Abstract
Cholesterol oxidation products (oxycholesterols) are produced from cholesterol by automatic and/or enzymatic oxidation of the steroidal backbone and side-chain. Oxycholesterols are present in plasma and serum, suggesting that oxycholesterols are related to the development and progression of various diseases. However, limited information is available about the absolute amounts of oxycholesterols in organs and tissues, and the physiological significance of oxycholesterols in the body. In the present study, we quantified the levels of 13 oxycholesterols in white adipose tissue (WAT) of mice and then evaluated correlations between each oxycholesterol level and WAT weight. The sum of the levels of 13 oxycholesterols in WAT (white adipose tissue) was 15.9 ± 3.4 μg/g of WAT weight and approximately 1 % of cholesterol level. Among oxycholesterols, the levels of 27-hydroxycholesterol (27-OH), an endogenous oxycholesterol produced by enzymatic oxidation, and the relative WAT weights were significantly negatively correlated. Next, we evaluated the effects of 27-OH on lipogenesis and adipogenesis in 3T3-L1 cells. TO901317 (TO), a potent and selective agonist for LXRα, significantly increased intracellular TAG contents, while 27-OH significantly reduced the contents to half when compared with control (DMSO) and completely abolished the effect of TO. In addition, 27-OH significantly reduced the mRNA levels of lipogenic (LXRα and FAS) and adipogenic genes (PPARγ and aP2) during adipocyte maturation of 3T3-L1 cells. In conclusion, our results indicate that 27-OH suppresses lipid accumulation by down-regulating lipogenic and adipogenic gene expression in 3T3-L1 cells.
Keywords: 27-Hydroxycholesterol, LXRα, PPARγ, Lipid accumulation, 3T3-L1 adipocytes
Introduction
Adipocytes are well recognized as endocrine cells that are controlled by energy intake and expenditure, and the endocrine function of adipocytes depends on adipocyte size. Large adipocytes [i.e. intracellular triacylglycerol (TAG) accumulation in adipocytes] have been shown to play crucial roles in the development of metabolic syndrome through secretion of fatty acids and adipocytokines such as tumor necrosis factor-α (Matsuzawa 2006). Thus, reduction or suppression of intracellular TAG accumulation in adipocytes is a target to prevent and/or alleviate obesity and metabolic syndrome.
The nuclear receptor liver X receptor alpha (LXRα) is predominantly expressed in central organs of lipid metabolism such as the liver and adipose tissue (Willy et al. 1995). Growing evidence indicates that LXRα has important regulatory roles in adipocytes (Laurencikiene and Rydén 2012). Most nuclear receptors are activated by endogenous lipophilic ligands, and LXRα is activated by oxycholesterols (Janowski et al. 1996; Schroepfer 2000). Oxycholesterols are produced from cholesterol by automatic and/or enzymatic oxidation of the steroidal backbone and side-chain. Oxycholesterols are present in plasma and serum, suggesting that oxycholesterols are related to the development and progression of various diseases such as cardiovascular disease and Alzheimer’s disease (Björkhem and Diczfalusy 2002; Sottero et al. 2009). However, limited information is available about the absolute amounts of oxycholesterols in adipose tissue and the physiological significance of oxycholesterols in adipose tissue and adipocytes.
In the present study, we quantified the levels of 13 oxycholesterols in white adipose tissue (WAT) of mice and then evaluated correlations between each oxycholesterol level and WAT weight. In addition, we evaluated the function of an oxycholesterol found a significant correlation on intracellular TAG accumulation in adipocytes.
