Abstract
Rumination is a maladaptive form of emotion regulation associated with psychopathology. Research with adults suggests that rumination covaries with diurnal cortisol rhythms, yet this has not been examined among adolescents. Here, we examine the day-to-day covariation between rumination and cortisol, and explore whether trait rumination is associated with alterations in diurnal cortisol rhythms among adolescent girls. Participants (N = 122) provided saliva samples 3 times per day over 3 days, along with daily reports of stress and rumination, questionnaires assessing trait rumination related to peer stress, and diagnostic interviews assessing depression and anxiety. Greater rumination than usual during the day was associated with lower cortisol awakening responses the following morning, but this effect was not significant after accounting for wake time and an objective measure of adherence to the saliva sampling protocol. Trait rumination was associated with lower average cortisol levels at waking and flatter diurnal slopes, accounting for wake time, protocol compliance, and other factors. These patterns may help to explain why rumination is related to the development of psychopathology.
Keywords: Cortisol, adolescence, rumination, HPA axis, stress
Rumination is a cognitive style of regulating one’s negative emotions by passively dwelling on them without taking action. Unsurprisingly, it has been associated with the development of psychopathology among adolescents (Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008, for review). Research with adults has linked rumination to indices of the hypothalamic–pituitary–adrenal (HPA) axis, one of the body’s primary stress-response systems (Zoccola & Dickerson, 2012, for review). Because disorders associated with rumination (e.g. depression) often first emerge in adolescence, it is important to understand whether stress physiology may be one mechanism underlying associations between rumination and subsequent psychopathology. Thus, the present study examines the relationship between rumination and diurnal cortisol rhythms among early adolescent girls.
Rumination is more common among females than males by early adolescence, and this may partially explain the gender difference in depression (Nolen-Hoeksema et al., 2008). One of the reasons that adolescence may be a particularly challenging time for girls is because of peer stress. Peers become increasingly important throughout adolescence, replacing parents as a primary agent of social support (Furman & Buhrmester, 1992). Adolescent girls, in particular, place a high value on peer relationships, resulting in stress when difficulties in these relationships arise, as often happens during this developmental period (Rose & Rudolph, 2006). The resulting stress may fuel rumination, placing girls at higher risk for depression and other forms of psychopathology.
Rumination may also alter the body’s stress-response systems. In her Response Styles Theory, Nolen-Hoeksema (1991) suggested that rumination may be associated with increased stress reactivity. Furthermore, the Perseverative Cognition Hypothesis (Brosschot, Gerin, & Thayer, 2006) posits that rumination may prolong physiological stress responses, including those involving the neuroendocrine system. According to this hypothesis, the time course is important, because once a stressful event has ended, cognitive perseveration can continually activate the stress response, resulting in higher allostatic load (McEwen, 1998) over time. These theories suggest that an examination of rumination and physiological stress processes may provide important information about how rumination affects the daily lives of adolescents.
One of the body’s primary stress response systems is the HPA axis, with cortisol as the main hormonal output and regulator of the system. Cortisol follows a diurnal pattern with peak secretion occurring approximately 30 minutes after waking (the cortisol awakening response; CAR), followed by a decline throughout the day (Clow, Hucklebridge, Stalder, Evans, & Thorn, 2010). The CAR appears to be more responsive to daily variation, whereas the diurnal slope (i.e. linear rate of decline in cortisol levels from waking to bedtime) appears to represent relatively more stable individual differences (Ross, Murphy, Adam, Chen, & Miller, 2014). Another metric often examined, area under the curve with respect to ground (AUCg; Pruessner, Hellhammer, Pruessner, & Lupien, 2003), estimates the total daily cortisol output; evidence regarding its stability has been inconsistent (e.g. Ross et al., 2014). Alterations in these diurnal cortisol indices have been associated with psychopathology in adolescence (e.g. Doane et al., 2013; Vrshek-Schallhorn et al., 2013).
If rumination does prolong the physiological stress response, as suggested by the Perseverative Cognition Hypothesis, we would expect to see covariation between rumination and cortisol output. On a day-to-day basis, rumination may be associated with alterations in cortisol patterns. In one study, adults who reported ruminating one day (i.e. greater daily rumination) showed an increased CAR the next day compared to adults who did not ruminate to the same degree (Zoccola, Dickerson, & Yim, 2011), offering preliminary support for this hypothesis. However, another study of adults showed that those who ruminated in the evening had a decreased CAR the next day (Cropley, Rydstedt, Devereux, & Middleton, 2015). Some studies examining the relationship between daily rumination and other indices of diurnal cortisol have offered support for the Perseverative Cognition Hypothesis. In one study, higher daily rumination was associated with higher daily cortisol output (averaged across 10 samples over 2 days; Huffziger et al., 2013). In another study, greater rumination regarding recent interpersonal difficulties predicted higher afternoon cortisol levels (McCullough, Orsulak, Brandon, & Akers, 2007).
