Abstract
Background
The role of thyroid hormones in diet-induced weight loss and subsequent weight regain is largely unknown.
Objectives
To examine the associations between thyroid hormones and changes in body weight and resting metabolic rate (RMR) in a diet-induced weight-loss setting.
Subjects/Methods
Data analysis was conducted among 569 overweight and obese participants aged 30–70 years with normal thyroid function participating in the 2-year POUNDS LOST randomized clinical trial. Changes in body weight and RMR were assessed during the 2-year intervention. Thyroid hormones (free triiodothyronine [T3], free thyroxine [T4], total T3, total T4, and thyroid stimulating hormone [TSH]), anthropometric measurements, and biochemical parameters were assessed at baseline, 6 months, and 24 months.
Results
Participants lost an average of 6.6 kg of body weight during the first 6 months and subsequently regained an average of 2.7 kg of body weight over the remaining period from 6–24 months. Baseline free T3 and total T3 were positively associated, whereas free T4 was inversely associated, with baseline body weight, body mass index, and RMR. Total T4 and TSH were not associated with these parameters. Higher baseline free T3 and free T4 levels were significantly associated with a greater weight loss during the first 6 months (P<0.05) after multivariate adjustments including dietary intervention groups and baseline body weight. Comparing extreme tertiles, the multivariate-adjusted weight loss ± standard error was −3.87±0.9 vs −5.39±0.9 kg for free T3 (P trend=0.02) and −4.09±0.9 vs −5.88±0.9 kg for free T4 (P trend=0.004). The thyroid hormones did not predict weight regain in 6–24 months. A similar pattern of associations was also observed between baseline thyroid hormones and changes in RMR. In addition, changes in free T3 and total T3 levels were positively associated with changes in body weight, RMR, body fat mass, blood pressure, glucose, insulin, triglycerides, and leptin at 6 months and 24 months (all P<0.05).
Conclusions
In this diet-induced weight-loss setting, higher baseline free T3 and free T4 predicted more weight loss, but not weight regain among overweight and obese adults with normal thyroid function. These findings reveal a novel role of thyroid hormones in body weight regulation and may help identify individuals more responsive to weight-loss diets.
Keywords: Obesity, Weight loss, Thyroid hormones, Resting Metabolic Rate, Dietary intervention, Leptin
Introduction
The rapidly growing prevalence of obesity has become a global public health concern because excessive weight gain predicts the incidence of several major chronic diseases, including diabetes, cardiovascular disease, and certain types of cancer.1, 2 Although there are many approaches to achieving weight loss, the maintenance of weight loss has become a major challenge.3–5 For instance, energy-restricted diets or other diets can lead to weight loss in a short term (e.g., approximately 6 months),6 but subsequent weight regain is typically observed for the majority of study participants.4, 7 Meanwhile, following the same dietary interventions, apparent between-individual variability in weight loss and weight regain has been observed,3, 8,9 although factors pertaining to this variability are largely unknown. Identifying such factors is critical for enhancing diet-induced weight loss and mitigating weight regain.
Thyroid hormones play an essential role in body weight regulation, mainly through regulating energy expenditure.10, 11 It is well established that thyroid dysfunction, including hyperthyroidism and hypothyroidism, leads to significant changes in body weight and resting metabolic rate (RMR).11, 12 However, whether thyroid hormones within the physiological range determine the amount of weight loss and regain is not well elucidated. A few observational prospective studies yielded mixed findings regarding the association of thyroid hormones or function with changes in body weight or body mass index (BMI).13–17 It is challenging to evaluate thyroid hormones in relation to weight change in observational studies because the causes of weight change are heterogeneous and often not well understood. In addition, few studies has examined RMR, a factor that is associated with both thyroid function and energy expenditure,11, 18 in relation to thyroid hormones during weight change. Thus far, evidence is scarce from weight-loss trials where the cause of weight change is known and homogeneous among individuals.
To fill this critical knowledge gap, we investigated thyroid hormones (free triiodothyronine [T3], free thyroxine [T4], total T3, total T4, and thyroid stimulating hormone [TSH]) in relation to changes in body weight, RMR, and metabolic parameters among overweight and obese adults with normal thyroid function, participating in the Prevention of Obesity Using Novel Dietary Strategies (POUNDS) LOST Study, a 2-year randomized clinical trial.9
Subjects and Methods
Study participants
The POUNDS LOST Study was a 2-year randomized clinical trial that compared the effects of four energy-reduced diets with different compositions of macronutrients (carbohydrate, fat, and protein) on body weight.9 A total of 811 overweight and obese men and women aged 30–70 years were randomly assigned to one of four diets which consisted of similar foods and met the guidelines for cardiovascular health. Each participant’s diet prescription represented a reduced caloric intake of 750 kcal/day from estimated energy needs, which were calculated from the RMR at baseline. All study participants had normal thyroid function defined as 0.1≤TSH≤4.5 mIU/L and 57.9≤ total T4< 169.9 nmol/L.19 The detailed study design and methods have been described elsewhere.9 At baseline examination, participants with diabetes treated with medications or with unstable cardiovascular disease and those using medications that affected body weight or had insufficient motivation were excluded. At 2 years, 80% of the participants (n=645) had completed the trial. The key findings of the POUNDS LOST Study were reported previously.9 Briefly, most of the weight loss was observed in the first 6 months, followed by a gradual weight regain from 6 to 24 months. The total amount of weight loss was not significantly different among diet groups.
The current analysis included 569 POUNDS LOST trial participants with available fasting serum samples, collected at baseline and 6 months. Of these individuals, 429 also provided blood samples at 2 years. The protocol was approved by the Institutional Review Board at Harvard T.H. Chan School of Public Health, Brigham and Women’s Hospital, and the Pennington Biomedical Research Center of the Louisiana State University System, as well as by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All participants provided written informed consent.
