Abstract
Background and aims
Buprenorphine-naloxone (BUP-NLX) can be used to manage prescription opioid addiction among persons with chronic pain, but post-treatment relapse is common and difficult to predict. This study estimated whether changes in pain over time and pain volatility during BUP-NLX maintenance would predict opioid use during the taper BUP-NLX taper.
Design
Secondary analysis of a multisite clinical trial for prescription opioid addiction, using data obtained during a 12-week BUP-NLX stabilization and 4-week BUP-NLX taper.
Setting
Community clinics affiliated with a national clinical trials network in 10 U.S. cities.
Participants
Subjects with chronic pain who entered the BUP-NLX taper phase (N = 125) with enrollment occurring from June, 2006 to July 2009 (52% male, 88% Caucasian, 31% married).
Measurements
Outcomes were weekly biologically-verified and self-reported opioid use from the 4-week taper phase. Predictors were estimates of baseline severity, rate of change, and volatility in pain from weekly self-reports during the 12-week maintenance phase.
Findings
Controlling for baseline pain and treatment condition, increased pain (OR = 2.38, p = .02) and greater pain volatility (OR = 2.43, p = .04) predicted greater odds of positive opioid urine screen during BUP-NLX taper. Increased pain (IRR = 1.40, p = .04) and greater pain volatility (IRR = 1.66, p = .009) also predicted greater frequency of self-reported opioid use.
Conclusions
Adults with chronic pain receiving outpatient treatment with buprenorphine-naloxone (BUP-NLX) for prescription opioid addiction have elevated risk for opioid use when tapering off of maintenance treatment. Those with relative persistence in pain over time and greater volatility in pain during treatment are less likely to sustain abstinence during BUP-NLX taper.
Keywords: chronic pain, prescription opiates, buprenorphine-naloxone, treatment outcomes, prediction
Prescription opioid addiction in adults with chronic pain has become increasingly common and problematic in many developed nations (1, 2). At the national level both opioid prescribing and opioid-related overdoses have accelerated drastically in the last 15-20 years (1, 3). Chronic pain patients are now prescribed opioids for longer durations and at higher doses than in previous decades, which places them at greater risk for physiological tolerance and potential addiction (4). Treatment of this population is complicated by complex medical and psychiatric problems that often intensify upon opioid withdrawal and prompt relapse (5). The optimization of treatment for this population is currently a national priority across diverse areas of interest including addiction treatment, pain management, and primary care (6).
Both clinical recommendations and empirical studies suggest buprenorphine-naloxone (BUP-NLX) is a viable pharmacotherapy for chronic pain patients with prescription opioid addiction. Compared to full opioid agonists, BUP-NLX offers improved safety and diminished abuse liability (7-10). Although not currently FDA-approved for pain indications, the analgesic benefits of BUP-NLX in patients with opioid addiction have been described (11-13). Empirical studies also suggest BUP-NLX maintenance can significantly reduce pain in this population (14-16), with one randomized trial finding no differences in pain between patients receiving six months of BUP-NLX vs. low-dose methadone (17). These studies suggest that BUP-NLX can be used to sufficiently manage pain in opioid-dependent populations. However, little research to date has examined whether individual differences in pain control during BUP-NLX might impact opioid use outcomes.
Because persistent pain is often associated with relapse following addiction treatment (18, 19), unresponsive pain during BUP-NLX maintenance could trigger a return to opioid use during or following treatment. In our prior analysis of data from a large clinical trial involving BUP-NLX maintenance and counseling, we found significant variability in patterns of pain during treatment that corresponded to treatment outcome (20). Among baseline pain severity, rate of change in pain over time, and volatility in pain, only pain volatility predicted outcomes at end of treatment, with greater volatility in pain during treatment related to reduced probability of opioid abstinence. Such fine-grained and dynamic aspects of pain may have unique predictive value for substance use outcomes, as the presence of chronic pain alone did not predict treatment outcomes in the same trial or other BUP-NLX treatment samples (21-24). Continued identification and validation of such predictive markers is a vital step towards identifying processes linked to individual relapse risk developing related strategies for improving treatment.