Habitual rumination may also be associated with diurnal cortisol. The literature examining the relationship between habitual (i.e. trait) rumination and the CAR, however, is mixed, with prior work finding either no relationship (e.g. Zoccola et al., 2011) or a negative relationship (e.g. Kuehner, Holzhauer, & Huffziger, 2007). Studies examining the relationship between trait rumination and other cortisol indices have also found inconsistent patterns. One study found no relationship between trait rumination and diurnal cortisol (Kuehner et al., 2007), while another found that trait rumination predicted greater evening, but not morning, cortisol (Rydstedt, Cropley, Devereux, & Michalianou, 2009), offering mixed support for the Perseverative Cognition Hypothesis. We are not aware of previous research examining the relationship between rumination and diurnal slope, but given that slope tends to be a relatively more stable index (Ross et al., 2014), we might expect it to be related to trait rumination.
Thus, although there is some indication that daily and trait rumination may be related to the CAR and other indices of the diurnal rhythm, research is limited and mixed regarding support for the Perseverative Cognition Hypothesis. Additionally, prior work has focused on adults, leaving questions about whether rumination and diurnal cortisol patterns are linked earlier in development, such as during adolescence.
This heterogeneity among findings could be due to several factors including measurement of rumination and cortisol (Zoccola & Dickerson, 2012). Rumination is often measured as a trait using a questionnaire where participants indicate how they typically respond when distressed, yet spontaneous use of rumination varies even among those scoring high on trait rumination (Moberly & Watkins, 2008). Daily measures of rumination are more consistently related to cortisol responses in studies utilising laboratory stressors (Zoccola & Dickerson, 2012). Additionally, trait rumination in response to stress is more consistently linked to cortisol than trait rumination in response to depression (Zoccola & Dickerson, 2012). Further, cortisol rhythms, although somewhat stable within individuals over time, can also vary on a daily basis (Ross et al., 2014). Thus, measuring diurnal rhythms across multiple days is advantageous. Many prior studies have relied on cortisol collected on only 1 day (e.g. Kuehner et al., 2007; Zoccola et al., 2011) or only one or two samples obtained each day (e.g. McCullough et al., 2007), the latter of which does not permit evaluation of all of the indicators (e.g. CAR, slope, AUCg) typically used to capture the diurnal rhythm.
In the present study, we attempted to overcome some of these methodological challenges by examining both spontaneous, daily rumination, as well as habitual, trait rumination, and their relationship to diurnal cortisol patterns utilising cortisol samples obtained over multiple days. We used a sample of adolescent girls, and conducted analyses with and without accounting for symptoms of depression and anxiety. Based upon the Perseverative Cognition Hypothesis (Brosschot et al., 2006), we expected to find day-today covariation in rumination and cortisol, such that higher levels of daily rumination (as compared to an individual’s typical level) would predict a greater CAR the following day. In an exploratory manner, we examined whether day-to-day associations between daily rumination and cortisol differed for individuals who were high or low on trait rumination. Based on the Perseverative Cognition Hypothesis, we also predicted that trait rumination would be associated with a larger AUCg. Given the relative stability of the diurnal slope over time (e.g. Ross et al., 2014) and prior research showing that flatter slopes are associated with adverse psychological and physical health outcomes (e.g. Doane et al., 2013), we predicted that greater trait rumination in response to peer stress would also be associated with flatter diurnal slopes.
Method
Participants and procedures
Participants were 122 early adolescent girls (M age = 12.39 years, SD = 0.76 years; 87% White) who completed the cortisol portion of a larger study examining biopsychosocial predictors of emotional disorders (total sample: N = 132 mother-adolescent dyads).1 Participants were recruited from two rural New England counties through advertisements or flyers; referrals and word-of-mouth; and local schools. Most families were middle to upper class (<$40,000 [17.6%]; $41,000–$60,000 [19.4%]; $61000–$100,000 [24.1%]; >$100,000 [38.9%]).
Participation included one laboratory visit during which girls completed diagnostic interviews and a packet of questionnaires, including a measure of pubertal status. They also completed a battery of online questionnaires at home, including a measure of trait rumination. Of the 122 girls who completed the laboratory visit and the cortisol collection, 108 completed the online questionnaires.2
Cortisol collection procedure
At the laboratory visit, participants were provided instructions and the materials for the cortisol collection; and scheduled to complete the collection on three consecutive weekdays within approximately one week of the visit (M = 7.48 days; SD = 8.86), avoiding atypical days such as vacations or birthdays. Three saliva samples were provided on each collection day: waking (M = 0.26 μg/dl, SD = 0.19, range = .007–1.800), 30 min after waking (M = 0.37 μg/dl, SD = 0.21, range = .002–1.177), and bedtime (M = 0.04 μg/dl, SD = 0.10, range = .000–.725). Following each sample, participants recorded the time and completed a diary assessment (see below). Cortisol was obtained non-invasively from saliva by passive drool using straws and 1.5 mL vials. Families were contacted the night before, and on the second day of, collection to answer questions and ensure that protocols were followed. Samples were returned via mail and stored at −20 degree Celsius and sent by courier on dry ice over 3 days to the Biochemisches Labor at the University of Trier, Germany to be assayed. On average, participants provided 8.81 (SD = 0.72) samples over the 3-day protocol.