Measurements
Serum free T3, free T4, total T3, total T4, and TSH were measured using a competitive electrochemiluminescence immunoassay on the Roche E Modular system (Roche Diagnostics, Indianapolis, IN). Fasting serum samples from the same participants were assayed in the same run by the same technicians in a random sequence. The intra-assay coefficient of variation (CV) ranged from 2.1–3.4% for free T3, 3.4–4.5% for total T3, 3.3–6.6% for free T4, 3.0–6.9% for total T4, and 1.8–5.4% for TSH. Serum leptin and soluble leptin receptor (sOB-R) were measured by an ultra-sensitive ELISA assay, an enzymatically amplified “two-step” sandwich-type immunoassay (R&D Systems, Minneapolis, MN). The intra-assay CV ranged from 3.5–5.4% for leptin and 5.3–8.6% for sOB-R. Fasting glucose, insulin, triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterolwere determined on the Synchron CX7 (Beckman Coulter, Brea CA), and hemoglobin A1C was determined on a Synchron CX5 (Beckman Coulter, Brea CA).9 Body weight and waist circumference (WC) were assessed in the morning before breakfast and after urinating at baseline, 6, 12, 18, and 24 months. Height was measured at baseline. BMI was calculated as weight in kilograms divided by the square of height in metres. After an overnight fasting, body composition was assessed by dual energy X-ray absorptiometry (DXA) (Hologic QDR 4500A bone densitometer, Hologic Inc).20 Blood pressure was measured by an automated device (Omron HEM907XL, Omron) at baseline, 6 months, and 24 months, and after an overnight fast, RMR was assessed using a Deltatrac II Metabolic Monitor (Datex-Ohmeda, Helsinki, Finland).21
Other covariates
Information on age, sex, race (White, Black, Hispanic, and other), smoking status (never, former, and current smoker), educational attainment (≤high school, some college, and >college), alcohol consumption (drinks/week), menopausal status (yes, no), hormone replacement therapy (yes, no) was collected by using a standardized questionnaire. The Baecke self-reported questionnaire was used to evaluate physical activity.22 A score was calculated based on the physical activity at work, sport during leisure time, and other physical activity during leisure time.22
Statistical analysis
Two primary outcomes were examined in the current study: 1) weight loss between baseline and 6 months and 2) weight change between 6 months and 24 months. The comparisons between participants included and the rest were examined by using the Student’s t test for normally distributed variables, the Wilcoxon rank-sum test for skewed variables, and the Chi-Square test for categorical variables. The associations between baseline thyroid hormones and changes in body weight, RMR, or body composition at 6 months and at 24 months were examined using linear regression (weight assessment at only 6 months and 24 months were used). The least-square (LS) means of changes in body weight and RMR according to tertile levels of baseline thyroid hormones were calculated. We adjusted potential confounders in statistical analysis, including baseline age, sex, race, smoking status, educational attainment, alcohol consumption, physical activity, menopausal status, hormone replacement therapy, the four diet intervention groups, and baseline body weight. Tests of linear trend across increasing tertile levels of thyroid hormones were examined by assigning a median value to each tertile and treating it as a continuous variable. We also tested the linear trend using the thyroid hormone levels as continuous variables. To evaluate the associations between baseline thyroid hormones and baseline body weight and metabolic parameters, as well as the associations between changes in thyroid hormones and changes in body weight and metabolic parameters (follow-up value minus baseline value), we calculated partial Spearman correlation coefficients (rs) after adjustment for the potential confounders mentioned above. In secondary analyses, we used linear mixed-effects models to examine the weight changes (0–24 months and 6–24 months) by tertiles of thyroid hormones, with an unstructured covariance matrix. Weight measurements at baseline, 6, 12, 18, and 24 months were included in the mixed-effect models. In addition, considering that participants with subclinical thyrotoxicosis/hyperthyroidism may affect the associations of interest, we further excluded the subjects with TSH <0.4 mIU/L (n=7) in a sensitivity analysis. A two-sided P <0.05 was considered statistically significant. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
Participants
The mean age of the 569 participants was 51.6 (SD 9.0) years, with a mean BMI of 32.6 (SD 3.8) kg/m2. On average, participants lost 6.6 kg of body weight during the first 6 months and subsequently regained an average of 2.7 kg body weight for the remaining period of 6–24 months, with no significant differences among the four diet groups. Compared with the participants who were not included in the current analysis because serum samples were lacking or insufficient, the participants who were included were slightly older (51.6 vs 49.3 years), more likely to be men, and had lower levels of educational attainment. No significant differences were otherwise observed in other characteristics, including body weight and RMR, as well as other parameters (Supplementary Table 1).
Relationships between thyroid hormones, body weight, and other assessments at baseline
The baseline characteristics of the participants according to tertiles of thyroid hormones are shown in Table 1. At baseline, there were significant inter-correlations between free T3, free T4, total T3, and total T4 (rs 0.17–0.81, all P<0.001). TSH was weakly associated with free T4 (r=−0.16, P<0.001) and total T4 (r=−0.09, P<0.05), and not associated with free T3 and total T3 (Supplementary Table 2). After adjusting for age, sex, and race, free T3 and total T3 were positively associated with body weight, BMI, and RMR (rs ranged from 0.10 to 0.21, all P<0.01) at baseline, whereas free T4 had weak, inverse correlations with body weight, BMI, and RMR (all rs were −0.09, P<0.05). Total T4 and TSH were not correlated with these measurements (Supplementary Table 2). In addition, free T3, total T3, and free T3/free T4 ratio were positively associated with body weight, WC, blood pressure, leptin, insulin, and triglycerides (all P<0.01). Free T4 had weak, inverse associations with whole body lean mass, glucose, leptin, and insulin (all P<0.05). In contrast, total T4 and TSH were not significantly associated with most of these parameters (Supplementary Table 2).
