Abstract
Monoclonal antibodies and antibody fragments are used for diverse diagnostic and therapeutic applications. We have investigated the secretory production of Fab fragments from insect cells cotransfected with plasmid vectors carrying heavy- and light-chain genes. In the present study, to promote the formation of the disulfide bond between the heavy and light chains, some positively charged amino acid residues were introduced near the cysteine residue for the disulfide bond at the C-terminus of CL, while some negatively charged amino acid residues were added near the cysteine residue for the disulfide bond at the C-terminus of CH1. This electrostatic steering led to an increase in Fab secretions from insect cells.
Keywords: Antibody, Fab fragment, Electrostatic steering, Charged amino acid, Insect cell
Introduction
Monoclonal antibodies and antibody fragments, such as Fab and scFv, have been employed in a variety of diagnostic and therapeutic applications. These are expressed and produced not only in mammalian cells, such as CHO cells (Kim et al. 2012; Mohan et al. 2008; Omasa et al. 2010) and 293T cells (Menzel et al. 2008; Puttikhunt et al. 2008), but also in insect cells (Furuta et al. 2012; Gilmartin et al. 2012; Palmberger et al. 2011; Yamaji et al. 2008), Escherichia coli (Katsuda et al. 2012; Schlapschy and Skerra 2011), and other organisms (Klatt and Konthur 2012).
Insect cells are easier to handle than mammalian cells because they do not require CO2 supplementation in the culture atmosphere and can be grown to high densities in suspension with a serum-free medium. Insect cells can also produce heterologous proteins including antibodies via post-translational processing and modifications similar to those performed in mammalian cells.
There have been several efforts to improve the productivity of antibody molecules, which include culture techniques (Ahn and Antoniewicz 2012; Amanullah et al. 2010; Kishishita et al. 2015; Quek et al. 2010; Reinhart et al. 2015), establishment of cell lines (Costa et al. 2010), coexpression of factors related to protein folding such as chaperone proteins (Nishimiya 2014; Schlapschy and Skerra 2011), optimization of codon usage (Carton et al. 2007; Colcher et al. 1998; Tiwari et al. 2010), and the selection of vectors (Davies et al. 2011; Li et al. 2007) and a signal sequence (Haryadi et al. 2015; Klatt and Konthur 2012; Kober et al. 2013).
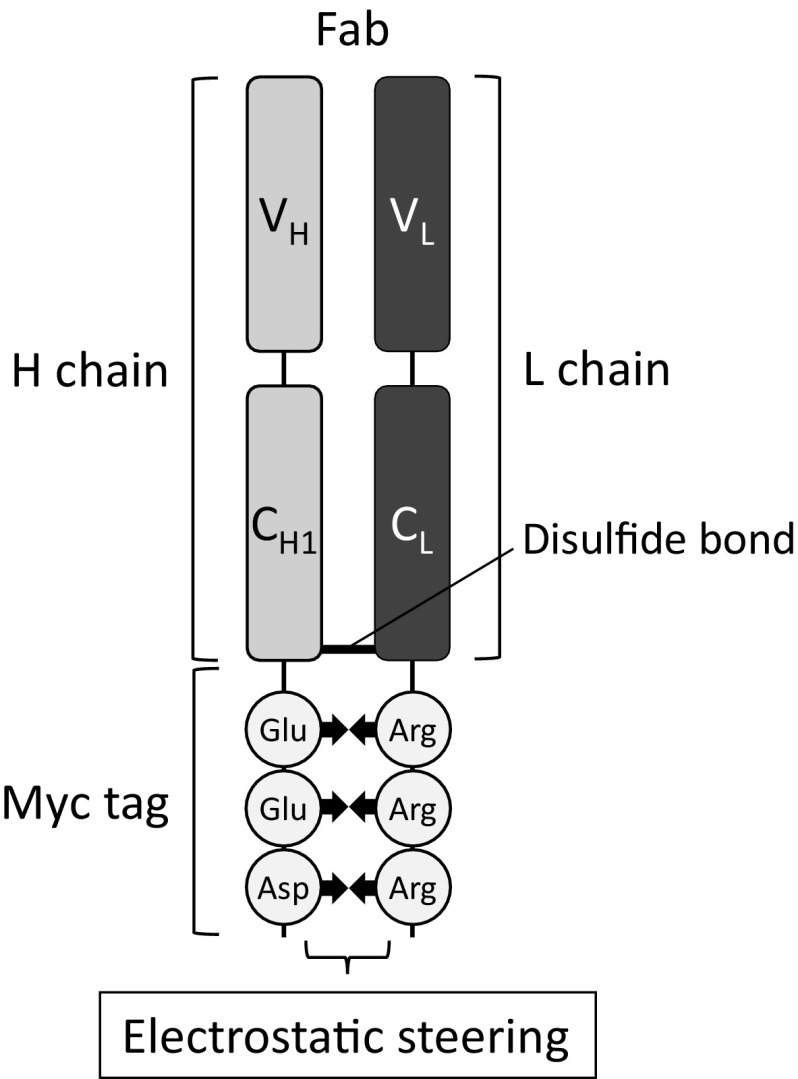
An IgG and an Fab fragment consist of two polypeptide chains: a heavy chain (Hc) and a light chain (Lc). Each chain is expressed independently, and both chains intracellularly associate by disulfide bond(s) and hydrogen bonds during the secretory process. A disulfide bond is formed between the cysteine residue at the C-terminal region of CH1 of Hc and the cysteine residue at the C-terminal region of CL of Lc (Fig. 1). We have investigated the secretory production of an Fab fragment from insect cells cotransfected with plasmid vectors containing Hc (Fd fragment) and Lc genes (Yamaji et al. 2008). In the present study, a Myc tag, which contained negatively charged amino acid residues including two glutamic acids and an aspartic acid near the C-terminus, was added near the cysteine residue for the disulfide bond at the C-terminus of the Hc of an Fab fragment. On the other hand, some positively charged arginine residues were introduced near the cysteine residue for the disulfide bond at the C-terminus of the Lc of the Fab fragment (Fig. 1). We examined whether the static electricity interaction between the Hc and the Lc would promote the formation of a disulfide bond and could improve the productivity of Fab fragment secretion from insect cells.
