [95% CI, 9.5–22.9]) and minimum SBP (−25.9 ± 14.5% in SA and −51.4 ± 17.1% in GA; difference between means: 25.5% [95% CI, 19.3–31.7]). Linear regression was then used to compare absolute differences in intraoperative BP changes from baseline between SA and GA patients as a sensitivity analysis. (Table 4) The crude estimates simply reflected the differences in BP and HR between the SA and GA patients, which were also displayed in Table 2. Subsequent linear regression models adjusted for covariates such as baseline hemodynamics (BP or HR), sex, age at the time of the procedure, preterm birth, and duration of the procedure. After adjusting for all covariates, infants with SA were found to have absolute mean intraoperative MAP values that were 12.4 mmHg (95% CI: 7.6 – 17.3 mmHg) higher than GA patients, minimum intraoperative MAPs that were 16.1 mmHg (95% CI: 10.9 – 21.3) higher than GA, and mean intraoperative SBP values that were 23.7 mmHg (95% CI: 17.2 – 30.3 mmHg) higher than GA patients, and minimum intraoperative SBP values that were 28.2 mmHg (95% CI: 20.6 – 35.8 mmHg) higher than GA patients.(Table 4) No differences were seen with intraoperative HR after adjustment for covariates.
Table 2.
Mean Preoperative and Intraoperative Vital Signs
| Spinal Anesthesia (n=51) mean ± SD | General Anesthesia (n=52) mean ± SD | Difference SA – GA (95% CI) | p-value | ||
|---|---|---|---|---|---|
| Preoperative | Blood Pressure (mmHg) | ||||
| Baseline SBP | 98.7 ± 17.6 | 95.9 ± 13.7 | 2.8 (−2.7 – 8.3) | 0.3 | |
| Baseline MAP | 70.5 ± 12.1 | 67.5 ± 9.6 | 3 (−1.2 – 7.3) | 0.2 | |
| Heart Rate (Beats per Minute) | |||||
| Baseline HR | 137.6 ± 17.0 | 141.5 ± 17.5 | −3.8 (−10.6 – 2.9) | 0.3 | |
|
| |||||
| Intraoperative | Blood Pressure (mmHg) | ||||
| Mean intraoperative SBP | 89.3 ± 13.5 | 70.5 ± 11.1 | 18.8 (14 – 23.6) | <0.0001 | |
| Maximum intraoperative SBP | 111.3 ± 18.6 | 102.3 ± 17.6 | 9 (2 – 16.1) | 0.01 | |
| Minimum intraoperative SBP | 72.5 ± 14.5 | 45.4 ± 13.3 | 27.1 (21.6 – 32.5) | <0.0001 | |
| Mean intraoperative MAP | 57.5 ± 9.9 | 49.2 ± 8.1 | 8.3 (4.8 – 11.8) | <0.0001 | |
| Maximum intraoperative MAP | 77.9 ± 14.9 | 84.8 ± 19.8 | −6.9 (−13.8 – −0.1) | 0.047 | |
| Minimum intraoperative MAP | 44 ± 9.8 | 29.8 ± 9 | 14.3 (10.6 – 17.9) | <0.0001 | |
| Heart Rate (Beats per Minute) | |||||
| Mean intraoperative HR | 158.3 ± 17.4 | 157.7 ± 13.1 | 0.6 (−5.4 – 6.6) | 0.8 | |
| Maximum intraoperative HR | 189.9 ± 18.7 | 200.8 ± 19.3 | −10.8 (−18.3 – −3.4) | 0.005 | |
| Minimum intraoperative HR | 127.7 ± 21.2 | 124.7 ± 25.1 | 3.1 (−6 – 12.1) | 0.5 | |
Table 3.