Table 1.
Baseline characteristics of the participants according to extreme tertiles of thyroid hormones
| Free T3 (pg/mL) | Total T3 (ng/mL) | Free T4 (pmol/mL) | Total T4 (nmol/mL) | TSH (mIU/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Tertile 1 <3.04 |
Tertile 3 >3.34 |
Tertile 1 <104.2 |
Tertile 3 >119.9 |
Tertile 1 <14.1 |
Tertile 3 >15.8 |
Tertile 1 <77.4 |
Tertile 3 >89.5 |
Tertile 1 <1.27 |
Tertile 3 >2.0 |
|
| N | 178 | 181 | 175 | 175 | 187 | 186 | 174 | 174 | 182 | 185 |
| Age (years) | 52.4 ± 8.6 | 50.8 ± 9.6 | 52.8 ± 8.5 | 51.0 ± 9.2 | 51.4 ± 8.8 | 51.5 ± 9.6 | 52.1 ± 8.6 | 50.9 ± 9.8 | 51.0 ± 9.4 | 52.4 ± 8.5 |
| Sex, men, % | 16.9 | 61.3 | 30.9 | 40.6 | 27.8 | 45.7 | 44.3 | 27.6 | 39.0 | 35.1 |
| Race, White, % | 76.4 | 88.4 | 81.1 | 81.1 | 75.9 | 84.9 | 82.2 | 81.0 | 73.6 | 87.0 |
| BMI (kg/m2) | 31.9 ± 4.0 | 33.4 ± 3.5 | 31.7 ± 3.8 | 33.5 ± 3.6 | 32.9 ± 4.0 | 32.5 ± 3.7 | 32.3 ± 3.8 | 32.5 ± 3.8 | 32.7 ± 3.8 | 32.8 ± 3.8 |
| Weight (kg) | 87.8 ± 15.1 | 98.3 ± 14.6 | 89.4 ± 15.2 | 95.5 ± 14.4 | 92.5 ± 16.2 | 93.2 ± 15.3 | 93.6 ± 16.0 | 91.5 ± 14.7 | 92.7 ± 15.6 | 93.2 ± 15.3 |
| Waist circumference (cm) | 98.4 ± 13.1 | 108.6 ± 11.7 | 100.1 ± 12.8 | 106.6 ± 12.1 | 102.7 ± 13.9 | 104.1 ± 12.3 | 103.8 ± 13.6 | 102.8 ± 12.2 | 104.0 ± 12.8 | 103.8 ± 13.3 |
| Resting metabolic rate (kcal/24h) | 1406 ± 260 | 1699 ± 303 | 1468 ± 295 | 1612 ± 295 | 1519 ± 303 | 1563 ± 300 | 1575 ± 324 | 1501 ± 260 | 1559 ± 310 | 1538 ± 292 |
| Education level, some college, % | 18.0 | 18.2 | 17.1 | 21.7 | 19.3 | 17.2 | 17.8 | 20.1 | 19.8 | 17.3 |
| Current smoker, yes, % | 3.4 | 4.4 | 1.7 | 3.4 | 2.7 | 1.6 | 2.9 | 2.3 | 3.3 | 2.7 |
| Alcohol consumption (drinks/week) | 2.4 ± 3.4 | 2.2 ± 2.9 | 3.0 ± 3.8 | 1.8 ± 2.7 | 2.3 ± 3.4 | 2.1 ± 2.7 | 3.1 ± 3.8 | 1.2 ± 1.9 | 2.3 ± 3.3 | 2.0 ± 2.8 |
| Physical activity | 1.58 ± 0.1 | 1.57 ± 0.1 | 1.60 ± 0.1 | 1.56 ± 0.1 | 1.57 ± 0.1 | 1.59 ± 0.1 | 1.59 ± 0.1 | 1.56 ± 0.1 | 1.58 ± 0.1 | 1.57 ± 0.1 |
| Systolic blood pressure (mmHg) | 117.4 ± 13.1 | 122.1 ± 12.1 | 119.0 ± 13.9 | 122.4 ± 13.6 | 119.8 ± 13.5 | 119.4 ± 12.0 | 119.7 ± 12.6 | 118.9 ± 12.6 | 120.2 ± 14.2 | 120.3 ± 14.0 |
| Diastolic blood pressure (mmHg) | 73.3 ± 8.7 | 77.7 ± 8.9 | 74.0 ± 9.3 | 78.2 ± 9.1 | 75.4 ± 9.4 | 76.0 ± 8.6 | 74.9 ± 9.1 | 75.7 ± 8.0 | 75.9 ± 9.6 | 75.7 ± 9.0 |
| Total fat mass (kg) * | 34.7 ± 7.7 | 35.1 ± 7.5 | 33.5 ± 8.0 | 36.4 ± 7.0 | 35.5 ± 8.2 | 34.9 ± 7.7 | 33.6 ± 7.7 | 35.8 ± 7.4 | 34.9 ± 7.9 | 35.7 ± 7.4 |
| Total lean mass (kg) * | 53.9 ± 11.5 | 64.8 ± 12.9 | 57.7 ± 12.0 | 60.5 ± 13.3 | 59.5 ± 13.5 | 60.1 ± 13.6 | 63.1 ± 13.5 | 57.0 ± 12.4 | 60.4 ± 13.6 | 60.4 ± 13.8 |
| Glucose (mg/dL) | 90.5 ± 11.6 | 93.8 ± 12.9 | 91.9 ± 12.2 | 93.4 ± 12.7 | 93.6 ± 13.4 | 92.3 ± 12.1 | 93.4 ± 12.1 | 91.0 ± 11.6 | 92.4 ± 12.5 | 93.3 ± 13.4 |
| Insulin (uU/mL) | 10.0 ± 6.2 | 15.0 ± 9.8 | 9.7 ± 5.5 | 14.9 ± 9.4 | 13.7 ± 9.4 | 11.4 ± 6.3 | 12.2 ± 8.9 | 12.7 ± 7.8 | 13.2 ± 9.7 | 12.1 ± 6.6 |
| Total cholesterol (mg/dL) | 245.0 ± 87.0 | 264.9 ± 121.8 | 249.4 ± 96.3 | 262.8 ± 113.7 | 265.0 ± 125.2 | 240.2 ± 90.7 | 256.9 ± 120.5 | 250.0 ± 99.0 | 250.9 ± 97.4 | 252.8 ± 108.5 |
| LDL cholesterol (mg/dL) | 129.7 ± 30.5 | 120.3 ± 32.5 | 128.4 ± 30.7 | 120.4 ± 31.8 | 127.4 ± 33.1 | 123.8 ± 30.6 | 123.4 ± 32.2 | 123.4 ± 30.2 | 122.0 ± 31.3 | 122.7 ± 29.4 |
| HDL cholesterol (mg/dL) | 54.4 ± 14.2 | 43.6 ± 12.1 | 52.3 ± 14.6 | 46.6 ± 13.5 | 49.9 ± 14.5 | 48.4 ± 14.0 | 48.1 ± 13.8 | 49.3 ± 13.8 | 48.3 ± 14.1 | 49.3 ± 13.8 |
| Triglycerides (mg/dL) | 124.9 ± 72.8 | 169.6 ± 93.0 | 124.5 ± 63.6 | 161.4 ± 94.1 | 149.1 ± 88.4 | 140.1 ± 73.3 | 142.4 ± 77.2 | 147.2 ± 83.0 | 138.7 ± 72.8 | 153.4 ± 91.1 |
| Leptin (ng/mL) | 31.3 ± 18.1 | 25.9 ± 18.9 | 25.9 ± 17.4 | 32.2 ± 20.1 | 32.8 ± 19.7 | 25.1 ± 17.5 | 25.5 ± 18.0 | 31.6 ± 19.0 | 27.8 ± 19.7 | 30.1 ± 18.2 |
| Leptin soluble receptor (ng/mL) | 21.7 ± 5.7 | 20.7 ± 5.3 | 22.0 ± 5.4 | 21.0 ± 5.8 | 20.5 ± 5.3 | 22.2 ± 6.1 | 21.1 ± 5.3 | 21.6 ± 5.9 | 20.9 ± 5.7 | 21.4 ± 5.1 |
Data are mean ± SD or percentage (%).
A number of 70–97 participants with missing values for total fat mass and total lean mass.
Baseline thyroid hormones and subsequent changes in body weight and RMR
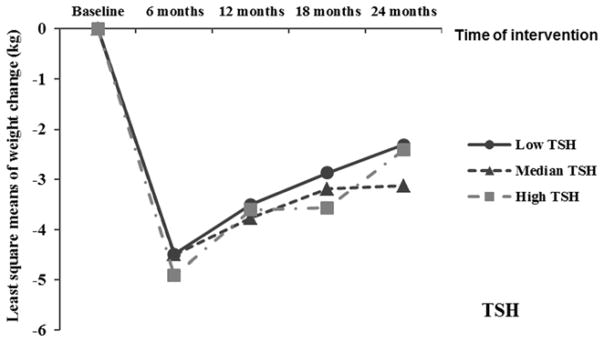
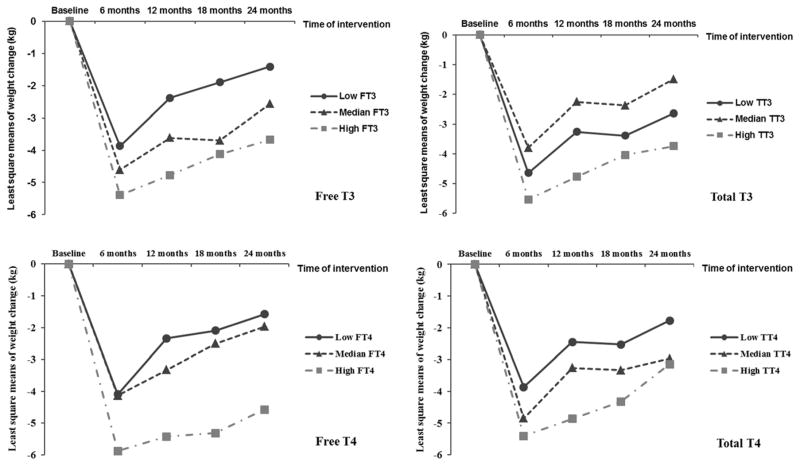
After adjustment of covariates, including diet groups, physical activity, and baseline body weight, higher baseline free T3, free T4 and total T4 levels were associated with a greater weight loss during the first 6 months (comparing extreme tertiles, the LS means of weight loss [kg] was −3.87±0.9 vs −5.39±0.9 for free T3; −4.09±0.9 vs −5.88±0.9 for free T4; and −3.86±0.9 vs −5.40±0.9 for total T4; all P trend≤0.01), but not in the weight regain period (comparing extreme tertiles, the LS means of weight regain [kg] was 2.15±0.9 vs 1.85±0.9 for free T3; 2.18±0.9 vs 1.57±0.9 for free T4; and 1.77±0.9 vs 2.60±0.9 for total T4; all P trend>0.05). For the entire 2-year study period, higher baseline free T3 and free T4 were associated with a greater weight loss (Table 2). The results were similar when free T3 and free T4 were treated as contionous variables (both P trend<0.05). When thyroid hormones were further mutually adjusted, the results remained largely unchanged (Table 2). Baseline TSH was not associated with changes in body weight during the period of weight loss or weight regain. The trajectory of changes in body weight according to baseline tertiles of thyroid hormones is shown in Figure 1. Moreover, using linear mixed-effects models, we estimated that, for the entire study period, higher baseline free T3 and free T4 levels were significantly associated with a higher weight loss rate (i.e., weight change per 6 months; (data not shown) and a greater change in body fat mass (Supplementary Table 3). In contrast, baseline body weight was not significantly associated with subsequent changes in these thyroid hormones. No association was observed between baseline leptin levels and weight change.