Fig. 1.
Schematic representation of electrostatic steering between the heavy chain (Hc) and the light chain (Lc) of an antibody Fab fragment
Materials and methods
Materials
All reagents were of the highest grade available and were acquired from Nacalai Tesque (Kyoto, Japan) unless otherwise indicated.
Plasmid construction
The plasmids encoding the Hc and Lc genes of the Fab fragment of 3A21 mouse anti-bovine RNaseA (Katakura et al. 1996) were gifts from Dr. Y. Kumada who is with the Kyoto Institute of Technology. The expression vector, pIHAneo (Yamaji et al. 2008), which was designed to add a 6 × histidine tag at the C-terminus of a target protein when a native stop codon for the gene is not included, was used. A Drosophila immunoglobulin heavy chain binding protein (BiP) signal sequence (Yamaji et al. 2008) was employed upstream of the Hc and Lc genes of the 3A21 Fab. Primers (Eurofins Genomics, Tokyo, Japan; or Life Technologies, Tokyo, Japan) used for the plasmid construction are shown in Table 1.
Table 1.
Sequence of oligonucleotides used for plasmid construction
| Primer | Sequence |
|---|---|
| BipHcXbaI | 5′-CTCTAGAGCATGAAGTTATGCATATTACTGGCCGTCGTGGCCTTTGTTGGCCTCTCGCTCGGGGATGTGCAGCTTCAGGA-3′ |
| BipHcSacII | 5′-TCCCCGCGGTTAGTGATGGTGATGGTGATGACTAGTACAATCCCTGG-3′ |
| BipLcXbaI | 5′-GC TCTAGA GCATGAAGTTATGCATATTACTGGCCGTCGTGGCCTTTGTTGGCCTCTCGCTCGGGGACATCAAGATGACCCAGTCT-3′ |
| BipLcSacII | 5′-TCCCCGCGGGGACTCGAGTGCGGCCGCACACTCATT-3′ |
| CLR1for | 5′-GTGTGCGGCCAGACTCGAGCACC-3′ |
| CLR1rev | 5′-GGTGCTCGAGTCTGGCCGCACAC-3′ |
| CLR3rev | 5′-TTCGAACCGCGGGGACTCGAGCCTACGTCTGGCCGCACACTC-3′ |
| CLR5rev | 5′-TTCGAACCGCGGGGACTCGAGGCGTCGCCTACGTCTGGCCGCAC-3′ |
| pIHAneofor | 5′-GACGGTATCGATAAGCTTGATAT-3′ |
| CH1mycrev | 5′-GAATTCCGCGGTCACAGATCCTCTTCTGAGATGAGTTTTTGTTCCTCGAGTGCGGCCGC-3′ |
Bold nucleotides encoding arginine, italic nucleotides encoding BiP signal sequence or Myc tag, underline restriction enzyme sites
The DNA fragment encoding the BiP signal sequence and the 3A21 Fab Hc gene was amplified with the primers, BipHcSacII and BipHcXbaI (Table 1), via PCR. The amplified fragment was digested with SacI (New England Biolabs, Ipswich, MA, USA) and XbaI (New England Biolabs) and inserted into pIHAneo between the SacI and XbaI sites. The resultant plasmid was designated pIHAneo/H. The DNA fragment encoding the BiP signal sequence and the 3A21 Lc gene was amplified with the primers, BipLcXbaI and BipLcSacII, via PCR, and was subcloned at the XbaI-SacII site of pIHAneo to give pIHAneo/L.
To construct a plasmid that would express the 3A21 Lc with one arginine residue added at the C-terminus, the procedure established for the QuikChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA) was utilized with a small modification. The full length of the pIHAneo/L with nucleotides encoding an arginine residue was amplified by PCR from the pIHAneo/L with the primers CLR1for and CLR1rev that included nucleotides encoding one arginine residue, and the PCR product was treated with DpnI (New England Biolabs) to digest the template pIHAneo/L. The resultant plasmid was designated pIHAneo/L/R1.