Mean Percentage Change in Intraoperative Blood Pressure and Heart Rate from Baseline for SA and GA patients
| Spinal Anesthesia (n=51) % Change ± SD | General Anesthesia (n=52) % Change ± SD | Difference SA – GA (95% CI) | p-value | |
|---|---|---|---|---|
| Baseline SBP versus | ||||
| Mean intraoperative SBP | −8.2 ± 16.8 | −24.2 ± 17.2 | 16.2 (9.5 – 22.9) | <0.0001 |
| Maximum intraoperative SBP | 14.4 ± 22.3 | 9 ± 25.1 | 5.4 (−3.9 – 14.7) | 0.25 |
| Minimum intraoperative SBP | −25.9 ± 14.5 | −51.4 ± 17.1 | 25.5 (19.3 – 31.7) | <0.0001 |
| Baseline MAP versus | ||||
| Mean intraoperative MAP | −16.3 ± 19.9 | −24.6 ± 19.3 | 8.4 (0.8 – 16) | 0.03 |
| Maximum intraoperative MAP | 13.4 ± 29 | 28.7 ± 36.6 | −15.3 (−28.2 – −2.3) | 0.02 |
| Minimum intraoperative MAP | −36.1 ± 16.7 | −54.5 ± 18.3 | 18.4 (11.5 – 25.2) | <0.0001 |
| Baseline HR versus | ||||
| Mean intraoperative HR | 16.4 ± 17.5 | 12.8 ± 13.8 | 3.6 (−2.5 – 9.8) | 0.2 |
| Maximum intraoperative HR | 39.8 ± 20.4 | 44 ± 22.3 | −4.2 (−12.6 – 4.1) | 0.3 |
| Minimum intraoperative HR | −6.1 ± 18.4 | −11 ± 18.7 | 4.9 (−2.3 – 12.2) | 0.2 |
Table 4.
Absolute Differences in Intraoperative Hemodynamic Values between SA and GA patients
| Difference in SA – GA | Crude Estimate(95% CI) | p-value | Adjusted Estimate1(95% CI) | p-value | Adjusted Estimate2(95% CI) | p-value | Adjusted Estimate3(95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Blood Pressure (mmHg) | ||||||||
| Mean intraoperative SBP | 18.8 (14 – 23.6) | <.0001 | 18.5 (13.6 – 23.3) | <.0001 | 18.3 (13.5 – 23) | <.0001 | 23.7 (17.2 – 30.3) | <.0001 |
| Maximum intraoperative SBP | 9 (2 – 16.1) | 0.01 | 8.5 (1.4 – 15.5) | 0.02 | 8.2 (1.2 – 15.2) | 0.02 | 18 (8.5 – 27.5) | 0.0003 |
| Minimum intraoperative SBP | 27.1 (21.6 – 32.5) | <.0001 | 26.5 (21.1 – 32) | <.0001 | 26.2 (20.8 – 31.6) | <.0001 | 28.2 (20.6 – 35.8) | <.0001 |
| Mean intraoperative MAP | 8.3 (4.8 – 11.8) | <.0001 | 8.2 (4.6 – 11.8) | <.0001 | 8.1 (4.6 – 11.6) | <.0001 | 12.4 (7.6 – 17.3) | <.0001 |
| Maximum intraoperative MAP | −6.9 (−13.8 – −0.1) | 0.047 | −7.1 (−14.1 – −0.2) | 0.044 | −7.7 (−14.5 – −0.8) | 0.03 | 0.7 (−8.5 – 10) | 0.9 |
| Minimum intraoperative MAP | 14.3 (10.6 – 17.9) | <.0001 | 14.2 (10.5 – 17.9) | <.0001 | 14.1 (10.4 – 17.8) | <.0001 | 16.1 (10.9 – 21.3) | <.0001 |
| Heart Rate (Beats per Minute) | ||||||||
| Mean intraoperative HR | 0.6 (−5.4 – 6.6) | 0.9 | 1.6 (−4.2 – 7.4) | 0.6 | 1.9 (−4 – 7.8) | 0.5 | 2.1 (−6.2 – 10.4) | 0.6 |
| Maximum intraoperative HR | −10.8 (−18.3 – −3.4) | 0.005 | −10.3 (−17.7 – −2.8) | 0.007 | −9.3 (−16.5 – −2.2) | 0.01 | −2.4 (−12.2 – 7.5) | 0.6 |
| Minimum intraoperative HR | 3.1 (−6 – 12.1) | 0.5 | 3.9 (−5.2 – 12.9) | 0.4 | 4.4 (−4.7 – 13.6) | 0.3 | −0.4 (−13.2 – 12.4) | 1.0 |
Adjusted for pre-operative hemodynamic value (blood pressure or heart rate)
Adjusted for pre-operative hemodynamic value, sex, age at time of procedure, preterm
Adjusted for pre-operative hemodynamic value, sex, age at time of procedure, preterm, and duration of procedure
As a further sensitivity analysis we restricted the cohort to only SA and GA patients with open pyloromyotomies in order to determine whether the BP differences were due to some GA children receiving laparoscopic procedures. When comparing the 19 GA patients at CUMC with an open pyloromyotomy with all 51 SA patients who also had an open pyloromyotomy, we found the hemodynamic differences between SA and GA patients remained the same. In this analysis, the average percent decrease from baseline in mean intraoperative SBP was −8.0 ± 17.0% for SA and −28.1 ± 16.9% for GA (difference between means: 20.1% [95% CI, 11–29.2]), and the decrease from baseline for intraoperative MAP was −16.2 ± 19.6% for SA and −27.9 ± 22.1% for GA (difference between means: 11.7% [95% CI, 0.8–22.5]).
