Abstract
Substitution of phenylalanine for tyrosine-809 in the human colony-stimulating factor 1 receptor (CSF-1R) inhibited its ability to transduce ligand-dependent mitogenic signals in mouse NIH 3T3 cells. When combined with an "activating" mutation at codon 301 that induces constitutive CSF-1R tyrosine kinase activity, the codon 809 mutation suppressed ligand-independent cell transformation. Comparative mapping of tryptic phosphopeptides from mutant and wild-type CSF-1R indicated that tyrosine-809 is a site of ligand-dependent receptor phosphorylation in vivo. The mutant receptor was active as a tyrosine kinase in vitro and in vivo, underwent CSF-1-dependent association with a phosphatidylinositol 3-kinase, and induced expression of the protooncogenes c-fos and junB, underscoring its ability to trigger some of the known cellular responses to CSF-1. The mutant receptor is likely to be impaired in its ability to interact with critical cellular effectors whose activity is required for mitogenesis.
Full text
PDF

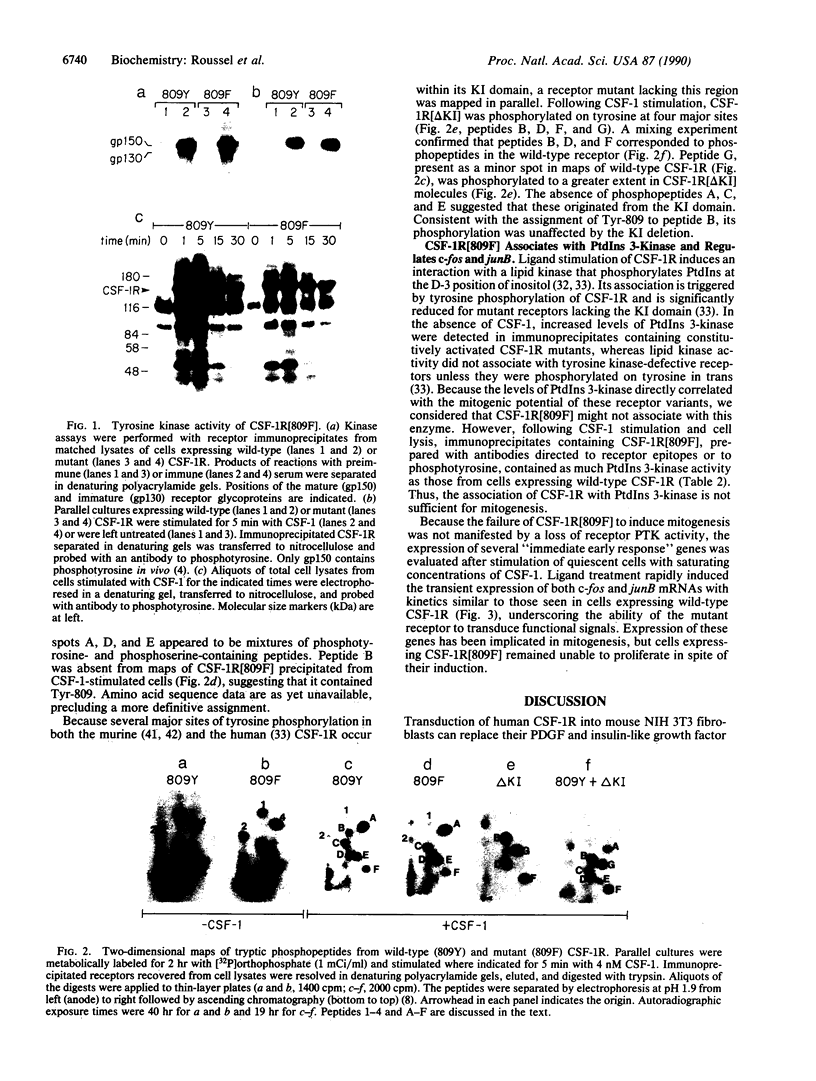


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Gonda M. A., Rettenmier C. W., Sherr C. J. Subcellular localization of glycoproteins encoded by the viral oncogene v-fms. J Virol. 1984 Sep;51(3):730–741. doi: 10.1128/jvi.51.3.730-741.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmun R. A., Look A. T., Roberts W. M., Roussel M. F., Seremetis S., Ohtsuka M., Sherr C. J. Monoclonal antibodies to the human CSF-1 receptor (c-fms proto-oncogene product) detect epitopes on normal mononuclear phagocytes and on human myeloid leukemic blast cells. Blood. 1989 Feb 15;73(3):827–837. [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Downing J. R., Margolis B. L., Zilberstein A., Ashmun R. A., Ullrich A., Sherr C. J., Schlessinger J. Phospholipase C-gamma, a substrate for PDGF receptor kinase, is not phosphorylated on tyrosine during the mitogenic response to CSF-1. EMBO J. 1989 Nov;8(11):3345–3350. doi: 10.1002/j.1460-2075.1989.tb08496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Rettenmier C. W., Sherr C. J. Ligand-induced tyrosine kinase activity of the colony-stimulating factor 1 receptor in a murine macrophage cell line. Mol Cell Biol. 1988 Apr;8(4):1795–1799. doi: 10.1128/mcb.8.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Roussel M. F., Sherr C. J. Ligand and protein kinase C downmodulate the colony-stimulating factor 1 receptor by independent mechanisms. Mol Cell Biol. 1989 Jul;9(7):2890–2896. doi: 10.1128/mcb.9.7.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990 Jan 25;343(6256):377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Williams L. T. A PDGF receptor domain essential for mitogenesis but not for many other responses to PDGF. Nature. 1988 Sep 1;335(6185):85–87. doi: 10.1038/335085a0. [DOI] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Rettenmier C. W., Rock C. O. Prostaglandin E2 inhibition of growth in a colony-stimulating factor 1-dependent macrophage cell line. J Biol Chem. 1990 Apr 25;265(12):6611–6616. [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell. 1989 Sep 22;58(6):1121–1133. doi: 10.1016/0092-8674(89)90510-2. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Ellis C., Pawson T., Cooper J. A. Binding of GAP to activated PDGF receptors. Science. 1990 Mar 30;247(4950):1578–1581. doi: 10.1126/science.2157284. [DOI] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Jinno A., Takagi N., Shibuya M. A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol Cell Biol. 1990 May;10(5):2261–2268. doi: 10.1128/mcb.10.5.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988 Mar 17;332(6161):272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hanafusa H. Association of the v-crk oncogene product with phosphotyrosine-containing proteins and protein kinase activity. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2638–2642. doi: 10.1073/pnas.87.7.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rapp U., Roberts T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rhee S. G., Williams L. T. Platelet-derived growth factor (PDGF)-dependent association of phospholipase C-gamma with the PDGF receptor signaling complex. Mol Cell Biol. 1990 May;10(5):2359–2366. doi: 10.1128/mcb.10.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka M., Roussel M. F., Sherr C. J., Downing J. R. Ligand-induced phosphorylation of the colony-stimulating factor 1 receptor can occur through an intermolecular reaction that triggers receptor down modulation. Mol Cell Biol. 1990 Apr;10(4):1664–1671. doi: 10.1128/mcb.10.4.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T. Non-catalytic domains of cytoplasmic protein-tyrosine kinases: regulatory elements in signal transduction. Oncogene. 1988 Nov;3(5):491–495. [PubMed] [Google Scholar]