To construct a plasmid that would express the Lc with three arginine residues added at the C-terminus (pIHAneo/L/R3), PCR was performed with the template pIHAneo/R1 and the primers pIHAneofor and CLR3rev that included nucleotides encoding three arginine residues. The amplified fragment was digested with SacI and XbaI and inserted into the pIHAneo/Lc between the SacI and XbaI sites using Ligation high ver. 2 (Toyobo, Osaka, Japan).
To construct a plasmid expressing the Lc with five arginine residues added at the C-terminus (pIHAneo/L/R5), PCR was performed with the template pIHAneo/L/R3 and the primers pIHAneofor and CLR5rev, which included nucleotides encoding five arginine residues. The procedure was the same as that for pIHAneo/L/R3.
To construct a plasmid encoding the 3A21 Fab Hc gene and a Myc tag at the C-terminus (pIHAneo/H/myc), PCR was performed with the template pIHAneo/H and the primers pIHAneofor and CH1mycrev including nucleotides encoding the Myc tag. The procedure was the same as that for pIHAneo/L/R3. In the pIHAneo/H/myc, the stop codon was included downstream of the nucleotides encoding the Myc tag. On the other hand, in the other expression plasmids, pIHAneo/H, pIHAneo/L, pIHAneo/L/R1, pIHAneo/L/R3, and pIHAneo/L/R5, no stop codon was included downstream of the inserted gene so that a 6 × histidine tag could be added at the C-terminus of the target protein.
Cell culture and transfection
Trichoplusia ni BTI-TN-5B1-4 (High Five) cells (Life Technologies) were maintained at 27 °C with a serum-free medium (Express Five SFM; Life Technologies), as described previously (Furuta et al. 2012). Cells (8 × 104 cells/well) were inoculated in 24-well cell culture plates. For cotransfection of the Hc and Lc genes, the plasmids of the Hc (300 ng/well) and Lc (600 ng/well) genes were added to polyethyleneimine “Max” (Mw 40,000; Polysciences, Warrington, PA, USA) (1.8 μg/well) in 150 mM of NaCl and incubated for 5 min. On the other hand, for transfection of the Lc gene alone, the plasmid of the Lc gene (600 ng/well) was added to polyethyleneimine (1.2 μg/well) and incubated. The mixture (20 μl/well) was added to the cells 45 min after the cell inoculation. The supernatants were collected 3 days after the transfection.
Enzyme-linked immunosorbent assay (ELISA)
Culture supernatants were analyzed by ELISA to evaluate an Fab fragment with the antigen-binding activity, as previously described (Furuta et al. 2012). ELISA plates were coated with bovine RNaseA (Sigma-Aldrich, St. Louis, MO, USA) as the antigen, and peroxidase-conjugated goat anti-mouse IgG (Promega, Madison, WI, USA) was used. The detections were carried out using the ELISA POD substrate TMB kit (Nacalai Tesque) according to the manufacture’s protocol.
Western blotting
The 6 × histidine tag encoded in the pIHAneo was detected. The culture supernatants were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using a 12.5 % gel under non-reducing conditions. The dry transfer was carried out using the iBlot 2 dry blotting system (Life Technologies) according to the manufacturer’s protocol. The following reaction was performed with rabbit anti-6 × His (Bethyl Laboratories, Boston, MA, USA) as the primary antibody and anti-rabbit IgG (Fc) AP conjugate (Promega) as the secondary antibody using the SNAP i.d. 2.0 protein detection system (Merck Millipore, Darmstadt, Germany). The detection was carried out with the BCIP/NBT color development substrate (Promega) according to the manufacturer’s protocol. The relative quantities of the detected bands were calculated using Image Lab 5.0 (Bio-Rad, Hercules, CA, USA).