Another consideration was that the location of BP cuff placement may have affected BP readings. Since all patients at UVM with SA had BP cuffs placed on the leg, we evaluated whether the site of the BP cuff affected our results by restricting the GA patients to only the 28 children with the BP cuff placed on their lower extremity. In this analysis, the average percent decrease from baseline in mean intraoperative SBP was −8.0 ± 17.0% for SA and −24.2 ± 17.0% for GA (difference between means: 16.2% [95% CI, 8.2–24.2]), and the decrease from baseline for intraoperative MAP was −16.2 ± 19.6% for SA and −24.9 ± 18.5% for GA (difference between means: 8.7% [95% CI, −0.3–17.7]).
To confirm that our results were not solely due to institutional variation, we also evaluated the five ASA 1 and 2 infants from UVM with available hemodynamic data where GA was the anesthetic of choice and SA was never attempted. The reason for GA in these patients was the preference of a new surgeon at UVM. In these GA patients the mean percentage decrease from baseline was −35.7 ± 21.1% for mean intraoperative MAP and −51.4 ± 22.4% for minimum intraoperative MAP. Decreases of −30.6 ± 13.2%, were found for mean intraoperative SBP and −48.8 ± 17% for minimum intraoperative SBP. While data for only five infants were available, these decreases in BP were more than those seen in SA infants at UVM and comparable to the GA infants at CUMC. We also compared the 16 children with SA who received a supplemental IV or volatile anesthetic with the 35 children with SA who did not receive one. In children receiving a supplement, the average percent decrease from baseline in mean intraoperative SBP was −9.4 ± 18.8% compared to −7.4 ± 13.2% in those without a supplement. For mean intraoperative MAP, the decrease was −17.1 ± 20.9% for those with a supplement and −15.8 ± 19.3% for those without a supplement. These differences were not found to be statistically significant.
A significant number of children from UVM (n=25) did not have baseline hemodynamic data recorded in the medical record and were excluded from analysis. To ensure that these excluded SA children did not differ from those SA children included in the study, we compared the mean intraoperative SBP values (89.3 ± 13.5 mmHg in included SA vs. 89.0 ± 11.6 in excluded SA, difference between means: 0.3 mmHg [95% CI, −6.7–6.2]) and mean intraoperative MAP values (57.5 ± 9.9 mmHg in included SA vs. 55.2 ± 8.9 in excluded SA, difference between means: −2.3 mmHg [95% CI, −7–2.4]).
Discussion
In the present study, otherwise healthy infants undergoing pyloromyotomy were found to have significantly lower intraoperative BPs with GA compared to SA, but similar intraoperative heart rates. The percentage drop in mean MAP values from baseline in GA patients was found to be 24.6% while in SA the decrease was 16.3%. This larger BP drop in GA vs. SA persisted even after adjusting for covariates and restricting to only open pyloromyotomy patients. As GA is currently still the standard technique for most pediatric surgical procedures, the safety of SA still needs to be confirmed. However, the need for improved intraoperative hemodynamic stability may be one of the situations in which SA is favored over GA.