The aim of this study was: 1) to estimate whether individual patterns of pain during BUP-NLX maintenance treatment would prospectively predict both biologically-verified and self-reported opioid use during the BUP-NLX taper. The focus on BUP-NLX taper phase was motivated by awareness of this transitional stage as associated with increased risk for opioid relapse (5), as well as an attempt to extend prior work that predicted end of treatment outcome (20). Additionally, knowledge of factors related to post-treatment opioid use among patients with chronic pain is scarce, despite the high prevalence of chronic pain among BUP-NLX patients (25). Using estimates of baseline pain, rate of change in pain over time, and weekly volatility in pain as primary predictors, we hypothesized that having more severe baseline pain, greater persistence in pain over time, and greater volatility in pain during BUP-NLX maintenance would predict greater probability and frequency of opioid use during the BUP-NLX taper.
Methods
Study Design
This IRB-exempt study was a secondary analysis of publicly-available data from POATS, a multisite clinical trial for treatment of prescription opioid dependence conducted in the National Drug Abuse Treatment Clinical Trials Network (clinicaltrials.gov identifier NCT00316277). Full details and main findings of the main study are in previous reports (23, 26). In brief, Phase 1 of POATS (N = 653) randomized participants to an enhanced counseling condition or standard medical management counseling during 4-week BUP-NLX detoxification. In Phase 2 (N = 360) participants who did not sustain abstinence in Phase 1 were re-randomized to standard or enhanced counseling during 12 weeks of BUP-NLX maintenance followed by a 4-week BUP-NLX taper. This current study uses data from Phase 2 only. Data was selected in order to measure multiple features of pain during BUP-NLX maintenance, and to use these indices to prospectively predict future opioid use when participants were tapered off of medication. Data from baseline assessments were tested as covariates. The primary predictors were features of pain obtained from the 12 weeks of BUP-NLX stabilization in Phase 2, while the outcomes were measures of opioid use assessed during the subsequent 4-week taper phase.
Sample
Participants met the inclusion criteria for POATS sample described in prior reports (26); they were at least 18 years old, met DSM-IV criteria for current prescription opioid dependence, were physiologically dependent on prescription opioids, and had no unstable medical or psychiatric conditions. Any participants currently prescribed opioids for pain were cleared for opioid detoxification from their prescribing physician prior to enrollment. Exclusion criteria included use of heroin on ≥ 4 days in the past month, any prior injection of heroin, and current physiological dependence on other substances, such as alcohol, sedatives, or stimulants. From the original full POATS sample (N = 653), 360 participants entered Phase 2, and 149 participants (41%) in the Phase 2 sample had chronic pain. The current sample includes Phase 2 participants with chronic pain who completed at least one outcome assessment during the taper phase (N = 125). Chronic pain was assessed during initial screening by patient self-report of having “greater than usual aches and pains” for at least three months and confirmed during medical screening. Aside from reporting greater current pain at baseline, the sample for the current study did not differ significantly from the remaining Phase 2 sample (i.e., those without chronic pain) on any demographic or clinical variables obtained at baseline (see Table 1 for descriptive statistics).
Table 1.
Demographic and clinical characteristics of adults with chronic pain who received 12 weeks of buprenorphine-naloxone and counseling for prescription opioid dependence and completed at least one follow-up visit during a 4-week taper (N = 125).
| Variable | % or M (SD) |
|---|---|
| Sex (% (n) male) | 52% (64) |
| Race (% (n) White) | 88% (109) |
| Years of education: M (SD) | 12.8 (2.4) |
| Marital status (% (n) currently married) | 31% (38) |
| Baseline pain severity (Rated 0-10): M (SD) | 4.5 (3.0) |
| Days of prescription opioid use in past 30: M (SD) | 27.9 (3.8) |
| Heroin use history (% (n) ever used) | 24% (29) |
| Prescription opioid route (% (n) ever used non-orally) | 87% (108) |
| Prescription opioid treatment (% (n) ever received treatment) | 30% (37) |
| Lifetime major depression (% (n) with diagnosis) | 40% (50) |
Measures
Pain severity
Current pain severity was assessed weekly during the 12-week BUP-NLX maintenance phase with a single self-report rating (Range = 0-10) on the full or abbreviated Brief Pain Inventory-Short Form (27). Weekly pain scores were used to obtain individualized estimates of pain intercept, pain slope, and pain volatility in analyses described below, which were then used as predictors of opioid use outcomes in predictive models.