Results and discussion
The effect of positively charged amino acids added at the C-terminus of CL on Fab production
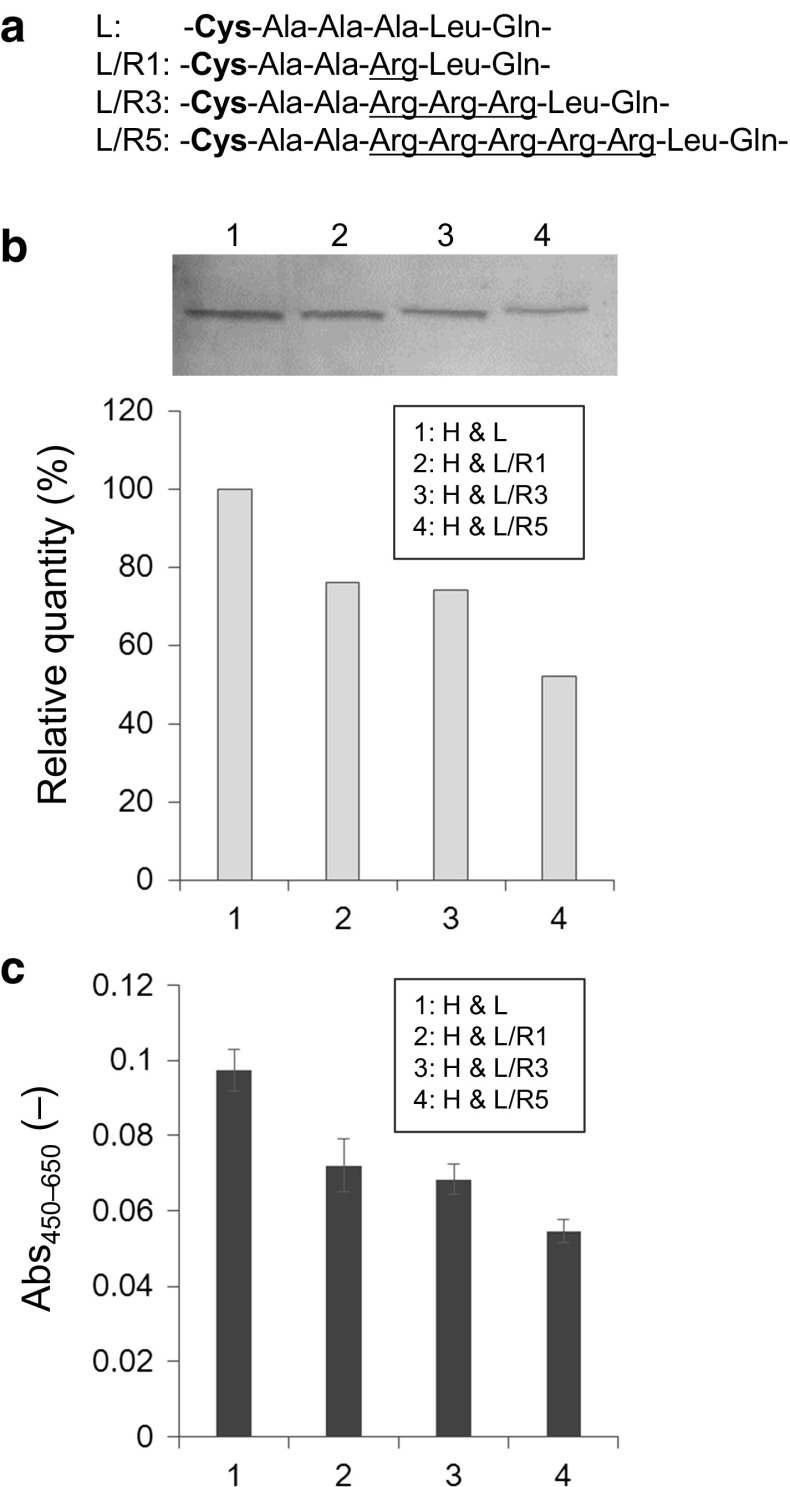
We constructed four types of 3A21 Lc expression plasmids: pIHAneo/L expressing the Lc without an arginine residue at the C-terminus of CL (L); and, pIHAneo/L/R1, pIHAneo/L/R3, and pIHAneo/L/R5, expressing the Lc with one, three, or five arginine residues at the C-terminus of CL (L/R1, L/R3, and L/R5), respectively. High Five cells were cotransfected with pIHAneo/H expressing the 3A21 Fab Hc (H) and one of the Lc expression plasmids, and we examined the secretory production of the 3A21 Fab fragment 3 days after cotransfection (Fig. 2).
Fig. 2.
Effect on Fab fragment secretion of adding arginine residues at the C-terminus of the Lc. a Amino acid sequence at the C-terminus of the Lc. Bold characters indicate the cysteine residue for a disulfide bond. The added arginine residues are underlined. b Fab fragments detected by western blotting. The quantity of the Fab fragment consisting of the Lc without an arginine residue (L) is indicated as 100 %. c Antigen-binding activities of Fab fragments detected by enzyme-linked immunosorbent assay (ELISA). Error bar = 1 SD (n = 3)
The quantities of the secreted Fab fragments were compared by western blotting under non-reducing conditions (Fig. 2b). The quantity was decreased when a positive charge was added to the Lc; the relative quantities of the Fab fragment consisting of the Lc with one, three, and five arginine residues at the C-terminus of CL (L/R1, L/R3, and L/R5) were 76, 74, and 52 %, respectively, of the Fab fragment consisting of the Lc without an arginine residue (L).
The antigen-binding activity of each Fab fragment was determined by ELISA (Fig. 2c). The ELISA data corresponded with the results of western blot analysis. The binding activities of Fab fragments were decreased with the number of arginine residues added at the C-terminus of CL; the relative activities of the Fab fragments consisting of L/R1, L/R3, and L/R5 was 71, 68, and 58 %, respectively, of the Fab fragment consisting of the Lc without an arginine residue (L).