Materials and methods
Materials
5-cholesten-3β,19-diol (19-hydroxycholesterol, 19-OH), 5-cholesten-3β,4β-diol (4β-hydroxycholesterol, 4β-OH), 5-cholesten-3β,7α-diol (7α-hydroxycholesterol, 7α-OH), 5-cholesten-3β,7β-diol (7β-hydroxycholesterol, 7β-OH), 5-cholesten-3β,22(R)-diol [22(R)-hydroxycholesterol, 22(R)-OH], 5-cholesten-3β,24-(S)-diol [22(S)-hydroxycholesterol, 22(S)-OH], 5-cholesten-3β,25-diol (25-hydroxycholesterol, 25-OH), 5-,25R-cholesten-3β,26-diol (27-hydroxycholesterol, 27-OH), 5α-cholestan-3β-ol-6-one (6-ketocholestanol, 6-keto), 5-cholesten-3β-ol-7-one (7-ketocholesterol, 7-keto), cholestan-5α,6α-epoxy-3β-ol (5α,6α-epoxycholesterol, 5,6α-epoxy), cholestan-5β,6β-epoxy-3β-ol (5β,6β-epoxycholesterol, 5,6β-epoxy), cholestan-3β,5α,6α-triol (α-cholestantriol, α-triol), and cholestan-3β,5α,6β-triol (β-cholestantriol, β-triol) were purchased from Steraloids Inc. (Newport, RI, USA). 5α-Cholestane was purchased from SIGMA-ALDRICH Japan K.K. (Tokyo, Japan). TO901317 (TO) was purchased from Cayman Chemical Company (Ann Anbor, MI, USA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco-BRL (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Life Technologies (Carlsbad, CA, USA). Ascorbic acid, dexamethasone, and 3-isobutyl-1-methylxanthine were purchased from Nacalai Tesque (Kyoto, Japan). Penicillin and streptomycin were purchased from Meiji Seika Pharma Co., Ltd. (Tokyo, Japan). Dimethyl sulfoxide (DMSO) and insulin were purchased from Wako Pure Chemicals (Tokyo, Japan).
Animal experiments
Six-week-old male C57BL/6 J mice (n = 8) were obtained from Charles River Laboratories Japan, Inc. (Yokohama, Japan). The mice were housed individually in plastic cages in a temperature-controlled room (21–23 °C) under a 12-h light/dark cycle. Experimental diet was prepared according to the AIN-76 formula (American Institute of Nutrition 1977) with some modifications and contained (g/kg): 200 casein, 150 corn starch, 100 olive oil, 50 cellulose, 35 mineral mixture (AIN-76), 10 vitamin mixture (AIN-76), 3 DL-methionine, 2 choline bitartrate, 0.5 cholesterol, and sucrose to 1000 g. The mice were initially fed a commercial diet (NMF, Oriental Yeast Co., Tokyo, Japan) for 3 days and then fed the experimental diet for 9 weeks. All the mice were allowed free access to water. The body weight for each mouse was measured every week and consumption of the feed for each mouse was measured every day. At the end of the feeding period, the mice were subjected to 6-h fasting, anesthetized with Sonmopentyl® (Kyoritsu Seiyaku Corporation, Tokyo, Japan) and then sacrificed by aortic exsanguination. Perirenal WAT was immediately excised and weighed. The samples were kept at −30 °C until analysis.
This experiment was conducted according to the Guidelines for Animal Experiments of Kyushu University (Fukuoka, Japan) and the law (no. 105) and notification (no. 6) of the government of Japan. The animal protocol was approved by the review committee of Kyushu University (authorization no. A22-159-3).