Neuroaxial anesthesia in neonates and infants is thought to be hemodynamically stable due either to a minimal impact on sympathetic activity, or a reflex reduction in vagal activity that balances the decreased sympathetic activity.(11) This has been seen in several case series published describing the hemodynamic stability of regional anesthesia in children, particularly in children with epidural anesthesia.(12–14) Few studies however have directly compared GA to SA in infants receiving the same procedure. One such clinical trial evaluating post-operative apnea after inguinal hernia surgery reported a minimum SBP 15.9 mmHg lower in general compared to regional anesthesia, which was composed of children receiving spinal blocks alone or in conjunction with caudal catheters or peripheral nerve blocks.(15) Our study builds on these results but differs as it includes children with SA only, involves pyloromyotomy procedures which are often more stimulating than inguinal hernia procedures, and also evaluates mean blood pressure values which are more representative of intraoperative hemodynamics than minimum values that occur at a single time point. To our knowledge, the present study is also the first such study to use automatically recorded vital signs in an electronic anesthetic record, which are likely more reliable than paper charting.
While other institutional differences in intraoperative anesthetic management could also account for these hemodynamic differences, such as fluid or vasopressor administration, or the use of high levels of volatile anesthetic, in this cohort, no vasopressors were used in any patients, and more fluid was actually administered to the GA patients than the SA patients. AIso, in the GA population, the mean intraoperative percentage of sevoflurane was 1.4%, which based on a MAC value of 3.2% for infants under 6 months of age, is a dose less than 0.5 MAC.(16) Of note, these higher BP values in SA patients were also found despite over 30% of the SA patients needing a dose of IV or inhaled anesthetic. Since this was an observational study, there was no standardized anesthetic for the GA patients, however the doses used are well within the range of those used in common clinical care. While the number of infants from UVM who had GA as the chosen anesthetic for pyloromyotomy was limited, the decreases in intraoperative BP values for those patients were similar to that found in GA patients at CUMC. This further suggests that the BP differences are associated with the anesthetic type, and not merely institutional differences.
While maintaining intraoperative hemodynamic stability is always preferred, what constitutes hypotension in infants is still unclear, as a consensus on the definition at this age does not exist.(17) While it is desirable to maintain BP within the limits of cerebral autoregulation, there are currently no definitive studies to offer guidance on what those limits are in children. Cerebral blood flow (CBF) studies in infants using transcranial Doppler (TCD) sonography have found decreases in CBF velocity (CBFV) associated with decreases in MAP under sevoflurane anesthesia, particularly when the MAP decreased by greater than 20% from baseline values.(18) However this was only found in one small study and decreases in CBFV have also been seen in infants under SA.(19) To complicate matters further, there is also a paucity of information regarding the influence of anesthesia, mechanical ventilation, and PaCO2 on cerebral blood circulation.(20) Most importantly, the long-term impact of a potential decrease in CBFV is unknown, as are the risks of treating it with fluids or pressors.(21, 22) While an SA technique could be used in infants where adherence to baseline BP is important, it is important to note that there are currently no studies that are adequately powered to evaluate serious and rare complications of spinal anesthesia in infants with pyloromyotomy, such as gastric aspiration.(9)
There were several limitations in our study that should be considered. While our cohorts were demographically similar healthy ASA 1 and 2 infants, there may be other unmeasured differences between the patients at the two hospitals. In order to account for this, we adjusted for a number of demographic and clinical covariates. While UVM and CUMC both used automated record keeping, UVM typically measured intraoperative BP every 5 minutes, while at CUMC, it was measured ever 3 minutes. In addition, at UVM, HR was measured every minute while at CUMC it was measured every 15 seconds. However this should not impact the comparisons between the mean hemodynamic variables. While intrathecal epinephrine was used in the SA group to prolong the block, it is unlikely to have accounted for the difference in BP, as intrathecal epinephrine for spinals has not been found to impact intraoperative heart rate or BP in children or adults.(23–25) Since different BP machines were used at each institution, it is possible that BP values at one institution may be systematically higher or lower than those at the other institution. The primary analysis however evaluated the change in BP from baseline for each child and does not directly compare BP values between institutions.