The effect of adding positively charged amino acids at the C-terminus of CL on Lc secretion
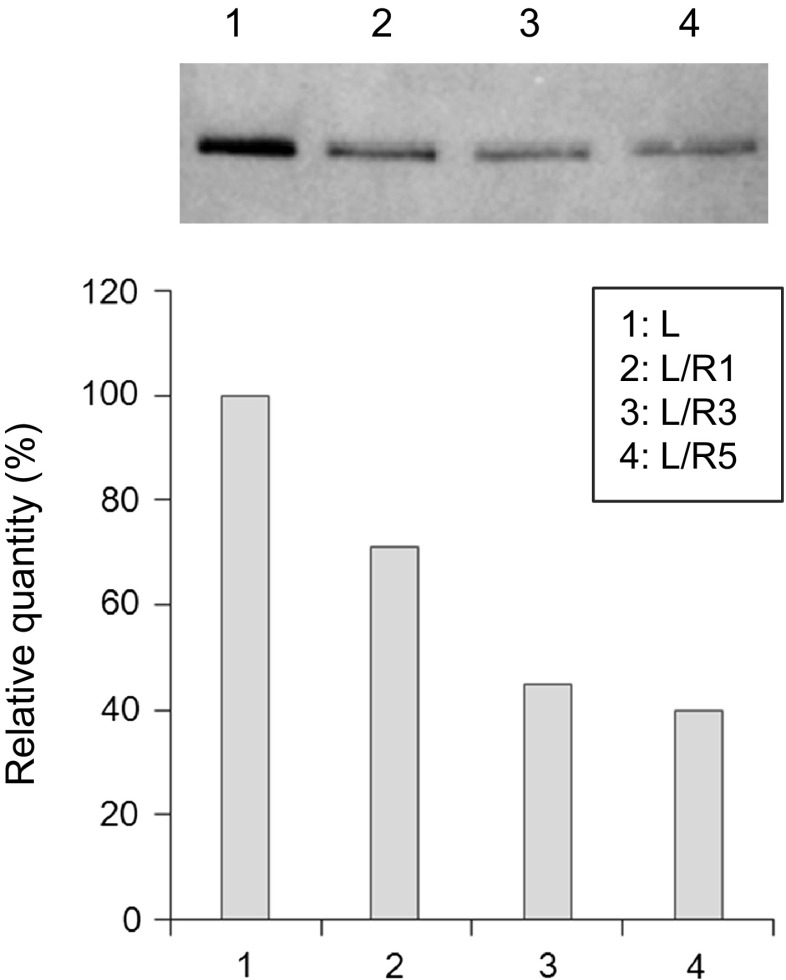
Both Lc(s) alone and Lc(s) associated with Hc(s) are secreted from insect cells the same as with mammalian cells, although Hc(s) alone are not secreted from these cells. To determine whether decreases in the secreted Fab fragment were caused by decreases in Lc secretion, High Five cells were transfected with each of the 3A21 Lc expression plasmids: pIHA/L, pIHA/L/R1, pIHA/L/R3, and pIHA/L/R5 (Fig. 3).
Fig. 3.
Effect on Lc secretion of adding a positive charge at the C-terminus of the Lc. Fab fragments were detected by western blotting. The quantity of L is indicated as 100 %
The quantities of the secretory Lc were compared by western blotting. The quantity was decreased with the addition of a positive charge to the Lc; the relative quantities of L/R1, L/R3, and L/R5 were 71, 45, and 40 %, respectively. These results show that a decrease in the secretory Fab fragment resulted from the decrease in secretion of positively charged Lc. This might have been caused by some interactions with negatively charged phosphate groups of nucleic acids and phospholipids in cells as well as in the cell membranes.
Secretory production of Fab containing negatively charged amino acids at the C-terminus of CH1 and positively charged amino acids at the C-terminus of CL
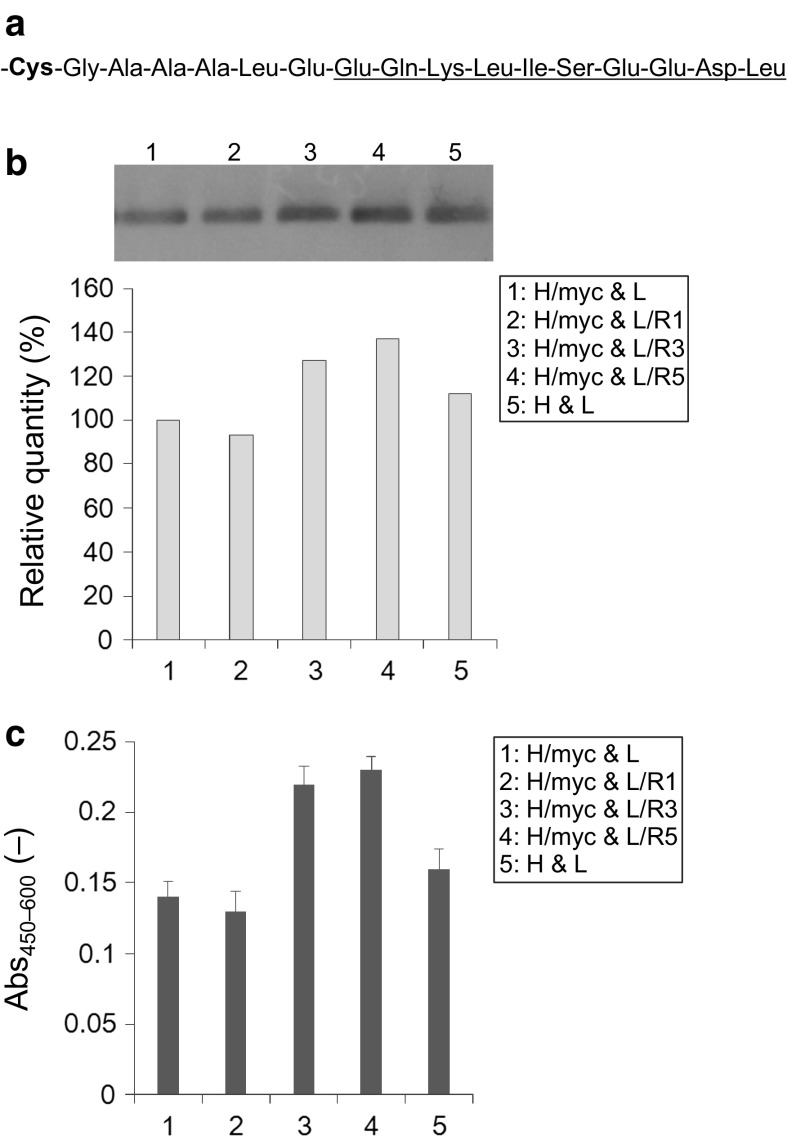
Finally, we examined whether the electrostatic steering between the negatively charged amino acids added at the C-terminus of CH1 and the positively charged amino acids added at the C-terminus of CL would facilitate an association between the Hc and the Lc of the 3A21 Fab. A Myc tag was added near the cysteine residue for the disulfide bond at the C-terminus end of CH1 using pIHAneo/H/myc. The Myc tag contains four negatively charged amino acids (three glutamic acids and one aspartic acid) but only one positively charged amino acid (a lysine), and three negatively charged amino acid residues (Glu–Glu–Asp) are located near the C-terminus (Fig. 4a). Hence, we assumed that the electrostatic steering between the negatively charged Myc tag at the C-terminus of the 3A21 Fab Hc and the arginine residues added at the C-terminus of the Lc could promote the formation of a disulfide bond for the association of the Hc and the Lc (Fig. 1). High Five cells were cotransfected with each pair of plasmids: pIHAneo/H/myc and pIHAneo/L, pIHAneo/H/myc and pIHAneo/L/R1, pIHAneo/H/myc and pIHAneo/L/R3, pIHAneo/H/myc and pIHAneo/L/R5, and pIHAneo/H and pIHAneo/L (Fig. 4).
Fig. 4.