Analysis of cholesterol and oxycholesterols in perirenal WAT of mice
Total lipids in perirenal WAT were extracted with chloroform:methanol (2:1, v/v) containing 0.01 % (w/v) butylated hydroxytoluene (BHT) (Nacalai Tesque, Kyoto, Japan). The levels of cholesterol in perirenal WAT were analyzed by gas chromatography (GC) using 5α-cholestane as an internal standard, as described previously (Matsuoka et al. 2014). The levels of oxycholesterols in perirenal WAT were analyzed according to the previous report (Ando et al. 2002) with a few modifications. After total lipids were saponified overnight, unsaponified lipids were extracted with hexane. The extracted lipids were applied to a Sep-pak Vac silica cartridge (Nihon Waters, Tokyo) to separate the oxycholesterols from cholesterol. The cartridge equilibrated with hexane was sequentially eluted with a solvent mixture composed of hexane and 2-propanol (1:200, v/v), and one composed of hexane and 2-propanol (3:7, v/v), which allowed for sequential elution of cholesterol, and 19-OH plus oxycholesterols, respectively. After removal of the oxycholesterol fraction-containing solvent under N2, the dried residues were converted to trimethylsilyl ethers for 30 min at room temperature. Determination of oxycholesterol was performed using GC–MS (Shimadzu GC-17A version 3 coupled with a SPB-1 fused silica capillary column, 60 m × 0.25 mm, 0.25 µm thickness, Spelco Inc., Bellefonte, PA, USA) connected to a QP5050A series mass-selective detector (Shimadzu). The following temperature program was applied with helium (high-purity 99.9999 %) as a carrier gas at a flow rate of 1.5 ml/min: 180 °C for 1 min; from 180 to 250 °C at 20 °C/min; from 250 to 290 °C at 5 °C/min; 290 °C for 32.5 min. The total run time was 45 min. The injector was operated in the split ratio of 1:5 and was kept at 300 °C, and the detector transfer line was kept at 300 °C. The individual oxycholesterol concentrations were measured with 19-OH as an internal standard. The mass spectrometer was operated in the electron impact (EI) mode (70 eV). A quantitative analysis was performed by the internal standard method for mass spectrometry in the selected ion monitoring mode. The monitored ion during the chromatographic run was varied as a function of time and the characteristic ion for each oxycholesterol was recorded. To quantify each oxycholesterol, calibration curves measuring the peak area of each oxycholesterol versus that of the internal standard were used. Peak identification was confirmed by the relative retention time and a mass spectral comparison with authentic standards.
Cell culture and treatment
3T3-L1 preadipocytes (JCRB9014) were maintained in the maintenance medium (DMEM supplemented with 10 % FBS, 200 μM ascorbic acid, 100 units/ml penicillin, and 100 μg/ml streptomycin) at 37 °C in 5 % CO2. After 3T3-L1 preadipocytes reached confluence, the cells were incubated for an additional 48 h. Then, the cells were differentiated in the differentiation medium (the maintenance medium supplemented with 0.25 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, and 10 μg/ml insulin) for 48 h. The medium was then replaced with the maturation medium (the maintenance medium supplemented with 5 μg/ml insulin), and changed to fresh medium every 2 days. 27-OH and TO were added to the maturation medium as DMSO solution. The final concentration of DMSO was adjusted to 1 % so as not to affect cell growth.
Measurement of intracellular triacylglycerol contents in 3T3-L1 adipocytes
Ten days after induction of differentiation, the cells were washed twice with ice-cold PBS buffer containing 0.02 % EDTA, harvested in ice-cold RIPA buffer, and lysed by sonication. Intracellular TAG contents in the lysates were measured using a commercial enzyme assay kit (Triglyceride E-Test from Wako Pure Chemicals). Protein contents in the lysates were measured as described previously (Lowry et al. 1951). Intracellular TAG contents were expressed as a relative value after normalization to protein contents.
Oil red O staining
Ten days after induction of differentiation, the cells were washed twice with PBS buffer, fixed with 10 % formalin for 1 h, washed twice with distilled water, and then stained with Oil Red O dye (Lipid assay kit from Cosmo Bio Co., Ltd., Tokyo, Japan) for 15 min. Stained cells were washed three times with distilled water, and then photographed.