Table 2.
Changes in body weight according to baseline tertiles of thyroid hormone
| Tertiles of Thyroid Hormone
|
P trend | |||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| Weight change (0–6 months), kg | ||||
|
| ||||
| Free T3 (n=537) | ||||
| Model 1* | −3.87±0.9 | −4.61±0.9 | −5.39±0.9 | 0.01 |
| Model 2† | −3.96±0.9 | −4.74±0.9 | −5.52±0.9 | 0.02 |
| Total T3 (n=525) | ||||
| Model 1* | −4.64±0.9 | −3.79±0.9 | −5.54±0.9 | 0.09 |
| Model 2† | −4.71±0.9 | −3.80±0.9 | −5.61±0.9 | 0.15 |
| Free T4 (n=558) | ||||
| Model 1* | −4.09±0.9 | −4.14±0.8 | −5.88±0.9 | 0.002 |
| Model 2† | −4.20±0.9 | −4.17±0.9 | −5.92±0.9 | 0.004 |
| Total T4 (n=522) | ||||
| Model 1* | −3.86±0.9 | −4.85±0.9 | −5.40±0.9 | 0.01 |
| Model 2† | −3.81±0.9 | −4.84±0.9 | −5.49±0.9 | 0.02 |
| TSH (n=556) | ||||
| Model 1* | −4.49±0.9 | −4.50±0.9 | −4.91±0.8 | 0.43 |
| Model 2† | −4.54±0.9 | −4.70±0.9 | −4.89±0.9 | 0.58 |
|
| ||||
| Weight change (6–24 months), kg | ||||
|
| ||||
| Free T3 (n=455) | ||||
| Model 1* | 2.15±0.9 | 2.08±0.9 | 1.85±0.9 | 0.65 |
| Model 2† | 2.22±0.9 | 2.11±0.9 | 1.88±0.9 | 0.64 |
| Total T3 (n=445) | ||||
| Model 1* | 1.91±0.9 | 2.22±0.9 | 2.02±0.9 | 0.89 |
| Model 2† | 1.94±0.9 | 2.21±0.9 | 2.07±0.9 | 0.89 |
| Free T4 (n=473) | ||||
| Model 1* | 2.18±0.9 | 2.13±0.9 | 1.57±0.9 | 0.30 |
| Model 2† | 2.19±0.9 | 2.26±0.9 | 1.60±0.9 | 0.35 |
| Total T4 (n=442) | ||||
| Model 1* | 1.77±0.9 | 1.82±0.9 | 2.60±0.9 | 0.19 |
| Model 2† | 1.59±0.9 | 1.76±0.9 | 2.73±0.9 | 0.13 |
| TSH (n=472) | ||||
| Model 1* | 2.30±0.9 | 1.17±0.9 | 2.44±0.9 | 0.41 |
| Model 2† | 2.45±0.9 | 1.35±0.9 | 2.40±0.9 | 0.67 |
|
| ||||
| Weight change (0–24 months), kg | ||||
|
| ||||
| Free T3 (n=455) | ||||
| Model 1* | −1.41±1.2 | −2.57±1.2 | −3.68±1.2 | 0.01 |
| Model 2† | −1.55±1.2 | −2.69±1.2 | −3.74±1.3 | 0.03 |
| Total T3 (n=445) | ||||
| Model 1* | −2.64±1.2 | −1.49±1.2 | −3.74±1.2 | 0.16 |
| Model 2† | −2.84±1.3 | −1.49±1.2 | −3.64±1.3 | 0.34 |
| Free T4 (n=473) | ||||
| Model 1* | −1.57±1.2 | −1.97±1.1 | −4.57±1.2 | <0.001 |
| Model 2† | −1.70±1.2 | −1.93±1.2 | −4.60±1.2 | <0.001 |
| Total T4 (n=442) | ||||
| Model 1* | −1.78±1.2 | −2.97±1.2 | −3.14±1.2 | 0.12 |
| Model 2† | −1.83±1.3 | −3.00±1.2 | −3.14±1.3 | 0.20 |
| TSH (n=472) | ||||
| Model 1* | −2.31±1.2 | −3.12±1.2 | −2.41±1.2 | 0.87 |
| Model 2† | −2.23±1.2 | −3.20±1.2 | −2.44±1.2 | 0.94 |
Data are least squared means ± SE. Weight change was calculated as difference between weight at baseline and weight at examinations;
Model 1, adjusted for age, sex, race, education, smoking status, alcohol consumption, physical activity ratio, menopausal status, hormone replacement therapy, diet groups, and baseline weight.
Model 2, further adjusted for other thyroid functional parameters.
Figure 1.
Trajectory of changes in body weight according to baseline tertiles of thyroid hormone. Data were least square means, adjusted for age, sex, race, education, smoking, alcohol consumption, physical activity, menopausal status, hormone replacement therapy, diet groups and baseline weight.
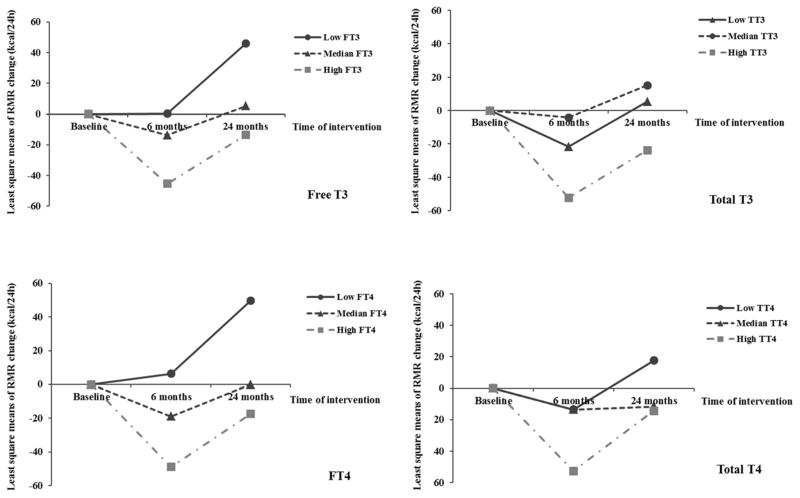
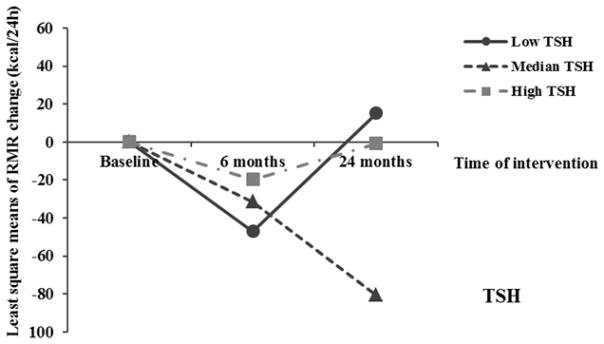
In addition, a similar pattern of associations was observed between baseline thyroid hormones and changes in RMR. Higher baseline free T3 and free T4 levels were significantly associated with a greater decrease in RMR during the weight-loss period, but not in the weight-regain period (Table 3 and Figure 2). When further adjusting for changes in lean mass, the associations remained significant during weight loss period (0–6 month), but did not reach statistical significance during the overall 24-month period, possibly due to the reduced statistical power (n≈170) (data not shown). Moreover, after multivariate adjustment of covariates including baseline thyroid hormones, higher baseline RMR was also associated with greater decrease in RMR during the weight loss period: comparing extreme tertiles of baseline RMR, change in RMR during the first 6 months was 32.2 (SE 22.5) vs −116.5 (SE 23.6) kcal/24h (P<0.001), and the Spearman correlation coefficient between baseline RMR and changes in RMR was −0.37 (P<0.001).