Opioid use
Opioid use was assessed weekly with a urine drug screen (UDS) and a calendar-assisted self-report interview (28). Results of each UDS were aggregated across all tested opioids (i.e, analgesics, illicit opioids, methadone) to provide a single dichotomous indicator of opioid use (0 = negative, 1 = positive) for each visit. Self-reported use data were used to measure opioid use frequency, coded as the number of days of opioid use in the past week, standardized into 7-day segments for analysis (Range = 0-7). Outcome variables in this study were UDS-confirmed opioid use and self-reported opioid use frequency assessed during the BUP-NLX taper (i.e., at weekly visits in week 13-16 post-randomization in Phase 2).
Baseline demographic and clinical characteristics
Baseline demographic and clinical covariates were selected according to previous literature and prior studies of this sample (Dreifuss et al., 2013). A brief demographics questionnaire and the Addiction Severity Index-Lite (29) assessed demographics. The Pain and Opiate Analgesic Use History (26) captured current and historical measures of opioid use, pain, and opioid dependence treatment, while the Composite International Diagnostic Interview (30) assessed lifetime major depression. Demographic covariates tested were sex, race, and marital status, while clinical covariates were Phase 1 treatment condition, Phase 2 treatment condition, history of heroin use, history of non-oral prescription opioid use, history of opioid dependence treatment, and lifetime major depression.
Statistical analyses
Model-based estimates of pain trajectories and volatility in this sample were described in detail in our previous work (20) and are reviewed here. A multilevel growth curve model was fit to weekly pain scores during BUP-NLX stabilization, controlling for fixed effects of time, sex, and weekly opioid use, and random effects for person (intercepts) and time (slope). Individual estimates of pain intercepts and time slopes were extracted from the model for subsequent use as predictor variables. Each individual's intercept and time slope (respectively) reflect baseline level and degree of change over time in pain. As shown in Figure 1 for conceptual illustration, participants with “low” estimated time slopes (25th percentile) had pain that decreased over time, while those with “high” slopes (75th percentile) had little overall change or slight increase in pain. From the multilevel pain score model, we also extracted an index of pain volatility, which captures the extent of each individual's week-to-week instability in pain. Following the methods of previous similar studies in smoking (31), we collected residuals of each individual's pain growth curve, converted the residuals to absolute values, and computed the average for a single pain volatility score. Because this score is derived from the absolute residuals of the multilevel growth curve model, it captures the extent that a given subject had pain scores that deviated either far above or far below their typical trajectory of pain during BUP-NLX stabilization. To illustrate volatility, Figure 1 displays the residuals from the pain score growth curves separately for participants with “low” volatility (≤ 25th percentile) and “high” volatility (≥ 75th percentile). Because the residuals reflect remaining variability in pain after accounting for each individual's pain growth curve, the plotted residuals display weekly “unaccounted for” variation in pain, as if each individual's pain growth curve was constant at 0. Figure 1 illustrates that individuals with lower pain volatility scores (lower quartile) had pain scores that adhered closely to their individual trajectory, while those with high pain scores (upper quartile) had pain scores that deviated more drastically from their individual pain trajectory. Standardized, continuous measures of pain intercept, time slope, and volatility were used as predictors in the primary analyses.
Figure 1.
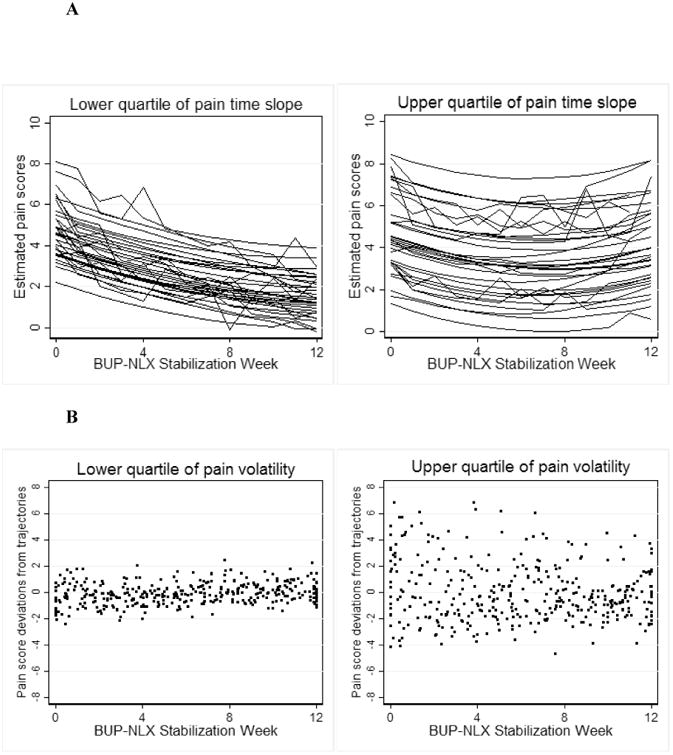
A. Estimated pain trajectories for participants in upper and lower quartile of pain time slope. Individual trajectories were estimated as a function of time, sex, and weekly opioid use, random person-level intercepts, and random time slopes.