Analysis of mRNA expression
Total RNA from 3T3-L1 adipocytes was isolated using a guanidinium thiocyanate/cesium chloride ultracentrifugation method (Chirgwin et al. 1979) and was reverse transcribed with a Transcriptor First Strand cDNA Synthesis kit (Roche, Berlin, Germany) to obtain cDNA. Quantitative real-time RT-PCR analysis was performed with a SYBR Premix EX Taq II kit (Takara, Shiga, Japan) and a real-time PCR system (Mx3000P qPCR system; Agilent Technologies, Santa Clara, CA, USA). Primer sequences used were as follows: liver X receptor alpha (LXRα) (forward primer, 5′-CCGACAGAGCTTCGTCCACA-3′; reverse primer, 5′-CCCACAGACACTGCACAG-3′), peroxisome proliferator-activated receptor gamma (PPARγ) (forward primer, 5′-GCCCTTTGGTGACTTTATGG-3′; reverse primer, 5′-CAGCAGGTTGTCTTGGATGT-3′), fatty acid synthase (FAS) (forward primer, 5′-TTGCTGGCACTACAGAATGC-3′; reverse primer, 5′-AACAGCCTCAGAGCGACAAT-3′), adipocyte fatty acid-binding protein (aP2) (forward primer, 5′-AAGACAGCTCCTCCTCGAAGGTT-3′; reverse primer, 5′-TGACCAAATCCCCATTTACGC-3′), β-actin (forward primer, 5′-AGCCATGTACGTAGCCATCC-3′; reverse primer, 5′-TCCCTCTCAGCTGTGGTGGTGAA-3′). Results were expressed as a relative value after normalization to β-actin expression.
Statistical analysis
All values are expressed as the mean ± standard error of mean (SEM). Linear regression analysis was used to assess the relation between oxycholesterol levels in WAT and relative WAT weights (Table 1). Comparisons among groups were performed by using one-way ANOVA followed by the Dunnett’s multiple comparison post hoc test (Fig. 2) or Tukey–Kramer multiple comparison post hoc test (Figs. 3, 4). Differences were considered significant at P < 0.05. Statistical analysis was performed using Excel 2011 (Microsoft, USA) with the add-in software Statcel 3 (Yanai 2011).
Table 1.
Oxycholesterol levels in white adipose tissue of mice and correlation coefficients between each oxycholesterol level and white adipose tissue weight
| Oxycholesterol levels | Correlation coefficient | ||
|---|---|---|---|
| (μg/g tissue) | r | P value | |
| Autoxidation | |||
| 7β-OH | 0.909 ± 0.404 | 0.560 | N.S. |
| 5,6β-epoxy | 2.15 ± 0.22 | −0.405 | N.S. |
| 5,6α-epoxy | 2.27 ± 0.26 | 0.218 | N.S. |
| β-triol | 1.93 ± 0.63 | 0.578 | N.S. |
| 6-keto | 1.24 ± 0.53 | 0.612 | N.S. |
| α-triol | 0.419 ± 0.217 | 0.536 | N.S. |
| 7-keto | 2.22 ± 0.29 | 0.466 | N.S. |
| Auto- and/or Enzymatic-oxidation | |||
| 7α-OH | 1.14 ± 0.58 | 0.541 | N.S. |
| 25-OH | 0.513 ± 0.262 | 0.541 | N.S. |
| Enzymatic oxidation | |||
| 4β-OH | 1.48 ± 0.41 | 0.392 | N.S. |
| 22(R)-OH | 0.100 ± 0.049 | 0.430 | N.S. |
| 24(S)-OH | 0.403 ± 0.120 | 0.454 | N.S. |
| 27-OH | 1.15 ± 0.07 | −0.727 | 0.041 |
| Total oxycholesterols | 15.9 ± 3.4 | 0.525 | N.S. |
The data are expressed as the mean ± SEM (n = 8)
4β-OH, 4β-hydroxycholesterol; 7α-OH, 7α-hydroxycholesterol; 7β-OH, 7β-hydroxycholesterol; 22(R)-OH, 22(R)-hydroxycholesterol; 22(S)-OH, 22(S)-hydroxycholesterol; 25-OH, 25-hydroxycholesterol; 27-OH, 27-hydroxycholesterol; 6-keto, 6-ketocholestanol; 7-keto, 7-ketocholesterol; 5,6α-epoxy, 5α,6α-epoxycholesterol; 5,6β-epoxy, 5β,6β-epoxycholesterol; α-triol, α-cholestantriol; β-triol, β-cholestantriol; N.S. not significant
Fig. 2.