To obtain an objective time of collection, the straws used in the passive drool were stored in a container with a MEMS 6TM (Aardex) track cap that records each time it is opened. Waking samples were considered compliant if track cap detected-time was within 15 min of self-reported wake time. Samples scheduled for 30 min after waking were considered compliant if provided between 23 and 37 min after the waking sample according to track cap data, and if the track cap was used (e.g. Doane & Zeiders, 2014). Given the importance of fidelity to the sampling protocol to accurately characterise the CAR (Kudielka, Broderick, & Kirschbaum, 2003), the CAR and cortisol levels at 30 min after waking were treated as missing for participants who failed to use the track cap (n = 31). For other diurnal cortisol outcomes (e.g. diurnal slope), dummy variables for individuals who did not use the track cap and specific non-compliant days were created, and tested as covariates (see below).
Measures
Cortisol
Samples were assayed for cortisol in duplicate, using a solid phase time-resolved fluorescence immunoassay with fluorometric endpoint detection (DELFIA; Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992). The intra-assay coefficients of variation ranged from 4.0% to 6.7% and the inter-assay coefficients of variation ranged from 7.1% to 9.0%. Four cortisol values were windsorised at 1.81 μg/dl and then all raw values were natural log transformed. In addition to cortisol levels at waking and 30 min after waking, three indices were considered as outcomes: a) CAR, using the formula for area under the curve with respect to increase (AUCi) for the two morning samples (Pruessner et al., 2003); b) hourly rate of change from waking to bedtime (diurnal slope), found by computing the difference between log transformed waking and bedtime cortisol values and dividing by total time awake (difference in time between first and last sample of the day), and c) total diurnal cortisol output, using the formula for area under the curve with respect to ground (AUCg) for all samples (Pruessner et al., 2003).
Trait rumination
Trait rumination was assessed with the 3-item rumination scale from the social stress version of Responses to Stress Questionnaire (RSQ; Connor-Smith, Compas, Wadsworth, Thomsen, & Saltzman, 2000). Participants indicated whether they had experienced peer problems over the past 3 months (e.g. being left out or rejected), and then reported how much they had engaged in various responses to the peer stressors, including rumination. The mean was used (α = .70).
Daily rumination
Daily rumination was assessed with one question in each bedtime diary report (Overall today, how much did you focus on your problems/stress?), rated on a 4-point scale (1 = not at all to 4 = a lot). Moberly and Watkins (2008) used this item and another (focus on feelings) to measure spontaneous rumination in an ecological momentary assessment study, and demonstrated construct validity via its association with trait rumination and predictive validity via its association with negative affect. We chose to use the single item regarding focus on problems, as it better reflects rumination regarding stress, in line with our measure of trait rumination.
Potential covariates
We also considered between-person and day-to-day factors that have been associated with diurnal cortisol in prior work as covariates. Models were tested with and without these covariates. Racial/ethnic background was represented with a dummy variable (0 = non-White, 1 = White). Parents’ income was assessed continuously as an indicator of socioeconomic status (1 = under $10,000/year to 6 = over $100,000/year). At the time of each collection, participants completed a dairy assessment including time of waking (in the waking diary report), caffeine use in the last hour (range = 4.4–7.1%), negative affect (negative subscale from the Positive and Negative Affect Schedule; Watson, Clark, & Tellegen, 1988; rated on a 5-point scale from 0 = very slightly or not at all to 4 = extremely), and perceived stress level (“How stressful was the most stressful event or problem you encountered in the last hour?” rated on a 5-point scale from 1 = not at all to 5 = very much). Due to limited frequency, birth control and nicotine use were not included. Dummy variables indicating adherence to the protocol were also included as predictors to test whether compliance problems influenced cortisol estimates, including whether participants used the track cap devices or whether the first two samples of the day were compliant. The dummy variable for track cap usage was not included in wake + 30 or CAR models because these outcomes were treated as missing if the track cap was not used.
We measured pubertal status with the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988). The 5 items, which assess growth spurt in height, skin and body hair changes, breast development, and age at menarche, are rated on a 4-point scale, from 1 = no development to 4 = development seems completed; except for menarche which is rated 1 or 4. The mean was used (α = .70).