Secretion of Fab fragments with a positive charge at the C-terminus of the Lc and a negative charge at the C-terminus of the Hc. a Amino acid sequence at the C-terminus of H/myc. Bold characters indicate the cysteine residue for a disulfide bond. The Myc tag is underlined. b Fab fragments detected by western blotting. The quantity of the Fab fragment consisting of H/myc and L is indicated as 100 %. c Antigen-binding activities of Fab fragments detected by ELISA. Error bar = 1 SD (n = 3)
The quantities of the secretory Fab fragment were compared by western blotting under non-reducing conditions (Fig. 4b). The quantity of the Fab fragment consisting of the Lc without an arginine residue (L) and the Hc with a C-terminal Myc tag (H/myc) was slightly lower than that of the Fab fragment consisting of L and the Hc without a Myc tag (H). The quantities of the Fab fragment consisting of H/myc were increased by adding either three or five positively charged arginine residues to the Lc; the relative quantity of the Fab fragment consisting of H/myc and L/R1 was 93 % of the Fab fragment consisting of H/myc and L, the relative quantity of the Fab fragment consisting of H/myc and L/R3 was 127 %, and the relative quantity of the Fab fragment consisting of H/myc and L/R5 was 137 %.
The antigen-binding activities of the Fab fragment consisting of H/myc were examined by ELISA (Fig. 4c). The result was similar to that obtained by western blotting; the relative activities of the Fab fragments consisting of H/myc and L/R1, L/R3, and L/R5 were 93, 157, and 164 % of the Fab fragment consisting of H/myc and L, respectively. This result indicates that the electrostatic steering between the C-terminus of CH1 and the C-terminus of CL did not affect the antigen-binding activity of the 3A21 Fab fragment.
Consequently, the increase in secretory Fab fragments by the electrostatic steering between the C-terminus of Hc and the C-terminus of Lc overcame the decrease in Fab secretion by adding positively charged amino acids to the Lc. Reportedly, the electrostatic steering was useful for the heterodimeric association when a bispecific antibody was produced (Igawa et al. 2010; Klein et al. 2012; Strop et al. 2012). On the other hand, the appropriate modifications of the charges in antibody molecules are known to promote the correct folding (Nichols et al. 2015; Perchiacca et al. 2014; Zhang et al. 2004), and then the correct folding is required for the secretion. Therefore, we concluded that the fine-tuning of the added charges for the H/L association was useful for antibody production. This procedure could be applied to both mammalian cells and E. coli, as well as to insect cells, for efficient antibody production.