B. Pain score deviations for participants in upper and lower quartile of pain volatility.
Separate multilevel models were used to examine the two outcome variables, opioid UDS and opioid use frequency, which were both assessed weekly during the BUP-NLX taper phase and nested within individuals. Multilevel logistic regression examined opioid UDS and multilevel Poisson regression examined opioid use frequency. Random intercepts accounted for person-level clustering of observations, while random time slopes allowed individual heterogeneity in the rate of change in opioid use over time during the taper phase. All available data was included, as missing data analyses revealed no significant differences on study variables between patients who completed a taper-phase visit and those who did not, supporting the missing-at-random assumption and the use of maximum-likelihood estimation. Preliminary models tested time, demographics, baseline clinical severity variables, and treatment condition as covariates, with any statistically significant covariates (p < .05) retained for subsequent models. Pain intercept, pain time slope, and pain volatility were then added to the model together as a set of person-level predictors, to test the independent predictive effects of pain intercept, pain time slope, and pain volatility. For the multilevel logistic and Poisson regression models, each coefficient estimate is expressed with an odds ratio (OR) or incidence-rate ratio (IRR), respectively. With standardized continuous predictors, these estimates reflect the differential probability of the outcome associated with a +/- SD difference in the predictor variable, expressed as difference in odds (positive drug screen) or incidence rate (days using opioids). All analyses were conducted in Stata 13.0 (32).
Results
Descriptives of opioid use during BUP-NLX taper
During the 4-week BUP-NLX taper phase, 407 observations of opioid UDS and opioid use frequency (each) were provided. Nearly the entire sample (91%) provided opioid use measures in Week 13 (114/125) but retention declined to 65% at Week 16 (81/125). Opioid use increased during the 4-week taper, from 22% of screens positive in Week 13 to 31% in Week 16. Opioid use frequency also increased over time during the 4-week taper, from a mean of 0.28 (SD = 0.68) days/week at Week 13 to 0.64 (SD = 1.49) at Week 16.
Covariate models of opioid use outcomes
Covariates tested for both opioid UDS and opioid use frequency included demographics, clinical covariates, and treatment condition. Only Phase 2 treatment condition predicted opioid UDS (OR = 0.11, p = .01, 95% CI [0.02, 0.58]). Receiving enhanced counseling reduced the probability of a positive UDS; participants in this condition had a lower overall proportion of positive UDS (18%) than those in standard counseling (32%). No other demographic or clinical covariates predicted opioid UDS. For the model of opioid use frequency, time was the only statistically significant covariate (IRR = 1.42, p = .001, 95% CI [1.15, 1.74]), indicative of a significant increase over time in opioid use frequency during the taper phase. Significant variance in random time slopes for both outcomes revealed individual heterogeneity in the rate of change over time. Treatment condition and linear time (fixed and random effects) were included as covariates in all subsequent models.
Prediction of opioid use outcomes from pain variables
Pain intercept, time slope, and volatility during BUP-NLX stabilization were tested as predictors of opioid use during BUP-NLX taper. Pain time slope (95% CI [1.13, 5.02]) and pain volatility (95% CI [1.03, 5.76]) both predicted opioid UDS (See Table 2). As shown in Figure 2 for illustration, the group of participants with at least one positive UDS during taper had greater levels of pain over time and greater pain volatility scores during BUP-NLX maintenance, compared to the group of participants without a positive UDS during taper. These independent effects indicated that both pain slopes and greater pain volatility predicted the likelihood of opioid use during BUP-NLX taper when controlling for the other factor. These effects also appeared to be clinically-significant. The ORs, which were estimated on standardized coefficients, indicated a two SD difference in pain volatility or pain time slope was associated (respectively) with 4.86 and 4.76 greater odds of opioid use during the taper. Treatment condition also remained statistically significant (95% CI [0.04, 0.80]), indicating enhanced medical management reduced the likelihood of opioid use during the taper in this sample with chronic pain, regardless of pain intercept, slope, and volatility.
