Abstract
Enterolactone (ENL) is formed by the conversion of dietary precursors like strawberry lignans via the gut microbiota. Urinary concentrations of lignan metabolites are reported to be significantly associated with a lower risk of Type 2 diabetes (T2D). In the present study, antidiabetic effect of ENL and its modes of action were studied in vitro and in vivo employing a rat skeletal muscle-derived cell line, L6 myocytes in culture, and T2D model db/db mice. ENL dose-dependently increased glucose uptake in L6 myotubes under insulin absent condition. This increase by ENL was canceled by compound C, an inhibitor of 5′-adenosine monophosphate-activated protein kinase (APMK). Activation (=phosphorylation) of AMPK and translocation of glucose transporter 4 (GLUT4) to plasma membrane in L6 myotubes were demonstrated by Western blotting analyses. Promotion by ENL of GLUT4 translocation to plasma membrane was also visually demonstrated by immunocytochemistry in L6 myoblasts that were transfected with glut4 cDNA-coding vector. T2D model db/db mice were fed the basal 20 % casein diet (20C) or 20C supplemented with ENL (0.001 or 0.01 %) for 6 weeks. Fasting blood glucose (FBG) levels were measured every week and intraperitoneal glucose tolerance test (IPGTT) was conducted. ENL at a higher dose (0.01 % in 20C) suppressed the increases in FBG levels. ENL was also demonstrated to improve the index of insulin resistance (HOMA-IR) and glucose intolerance by IPGTT in db/db mice. From these results, ENL is suggested to be an antidiabetic chemical entity converted from dietary lignans by gut microbiota.
Keywords: AMPK, Enterolactone, GLUT4, L6 myocytes, db/db mice
Introduction
The mammalian lignans enterolactone (ENL) and enterodiol are commonly found in blood and urine of pigs (Bach Knudsen et al. 2003) and humans (Setchell et al. 2014). They are formed by the conversion of dietary precursor lignans from, for instance, strawberry (Mazur et al. 2000) and rye (Bach Knudsen et al. 2003) via the intestinal microbiota (Setchell et al. 1981). ENL has been thought to be the major biologically active lignan, and suggested to be associated with low risk of acute coronary events (Vanharanta et al. 1999) and breast cancer (Saarinen et al. 2007). We have found that ENL more strongly suppresses the proliferation/growth of AH109A hepatoma cells in vitro and in vivo than does its precursor lignan hydroxymatairesinol (Miura et al. 2007), suggesting that ENL is a chemical entity showing various bioactivities of lignans. Urinary concentrations of lignan metabolites, especially enterolactone, are reported to be significantly associated with a lower risk of Type 2 diabetes (T2D) in humans (Sun et al. 2014).
In the present study, antidiabetic effect of ENL and its modes of action were studied both in vitro and in vivo employing rat skeletal muscle-derived L6 myocytes in culture and T2D model db/db mice, respectively.
Materials and methods
Materials
(±)-Entelolactone (ENL) was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). A rat skeletal muscle-derived cell line of L6 myoblasts was obtained from American Type Culture Collection (Manassas, VA, USA; ATCC® number CRL-1458). Dulbecco’s modified Eagle medium (DMEM) was from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). Fetal bovine serum (FBS) was from JRH Biosciences (Lenexa, KS, USA). Streptomycin and penicillin G were purchased from Nacalai Tesque Inc. (Kyoto, Japan). Bovine serum albumin (BSA, fatty acid-free) and Triton X-100 were from Sigma-Aldrich Co. (St. Louis, MO, USA). Compound C was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). 5-Aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) was from Toronto Research Chemicals (Toronto, ON, Canada). Insulin assay kit was from Morinaga Institute of Biological Science, Inc. (Yokohama, Japan). Glucose and triglyceride (TG) assay kits (Glucose CII-Test Wako and Triglyceride E-test Wako, respectively) were from Wako Pure Chemical Industries. Serum lipid peroxide was estimated as thiobarbituric acid-reactive substances (TBARS) with a commercial kit (TBARS Assay Kit) (ZeptoMetrix Corporation, Buffalo, NY, USA). Anti-phospho-AMPKα (Thr172) and anti-AMPK antibodies were purchased from Cell Signaling Technology, Inc. (Beverley, MA, USA). Anti-GLUT4 antibody was from AbD Serotech (Oxford, UK) and anti-Na+K+-ATPase α-1 antibody from Millipore (Billerica, MA, USA). Horseradish peroxidase-conjugated anti-rabbit IgG antibody was obtained from Amersham Biosciences (Little Chalfont, UK). Expression vector pFN21A (HaloTag®7) and anti-HaloTag® rabbit polyclonal antibody were from Promega KK (Tokyo, Japan). Anti-caveolin-3 goat polyclonal IgG and FITC-conjugated anti-goat IgG were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Alexa Fluor 555-conjugated anti-rabbit IgG was from Invitogen (Carlsbad, CA, USA). All other chemicals were of the best grade commercially available, unless otherwise noted. Plastic multiwell plates and tubes were obtained from Nunc A/S (Roskilde, Denmark), or Sumitomo Bakelite Co., Ltd., (Tokyo, Japan).