Table 3.
Changes in resting metabolic rate according to baseline tertiles of thyroid hormone
| Tertiles of Thyroid Hormone
|
P trend | |||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| Resting metabolic rate change, kcal/day (0–6months) | ||||
|
| ||||
| Free T3 (n=503) | ||||
| Model 1* | 0.23±21.3 | −13.7±21.6 | −45.4±21.2 | 0.003 |
| Model 2† | −14.7±21.4 | −20.2±21.4 | −47.7±21.4 | 0.04 |
| Total T3 (n=491) | ||||
| Model 1* | −21.7±21.6 | −4.20±21.4 | −52.3±21.2 | 0.02 |
| Model 2† | −29.7±21.9 | −7.72±21.1 | −45.5±21.5 | 0.26 |
| Free T4 (n=523) | ||||
| Model 1* | 6.33±21.3 | −18.9±20.3 | −48.9±21.0 | <0.001 |
| Model 2† | −3.95±21.9 | −26.4±20.8 | −51.37±21.5 | 0.001 |
| Total T4 (n=489) | ||||
| Model 1* | −13.5±21.2 | −13.7±21.1 | −52.6±21.1 | 0.01 |
| Model 2† | −15.8±21.7 | −15.1±21.2 | −52.6±21.6 | 0.03 |
| TSH (n=520) | ||||
| Model 1* | −29.8±21.3 | −11.1±21.3 | −21.8±21.2 | 0.80 |
| Model 2† | −33.5±21.3 | −18.9±21.2 | −28.0±21.2 | 0.90 |
|
| ||||
| Resting metabolic rate change, kcal/day (6–24 months) | ||||
|
| ||||
| Free T3 (n=356) | ||||
| Model 1* | 24.8±37.9 | 11.3±36.6 | 12.3±36.2 | 0.66 |
| Model 2† | 29.1±38.4 | 4.1±36.7 | 4.5±36.7 | 0.42 |
| Total T3 (n=348) | ||||
| Model 1* | 7.16±37.2 | 5.74±37.8 | 10.8±36.6 | 0.88 |
| Model 2† | 10.8±38.1 | 10.9±37.6 | 11.1±37.1 | 0.99 |
|
| ||||
| Free T4 (n=370) | ||||
| Model 1* | 18.4±37.6 | 13.4±36.7 | 20.1±37.2 | 0.94 |
| Model 2† | 13.8±37.4 | 8.10±36.7 | 9.04±36.9 | 0.85 |
| Total T4 (n=346) | ||||
| Model 1* | −0.53±36.8 | 1.60±36.6 | 27.3±36.9 | 0.26 |
| Model 2† | −0.77±37.5 | 3.67±36.6 | 29.9±37.8 | 0.30 |
| TSH (n=368) | ||||
| Model 1* | 53.8±36.4 | −11.5±36.0 | 5.46±36.6 | 0.19 |
| Model 2† | 42.5±36.7 | −16.5±36.3 | 0.60±37.1 | 0.29 |
|
| ||||
| Resting metabolic rate change, kcal/day (0–24 months) | ||||
|
| ||||
| Free T3 (n=363) | ||||
| Model 1* | 45.9±39.2 | 5.09±38.2 | −13.6±37.9 | 0.04 |
| Model 2† | 32.6±39.7 | −9.84±38.2 | −24.2±38.6 | 0.07 |
| Total T3 (n=355) | ||||
| Model 1* | 5.24±38.5 | 15.0±39.4 | −23.8±38.3 | 0.26 |
| Model 2† | −1.83±39.6 | −15.5±39.4 | −15.3±39.1 | 0.61 |
| Free T4 (n=377) | ||||
| Model 1* | 49.7±39.0 | −0.04±38.6 | −17.4±39.0 | 0.01 |
| Model 2† | 36.4±38.4 | −13.9±38.2 | −31.4±38.5 | 0.01 |
| Total T4 (n=353) | ||||
| Model 1* | 17.6±38.3 | −11.6±38.2 | −14.4±38.6 | 0.23 |
| Model 2† | 20.7±39.3 | −10.6±38.3 | −17.4±39.8 | 0.23 |
| TSH (n=375) | ||||
| Model 1* | 24.0±39.0 | −0.34±38.3 | 5.99±39.0 | 0.65 |
| Model 2† | 10.6±38.7 | −14.8±38.0 | −4.38±39.0 | 0.77 |
Data are least square means ± SE. RMR change was defined as difference between RMR assessment at baseline and RMR assessments at follow-up examinations;
Model 1, adjusted for age, sex, race, education, smoking, alcohol consumption, physical activity, menopausal status, hormone replacement therapy, diet groups, and baseline resting metabolic rate.
Model 2, further adjusted for other thyroid functional parameters.
Figure 2.
Trajectory of changes in RMR according to baseline tertiles of thyroid hormone. Data were least square means, adjusted for age, sex, race, education, smoking, alcohol consumption, physical activity, menopausal status, hormone replacement therapy, diet groups and baseline RMR.
Changes in thyroid hormones, body weight, and metabolic parameters
At 6 months and 2 years, after multivariate adjustment, changes in free T3 and total T3 levels were positively associated with changes in body weight and metabolic parameters, including RMR, leptin, blood pressure, glucose, insulin, and triglycerides (all P trend<0.05). In contrast, no such patterns of association were observed for free T4, total T4, and TSH (Table 4). When percentage changes were used in the analyses, the results were mostly similar. In addition, no associations were observed between thyroid hormones at 6 months and changes in body weight over the remaining period from 6–24 months (Supplementary Table 4). Changes in RMR were significantly associated with changes in body weight during the period of weight loss (r=0.41, P<0.001) and weight regain (r=0.20, P<0.001). A greater reduction in RMR during the first 6 months was significantly associated with a greater weight regain from 6–24 months (r=−0.10, P<0.05). Supplementary Table 5 shows the levels of metabolic parameters (e.g., thyroid hormones, blood pressure, glucose, insulin, triglycerides, cholesterols, and leptin) at baseline, 6 months, and 24 months. Most of the parameters significantly decreased during the weight-loss period and then modestly increased over the weight-regain period.
Table 4.