Table 2.
Results of multilevel models predicting opioid use during four-week buprenorphine-naloxone taper.
| Variables | Opioid UDS | Days/week using opioids | ||||||
|---|---|---|---|---|---|---|---|---|
| Covariate model | Full model | Covariate model | Full model | |||||
| OR | p | OR | p | IRR | p | IRR | p | |
| Taper week | 1.35 | .20 | 1.31 | 0.27 | 1.41 | .001 | 1.40 | .001 |
| Enhanced vs. standard counseling | 0.13 | .01 | 0.17 | 0.03 | 0.48 | .05 | 0.58 | .12 |
| Pain intercept | 1.13 | 0.44 | 1.16 | .42 | ||||
| Pain time slope | 2.38 | 0.02 | 1.40 | .04 | ||||
| Pain volatility | 2.43 | 0.04 | 1.66 | .009 | ||||
Figure 2.
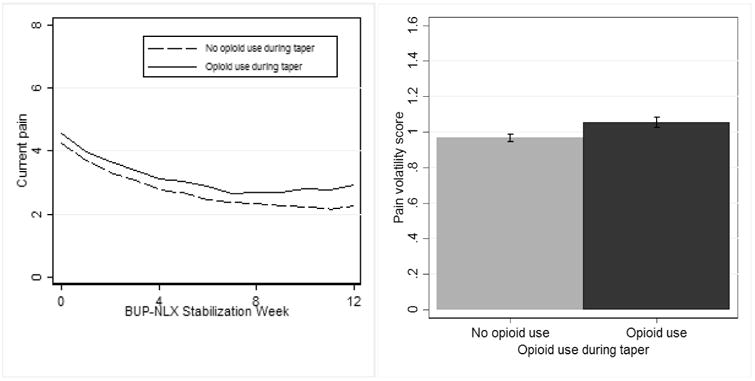
Participants with relatively greater pain over time and greater pain volatility during buprenorphine-naloxone stabilization were more likely to use opioids during the 4-week taper.
Results of models use to predict opioid use frequency were similar. Both pain time slope (95% CI [1.02, 1.97]) and pain volatility (95% CI [1.20, 2.58]) independently predicted opioid use frequency during the taper phase (see Table 2), with greater levels of pain over time and greater pain volatility during BUP-NLX maintenance predicting greater frequency of opioid use during BUP-NLX taper. As shown in Figure 3, the group of participants reporting opioid use multiple days per week during taper also had the greatest persistence in pain and the greatest volatility in pain during BUP-NLX maintenance. These effects also appeared to be clinically-significant, as the IRRs indicated a two SD difference in pain volatility or pain time slope was associated (respectively) with 2.80 and 3.32 increase in the rate of opioid use (days using per week). Linear time also remained statistically significant (95% CI [1.15, 1.73]), indicative of the overall increase in opioid use frequency during the taper phase.
Figure 3.
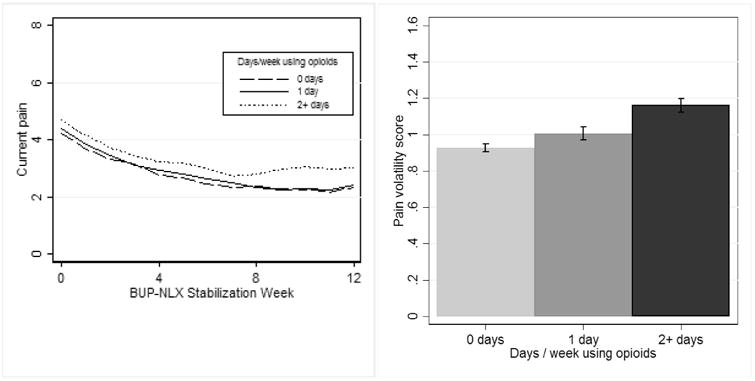
Patients with greater pain over time and greater pain volatility during 12 weeks of buprenorphine-naloxone stabilization reported more days/week using opioids during the subsequent 4-week taper.