Glucose uptake by L6 myotubes in culture
L6 Myoblasts were maintained in DMEM supplemented with 10 % (v/v) FBS, streptomycin (100 µg/ml), and penicillin G (100 IU/ml) (10 % FBS/DMEM) under atmosphere of 5 % CO2/95 % humidified air at 37 °C as previously described (Yagasaki et al. 2003). Effect of ENL on glucose uptake in L6 myotubes was investigated according to the procedure as described previously (Kawano et al. 2009) with slight modifications (Son et al. 2015). In brief, L6 myoblasts (5 × 104 cells/well) were grown in Nunc 24-place multiwell plates and cultured for 11 days to form myotubes. The myotubes were preincubated for 2 h in Krebs–Henseleit buffer (pH 7.4) containing 0.1 % BSA, 10 mM Hepes and 2 mM sodium pyruvate (KHH buffer). The myotubes were then incubated in KHH buffer (0.4 ml) containing 11 mM glucose in the absence or presence of ENL (0–100 μM) for 4 h. ENL was dissolved in absolute ethanol. The final ethanol concentration in experimental media was 0.1 % (v/v). Glucose concentrations in KHH buffer were determined with a glucose assay kit. The amounts of glucose uptake were calculated from the differences in glucose concentrations between before and after incubation.
Western blotting analyses
Western blotting analyses for phosphorylation of 5′-adenosine monophosphate-activated protein kinase (AMPK) was performed as described previously (Minakawa et al. 2011; Son et al. 2015). Briefly, L6 myoblasts (5 × 105 cells/dish) were cultured in Nunc 60 mm dishes and grown for 11 days to form myotubes. The myotubes were incubated 2 h in KHH buffer. They were then incubated in KHH buffer containing 11 mM glucose in the absence or presence of ENL for 30, 60, 120 and 240 min. The cell lysates were subjected to Western blotting analyses for AMPK and phospho-AMPK.
From L6 myotubes differentiated in 60 mm dishes and treated with ENL, plasma membrane fractions were obtained by the methods described previously (Nishiumi and Ashida 2007) with slight modifications. Then, Western blotting analyses for GLUT4 and Na+K+-ATPase were conducted as described previously (Minakawa et al. 2011).
Transfection of HaloTag®-glut4 expression vector (pFN21A-rat glut4) into L6 myoblasts and immunocytochemistry
Effect of ENL on GLUT4 translocation to plasma membrane was also examined by the visualizing method according to the procedure previously described (Minakawa et al. 2012). To transfect the expression vector (HaloTag®-glut4 vector), L6 myoblasts (5 × 104 cells/well) were cultured in an 8-well chamber slide (Nunc) for immunocytochemistry. To support cell attachment and growth, the 8-well chamber slide was coated with collagen (Nitta Gelatin, Osaka, Japan). After 24 h, at an approximately 60 % confluency, they were transfected with the expression vector or control vector using FuGENE 6 (Roche Diagnostics, IN, USA), according to the manufacturer’s instruction. The amount of transfected DNA was 0.2 μg/well. Cells were used for immunocytochemistry 36 h after transfection, using appropriate antibodies and Axiovert 200 M microscope (Carl Zeiss, Oberkochen, Germany), as already mentioned (Minakawa et al. 2012).
