Summary
What is known and objective
Despite the known significant drug-drug interaction between isavuconazole and tacrolimus there are no recommendations on dose-adjustment when these drugs are given concomitantly. We report on a patient with a mediastial Aspergillus fumigatus infection resistant to posaconazole and describe how she was successfully managed with isavuconazole therapeutic drug-level monitoring.
Case description
Our patient presented with a mediastial Aspergillus fumigatus infection, two years after lung transplantation. A. fumigatus was resistant to posaconazole and the patient had intolerance to voriconazole shown by elevated transaminases. The patient was given isavuconazole with drug-level monitoring. She was managed successfully with no adverse event. Tacrolimus concentration continued to increase after more than 2 weeks of therapy, and required a further reduction to 72% of the usual dose to maintain the target concentrations over a 8 week period.
What is new and Conclusion
When isavuconazole is given to patients on tacrolimus, the dose of the latter will need considerable reduction. We would suggest an initial 50% reduction and recommend close weekly monitoring of tacrolimus concentration. Further dose decreases of 25–50% may be required
Keywords: drug-drug interaction, therapeutic drug monitoring, new antifungal agent
What is Known and Objective
Isavuconazole is a broad-spectrum azole antifungal approved in March 2015 by the Food and Drug Administration (FDA) for the treatment of invasive mucormycosis and invasive aspergillosis. Anti-infectives such as azole antifungals, frequently used in hematopoietic stem cell and solid organ transplant patients receiving immunosuppressant therapies, can lead to significant drug-drug interactions. There are various warnings and dosing recommendations for concomitant administration of antifungal agents and immunosuppressive agents. The labeling of isavuconazole at initial approval recommends monitoring the concentration of tacrolimus when co-administered with isavuconazole. However, no specific recommendations are given.1,2
Case Description
Our patient is a 30-year-old female with autosomal dominant Hyper IgE syndrome (AD-HIES). AD-HIES is a rare primary immune deficiency characterized by eczema, recurrent skin and pulmonary infections, elevated serum IgE, and various abnormalities of connective tissue, bone, and vasculature.3 By the age of 27, she had had repeated pulmonary infections leading to large bilateral pneumatoceles with severe obstructive and restrictive lung disease requiring continuous positive airway pressure and oxygen. She had chronic pulmonary infection or colonization with Mycobacteria abscessus, Stenotrophomonas maltophilia, and Aspergillus fumigatus for which she was treated with chronic posaconazole as well as other antimicrobials. She underwent a successful bilateral lung transplant in September 2013. Three months post-transplant, she developed an A. fumigatus infection of the right-sided bronchial anastomosis and was treated with various antifungals including oral (PO) posaconazole, intravenous (IV) caspofungin, aerosolized amphotericin B, and aerosolized voriconazole. Systemic voriconazole was avoided given significant hepatotoxicity with her two previous courses. Six months post-transplant, posaconazole-resistant A. fumigatus abscesses were noted in the chest wall and supradiaphram and she was treated with IV liposomal amphotericin B and IV caspofungin. In early 2015, a right-sided mediastinal mass obliterating the right pulmonary artery was noted on chest imaging performed due to elevated inflammatory markers. A biopsy of the mass revealed A. fumigatus and antifungal susceptibility testing showed posaconazole and itraconazole were inactive (MICs >16 mcg/mL), and MICs of voriconazole, caspofungin, and amphotericin B were 0.5, 0.125, and 2 mcg/mL, respectively. Isavuconazole susceptibility testing was performed by The Fungus Testing Laboratory in San Antonio, Texas and the isavuconazole MIC was 1 mcg/mL.
Treatment was initiated with IV caspofungin and IV liposomal amphotericin B with improvement in inflammatory markers and gradual improvement in the size of the mass. However, liposomal amphotericin B was poorly tolerated and isavuconazole was obtained via the FDA’s expanded access mechanism and started 4.5 weeks after starting liposomal amphotericin B and caspofungin. Isavuconazole was administered as 200 mg PO three times daily for 2 days, followed by 200 mg PO once daily. All doses were administered as an inpatient with documentation of all doses received. Liposomal amphotericin B was discontinued one week after starting isavuconazole and she continued on caspofungin. Eight weeks after starting isavuconazole, continued improvement of the Aspergillus infection was observed. Liver function tests remained stable and the only potentially associated adverse effect of the isavuconazole was hair thinning.
At the time isavuconazole was started, her concomitant medications included: liposomal amphotericin B 5 mg/kg/day once daily, tacrolimus 3.5 mg twice daily, caspofungin 50 mg daily, azithromycin 250 mg three times weekly, ferrous sulfate 325 mg daily, hydrocortisone 20 mg and 10 mg in the morning and evening respectively, metoprolol tartrate 50 mg twice daily, sulfamethoxazole/trimethoprim 400/80 mg 1 tablet daily and omeprazole 40 mg twice daily.