Discussion
In this study we identified characteristics of pain during treatment for prescription opioid addiction that predicted post-treatment opioid use in persons with chronic pain. Higher levels of pain over time and greater volatility in pain during BUP-NLX maintenance and counseling independently predicted both biologically-verified and self-reported opioid use during the BUP-NLX taper. The course of BUP-NLX treatment for opioid dependence often has a fixed duration, with the transition off opioid maintenance associated with elevated rates of relapse (33). However, the specific factors that contribute to relapse during BUP-NLX treatment are not well-understood, especially in patients with chronic pain who comprise substantial and increasing portions of the treatment population (25). In this study of patients with chronic pain, those with relative persistence in pain and greater volatility in pain during BUP-NLX maintenance had the greatest odds of opioid use and also used opioids more frequently during the BUP-NLX taper. These findings provide preliminary evidence that persistent or erratic pain during prescription opioid addiction treatment may impact risk for opioid use during withdrawal of opioid maintenance therapy in adults with chronic pain.
Our findings suggest that temporal aspects of pain response could potentially be used to identify high-risk patients who warrant additional interventions to stabilize pain prior to tapering from opioid maintenance. In prior studies of substance use treatment patients with chronic pain or severe pain at baseline had the greatest rates of post-treatment relapse (18, 19), but findings from similar studies of BUP-NLX for opioid dependence have been mixed (22-24). Our study revealed that among opioid-dependent adults with chronic pain, the more dynamic aspects of pain predicted future opioid use more reliably than the static indicator of baseline pain severity. While replication in other samples is necessary, these findings could potentially be used to guide treatment of other substance use disorders, given the strong overlap between chronic pain and use of other substances such as alcohol and illicit opiates (34, 35).
An additional, unexpected finding was that the enhanced counseling condition produced lower rates of opioid use during the BUP-NLX taper than standard counseling condition. Treatment condition did not impact rates of multi-week abstinence at treatment endpoint or 2-month follow-up in this sample (23). The current study differs from the primary study by (1) including only patients with chronic pain, (2) focusing only on the taper phase, and (3) predicting observed measures of opioid use instead of a composite measure of sustained abstinence. Enhanced medical management may have been particularly helpful for preventing relapse during the BUP-NLX taper for participants with chronic pain, perhaps by providing additional coping skills for managing aversive symptoms that arise during the taper. Persons with chronic pain comprise a substantial amount of adults treated for opioid dependence (25), thus research should continue to develop more effective therapies for improving treatment outcomes in this sub-population.
Because persistence and volatility in pain predicted future opioid use, our findings provide a rationale to investigate specific mechanisms that explain individual differences in pain response during opioid maintenance treatment for prescription opioid addiction and chronic pain. These individual changes in pain may reflect underlying biomedical pathology, life stress and mood, or genetic factors that influence pain sensitivity or biological response to opioid maintenance medications (36, 37). Central and peripheral inflammatory mechanisms may play a critical role in both pain perception and substance use, including substance-related reward (38). Further translational investigations are needed to specify underlying mechanisms and further personalize interventions for patients with chronic pain and prescription opioid addiction.
Our findings should be interpreted in light of several limitations. This study was a secondary analysis in which we tested hypotheses outside the scope of the original clinical trial. As such, these findings are preliminary and in need of confirmation through replication or a prospective design. These analyses also involved a subset of the original sample (participants with chronic pain who were retained after BUP-NLX detoxification and maintenance), so these findings may not generalize to the general population of prescription opioid-dependent adults who seek treatment. A potential concern is the accelerated rate of attrition during the taper phase which led to a steep increase in missing data, although confidence in the results is bolstered by our use of modern estimation procedures that are generally more robust to missing data than alternative approaches (39). The original clinical trial was conducted in community clinics connected to an established clinical trials research network, therefore the frequency and quality of clinical services provided in this study may differ from those typically available to this population. Because this sample was recruited for treatment of prescription opioid addiction, these findings may not generalize to adults with chronic pain patients who do not meet diagnostic thresholds for prescription opioid addiction or aren't seeking treatment.
In conclusion, we found that features of individual trajectories of pain during BUP-NLX maintenance predicted opioid use during BUP-NLX taper in patients with prescription opioid addiction and chronic pain. In adults with chronic pain receiving treatment for prescription opioid addiction, those who have relatively persistent or volatile pain during BUP-NLX maintenance are at greater risk for resuming opioid use while tapering off BUP-NLX. These findings suggest that stabilizing and/or reducing subjective pain prior to discontinuation of BUP-NLX maintenance may be a means to improve treatment outcomes in this population. Future research should examine dynamic aspects of pain response, perhaps while supplementing opioid maintenance treatment with behavioral therapies or medications that target pain, to determine whether stabilizing pain improves longer-term outcomes of prescription opioid addiction treatment in adults with chronic pain.