Acknowledgments
The authors thank Dr. Y. Kumada of the Kyoto Institute of Technology for providing us the plasmids encoding the Hc and Lc genes of the 3A21 Fab fragment. This research was partially supported by the programs for developing key technologies for discovering and manufacturing pharmaceuticals used for next-generation treatments and diagnoses from both the Ministry of Economy, Trade and Industry, Japan (METI) and the Japan Agency for Medical Research and Development (AMED).
References
- Ahn WS, Antoniewicz MR. Towards dynamic metabolic flux analysis in CHO cell cultures. Biotechnol J. 2012;7:61–74. doi: 10.1002/biot.201100052. [DOI] [PubMed] [Google Scholar]
- Amanullah A, Otero JM, Mikola M, Hsu A, Zhang J, Aunins J, Schreyer HB, Hope JA, Russo AP. Novel micro-bioreactor high throughput technology for cell culture process development: reproducibility and scalability assessment of fed batch CHO cultures. Biotechnol Bioeng. 2010;106:57–67. doi: 10.1002/bit.22664. [DOI] [PubMed] [Google Scholar]
- Carton JM, Sauerwald T, Hawley-Nelson P, Morse B, Peffer N, Beck H, Lu J, Cotty A, Amegadzie B, Sweet R. Codon engineering for improved antibody expression in mammalian cells. Protein Expr Purif. 2007;55:279–286. doi: 10.1016/j.pep.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Colcher D, Pavlinkova G, Beresford G, Booth BJ, Choudhury A, Batra SK. Pharmacokinetics and biodistribution of genetically-engineered antibodies. Q J Nucl Med. 1998;42:225–241. [PubMed] [Google Scholar]
- Costa AR, Rodrigues ME, Henriques M, Azeredo J, Oliveira R. Guidelines to cell engineering for monoclonal antibody production. Eur J Pharm Biopharm. 2010;74:127–138. doi: 10.1016/j.ejpb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Davies SL, O’Callaghan PM, McLeod J, Pybus LP, Sung YH, Rance J, Wilkinson SJ, Racher AJ, Young RJ, James DC. Impact of gene vector design on the control of recombinant monoclonal antibody production by Chinese hamster ovary cells. Biotechnol Prog. 2011;27:1689–1699. doi: 10.1002/btpr.692. [DOI] [PubMed] [Google Scholar]
- Furuta T, Ogawa T, Yamaji H. Production of antibody fragments using the baculovirus–insect cell system. Methods Mol Biol. 2012;907:371–387. doi: 10.1007/978-1-61779-974-7_22. [DOI] [PubMed] [Google Scholar]
- Gilmartin AA, Lamp B, Rumenapf T, Persson MA, Rey FA, Krey T. High-level secretion of recombinant monomeric murine and human single-chain Fv antibodies from Drosophila S2 cells. Protein Eng Des Sel. 2012;25:59–66. doi: 10.1093/protein/gzr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haryadi R, Ho S, Kok YJ, Pu HX, Zheng L, Pereira NA, Li B, Bi X, Goh LT, Yang Y, Song Z. Optimization of heavy chain and light chain signal peptides for high level expression of therapeutic antibodies in CHO cells. PLoS One. 2015;10:e0116878. doi: 10.1371/journal.pone.0116878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa T, Tsunoda H, Kikuchi Y, Yoshida M, Tanaka M, Koga A, Sekimori Y, Orita T, Aso Y, Hattori K, Tsuchiya M. VH/VL interface engineering to promote selective expression and inhibit conformational isomerization of thrombopoietin receptor agonist single-chain diabody. Protein Eng Des Sel. 2010;23:667–677. doi: 10.1093/protein/gzq034. [DOI] [PubMed] [Google Scholar]
- Katakura Y, Kobayashi E, Kurokawa Y, Omasa T, Fujiyama K, Suga KI. Cloning of cDNA and characterization of anti-RNase A monoclonal antibody 3A21. J Ferment Bioeng. 1996;82:312–314. doi: 10.1016/0922-338X(96)88826-X. [DOI] [Google Scholar]
- Katsuda T, Sonoda H, Kumada Y, Yamaji H. Production of antibody fragments in Escherichia coli. Methods Mol Biol. 2012;907:305–324. doi: 10.1007/978-1-61779-974-7_18. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim YG, Lee GM. CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol Biotechnol. 2012;93:917–930. doi: 10.1007/s00253-011-3758-5. [DOI] [PubMed] [Google Scholar]
- Kishishita S, Katayama S, Kodaira K, Takagi Y, Matsuda H, Okamoto H, Takuma S, Hirashima C, Aoyagi H. Optimization of chemically defined feed media for monoclonal antibody production in Chinese hamster ovary cells. J Biosci Bioeng. 2015;120:78–84. doi: 10.1016/j.jbiosc.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Klatt S, Konthur Z. Secretory signal peptide modification for optimized antibody-fragment expression-secretion in Leishmania tarentolae. Microb Cell Fact. 2012;11:97. doi: 10.1186/1475-2859-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Sustmann C, Thomas M, Stubenrauch K, Croasdale R, Schanzer J, Brinkmann U, Kettenberger H, Regula JT, Schaefer W. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs. 2012;4:653–663. doi: 10.4161/mabs.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober L, Zehe C, Bode J. Optimized signal peptides for the development of high expressing CHO cell lines. Biotechnol Bioeng. 2013;110:1164–1173. doi: 10.1002/bit.24776. [DOI] [PubMed] [Google Scholar]