Current (past month) and lifetime depressive and anxiety symptoms were assessed using the Schedule for Affective Disorders and Schizophrenia for school-aged children-present and lifetime version (Kaufman et al., 1997). Symptoms were rated on a 4-point scale from 0 = no symptoms to 3 = meets DSM-IV criteria. Inter-rater reliability (assessed via electronic recordings for 27% of interviews) was good (inter-class correlations [ICCs]: M = .89). Depression and anxiety composites were formed by averaging current and lifetime depressive and anxiety symptoms, respectively.
Data analytic plan
Preliminary analyses examined descriptive statistics, zero-order correlations, and the impact of compliance (e.g. timing, track cap use) on cortisol estimates. Multilevel models were estimated in Mplus 7 (Muthén & Muthén, 1998–2012) using maximum likelihood estimation with robust standard errors to account for the nested nature of the data (days nested within persons). This approach allowed for the examination of between- and within-person variation in cortisol and was consistent with current recommendations for handling missing data (Baraldi & Enders, 2010). Diurnal cortisol outcomes were treated as continuous variables and unstandardised coefficients are reported.
A series of two-level models were tested separately for each of the five cortisol outcomes (waking cortisol, wake + 30 cortisol, CAR, diurnal slope, and diurnal AUCg). First, we conducted unconditional models with no predictors to assess between- and within-person variance present for each outcome. Next, we tested random intercept models in which diary-reported rumination was entered as a Level 1 (day-to-day) predictor of daily differences in cortisol. Based on the timing of bedtime diary reports, we focused on lagged associations to examine whether ruminating more than usual during the day prior to saliva sampling was associated with variation in cortisol. Likelihood ratio chi-square difference tests (i.e. nested model tests) indicating the day-to-day associations between rumination and cortisol did not significantly vary across individuals for waking cortisol, wake + 30 cortisol, diurnal slope, or diurnal AUCg (ps > .10). However, the day-to-day association between daily rumination and CAR varied significantly across individuals, χ2(2) = 6.26, p = .04. Thus, random slope models were used to account for between-person variance in this day-to-day association. We then added day-specific covariates at Level 1 (e.g. wake time) and individual factors at Level 2 (e.g. race/ethnicity) that could influence cortisol estimates or account for associations between rumination and cortisol. Continuous predictors at Level 1 were centred within-person (i.e. daily scores subtracted from individual averages) and Level 2 predictors were grand-mean centred. Dummy coded variables (e.g. race/ethnicity, compliance) were not centred for ease of interpretation. Please see below for an example equation using CAR as an outcome:
Finally, we estimated means-as-outcome models in which trait rumination was entered as a Level 2 (between-person) predictor of intercepts (average cortisol parameters), followed by more complex models with potential covariates at both levels. Example equation using waking cortisol as an outcome:
Results
Preliminary analyses
See Table 1 for descriptive statistics and zero-order correlations. Average daily rumination was significantly related to trait rumination (r = .31, p < .01), but unrelated to the cortisol indices (ps > .05). Greater trait rumination was associated with lower waking cortisol (r = −.20, p = .04), but not with other cortisol indices (ps > .05). Notably, average daily and trait rumination were each positively correlated with age.
Table 1.
Descriptive statistics and zero-order correlations.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Average waking cortisol | – | |||||||||||||||
| 2. Average wake + 30 cortisol | .36** | – | ||||||||||||||
| 3. CAR | −.62** | .38** | – | |||||||||||||
| 4. Diurnal cortisol slope | −.30** | −.17 | .10 | – | ||||||||||||
| 5. Diurnal AUCg (μg/dl) | .29** | .35** | .14 | .50** | – | |||||||||||
| 6. Average night before stress | −.14 | −.02 | .06 | .08 | .01 | – | ||||||||||
| 7. Average daily rumination | −.04 | −.02 | −.05 | .04 | .02 | .24** | – | |||||||||
| 8. Average daily NA | −.10 | .06 | .13 | .02 | .01 | .18† | .41** | – | ||||||||
| 9. White (1 = yes) | −.01 | .14 | −.01 | −.19* | −.07 | −.01 | .10 | −.04 | – | |||||||
| 10. Age (years) | .03 | .02 | −.05 | .14 | .12 | .18† | .24** | .14 | .25** | – | ||||||
| 11. Trait rumination | −.20* | −.12 | .15 | .13 | .07 | .09 | .31** | .29** | .15 | .25** | – | |||||
| 12. Family income | .20* | .08 | −.14 | −.04 | .10 | .08 | .03 | −.19* | .13 | .05 | .05 | – | ||||