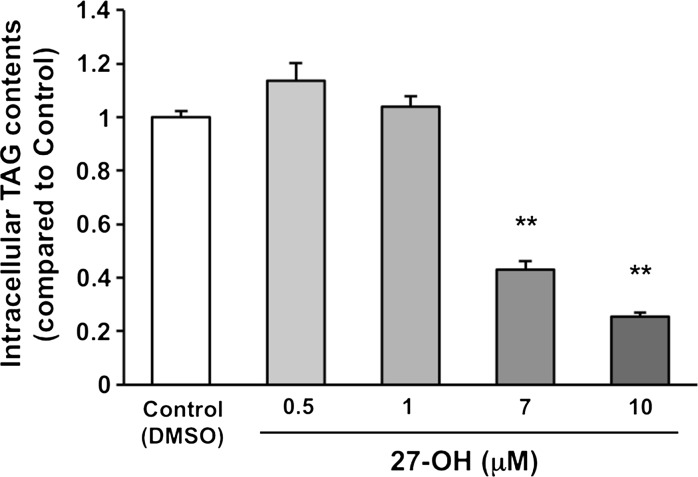
27-OH suppresses intracellular TAG accumulation during adipocyte maturation of 3T3-L1 cells. The cells were treated with DMSO (the vehicle control), 0.5, 1, 7, and 10 μM 27-OH for 10 days after induction of differentiation. TAG level of the vehicle control was arbitrarily set at 1. Data are presented as the mean ± SEM (n = 5). Columns marked with asterisk(s) are significantly different from the control (**P < 0.01). DMSO, dimethyl sulfoxide; 27-OH, 27-hydroxycholesterol; TAG; triacylglycerol
Fig. 3.
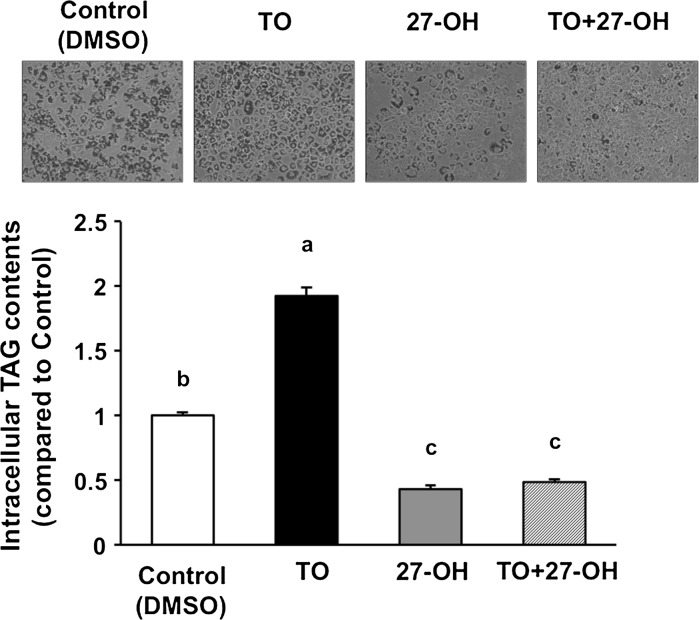
Intracellular TAG accumulation by the induction of TO is cancelled by the addition of 27-OH. The cells were treated with DMSO (the vehicle control), 1 μM TO, 7 μM 27-OH, and 1 μM TO + 7 μM 27-OH for 10 days after induction of differentiation. The cells were stained with Oil Red O dye. Intracellular TAG contents were measured. TAG level of the vehicle control was arbitrarily set at 1. Data are presented as the mean ± SEM (n = 5). Columns marked with different letters are significantly different at P < 0.05. DMSO, dimethyl sulfoxide; 27-OH, 27-hydroxycholesterol; TAG, triacylglycerol; TO, TO901317
Fig. 4.