Animal experiments and diets
Animal experiments were conducted in accordance with guidelines established by the Animal Care and Use Committee of Tokyo University of Agriculture and Technology and were approved by this committee (ethics approval number: 22–17). Male db/db and its misty (m/m) normal mice (5 weeks of age) were purchased from Charles River Japan (Kanagawa, Japan). Animals were individually housed in stainless-steel cages with wire bottoms in an air-conditioned room with a temperature of 22 ± 2 °C, a relative humidity of 60 ± 5 %, and an 8:00–20:00 light cycle. All mice were maintained on a stock CE-2 pellet diet (CLEA Japan, Tokyo), for 3 days and thereafter on a basal 20 % casein diet (20C) for 4 days. The composition of the 20C diet (AIN-93G formula) was described previously (Kawano et al. 2009). After preliminary feeding of CE-2 and 20C for 1 week, mice were deprived of their diet at 9:00 but allowed free access to water until blood collection from tail vein 3 h later. After determination of blood glucose concentrations with a commercial kit as described previously (Minakawa et al. 2011), db/db mice were divided into three groups of similar fasting blood glucose (FBG) levels and body weights (0 week). Diabetic mice of each of the three groups were given either the 20C diet as the diabetic control (CNT) group, 20C diet supplemented with 0.001 % ENL (ENL-L) or 20C diet supplemented with 0.01 % ENL (ENL-H) for 6 weeks. Likewise, misty mice were given the 20C diet as the normal (NOR) group for 6 weeks. Water and each diet were given ad libitum except for the experiments to determine FBG levels, which were conducted every week 3 h after fasting. At the end of 6th week of feeding, blood for final FBG determination was collected from tail vein. Thereafter whole blood was collected from all mice by cardiac puncture under Somnopentyl anesthesia and serum samples were prepared. Serum glucose, insulin, TG and TBARS levels were determined with commercial kits. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from glucose and insulin concentrations; HOMA-IR = fasting glucose level (mg/dl) × fasting insulin level (ng/ml)/405 (Lee et al. 2010).
Intraperitoneal glucose tolerance test
Intraperitoneal glucose tolerance test (IPGTT) was performed as described previously (Kawano et al. 2009) after the determination of FBG levels at the 5th week of feeding. Two (CNT and ENL-H) groups of db/db mice were deprived of their diets at 20:00 but allowed free access to water. After fasted for 15 h, ENL was orally given to mice of ENL-H group at a dose of 1 mg/ml of 0.1 % carboxymethyl cellulose sodium salt (CMC) solution/100 g body weight. Likewise, 0.1 % CMC solution alone (1 ml/100 g body weight) was orally given to mice of CNT group as a vehicle. Blood was collected from the tail vein of db/db mice 2 h later (0 min). Immediately after blood collection, diabetic mice received an intraperitoneal injection of glucose (0.2 g/ml/100 g body weight). Blood samples were successively collected at appropriate time intervals (30, 60, 90, 120 and 180 min), and blood glucose levels were determined as previously described (Kawano et al. 2009).
Statistical analyses
Data are expressed as means ± standard errors of means (SEM). Multigroup comparisons were conducted by one-way analysis of variance followed by Tukey–Kramer multiple comparisons test and differences between two group means were compared by Student’s t test. Values of p < 0.05 were considered statistically significant.
Results
Effect of ENL on glucose uptake in cultured L6 myotubes
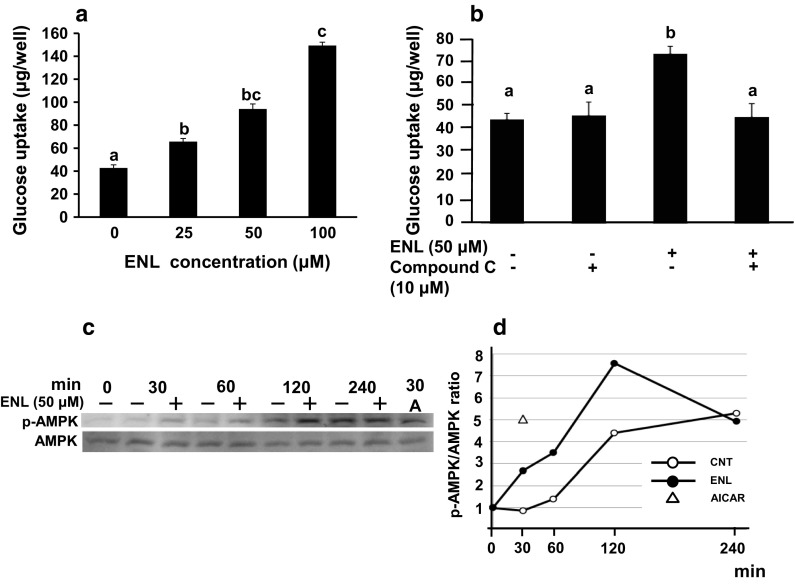
As shown in Fig. 1a, ENL dose-dependently and significantly increased glucose uptake in L6 myotubes at concentrations up to 100 μM in the absence of insulin. Compound C, an AMPK inhibitor (Zhou et al. 2001), significantly canceled the ENL-induced promotion of glucose uptake at a concentration of 10 μM (Son et al. 2013), where compound C alone exerted no influence on glucose uptake (Fig. 1b). This result strongly suggested an involvement of AMPK in ENL-stimulated glucose uptake.