- Roberts W. M., Look A. T., Roussel M. F., Sherr C. J. Tandem linkage of human CSF-1 receptor (c-fms) and PDGF receptor genes. Cell. 1988 Nov 18;55(4):655–661. doi: 10.1016/0092-8674(88)90224-3. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Sherr C. J. Transforming activities of human CSF-1 receptors with different point mutations at codon 301 in their extracellular domains. Oncogene. 1990 Jan;5(1):25–30. [PubMed] [Google Scholar]
- Roussel M. F., Dull T. J., Rettenmier C. W., Ralph P., Ullrich A., Sherr C. J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Rettenmier C. W., Look A. T., Sherr C. J. Cell surface expression of v-fms-coded glycoproteins is required for transformation. Mol Cell Biol. 1984 Oct;4(10):1999–2009. doi: 10.1128/mcb.4.10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Sherr C. J. Mouse NIH 3T3 cells expressing human colony-stimulating factor 1 (CSF-1) receptors overgrow in serum-free medium containing human CSF-1 as their only growth factor. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7924–7927. doi: 10.1073/pnas.86.20.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinsson L., Ek B., Mellström K., Claesson-Welsh L., Heldin C. H. Deletion of the kinase insert sequence of the platelet-derived growth factor beta-receptor affects receptor kinase activity and signal transduction. Mol Cell Biol. 1990 Feb;10(2):801–809. doi: 10.1128/mcb.10.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J. Colony-stimulating factor-1 receptor. Blood. 1990 Jan 1;75(1):1–12. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Shurtleff S. A., Downing J. R., Rock C. O., Hawkins S. A., Roussel M. F., Sherr C. J. Structural features of the colony-stimulating factor 1 receptor that affect its association with phosphatidylinositol 3-kinase. EMBO J. 1990 Aug;9(8):2415–2421. doi: 10.1002/j.1460-2075.1990.tb07417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley P., Kazlauskas A., Cooper J. A., Rohrschneider L. R. Macrophage colony-stimulating factor-induced tyrosine phosphorylation of c-fms proteins expressed in FDC-P1 and BALB/c 3T3 cells. Mol Cell Biol. 1990 Jun;10(6):2528–2538. doi: 10.1128/mcb.10.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. R., Reedijk M., Rothwell V., Rohrschneider L., Pawson T. The unique insert of cellular and viral fms protein tyrosine kinase domains is dispensable for enzymatic and transforming activities. EMBO J. 1989 Jul;8(7):2029–2037. doi: 10.1002/j.1460-2075.1989.tb03611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushinski R. J., Stanley E. R. The regulation of mononuclear phagocyte entry into S phase by the colony stimulating factor CSF-1. J Cell Physiol. 1985 Feb;122(2):221–228. doi: 10.1002/jcp.1041220210. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Druker B., Morrison D., Cantley L., Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989 Dec 7;342(6250):699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989 Jul;9(7):2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E. F., Rettenmier C. W., Look A. T., Sherr C. J. The v-fms oncogene induces factor independence and tumorigenicity in CSF-1 dependent macrophage cell line. 1986 Nov 27-Dec 3Nature. 324(6095):377–380. doi: 10.1038/324377a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987 Oct 1;247(1):165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford J., McAuliffe A., Rohrschneider L. R. Activation of the feline c-fms proto-oncogene: multiple alterations are required to generate a fully transformed phenotype. Cell. 1988 Dec 23;55(6):965–977. doi: 10.1016/0092-8674(88)90242-5. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yeung Y. G., Jubinsky P. T., Sengupta A., Yeung D. C., Stanley E. R. Purification of the colony-stimulating factor 1 receptor and demonstration of its tyrosine kinase activity. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1268–1271. doi: 10.1073/pnas.84.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer P., Hunter T. Identification of tyrosine 706 in the kinase insert as the major colony-stimulating factor 1 (CSF-1)-stimulated autophosphorylation site in the CSF-1 receptor in a murine macrophage cell line. Mol Cell Biol. 1990 Jun;10(6):2991–3002. doi: 10.1128/mcb.10.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]