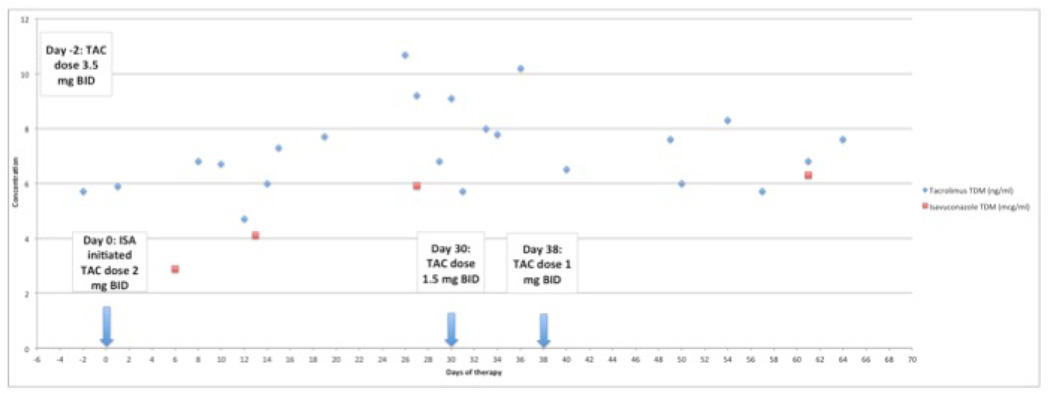
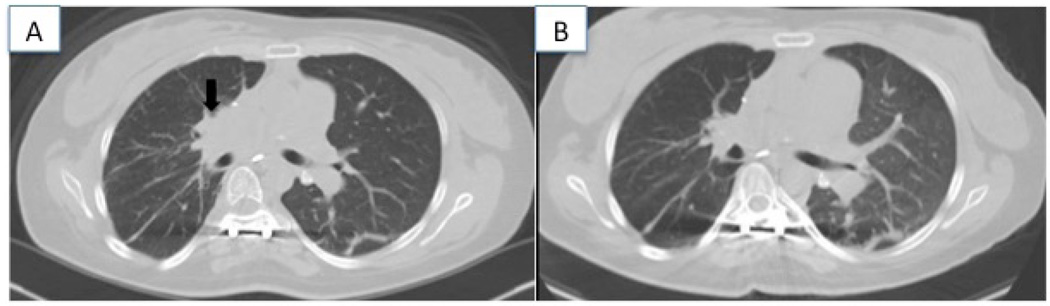
Upon initiation of isavuconazole, the tacrolimus dose was preemptively dose-reduced by 43% from 3.5 mg twice daily to 2 mg twice daily to maintain tacrolimus concentrations within a target range of 6–8 ng/mL. Tacrolimus concentrations were measured at least twice weekly, while trough isavuconazole concentrations were obtained at 1, 2, 4 and 8 weeks. Isavuconazole concentrations were determined using Liquid Chromatography- Tandem Mass Spectrometry (LC-MS/MS) and are included (Astellas Research Institute of America; Skoki, Illinois and Viracor-IBT Laboratories; Lee’s Summit, Missouri). Over the course of 8 weeks, trough concentrations of both isavuconazole and as tacrolimus increased. The tacrolimus dose required further reduction to maintain concentrations within the target range. By 8 weeks, the tacrolimus dose was reduced from the original dose by a total of 72% and isavuconazole steady-state concentrations were reached between weeks 2 and 4. The tacrolimus dose reduction and concentrations of tacrolimus and isavuconazole are shown in Figure 1. During this time all her laboratory results were within normal ranges. The patient’s Chest CT imagings are shown in Figure 2 prior to and after treatment.
Figure 1.
Concentration of isavuconazole (ISA) and tacrolimus (TAC)
Figure 2.
Chest CT imaging at presentation (A) showing a mediastinal mass, and after 4 months of therapy (B) showing improvement in the size of the mass.
Isavuconazole has a long terminal half life (~130 hours) and large volume of distribution (~ 450 L), and thus, current approved initial dosing is to administer loading doses of 200mg three times daily for 2 days, followed by 200mg daily thereafter. Isavuconazole is both a sensitive substrate as well as a moderate CYP3A4 inhibitor in vitro. In a drug interaction study, isavuconazole given at the approved dosing regimen for 5 days totaled led to a 2.25-fold increase in exposure following a 5mg dose of tacrolimus, a substrate of CYP3A4.1,2,4 Therefore, the manufacturer’s labeling recommends using caution when co-administrating tacrolimus and isavuconazole. Data from our patient case and other studies lead us to believe that even with administration of isavuconazole loading doses for the first two days, a true 90% steady state is not reached until at least 1–2 of weeks of therapy.
Many antifungal agents show similar drug-drug interactions with tacrolimus and their concomitant use is accompanied by a specific recommended dose-reduction. Recommendations for fluconazole, itraconazole, voriconazole and posaconazole suggest tacrolimus dose reduction of 40, 50–60, 66, 75–80%, respectively.5 However, at this time no recommendations exist for the concomitant administration of isavuconazole and tacrolimus.
What is new and Conclusion
Based on our experience of this case and the increased tacrolimus exposure seen in one reported drug-drug interaction study, we would suggest an initial 50% reduction in dose of tacrolimus and recommend close weekly monitoring of tacrolimus concentration. Further dose decreases of 25–50% may be required over an 8-week period.
Acknowledgments
This research was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Footnotes
Publication Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy of the U.S. Government.
References
- 1.FDA briefing document: Anti-infective Drugs Advisory Committee Meeting January 22, 2015. [Accessed May 7, 2015]; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM430747.pdf.
- 2.Prescribing information. Northbrook, IL: Astellas Pharma US; [Accessed May 7, 2015]. Cresemba (Isavuconazonium sulfate) [Google Scholar]
- 3.Freeman AF, Holland SM. Clinical manifestations, etiology, and pathogenesis of the hyper-IgE syndromes. Pediatr Res. 2009;65:32R–37R. doi: 10.1203/PDR.0b013e31819dc8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai A, Zadeikis N, Pearlman H, et al. Effect of multiple doses of isavuconazole on the pharmacokinetics of CYP3A4 substrate tacrolimus in healthy subjects. Clin Pharmacol Ther. 2013;93:S39–S40. [Google Scholar]
- 5.Dodds-Ashley E. Drug Interactions With Azoles in Transplant Recipients. Pharmacotherapy. 2010;30(8):842–854. doi: 10.1592/phco.30.8.842. [DOI] [PubMed] [Google Scholar]