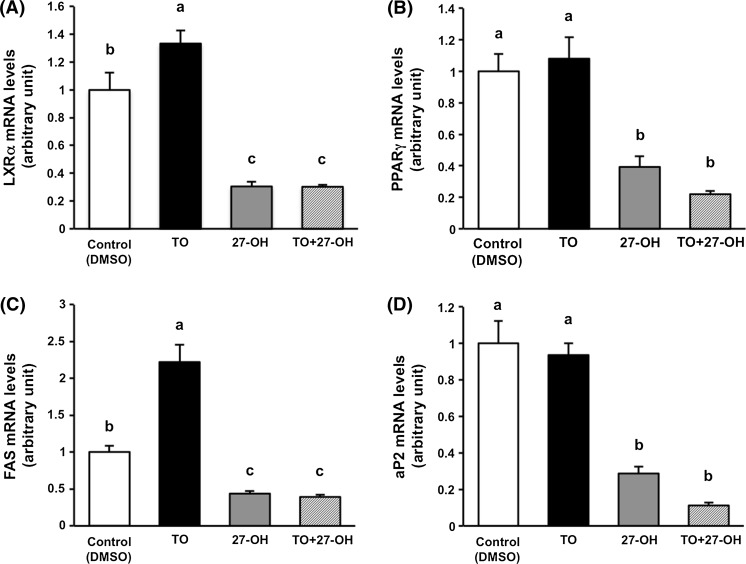
27-OH suppresses lipogenesis and adipogenesis of 3T3-L1 cells. The cells were treated with DMSO (the vehicle control), 1 μM TO, 7 μM 27-OH, and 1 μM TO + 7 μM 27-OH for 10 days after induction of differentiation. Each gene expression level (LXRα, PPARγ, FAS, and aP2) was normalized to the β-actin level. Each gene expression of the vehicle control was arbitrarily set at 1. Data are presented as the mean ± SEM (n = 5). Columns marked with different letters are significantly different at P < 0.05. aP2, adipocyte fatty acid-binding protein; DMSO, dimethyl sulfoxide; FAS, fatty acid synthase; LXRα, liver X receptor alpha; 27-OH, 27-hydroxycholesterol; PPARγ, peroxisome proliferator-activated receptor gamma; TAG, triacylglycerol; TO, TO901317
Results and discussion
27-Hydroxycholesterol levels in WAT are negatively correlated with relative WAT weights
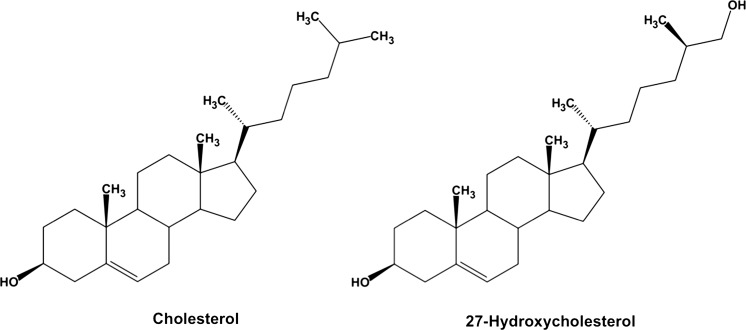
In the present study, after a 9 week feeding period, final body weights, perirenal WAT weights, relative perirenal WAT weights, and cholesterol levels in WAT of C57BL/6 J mice were 27.8 ± 0.6 g, 0.290 ± 0.009 g, 1.04 ± 0.02 g/100 g of body weight, and 1.42 ± 0.02 mg/g of WAT weight (mean ± SEM), respectively. Knowledge of the levels of oxycholesterols in tissues including adipose tissue is very limited. We thereby measured the levels of 13 oxycholesterols in WAT of mice. As shown in Table 1, oxycholesterol levels (μg/g tissue) were as follows (in order of decreasing mean values): 5,6α-epoxy (2.27), 7-keto (2.22), 5,6β-epoxy (2.15), β-triol (1.93), 4β-OH (1.48), 6-keto (1.24), 27-OH (1.15), 7α-OH (1.14), 7β-OH (0.909), 25-OH (0.513), α-triol (0.419), 24(S)-OH (0.403), 22(R)-OH (0.100). Total oxycholesterol level in WAT was 15.9 ± 3.4 μg/g of WAT weight and approximately 1 % of the cholesterol level. To understand physiological significance of oxycholesterols in WAT, we assessed correlations between each oxycholesterol level and relative WAT weight (Table 1). The levels of 27-OH, an endogenous oxycholesterol produced from cholesterol by the mitochondrial enzyme sterol 27-hydroxylase (CYP27A1) (Fig. 1), and the relative WAT weights were significantly negatively correlated (r = −0.727, P < 0.05), suggesting that 27-OH levels in WAT regulates adipose tissue mass and intracellular TAG contents. On the other hand, cholesterol levels in WAT did not correlate with the relative WAT weights (data not shown).