Acknowledgments
The analysis, interpretation, and preparation of this study was supported by National Institute on Drug Abuse (NIDA) grants 5T32 DA026400, 5R01 DA030577, 5R01 DA035054, and 3U10DA01304. The design, conduct, data collection, and management of the original clinical trial providing the data for this study was conducted in the NIDA Clinical Trials Network (CTN) and was supported by NIDA CTN grants 2U10DA015831, 2U10DA013045, 2U10DA015815, 2U10DA013727, 2U10DA020036, 2U10DA013035, 2U10DA013714, and 5U10DA013732. The sponsor of the original clinical, the NIDA Center for the CTN, collaborated in the design and conduct of the original trial but was not involved in the conceptualization of this study. The NIDA CTN publications committee is acknowledged for their review and feedback provided for this manuscript.
Declarations: Dr. Shoptaw and Dr. Heinzerling have received clinical research supplies from Pfizer and Medicinova. Dr. Ling has served as consultant to Reckitt Benckiser and Titan Pharmaceuticals, and has received unrestricted educational and research grants through UCLA from Reckitt Benckiser.
References
- 1.Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154(1):S94–100. doi: 10.1016/j.pain.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanch B, Pearson SA, Haber PS. An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br J Clin Pharmacol. 2014;78:1159–1166. doi: 10.1111/bcp.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician. 2014;17:E119–128. [PubMed] [Google Scholar]
- 4.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000-2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, Thomas C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction. 2009;104:256–265. doi: 10.1111/j.1360-0443.2008.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling W, Mooney L, Hillhouse M. Prescription opioid abuse, pain and addiction: clinical issues and implications. Drug Alcohol Rev. 2011;30:300–305. doi: 10.1111/j.1465-3362.2010.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamei J, Saitoh A, Suzuki T, Misawa M, Nagase H, Kasuya Y. Buprenorphine exerts its antinociceptive activity via mu 1-opioid receptors. Life Sci. 1995;56:PL285–290. doi: 10.1016/0024-3205(95)00078-x. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinic. Drug Alcohol Depend. 2003;70:S13–27. doi: 10.1016/s0376-8716(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 9.Dahan A. Opioid-induced respiratory effects: new data on buprenorphine. Palliat Med. 2006;20S1:S3–8. [PubMed] [Google Scholar]
- 10.Jones HE. Practical considerations for the clinical use of buprenorphine. Sci Pract Perspect. 2004;2:4–20. doi: 10.1151/spp04224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen KY, Chen L, Mao J. Buprenorphine-Naloxone Therapy in Pain Management. Anesthesiology. 2014;120:1262–1274. doi: 10.1097/ALN.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen K, Gutierrez A, Haller D, Potter JS. Sublingual buprenorphine for chronic pain: a survey of clinician prescribing practices. The Clinical journal of pain. 2014;30:295–300. doi: 10.1097/AJP.0b013e318298ddad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenblum A, Cruciani RA, Strain EC, Cleland CM, Joseph H, Magura S, et al. Sublingual buprenorphine/naloxone for chronic pain in at-risk patients: development and pilot test of a clinical protocol. J Opioid Manag. 2012;8:369–382. doi: 10.5055/jom.2012.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roux P, Sullivan MA, Cohen J, Fugon L, Jones JD, Vosburg SK, et al. Buprenorphine/naloxone as a promising therapeutic option for opioid abusing patients with chronic pain: reduction of pain, opioid withdrawal symptoms, and abuse liability of oral oxycodone. Pain. 2013;154:1442–1448. doi: 10.1016/j.pain.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pade PA, Cardon KE, Hoffman RM, Geppert CM. Prescription opioid abuse, chronic pain, and primary care: a Co-occurring Disorders Clinic in the chronic disease model. J Subst Abuse Treat. 2012;43:446–450. doi: 10.1016/j.jsat.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Daitch J, Frey ME, Silver D, Mitnick C, Daitch D, Pergolizzi J., J Conversion of chronic pain patients from full-opioid agonists to sublingual buprenorphine. Pain Physician. 2012;15:ES59–66. [PubMed] [Google Scholar]