Partial Spearman correlation coefficients among changes in thyroid hormones, body weight, and metabolic parameters
| 0–6 months | ΔFree T3 (0–6m) (n=528) | ΔTotal T3 (0–6m) (n=505) | ΔFree T4 (0–6m) (n=552) | ΔTotal T4 (0–6m) (n=499) | ΔTSH (0–6m) (n=547) |
|---|---|---|---|---|---|
| ΔWeight (0–6 m) | 0.29*** | 0.30*** | −0.05 | 0.06 | 0.05 |
| ΔResting metabolic rate (0–6 m) | 0.24*** | 0.25*** | 0.03 | 0.04 | −0.04 |
| ΔSystolic blood pressure (0–6 m) | 0.14** | 0.19*** | 0.04 | 0.07 | −0.02 |
| ΔDiastolic blood pressure (0–6 m) | 0.14** | 0.20*** | 0.04 | 0.10* | 0.01 |
| ΔGlucose (0–6 m) | 0.11* | 0.09* | 0.03 | 0.02 | −0.01 |
| ΔInsulin (0–6 m) | 0.16*** | 0.20*** | −0.05 | 0.02 | −0.01 |
| ΔTriglycerides (0–6 m) | 0.19*** | 0.23*** | −0.06 | 0.05 | 0.17*** |
| ΔTotal cholesterol (0–6 m) | 0.03 | 0.03 | −0.02 | 0.05 | −0.03 |
| ΔWhole body fat mass (0–6 m) † | 0.28*** | 0.33*** | 0.01 | 0.17* | 0.07 |
| ΔWhole body lean mass (0–6 m) † | 0.21*** | 0.32*** | −0.09 | −0.004 | 0.07 |
| ΔLeptin (0–6 m) | 0.17*** | 0.24*** | −0.13** | 0.03 | 0.09* |
| ΔSoluble leptin receptor (0–6 m) | −0.08 | −0.06 | 0.06 | 0.12** | 0.03 |
|
| |||||
| 6–24 months | ΔFree T3 (0–6m) (n=448) | ΔTotal T3 (0–6m) (n=429) | ΔFree T4 (0–6m) (n=469) | ΔTotal T4 (0–6m) (n=424) | ΔTSH (0–6m) (n=465) |
|
| |||||
| ΔWeight (6–24 m) | −0.03 | −0.006 | 0.06 | 0.05 | 0.09 |
| ΔResting metabolic rate (6–24 m) | −0.08 | −0.02 | −0.002 | 0.03 | 0.08 |
| ΔSystolic blood pressure (6–24 m) | −0.05 | −0.06 | −0.03 | −0.09 | 0.01 |
| ΔDiastolic blood pressure (0–6 m) | −0.04 | −0.09 | −0.02 | −0.13* | −0.03 |
| ΔGlucose (6–24 m) | 0.04 | 0.01 | 0.02 | 0.008 | −0.05 |
| ΔInsulin (6–24 m) | 0.04 | 0.009 | 0.02 | 0.005 | −0.001 |
| ΔTriglycerides (6–24 m) | −0.09 | −0.10* | 0.04 | −0.01 | 0.03 |
| ΔCholesterol (6–24 m) | −0.10* | −0.10* | 0.01 | −0.05 | 0.07 |
| ΔWhole body fat mass (6–24 m) † | −0.03 | 0.03 | 0.01 | 0.03 | 0.02 |
| ΔWhole body lean mass (6–24 m) † | −0.07 | −0.007 | −0.06 | 0.004 | 0.09 |
| ΔLeptin (6–24 m) | −0.07 | −0.11* | 0.07 | −0.05 | 0.11* |
| ΔSoluble leptin receptor (6–24 m) | −0.003 | −0.04 | −0.02 | −0.11* | −0.10 |
|
| |||||
| 6–24 months | ΔFree T3 (6−24m) (n=415) | ΔTotal T3 (6−24m) (n=388) | ΔFree T4 (6−24m) (n=425) | ΔTotal T4 (6−24m) (n=377) | ΔTSH (6−24m) (n=422) |
|
| |||||
| ΔWeight (6–24 m) | 0.25*** | 0.26*** | −0.13** | −0.005 | 0.04 |
| ΔResting metabolic rate (6–24 m) | 0.15** | 0.18** | −0.09 | 0.03 | −0.01 |
| ΔSystolic blood pressure (6–24 m) | 0.12* | 0.12* | −0.02 | 0.01 | 0.004 |
| ΔDiastolic blood pressure (0–6 m) | 0.16** | 0.18*** | 0.01 | 0.11* | 0.08 |
| ΔGlucose (6–24 m) | 0.04 | 0.05 | −0.004 | 0.01 | 0.04 |
| ΔInsulin (6–24 m) | 0.09 | 0.14** | −0.13** | 0.005 | 0.03 |
| ΔTriglycerides (6–24 m) | 0.14** | 0.15** | −0.12* | 0.06 | 0.03 |
| ΔCholesterol (6–24 m) | 0.05 | −0.04 | −0.05 | 0.03 | 0.05 |
| ΔWhole body fat mass (6–24 m) † | 0.29*** | 0.20* | −0.05 | 0.09 | 0.02 |
| ΔWhole body lean mass (6–24 m) † | 0.14 | 0.18* | −0.22** | −0.08 | 0.02 |
| ΔLeptin (6–24 m) | 0.23*** | 0.28*** | −0.09 | 0.07 | 0.04 |
| ΔSoluble leptin receptor (6–24 m) | 0.01 | −0.03 | 0.17*** | 0.13* | 0.04 |
|
| |||||
| 0–24 months | ΔFree T3 (0–24m) (n=398) | ΔTotal T3 (0–24m) (n=369) | ΔFree T4 (0–24m) (n=420) | ΔTotal T4 (0–24m) (n=359) | ΔTSH (0–24m) (n=418) |
|
| |||||
| ΔWeight (0–24 m) | 0.32*** | 0.41*** | −0.13** | 0.10 | 0.11 |
| ΔResting metabolic rate (0–24 m) | 0.16** | 0.26*** | −0.03 | 0.12* | −0.02 |
| ΔSystolic blood pressure (0–24 m) | 0.17*** | 0.19*** | −0.03 | 0.06 | −0.0003 |
| ΔDiastolic blood pressure (0–6 m) | 0.17*** | 0.22*** | −0.02 | 0.09 | −0.003 |
| ΔGlucose (0–24 m) | 0.17*** | 0.18*** | 0.004 | 0.10 | −0.07 |
| ΔInsulin (0–24 m) | 0.24*** | 0.35*** | −0.09 | 0.14** | 0.03 |
| ΔTriglycerides (0–24 m) | 0.14** | 0.16** | −0.13** | 0.07 | 0.15** |
| ΔCholesterol (0–24 m) | 0.03 | −0.02 | 0.007 | 0.10 | 0.08 |
| ΔWhole body fat mass (0–24 m) † | 0.34*** | 0.44*** | −0.02 | 0.24*** | 0.08 |
| ΔWhole body lean mass (0–24 m) † | 0.24*** | 0.36*** | −0.20** | 0.09 | 0.12 |
| ΔLeptin (0–24 m) | 0.20*** | 0.30*** | −0.12* | 0.07 | 0.20*** |
| ΔSoluble leptin receptor (0–24 m) | −0.07 | −0.15** | 0.10* | 0.06 | −0.03 |
Values were adjusted for age, sex, race, education, smoking, alcohol consumption, physical activity, menopausal status, hormone replacement therapy, and diet groups. Δ= follow-up value –baseline value.
P<0.05;
P<0.01;
P<0.001;
further adjusted for whole body lean mass (for whole body fat mass) or whole body fat mass (for whole body lean mass); n=293 for 6 months, and n=177 for 24 months.