- Li J, Menzel C, Meier D, Zhang C, Dubel S, Jostock T. A comparative study of different vector designs for the mammalian expression of recombinant IgG antibodies. J Immunol Methods. 2007;318:113–124. doi: 10.1016/j.jim.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Menzel C, Schirrmann T, Konthur Z, Jostock T, Dubel S. Human antibody RNase fusion protein targeting CD30+ lymphomas. Blood. 2008;111:3830–3837. doi: 10.1182/blood-2007-04-082768. [DOI] [PubMed] [Google Scholar]
- Mohan C, Kim YG, Koo J, Lee GM. Assessment of cell engineering strategies for improved therapeutic protein production in CHO cells. Biotechnol J. 2008;3:624–630. doi: 10.1002/biot.200700249. [DOI] [PubMed] [Google Scholar]
- Nichols P, Li L, Kumar S, Buck PM, Singh SK, Goswami S, Balthazor B, Conley TR, Sek D, Allen MJ. Rational design of viscosity reducing mutants of a monoclonal antibody: hydrophobic versus electrostatic inter-molecular interactions. MAbs. 2015;7:212–230. doi: 10.4161/19420862.2014.985504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimiya D. Proteins improving recombinant antibody production in mammalian cells. Appl Microbiol Biotechnol. 2014;98:1031–1042. doi: 10.1007/s00253-013-5427-3. [DOI] [PubMed] [Google Scholar]
- Omasa T, Onitsuka M, Kim WD. Cell engineering and cultivation of Chinese hamster ovary (CHO) cells. Curr Pharm Biotechnol. 2010;11:233–240. doi: 10.2174/138920110791111960. [DOI] [PubMed] [Google Scholar]
- Palmberger D, Rendic D, Tauber P, Krammer F, Wilson IB, Grabherr R. Insect cells for antibody production: evaluation of an efficient alternative. J Biotechnol. 2011;153:160–166. doi: 10.1016/j.jbiotec.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Perchiacca JM, Lee CC, Tessier PM. Optimal charged mutations in the complementarity-determining regions that prevent domain antibody aggregation are dependent on the antibody scaffold. Protein Eng Des Sel. 2014;27:29–39. doi: 10.1093/protein/gzt058. [DOI] [PubMed] [Google Scholar]
- Puttikhunt C, Keelapang P, Khemnu N, Sittisombut N, Kasinrerk W, Malasit P. Novel anti-dengue monoclonal antibody recognizing conformational structure of the prM-E heterodimeric complex of dengue virus. J Med Virol. 2008;80:125–133. doi: 10.1002/jmv.21047. [DOI] [PubMed] [Google Scholar]
- Quek LE, Dietmair S, Kromer JO, Nielsen LK. Metabolic flux analysis in mammalian cell culture. Metab Eng. 2010;12:161–171. doi: 10.1016/j.ymben.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Reinhart D, Damjanovic L, Kaisermayer C, Kunert R. Benchmarking of commercially available CHO cell culture media for antibody production. Appl Microbiol Biotechnol. 2015;99:4645–4657. doi: 10.1007/s00253-015-6514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlapschy M, Skerra A. Periplasmic chaperones used to enhance functional secretion of proteins in E. coli. Methods Mol Biol. 2011;705:211–224. doi: 10.1007/978-1-61737-967-3_12. [DOI] [PubMed] [Google Scholar]
- Strop P, Ho WH, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, Rickert M, Appah CT, Pascua E, Radcliffe T, Sutton J, Chaparro-Riggers J, Chen W, Casas MG, Chin SM, Wong OK, Liu SH, Vergara G, Shelton D, Rajpal A, Pons J. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol. 2012;420:204–219. doi: 10.1016/j.jmb.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Tiwari A, Sankhyan A, Khanna N, Sinha S. Enhanced periplasmic expression of high affinity humanized scFv against Hepatitis B surface antigen by codon optimization. Protein Expr Purif. 2010;74:272–279. doi: 10.1016/j.pep.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Yamaji H, Manabe T, Watakabe K, Muraoka M, Fujii I, Fukuda H. Production of functional antibody Fab fragment by recombinant insect cells. Biochem Eng J. 2008;41:203–209. doi: 10.1016/j.bej.2008.04.017. [DOI] [Google Scholar]
- Zhang YB, Howitt J, McCorkle S, Lawrence P, Springer K, Freimuth P. Protein aggregation during overexpression limited by peptide extensions with large net negative charge. Protein Expr Purif. 2004;36:207–216. doi: 10.1016/j.pep.2004.04.020. [DOI] [PubMed] [Google Scholar]