Fig. 1.
The chemical structures of cholesterol and 27-hydroxycholesterol
27-Hydroxycholesterol suppresses intracellular TAG accumulation during adipocyte maturation of 3T3-L1 cells
CYP27A1 is widely expressed throughout different tissues with highest levels not only in liver and muscle but also in kidney, intestine, lung, and so on (Bikle 2001). Although 27-OH is generated by CYP27A1, limited information is available about the physiological significance of 27-OH in the body. Based on the results of Table 1, we evaluated the effect of 27-OH on lipid accumulation in 3T3-L1 cells. The results showed that 27-OH dose-dependently suppressed intracellular TAG accumulation during adipocyte maturation of the cells (Fig. 2). Endogenous oxycholesterols act as ligands for LXRα (Janowski et al. 1996; Schroepfer 2000). LXRα is highly expressed in the liver and adipose tissue (Willy et al. 1995) and regulates expression of lipogenic genes (Kim and Spiegelman 1996; Joseph et al. 2002). Thus, we suggest that the suppression of TAG accumulation in WAT by 27-OH may contribute to its antagonistic action on LXRα.
Intracellular TAG accumulation by the induction of TO901317, a potent and selective agonist for LXRα, is cancelled by the addition of 27-hydroxycholesterol
It is well recognized that TO is a potent and selective agonist for LXRα (Schultz et al. 2000). LXRα activation accelerates lipid accumulation in adipocytes (Juvet et al. 2003). To examine further the effect of 27-OH on lipid accumulation in 3T3-L1 cells, we investigated whether 27-OH suppresses intracellular TAG accumulation induced by TO treatment. TO treatment significantly increased intracellular TAG contents by twofold, while 27-OH treatment significantly reduced the contents to half when compared with control and completely abolished the effect of TO (Fig. 3).
27-Hydroxycholesterol suppresses lipogenesis and adipogenesis in 3T3-L1 cells
To gain insight into the effect of 27-OH on lipogenesis and adipogenesis during adipocyte maturation of 3T3-L1 cells, we analyzed the expression levels of mRNA related to them. TO significantly increased LXRα mRNA levels, whereas 27-OH significantly reduced the mRNA levels when compared with control and completely abolished the effect of TO (Fig. 4A). LXRα also directly regulates FAS expression through interaction with its promoter (Joseph et al. 2002). Consistent with the observed dynamics of LXRα mRNA, 27-OH markedly reduced FAS mRNA levels and completely abolished the effect of TO (Fig. 4C).
Several stimuli that interfere with adipogenesis, such as trans10, cis12-conjugated linoleic acid (Granlund et al. 2005) and ultraviolet light A (Lee et al. 2010) reduce not only the expression of LXRα but also simultaneously downregulate the expression of PPARγ, a master regulator of adipogenesis. As shown in Fig. 4B, PPARγ mRNA levels were also significantly reduced by 27-OH treatment, the results that is in agreement with previous studies. In addition, 27-OH significantly reduced the mRNA levels of aP2, a PPARγ target gene and also called fatty acid binding protein 4 (FABP4) (Fig. 4D). PPARγ and CCAAT/enhancer-binding protein alpha (C/EBPα) cooperatively regulate adipogenesis (Lefterova et al. 2008). Recently, it has been reported that 27-OH treatment exhibited anti-adipogenic activity through the down-regulation of C/EBPα and FABP4 mRNA levels in 3T3-L1 cells (Li et al. 2014). Our results provide supportive evidence for the previous study (Li et al. 2014). Further studies are necessary to clarify the mechanisms of down-regulating gene expression related to lipogenesis and adipogenesis by 27-OH.