Our findings show that SA performed in a group of healthy infants undergoing pyloromyotomy results in reduced changes in BP from baseline and significantly higher BP readings in SA compared to GA patients. Further research is needed to assess whether any clinical impact of these hemodynamic differences between GA and SA exists.
What is already known
Hypotension and bradycardia are known side effects of spinal anesthesia in pregnant women undergoing cesarean section and adults undergoing other surgical procedures.
Few studies however have directly compared hemodynamic differences between general and spinal anesthesia in children.
What this article adds
Healthy infants undergoing pyloromyotomy with spinal anesthesia have significantly higher intraoperative blood pressures than those who had general anesthesia.
Acknowledgments
We acknowledge Howard Andrews, PhD and the Columbia University Data Collection Center (DCC) as well as the Irving Institute for their support of the DCC.
Funding: This work was supported in part by the Carl Koller Memorial Research grant from the American Society of Regional Anesthesia and Pain Medicine (ASRA) (Pittsburg, PA). Dr. Caleb Ing is supported by the Agency for Healthcare Research and Quality (AHRQ) under award number K08HS022941. The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures: None.
References
- 1.Bosenberg A. Benefits of regional anesthesia in children. Paediatr Anaesth. 2012;22:10–18. doi: 10.1111/j.1460-9592.2011.03691.x. [DOI] [PubMed] [Google Scholar]
- 2.Wolf AR, Doyle E, Thomas E. Modifying infant stress responses to major surgery: spinal vs extradural vs opioid analgesia. Paediatr Anaesth. 1998;8:305–311. doi: 10.1046/j.1460-9592.1998.00239.x. [DOI] [PubMed] [Google Scholar]
- 3.von Ungern-Sternberg BS, Regli A, Frei FJ, et al. The effect of caudal block on functional residual capacity and ventilation homogeneity in healthy children. Anaesthesia. 2006;61:758–763. doi: 10.1111/j.1365-2044.2006.04720.x. [DOI] [PubMed] [Google Scholar]
- 4.Rappaport BA, Suresh S, Hertz S, et al. Anesthetic Neurotoxicity - Clinical Implications of Animal Models. N Engl J Med. 2015;372:796–797. doi: 10.1056/NEJMp1414786. [DOI] [PubMed] [Google Scholar]
- 5.Kachko L, Simhi E, Tzeitlin E, et al. Spinal anesthesia in neonates and infants - a single-center experience of 505 cases. Paediatr Anaesth. 2007;17:647–653. doi: 10.1111/j.1460-9592.2007.02194.x. [DOI] [PubMed] [Google Scholar]
- 6.Williams RK, Adams DC, Aladjem EV, et al. The safety and efficacy of spinal anesthesia for surgery in infants: the Vermont Infant Spinal Registry. Anesth Analg. 2006;102:67–71. doi: 10.1213/01.ANE.0000159162.86033.21. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter RL, Caplan RA, Brown DL, et al. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992;76:906–916. doi: 10.1097/00000542-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Rout CC, Rocke DA. Prevention of hypotension following spinal anesthesia for cesarean section. Int Anesthesiol Clin. 1994;32:117–135. [PubMed] [Google Scholar]
- 9.Ing C, Sun LS, Friend AF, et al. Adverse Events and Resource Utilization After Spinal and General Anesthesia in Infants Undergoing Pyloromyotomy. Reg Anesth Pain Med. 2016 doi: 10.1097/AAP.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devitt JH, Rapanos T, Kurrek M, et al. The anesthetic record: accuracy and completeness. Can J Anaesth. 1999;46:122–128. doi: 10.1007/BF03012545. [DOI] [PubMed] [Google Scholar]