In a sensitivity analysis, after further excluding the participants with TSH <0.4 mIU/L (n=7), the main results remained largely unchanged (Supplementary Tables 6 and 7). In addition, further adjustment for participants’ compliance did not significantly change the findings. Moreover, when the analysis was stratified by sex and smoking status, the results were mostly similar with no significant interaction observed, although some of the associations lacked statistical significance due to reduced power (data not shown).
Discussion
In this analysis conducted among overweight or obese individuals with normal thyroid function, we demonstrated that higher levels of baseline free T3 and free T4 were significantly associated with a greater weight loss at 6 months and at 24 months, induced by weight-loss diets. In contrast, TSH levels did not predict weight loss or weight regain in this analysis. In addition, changes in free T3 and total T3 levels, but not free T4 and total T4 or TSH, were positively associated with changes in body weight and metabolic parameters, including RMR, blood pressure, triglycerides, and leptin.
In the current study, free T3, free T3/free T4 ratio, and total T3 were positively associated, whereas free T4 was negatively associated, with baseline body weight, BMI, and RMR. Total T4 and TSH were not significantly associated with these variables. These relationships were observed in some previous studies among euthyroid participants as well.23–27 For example, among 2,524 euthyroid men and women (mean age 46 years), Roef et al. demonstrated that free T3, free T3/free T4 ratio, and total T3 were positively associated with BMI, WC, and metabolic syndrome components, whereas free T4 had only a weak inverse associations with BMI and WC. In addition, TSH was positively associated with blood pressure and triglycerides, but not with BMI and WC.23 In contrast to our findings, some studies demonstrated that TSH was positively associated with BMI or body weight,13, 17, 28–30 and some studies reported no associations between free T4 and BMI.28, 31 These discrepant findings might be attributed to the difference in characteristics of the study participants, including age, gender, BMI, and smoking status.
The relationship of thyroid hormones and function with weight change was explored in several prospective observational studies, although the results were quite mixed.13–16 In a community-based study among 1,944 adults (18–65 years), Bjergved et al. reported that baseline TSH levels were not associated with body weight changes, whereas changes in serum TSH were significantly associated with weight change.15 Similarly, in the Framingham Offspring Study (n=2407) and the Nord-Trøndelag Health Study Study (n=15,020), TSH concentrations at baseline were not associated with subsequent weight change, but changes in TSH was positively correlated with changes in body weight in men and women with normal thyroid function.13, 16 However, Nyrnes et al. observed this pattern of associations only in non-smokers.17 In another small prospective study (n=89) among Pima Indians (mean age 29 years), baseline free T3, but not free T4 or TSH, was significantly associated with absolute weight change (rs= −0.27) and annual percentage of weight change (rs=−0.28).14 Although the exact reason for these mixed findings is unknown, it should be noted that in these observational studies, the causes of weight change were not quite clear among individuals, and the associations may be subject to confounding in an observational setting. Moreover, most of these observational studies did not account for some important covariates, including dietary factors and physical activity.
To our knowledge, the current investigation was among the first studies that examined the role of thyroid hormones in weight change in a controlled clinical trial. In a small intervention trial among 47 overweight subjects through education/counseling to emphasize lifestyle change (including diet and behavioral modification), TSH and total T3 were significantly associated with fat mass at baseline.32 After approximately a 9-month follow up, Agnihothri et al. found that total T3 change was positively associated with body weight change (rs=0.3, P<0.01),32 although they did not report the associations between baseline thyroid hormones and weight change. In another 2-year longitudinal study consisted of 477 euthyroid obese children, Wolters et al. observed that higher baseline free T3, but not free T4, was significantly associated with a greater decrease in BMI during 1-year lifestyle intervention for weight-loss, although TSH also predicted BMI reduction in this study.33 In addition, changes in TSH and free T3 during the intervention period were inversely associated with the BMI change during 1-year follow up after the intervention.33 In contrast, after multivariate adjustment, our analysis among overweight adults found that higher baseline levels of free T3 and free T4, but not TSH, were associated with a greater weight loss at 6 months. Moreover, during the period of weight loss and weight regain, changes in free T3 and total T3 were positively associated with changes in body weight. The discrepancies between our study and the study of Wolters et al. might be explained by the fact that there are significant differences in the levels of thyroid hormones and RMR between children and adults.34–37 There are dynamic changes of thyroid hormones during child development, whereas such changes are absent in adults. For instance, the levels of T3 are heightened in childhood until approximately 10 years old and then decline steadily until maturity.34
Accumulating evidence has suggested that RMR plays a vital role in weight maintenance.18, 38, 39 A recent study by Erin et al. showed that after “The Biggest Loser” competition (30 weeks), participants had a nearly 23% reduction of RMR, and after a 6-year follow up, RMR remained suppressed at similar levels as at the end of the competition, suggesting that potential metabolic adaptation might result in weight regain.39 In the POUNDS LOST Study, RMR also declined significantly during the weight loss period. Being consistent with previous studies,18, 38 the decrease of RMR at 6 months was significantly associated with weight regain over the remaining period from 6 to24 months (r=−0.10, P<0.05). Our study further elucidated that the baseline thyroid hormones predicted changes of RMR similar to those of body weight.
It is known that thyroid hormones regulate the RMR and thus body weight through increasing ATP production and by generating ion gradients including Na+/K+ and Ca2+ gradients.11, 40, 41 The mechanisms underlying our findings are not well understood. We suspect that euthyroid individuals with high free T3 or free T4, and thus high RMR, might be more sensitive to thyroid hormone regulation and more responsive to energy-reduction. Therefore, when weight loss begins, individuals with higher free T3 or free T4 tend to respond promptly and lose more weight. Nevertheless, more research is warranted to clarify the underlying mechanism between normal thyroid hormone function and body weight change, especially among overweight and obese individuals.
One strength of our study was the analysis of a 2-year diet-induced weight loss trial, which could help alleviate the potential influence of unknown confounders. Moreover, repeated measurements of body weight, thyroid hormones, RMR, and metabolic biomarkers could facilitate the exploration of the relationships between changes in these parameters during the process of weight loss and weight regain. Furthermore, detailed information on covariates such as physical activity and hormone replacement therapy was collected to facilitate multivariate adjustment in our study.
Several limitations should also be discussed. First, the drop-out rate was 20% in our 2-year intervention, although no significant differences were demonstrated in body weight and other parameters between the included and excluded participants. Second, other hormones or peptides such as ghrelin and cholecystokinin that might influence weigh change were not examined.4 More research is needed to investigate whether these hormones or peptides would influence the associations between thyroid hormones and weight loss. Lastly, our participants were relatively homogeneous in terms of their body fatness and health status. Whether these findings can be generalized to populations with other characteristics is unknown.
In conclusion, in a diet-induced weight-loss setting, free T3 and free T4 levels at baseline predict weight loss, but not weight regain among overweight and obese individuals with normal thyroid function. Our findings suggest that euthyroid overweight and obese individuals with relatively higher free T3 and free T4 levels might benefit more from a diet intervention strategy for weight loss.
Supplementary Material
Acknowledgments
We thank the participants in the trial for their dedication and contribution to the research.
Funding: This research was supported by NIH grants ES022981, ES021372, the National Heart, Lung, and Blood Institute (HL073286), and the General Clinical Research Center, National Institutes of Health (RR-02635). Qi Sun was supported by a career development award, R00-HL098459, from the National Heart, Lung, and Blood Institute. Gang Liu was supported by the International Postdoctoral Exchange Fellowship Program 2015 by the Office of China Postdoctoral Council.