| 13. Depressive symptoms | −.05 | −.02 | .13 | −.02 | .05 | −.09 | −.01 | .19* | .01 | .14 | .14 | −.19* | – | |||
| 14. Anxiety symptoms | −.10 | .18† | .20† | .07 | .15† | .10 | .19* | .16† | .04 | .17 | .17† | −.11 | .27** | – | ||
| 15. Pubertal status | −.01 | .04 | −.01 | .24** | .24** | .07 | .05 | .15† | −.02 | .43** | .43** | −.17† | .18* | .19* | – | |
| 16. Average wake time | −.23* | −.33** | −.09 | .17† | −.37** | .01 | .06 | .07 | −.27** | .12 | −.06 | −.02 | .05 | −.03 | .06 | – |
| Ma | −1.59 | −1.17 | 0.09 | −0.18 | 75.68 | 2.51 | 1.87 | 0.29 | 87.70 | 12.39 | 2.11 | 4.69 | 0.27 | 0.39 | 2.66 | 7.40 |
| SD | 0.63 | 0.51 | 0.20 | 0.08 | 12.18 | 0.68 | 0.60 | 0.33 | – | 0.76 | 0.78 | 1.39 | 0.52 | 0.32 | 0.60 | 1.55 |
| Minimum | −4.67 | −2.72 | −0.69 | −0.40 | 41.91 | 1.00 | 1.00 | 0.00 | – | 10.83 | 1.00 | 1.00 | 0.00 | 0.00 | 1.20 | 4.83 |
| Maximum | −0.25 | −0.18 | 0.83 | 0.04 | 122.13 | 3.89 | 3.33 | 1.63 | – | 15.00 | 4.00 | 6.00 | 2.50 | 1.43 | 3.70 | 12.01 |
Notes: N = 122 for waking cortisol, diurnal slope, and AUCg. N = 91 for wake + 30 cortisol and CAR after excluding participants who did not adhere to saliva protocol. Cortisol values were transformed using the natural log function. Parent income ranges from 1 = under $10,000/year to 6 = over $100,000/year. NA = negative affect. Wake time reported in hours with decimals representing portion of hour. Depressive and anxiety symptoms reflect composite of past (lifetime) and current (past month) symptoms (standardised).
Mean for continuous variables and percentage of sample for dichotomous variables.
p < .10.
p < .05.
p < .01.
Unconditional models with no predictors for each of the five outcomes were conducted to compute ICCs, which quantify the proportion of person-level variance for nested cortisol data: ICCwakingcort = .41, ICCwake30 = .24, ICCCAR = .11, ICCslope = .23, ICCAUCg = .38. By considering the residual variances for these variables (1 – ICC), day-to-day influences accounted for approximately 59% of the variance in waking cortisol, 76% for wake + 30 cortisol, 89% for CAR, 77% for diurnal slope, and 62% for diurnal AUCg.
Day-to-day variability in rumination and cortisol
On average, within-person changes in daily rumination were not associated with waking cortisol the next day (B = 0.092, p = .13), wake + 30 cortisol (B = 0.020, p = .75), diurnal slope (B = −.005, p = .63) or AUCg (B = −0.357, p = .75). Results were similar when adjusting for significant correlates of cortisol parameters, including wake time, non-compliance, race/ethnicity, family income, pubertal status, and track cap usage, and when additionally adjusting for symptoms of anxiety and depression, daily caffeine use, daily negative affect, and perceived stress level at bedtime, none of which significantly contributed to prediction. Within-person increases in prior night rumination were associated with lower CAR, B = −0.027, p < .001. However, this association was not significant (p = .43) after adjusting for other factors that accounted for significant variance in cortisol, including wake time, non-compliance, race/ethnicity, family income, and pubertal status. Of note, there were no significant within-person associations between daily rumination and cortisol parameters from the same day (ps > .39).
Given that there was significant between-person variance in the day-to-day association between prior night rumination and CAR, we conducted exploratory analyses to consider whether individual differences in trait rumination might account for this individual variation. Specifically, we used trait rumination as a Level 2 (between-person) predictor of the extent to which the day-to-day association between state rumination and CAR differed across individuals (i.e. cross-level interaction). Trait rumination did not significantly predict individual variation in this day-to-day association with, B = 0.030, p = .31, or without covariates, B = 0.048, p = .21.
Individual differences in trait rumination and cortisol
In models with a single between-person predictor, individual differences in trait rumination were significantly associated with lower average cortisol levels at waking, B = −0.162, p = .04, but not wake + 30 cortisol, CAR, diurnal slope, or AUCg (ps > .15). After adjusting for wake time, non-compliance, race/ethnicity, family income, and pubertal status, the association remained significant for waking cortisol (p = .03) and greater trait rumination significantly predicted flatter diurnal cortisol slopes, B = 0.017, p = .04 (see Table 2 for full results and Figure 1 for a visual representation). Results were also similar when also adjusting for symptoms of anxiety and depression, daily caffeine use, daily negative affect, and perceived stress level at bedtime, none of which significantly contributed to prediction.
Table 2.