- 11.Oberlander TF, Berde CB, Lam KH, et al. Infants tolerate spinal anesthesia with minimal overall autonomic changes: analysis of heart rate variability in former premature infants undergoing hernia repair. Anesth Analg. 1995;80:20–27. doi: 10.1097/00000539-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Bosenberg AT. Epidural analgesia for major neonatal surgery. Paediatr Anaesth. 1998;8:479–483. doi: 10.1046/j.1460-9592.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 13.Shenkman Z, Johnson VM, Zurakowski D, et al. Hemodynamic changes during spinal anesthesia in premature infants with congenital heart disease undergoing inguinal hernia correction. Paediatr Anaesth. 2012;22:865–870. doi: 10.1111/j.1460-9592.2012.03873.x. [DOI] [PubMed] [Google Scholar]
- 14.Rukewe A, Alonge T, Fatiregun A. Spinal anesthesia in children: no longer an anathema! Paediatr Anaesth. 2010;20:1036–1039. doi: 10.1111/j.1460-9592.2010.03431.x. [DOI] [PubMed] [Google Scholar]
- 15.Davidson AJ, Morton NS, Arnup SJ, et al. Apnea after Awake Regional and General Anesthesia in Infants: The General Anesthesia Compared to Spinal Anesthesia Study-Comparing Apnea and Neurodevelopmental Outcomes, A Randomized Controlled Trial. Anesthesiology. 2015 doi: 10.1097/ALN.0000000000000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerman J, Sikich N, Kleinman S, et al. The pharmacology of sevoflurane in infants and children. Anesthesiology. 1994;80:814–824. doi: 10.1097/00000542-199404000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Nafiu OO, Voepel-Lewis T, Morris M, et al. How do pediatric anesthesiologists define intraoperative hypotension? Paediatr Anaesth. 2009;19:1048–1053. doi: 10.1111/j.1460-9592.2009.03140.x. [DOI] [PubMed] [Google Scholar]
- 18.Rhondali O, Mahr A, Simonin-Lansiaux S, et al. Impact of sevoflurane anesthesia on cerebral blood flow in children younger than 2 years. Paediatr Anaesth. 2013;23:946–951. doi: 10.1111/pan.12166. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet MP, Larousse E, Asehnoune K, et al. Spinal anesthesia with bupivacaine decreases cerebral blood flow in former preterm infants. Anesth Analg. 2004;98:1280–1283. doi: 10.1213/01.ane.0000108962.37210.69. table of contents. [DOI] [PubMed] [Google Scholar]
- 20.McCann ME, Schouten AN. Beyond survival; influences of blood pressure, cerebral perfusion and anesthesia on neurodevelopment. Paediatr Anaesth. 2014;24:68–73. doi: 10.1111/pan.12310. [DOI] [PubMed] [Google Scholar]
- 21.Fanaroff JM, Wilson-Costello DE, Newman NS, et al. Treated hypotension is associated with neonatal morbidity and hearing loss in extremely low birth weight infants. Pediatrics. 2006;117:1131–1135. doi: 10.1542/peds.2005-1230. [DOI] [PubMed] [Google Scholar]
- 22.Pellicer A, Bravo MC, Madero R, et al. Early systemic hypotension and vasopressor support in low birth weight infants: impact on neurodevelopment. Pediatrics. 2009;123:1369–1376. doi: 10.1542/peds.2008-0673. [DOI] [PubMed] [Google Scholar]
- 23.Fosel T, Wilhelm W, Gruness V, et al. Spinal anesthesia in infancy using bupivacaine 0.5%. The effect of an adrenaline addition on duration and hemodynamics. Anaesthesist. 1994;43:26–29. doi: 10.1007/s001010050030. [DOI] [PubMed] [Google Scholar]
- 24.Phero JC, Bridenbaugh PO, Edstrom HH, et al. Hypotension in spinal anesthesia: a comparison of isobaric tetracaine with epinephrine and isobaric bupivacaine without epinephrine. Anesth Analg. 1987;66:549–552. [PubMed] [Google Scholar]
- 25.Racle JP, Benkhadra A, Poy JY, et al. Effect of increasing amounts of epinephrine during isobaric bupivacaine spinal anesthesia in elderly patients. Anesth Analg. 1987;66:882–886. [PubMed] [Google Scholar]