Footnotes
Conflict of Interest: The authors declare that there is no conflict of interested associated with this manuscript.
References
- 1.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–25. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 2.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–14. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 3.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6(1):67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 4.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 5.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 6.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–67. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R581–600. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes (Lond) 2008;32(1):177–84. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 9.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316(2):165–71. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–82. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoogwerf BJ, Nuttall FQ. Long-term weight regulation in treated hyperthyroid and hypothyroid subjects. Am J Med. 1984;76(6):963–70. doi: 10.1016/0002-9343(84)90842-8. [DOI] [PubMed] [Google Scholar]
- 13.Fox CS, Pencina MJ, D’Agostino RB, Murabito JM, Seely EW, Pearce EN, et al. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008;168(6):587–92. doi: 10.1001/archinte.168.6.587. [DOI] [PubMed] [Google Scholar]
- 14.Ortega E, Pannacciulli N, Bogardus C, Krakoff J. Plasma concentrations of free triiodothyronine predict weight change in euthyroid persons. Am J Clin Nutr. 2007;85(2):440–5. doi: 10.1093/ajcn/85.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjergved L, Jorgensen T, Perrild H, Laurberg P, Krejbjerg A, Ovesen L, et al. Thyroid function and body weight: a community-based longitudinal study. PLoS One. 2014;9(4):e93515. doi: 10.1371/journal.pone.0093515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svare A, Nilsen TI, Bjoro T, Asvold BO, Langhammer A. Serum TSH related to measures of body mass: longitudinal data from the HUNT Study, Norway. Clin Endocrinol (Oxf) 2011;74(6):769–75. doi: 10.1111/j.1365-2265.2011.04009.x. [DOI] [PubMed] [Google Scholar]
- 17.Nyrnes A, Jorde R, Sundsfjord J. Serum TSH is positively associated with BMI. Int J Obes (Lond) 2006;30(1):100–5. doi: 10.1038/sj.ijo.0803112. [DOI] [PubMed] [Google Scholar]
- 18.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318(8):467–72. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 19.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87(2):489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 20.Tirosh A, de Souza RJ, Sacks F, Bray GA, Smith SR, LeBoff MS. Sex Differences in the Effects of Weight Loss Diets on Bone Mineral Density and Body Composition: POUNDS LOST Trial. J Clin Endocrinol Metab. 2015;100(6):2463–71. doi: 10.1210/jc.2015-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jonge L, Bray GA, Smith SR, Ryan DH, de Souza RJ, Loria CM, et al. Effect of diet composition and weight loss on resting energy expenditure in the POUNDS LOST study. Obesity (Silver Spring) 2012;20(12):2384–9. doi: 10.1038/oby.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 23.Roef GL, Rietzschel ER, Van Daele CM, Taes YE, De Buyzere ML, Gillebert TC, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. 2014;24(2):223–31. doi: 10.1089/thy.2013.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassols J, Prats-Puig A, Soriano-Rodriguez P, Garcia-Gonzalez MM, Reid J, Martinez-Pascual M, et al. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab. 2011;96(12):3717–23. doi: 10.1210/jc.2011-1784. [DOI] [PubMed] [Google Scholar]
- 25.Shon HS, Jung ED, Kim SH, Lee JH. Free T4 is negatively correlated with body mass index in euthyroid women. Korean J Intern Med. 2008;23(2):53–7. doi: 10.3904/kjim.2008.23.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makepeace AE, Bremner AP, O’Leary P, Leedman PJ, Feddema P, Michelangeli V, et al. Significant inverse relationship between serum free T4 concentration and body mass index in euthyroid subjects: differences between smokers and nonsmokers. Clin Endocrinol (Oxf) 2008;69(4):648–52. doi: 10.1111/j.1365-2265.2008.03239.x. [DOI] [PubMed] [Google Scholar]
- 27.Taylor PN, Richmond R, Davies N, Sayers A, Stevenson K, Woltersdorf W, et al. Paradoxical Relationship Between Body Mass Index and Thyroid Hormone Levels: A Study Using Mendelian Randomization. J Clin Endocrinol Metab. 2016;101(2):730–8. doi: 10.1210/jc.2015-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitahara CM, Platz EA, Ladenson PW, Mondul AM, Menke A, Berrington de Gonzalez A. Body fatness and markers of thyroid function among U.S. men and women. PLoS One. 2012;7(4):e34979. doi: 10.1371/journal.pone.0034979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knudsen N, Laurberg P, Rasmussen LB, Bulow I, Perrild H, Ovesen L, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90(7):4019–24. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 30.Lee JJ, Pedley A, Marqusee E, Sutherland P, Hoffmann U, Massaro JM, et al. Thyroid Function and Cardiovascular Disease Risk Factors in Euthyroid Adults: A Cross-Sectional and Longitudinal Study. Clin Endocrinol (Oxf) 2016 doi: 10.1111/cen.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manji N, Boelaert K, Sheppard MC, Holder RL, Gough SC, Franklyn JA. Lack of association between serum TSH or free T4 and body mass index in euthyroid subjects. Clin Endocrinol (Oxf) 2006;64(2):125–8. doi: 10.1111/j.1365-2265.2006.02433.x. [DOI] [PubMed] [Google Scholar]
- 32.Agnihothri RV, Courville AB, Linderman JD, Smith S, Brychta R, Remaley A, et al. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid. 2014;24(1):19–26. doi: 10.1089/thy.2013.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolters B, Lass N, Reinehr T. TSH and free triiodothyronine concentrations are associated with weight loss in a lifestyle intervention and weight regain afterwards in obese children. Eur J Endocrinol. 2013;168(3):323–9. doi: 10.1530/EJE-12-0981. [DOI] [PubMed] [Google Scholar]
- 34.Corcoran JM, Eastman CJ, Carter JN, Lazarus L. Circulating thyroid hormone levels in children. Arch Dis Child. 1977;52(9):716–20. doi: 10.1136/adc.52.9.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westgren U, Burger A, Ingemansson S, Melander A, Tibblin S, Wahlin E. Blood levels of 3,5,3′-triiodothyronine and thyroxine: differences between children, adults, and elderly subjects. Acta Med Scand. 1976;200(6):493–5. doi: 10.1111/j.0954-6820.1976.tb08271.x. [DOI] [PubMed] [Google Scholar]
- 36.Ehrenkranz J, Bach PR, Snow GL, Schneider A, Lee JL, Ilstrup S, et al. Circadian and Circannual Rhythms in Thyroid Hormones: Determining the TSH and Free T4 Reference Intervals Based Upon Time of Day, Age, and Sex. Thyroid. 2015;25(8):954–61. doi: 10.1089/thy.2014.0589. [DOI] [PubMed] [Google Scholar]
- 37.Molnar D, Schutz Y. The effect of obesity, age, puberty and gender on resting metabolic rate in children and adolescents. Eur J Pediatr. 1997;156(5):376–81. doi: 10.1007/s004310050618. [DOI] [PubMed] [Google Scholar]
- 38.Saltzman E, Roberts SB. The role of energy expenditure in energy regulation: findings from a decade of research. Nutr Rev. 1995;53(8):209–20. doi: 10.1111/j.1753-4887.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 39.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 2016 doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haber RS, Loeb JN. Stimulation of potassium efflux in rat liver by a low dose of thyroid hormone: evidence for enhanced cation permeability in the absence of Na,K-ATPase induction. Endocrinology. 1986;118(1):207–11. doi: 10.1210/endo-118-1-207. [DOI] [PubMed] [Google Scholar]
- 41.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86(2):435–64. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.