Multilevel regression estimates predicting cortisol from trait rumination and covariates.
| Fixed effects | Waking cortisol
|
Wake + 30 cortisol
|
CAR
|
Diurnal cortisol slope
|
Diurnal AUCg
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Est. | SE | Est. | SE | Est. | SE | Est. | SE | Est. | SE | |
| Intercept: average level, γ00 | −1.763** | 0.146 | −1.359** | 0.120 | 0.167** | 0.056 | −0.119** | 0.021 | 80.813** | 3.576 |
| Level 1 (day-specific) | ||||||||||
| Wake time, γ10 | −0.180* | 0.085 | −0.196** | 0.062 | 0.036 | 0.044 | 0.015 | 0.012 | −4.509** | 0.871 |
| Track cap non-compliance, γ20 | 0.257* | 0.117 | −0.002 | 0.101 | −0.084* | 0.037 | −0.023† | 0.014 | −1.173 | 1.862 |
| Level 2 (person-specific) | ||||||||||
| Trait rumination, γ01 | −0.169* | 0.079 | −0.084 | 0.065 | 0.040 | 0.030 | 0.017* | 0.008 | 1.491 | 1.326 |
| White race/ethnicity, γ02 | −0.020 | 0.125 | 0.212* | 0.098 | 0.001 | 0.045 | −0.053** | 0.018 | −3.820 | 3.750 |
| Family income, γ03 | 0.089† | 0.048 | 0.034 | 0.045 | −0.025 | 0.023 | 0.002 | 0.006 | 1.177 | 0.766 |
| Pubertal status, γ04 | 0.020 | 0.091 | 0.038 | 0.091 | −0.015 | 0.035 | 0.030** | 0.011 | 5.454** | 1.993 |
| No track cap, γ05 | −0.174 | 0.141 | 0.013 | 0.016 | −2.838 | 2.975 | ||||
| Random effects | ||||||||||
| (L1 residual variance) | 0.351** | 0.057 | 0.390** | 0.123 | 0.090** | 0.027 | 0.009** | 0.001 | 145.200** | 35.153 |
| (L2 residual variance) | 0.233** | 0.076 | 0.122** | 0.034 | 0.009 | 0.006 | 0.002* | 0.001 | 83.649** | 21.523 |
Notes: 359 days nested within 122 individuals. Wake time within-person centred and all continuous Level 2 predictors grand-mean centred. Noncompliance, (1 = not compliant with sampling procedures), White race/ethnicity (1 = White), and no track cap (1 = did not use track cap) dummy coded. Outcomes calculated from log-based cortisol values (μg/dl). Est. = partial regression coefficient estimate. SE = robust standard error.
p < .10.
p < .05.
p < .01.
Figure 1.
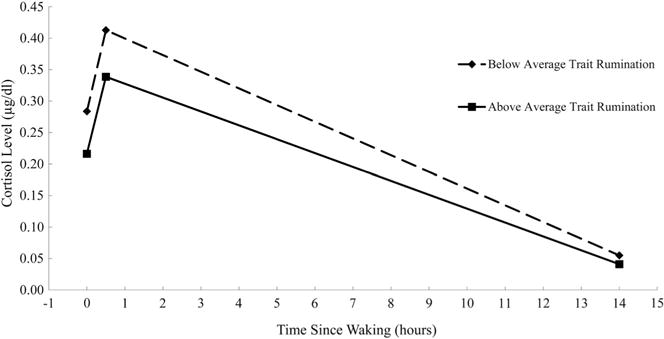
Diurnal cortisol levels plotted at waking, approximately 30 min after waking, and at bedtime for girls 1 SD above (n = 20) and below (n = 25) the sample mean of trait rumination.
Results were highly similar when we entered daily rumination at Level 1 and trait rumination at Level 2 in the models simultaneously with covariates. There were no significant within-person associations of daily rumination with cortisol parameters (ps > .15), but individual differences in trait rumination remained a significant predictor of lower average cortisol levels at waking, B = −0.170, p = .03, and flatter diurnal cortisol slopes, B = 0.017, p = .04.
We also tested whether between-person differences in average daily rumination (assessed using an individual’s average from available diary reports) were related to average cortisol parameters; none of these associations were significant (ps > .60).
Discussion
This study examined daily covariation between rumination and diurnal cortisol, as well as trait associations between rumination and typical diurnal patterns in a community sample of early adolescent girls. This is the first study examining relations between rumination and diurnal cortisol patterns in adolescent girls. Research examining these relations among adults has been mixed. To overcome some potential methodological issues that may account for mixed findings in previous research, we assessed cortisol over 3 days, used track caps to objectively assess adherence to the sampling protocol, and adjusted for non-compliance in analyses.