In conclusion, the present study indicates that 27-hydroxycholesterol suppresses intracellular TAG accumulation by down-regulating lipogenic and adipogenic gene expression during adipocyte maturation of 3T3-L1 cells. Whether increased circulating level of 27-OH and/or increased synthesis of 27-OH in WAT reduce lipid accumulation in WAT and then prevent obesity and metabolic syndrome, would be of great interest for future study.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers 21580144, 23780138.
Authors’ contributions
B.S. wrote the manuscript. B.S., K.K., Y.H., Y.N., Y.F. and LT.T. participated in the experimental work and collected and analyzed data. B.S. and M.S. contributed to the study design, supervised the study, and commented on the manuscript. All authors have read and approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
None.
References
- American Institute of Nutrition Report of the American Institute of Nurtition ad hoc Committee on Standards for Nutritional Studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- Ando M, Tomoyori H, Imaizumi K. Dietary cholesterol-oxidation products accumulate in serum and liver in apolipoprotein E-deficient mice, but do not accelerate atherosclerosis. Br J Nutr. 2002;88:339–345. doi: 10.1079/BJN2002670. [DOI] [PubMed] [Google Scholar]
- Bikle D. Vitamin D regulation of immune function. In: Litwack G, editor. Vitamins and the immune system. Amsterdam: Elsevier; 2001. pp. 1–21. [Google Scholar]
- Björkhem I, Diczfalusy U. Oxysterols: friends, foes, or just fellow passengers? Arterioscler Thromb Vasc Biol. 2002;22:734–742. doi: 10.1161/01.ATV.0000013312.32196.49. [DOI] [PubMed] [Google Scholar]
- Chirgwin JM, Przbyla AE, McDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Granlund L, Pedersen JI, Nebb HI. Impaired lipid accumulation by trans10, cis12 CLA during adipocyte differentiation is dependent on timing and length of treatment. Biochim Biophys Acta. 2005;1687:11–22. doi: 10.1016/j.bbalip.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TF, Tontonoz P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- Juvet LK, Andresen SM, Schuster GU, Dalen KT, Tobin KA, Hollung K, Haugen F, Jacinto S, Ulven SM, Bamberg K, Gustafsson JA, Nebb HI. On the role of liver X receptors in lipid accumulation in adipocytes. Mol Endocrinol. 2003;17:172–182. doi: 10.1210/me.2001-0210. [DOI] [PubMed] [Google Scholar]
- Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- Laurencikiene J, Rydén M. Liver X receptors and fat cell metabolism. Int J Obes (Lond) 2012;36:1494–1502. doi: 10.1038/ijo.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lee J, Jung E, Kim YS, Roh K, Jung KH, Park D. Ultraviolet A regulates adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells via up-regulation of Kruppel-like factor 2. J Biol Chem. 2010;285:32647–32656. doi: 10.1074/jbc.M110.135830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr, Liu XS, Lazar MA. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Daly E, Campioli E, Wabitsch M, Papadopoulos V. De novo synthesis of steroids and oxysterols in adipocytes. J Biol Chem. 2014;289:747–764. doi: 10.1074/jbc.M113.534172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Matsuoka R, Shirouchi B, Kawamura S, Baba S, Shiratake S, Nagata K, Imaizumi K, Sato M. Dietary egg white protein inhibits lymphatic lipid transport in thoracic lymph duct-cannulated rats. J Agric Food Chem. 2014;62:10694–10700. doi: 10.1021/jf502741b. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Therapy Insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006;3:35–42. doi: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottero B, Gamba P, Gargiulo S, Leonarduzzi G, Poli G. Cholesterol oxidation products and disease: an emerging topic of interest in medicinal chemistry. Curr Med Chem. 2009;16:685–705. doi: 10.2174/092986709787458353. [DOI] [PubMed] [Google Scholar]
- Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- Yanai H. Statcel 3—the usefull add-in software forms on Excel. 3. Tokyo: OMS; 2011. [Google Scholar]