- 17.Neumann AM, Blondell RD, Jaanimagi U, Giambrone AK, Homish GG, Lozano JR, et al. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexistent opioid addiction. J Addict Dis. 2013;32:68–78. doi: 10.1080/10550887.2012.759872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson MJ, PaascheOrlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction. 2007;102:752–760. doi: 10.1111/j.1360-0443.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- 19.Caldeiro RM, Malte CA, Calsyn DA, Baer JS, Nichol P, Kivlahan DR, et al. The association of persistent pain with out-patient addiction treatment outcomes and service utilization. Addiction. 2008;103:1996–2005. doi: 10.1111/j.1360-0443.2008.02358.x. [DOI] [PubMed] [Google Scholar]
- 20.Worley MJ, Heinzerling KG, Shoptaw S, Ling W. Pain volatility and prescription opioid addiction treatment outcomes in patients with chronic pain. Experimental and clinical psychopharmacology. 2015;23:428–435. doi: 10.1037/pha0000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter JS, Chakrabarti A, Domier CP, Hillhouse MP, Weiss RD, Ling W. Pain and continued opioid use in individuals receiving buprenorphine-naloxone for opioid detoxification: secondary analyses from the Clinical Trials Network. J Subst Abuse Treat. 2010;38:S80–86. doi: 10.1016/j.jsat.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox AD, Sohler NL, Starrels JL, Ning Y, Giovanniello A, Cunningham CO. Pain is not associated with worse office-based buprenorphine treatment outcomes. Subst Abus. 2012;33:361–365. doi: 10.1080/08897077.2011.638734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakrabarti A, Woody GE, Griffin ML, Subramaniam G, Weiss RD. Predictors of buprenorphine-naloxone dosing in a 12-week treatment trial for opioid-dependent youth: secondary analyses from a NIDA Clinical Trials Network study. Drug Alcohol Depend. 2010;107:253–256. doi: 10.1016/j.drugalcdep.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barry DT, Savant JD, Beitel M, Cutter CJ, Moore BA, Schottenfeld RS, et al. Pain and associated substance use among opioid dependent individuals seeking office-based treatment with buprenorphine-naloxone: a needs assessment study. Am J Addict. 2013;22:212–217. doi: 10.1111/j.1521-0391.2012.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss RD, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, et al. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): rationale, design, and methodology. Contemp Clin Trials. 2010;31:189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Sobell LC, Sobell MB. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 29.Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG. Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug Alcohol Depend. 2007;87:297–302. doi: 10.1016/j.drugalcdep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 31.Cofta-Woerpel L, McClure JB, Li Y, Urbauer D, Cinciripini PM, Wetter DW. Early cessation success or failure among women attempting to quit smoking: trajectories and volatility of urge and negative mood during the first postcessation week. J Abnorm Psychol. 2011;120:596–606. doi: 10.1037/a0023755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.StataCorp. Stata: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 33.Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: a review. Drug Alcohol Depend. 2011;119:1–9. doi: 10.1016/j.drugalcdep.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blinderman CD, Sekine R, Zhang B, Nillson M, Shaiova L. Methadone as an analgesic for patients with chronic pain in methadone maintenance treatment programs (MMTPs) Journal of opioid management. 2009;5:107–114. doi: 10.5055/jom.2009.0012. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Parraga GT, Lopez-Martinez AE. The Contribution of Posttraumatic Stress Symptoms to Chronic Pain Adjustment. Health Psychol. 2013;33:958–967. doi: 10.1037/hea0000040. [DOI] [PubMed] [Google Scholar]
- 37.Kwon JK, Chang IH. Pain, catastrophizing, and depression in chronic prostatitis/chronic pelvic pain syndrome. Int Neurourol J. 2013;17:48–58. doi: 10.5213/inj.2013.17.2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sturmer T, Raum E, Buchner M, Gebhardt K, Schiltenwolf M, Richter W, et al. Pain and high sensitivity C reactive protein in patients with chronic low back pain and acute sciatic pain. Ann Rheum Dis. 2005;64:921–925. doi: 10.1136/ard.2004.027045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallgren KA, Witkiewitz K, Kranzler HR, Falk DE, Litten RZ, O'Malley SS, et al. Missing Data in Alcohol Clinical Trials with Binary Outcomes. Alcohol Clin Exp Res. 2016;40:1548–1557. doi: 10.1111/acer.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]