The CAR is more responsive to contextual factors that vary from day to day (Ross et al., 2014); thus, we predicted that daily (but not trait) rumination would be associated with an increased CAR. Daily rumination was associated with the CAR, but in an unexpected direction: greater than average daily rumination predicted a lower CAR the next day. Importantly, daily rumination was no longer associated with the CAR after accounting for several covariates, such as wake time and non-compliance, suggesting caution in interpreting this finding. Nonetheless, this finding adds to a mixed literature regarding the direction of the association between daily rumination and the CAR, with prior studies with adult non-clinical samples finding that between-person differences in daily rumination were associated with an increased (Zoccola et al., 2011) or decreased (Cropley et al., 2015) CAR. Both prior studies examined rumination levels between individuals, while we examined within-person variability (i.e. whether ruminating more than one’s average level predicted the CAR). Although the Perseverative Cognition Hypothesis (Brosschot et al., 2006) would likely suggest that greater rumination would be associated with an increased CAR, any deviation from typical functioning in the diurnal rhythm may indicate dysfunction. The increase in cortisol in the first 30 minutes after waking (the CAR) is a normative pattern that may give individuals a “boost” to prepare for perceived daily demands (Adam, Hawkley, Kudielka, & Cacioppo, 2006). In line with this, our finding that greater daily rumination predicted a decreased CAR the next day may suggest that ruminating disrupts the body’s ability to prepare for perceived demands. Consistent with predictions, daily variation in rumination was not associated with other indices of the diurnal rhythm including the diurnal slope, which has demonstrated greater stability and thus is less likely to be influenced by daily contextual factors (e.g. Ross et al., 2014).
Given the relative stability of the diurnal slope over time (Ross et al., 2014) and prior research showing that flatter slopes are associated with adverse psychological and physical health outcomes (e.g. Doane et al., 2013), we predicted and found that habitual (i.e. trait) rumination in response to peer stress was associated with flatter slopes, suggesting less of a decline in cortisol throughout the day in girls who habitually ruminate. Higher trait rumination was also associated with lower waking cortisol, suggesting that trait rumination was associated with lower (rather than elevated) flatter slopes in our sample. This could indicate hypoactivation of the HPA axis, a potential result of chronic stress, reflecting allostatic load (McEwen, 1998), though more research has focused on elevated, flatter slopes than on lower, flatter slopes (Miller, Chen, & Zhou, 2007).
Counter to predictions, trait rumination did not predict greater daily output (measured by AUCg). Although our results appear to run counter to the Perseverative Cognition Hypothesis (Brosschot et al., 2006), the hypothesis may be better reflected in reactivity to acute stressors, (e.g. those detected in laboratory settings and over shorter time frames). In fact, HPA-axis studies that find the most support for the Perseverative Cognition Hypothesis involve increased cortisol response and/or delayed cortisol recovery following acute stress (Ottaviani et al., 2015). This reactivity literature highlights a psychological mechanism underlying specific HPA responses to acute stressors, whereas the current study examined both state and trait rumination in relation to diurnal cortisol patterns, which reflect typical daily functioning of the HPA axis and not reactivity per se. Although frequent activation of the HPA axis in response to stress may contribute to a flattening of the diurnal rhythm over time (Miller et al., 2007), our sample comprised otherwise healthy early adolescent girls; it remains unclear whether rumination is associated with acute cortisol reactivity and diurnal cortisol activity in a similar fashion.
This study is the first to examine the relationship between rumination and diurnal cortisol patterns among a sample of adolescent girls and across multiple time scales. Because rumination is associated with several forms of psychopathology that often emerge during adolescence (Nolen-Hoeksema et al., 2008), it is important to understand potential physiological mechanisms in the developmental period before the onset of psychopathology. Our results linking trait rumination to lower, flatter diurnal slopes reflects the link between rumination and diurnal cortisol patterns even after accounting for symptoms of psychopathology. Moreover, our trait rumination measure focused specifically on rumination related to peer stress; peer relationships are highly valued among adolescents, especially girls (Furman & Buhrmester, 1992; Rose & Rudolph, 2006). Additionally, we assessed compliance with objective indicators to ensure the quality of our measurement, and we sampled over 3 days.
Several limitations merit note. First, we used a small, self-selected sample of girls from the community, which may limit generalisability. It will be important for future research to examine the relationships between cortisol and rumination in clinical samples, for example, in order to investigate possible interactive effects between depressive symptoms and rumination on cortisol outcomes. Second, although we used a track-cap to assess compliance to the saliva sampling procedure, we used self-reported wake time rather than a more objective indicator (e.g. actigraphy). Third, because our trait rumination measure was specific to interpersonal problems, we do not know whether results will generalise to habitual rumination about other types of stressors. Finally, although our daily and trait measures of rumination both focused on stress, the one-item daily measure tapped rumination about general stress, while the trait measure tapped rumination related to peer-focused stress. It would have been optimal to have a more parallel (i.e. peer stress focused) measure of daily rumination to better compare effects of daily and habitual rumination.
An important next step in this line of research will be to examine the prospective relationships among rumination, diurnal cortisol patterns, and psychopathology. The diurnal cortisol patterns that were concurrently associated with rumination in the present study have been found to predict psychopathology in adolescence (e.g. Vrshek-Schallhorn et al., 2013). Although it is possible that these patterns are simply correlates, it is likely that they are mechanistically linked; for example, diurnal patterns may mediate the association between rumination and health outcomes. Understanding these relationships will be important for prevention and treatment given rumination’s role as a transdiagnostic risk factor for the development of psychopathology and other health concerns such as self-injury, substance use, compliance with medical treatment, and cardiovascular health (Nolen-Hoeksema et al., 2008).
Acknowledgments
We thank the families who generously gave their time to participate in this project as well as the staff of the Williams College Youth Emotion Center.
Funding
This research was supported by institutional funds from Williams College. MRS was supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. [DGE-1311230], and LDD was supported by a William T. Grant Foundation Scholar Award and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD079520.
Footnotes
Notes
There were not significant differences between those who did and did not complete the cortisol assessment on child age, family income, pubertal status, trait rumination, or depressive and anxiety symptoms (ps > .10). One father, who identified himself as the primary caregiver, participated with his daughter in the present study. In addition, three sibling pairs were included. However, results were identical when the father–daughter dyad and when one of the siblings were excluded. Full results upon request.
There were not significant differences between those who did and did not complete the online questionnaires on child age, family income, pubertal status, depressive and anxiety symptoms or the cortisol indices (ps > .10).
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Michael R. Sladek http://orcid.org/0000-0003-1697-438X
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience – cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldi AN, Enders CK. An introduction to modern missing data analyses. Journal of School Psychology. 2010;48(1):5–37. doi: 10.1016/j.jsp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60(2):113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neuroscience & Biobehavioral Reviews. 2010;35(1):97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, Saltzman H. Responses to stress in adolescence: Measurement of coping and involuntary stress responses. Journal of Consulting and Clinical Psychology. 2000;68(6):976–992. [PubMed] [Google Scholar]
- Cropley M, Rydstedt LW, Devereux JJ, Middleton B. The relationship between work-related rumination and evening and morning salivary cortisol secretion. Stress and Health. 2015;31(2):150–157. doi: 10.1002/smi.2538. [DOI] [PubMed] [Google Scholar]
- Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, Adam EK. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and Psychopathology. 2013;25(03):629–642. doi: 10.1017/S0954579413000060. [DOI] [PubMed] [Google Scholar]
- Doane LD, Zeiders KH. Contextual moderators of momentary cortisol and negative affect in adolescents’ daily lives. Journal of Adolescent Health. 2014;54(5):536–542. doi: 10.1016/j.jadohealth.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology. 1992;43(7):683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Furman W, Buhrmester D. Age and sex differences in perceptions of networks of personal relationships. Child Development. 1992;63(1):103–115. doi: 10.1111/j.1467-8624.1992.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Huffziger S, Ebner-Priemer U, Zamoscik V, Reinhard I, Kirsch P, Kuehner C. Effects of mood and rumination on cortisol levels in daily life: An ambulatory assessment study in remitted depressed patients and healthy controls. Psychoneuroendocrinology. 2013;38(10):2258–2267. doi: 10.1016/j.psyneuen.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine. 2003;65(2):313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kuehner C, Holzhauer S, Huffziger S. Decreased cortisol response to awakening is associated with cognitive vulnerability to depression in a nonclinical sample of young adults. Psychoneuroendocrinology. 2007;32(2):199–209. doi: 10.1016/j.psyneuen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Orsulak P, Brandon A, Akers L. Rumination, fear, and cortisol: An in vivo study of interpersonal transgressions. Health Psychology. 2007;26(1):126–132. doi: 10.1037/0278-6133.26.1.126. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moberly NJ, Watkins ER. Ruminative self-focus and negative affect: An experience sampling study. Journal of Abnormal Psychology. 2008;117(2):314–323. doi: 10.1037/0021-843X.117.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 7th. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100(4):569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, Brosschot JF. Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin. 2015;142:231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosomatic Medicine. 2003;65(1):92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin. 2006;132(1):98–131. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KM, Murphy ML, Adam EK, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydstedt LW, Cropley M, Devereux JJ, Michalianou G. The effects of gender, long-term need for recovery and trait inhibition-rumination on morning and evening saliva cortisol secretion. Anxiety, Stress, & Coping. 2009;22(4):465–474. doi: 10.1080/10615800802596378. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, Adam EK. The cortisol awakening response predicts major depression: Predictive stability over a 4-year follow-up and effect of depression history. Psychological Medicine. 2013;43(03):483–493. doi: 10.1017/S0033291712001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS. Assessing the relationship between rumination and cortisol: A review. Journal of Psychosomatic Research. 2012;73(1):1–9. doi: 10.1016/j.jpsychores.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, Yim IS. Trait and state perseverative cognition and the cortisol awakening response. Psychoneuroendocrinology. 2011;36(4):592–595. doi: 10.1016/j.psyneuen.2010.10.004. [DOI] [PubMed] [Google Scholar]