Fig. 1.
Effect of ENL on glucose uptake and AMPK phosphorylation in cultured L6 myotubes. a Glucose uptake for 4 h was measured in 11-day-old L6 myotubes without or with ENL (0, 25, 50, 100 µM). b Effect of 10 µM compound C, an AMPK inhibitor, on ENL-induced increase in glucose uptake. Each value represents the mean ± SEM for six wells. Values not sharing a common letter are significantly different at p < 0.05 by Tukey–Kramer multiple comparisons test. c Western blotting analyses for time-dependent AMPK phosphorylation. Western blotting was conducted with anti-phospho-AMPK and anti-AMPK antibodies. d p-AMPK/AMPK ratio was calculated from c. A AICAR, AMPK 5′-adenosine monophosphate-activated protein kinase, CNT control (no ENL treatment), ENL enterolactone
Effect of ENL on phosphorylation of AMPK in cultured L6 myotubes
L6 myotubes were treated with 50 μM of ENL for 30, 60, 120 and 240 min. The cells were also treated with AICAR (1 mM), a well-known activator of AMPK, for 30 min as a positive control agent (Fig. 1c). ENL stimulated the phosphorylation of AMPK, that is, ENL raised the ratio of phosphorylated AMPK to AMPK, p-AMPK/AMPK, from 30 min after treatment, which peaked after 120 min (Fig. 1d). These results suggested promotion by ENL of GLUT4 translocation to plasma membrane of L6 myocytes.
Effect of ENL on translocation of GLUT4 to plasma membrane of cultured L6 myocytes
To explore regulatory mechanisms for promotion of glucose uptake by ENL, we determined the translocation of GLUT4, known as a main glucose transporter in the skeletal muscle system, by Western blotting. Na+K+-ATPase was employed as a plasma membrane marker (Fig. 2a). ENL treatment of L6 myotubes for 30 min at 50 μM stimulated the translocation of GLUT4 to plasma membrane of L6 myotubes (Fig. 2a, upper electrophoretic profile). The ratio of GLUT4 to Na+K+-ATPase (GLUT4/Na+K+-ATPase) significantly increased in ENL-treated cells (Fig. 2a, lower graph). Furthermore, the effect of ENL on GLUT4 translocation was visually confirmed by immunocytochemistry. We employed L6 myoblasts to transfect the expression vector (HaloTag®-glut4 vector), because transfection efficiency into L6 myotubes was very low (Minakawa et al. 2012). Caveolin-3 is involved in spatial and temporal regulation of GLUT4 translocation to plasma membrane and hence glucose uptake in skeletal muscle cells (Fecchi et al. 2006). Figure 2b (top, red) shows cellular localization of Halo-GLUT4 protein [GLUT4–ENL(−) and GLUT4–ENL(+)], Fig. 2b (center, green) shows cellular localization of caveolin-3, and Fig. 2b (bottom, yellowish) shows their merging. In the absence of ENL, Halo-GLUT4 protein and caveolin-3 were expressed in whole area and partially in the cell surface regions in L6 myoblast transfected with HaloTag®-glut4 vector as shown in Fig. 2b [left, ENL(−)]. ENL treatment at 50 μM for 30 min strengthened co-localization of GLUT4 protein and caveolin-3 in the plasma membrane compartment as shown by yellowish color in Fig. 2b [right, ENL(+)].
Fig. 2.
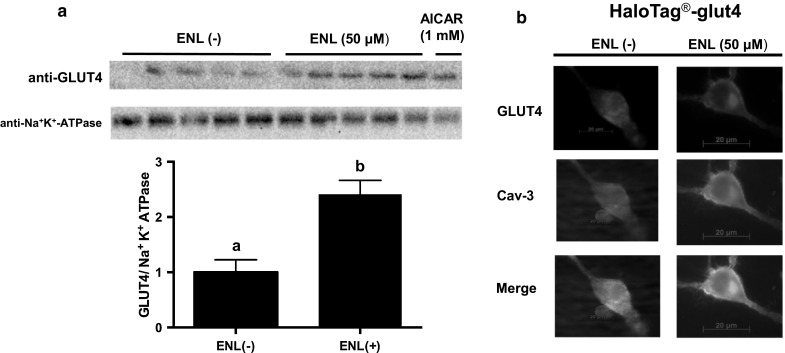
Effect of ENL on GLUT4 translocation to plasma membrane in cultured L6 myocytes. a Western blotting analyses of GLUT4 and Na+K+-ATPase (upper) and the ratio of GLUT4 to Na+K+-ATPase in L6 myotubes (lower). Each value represents the mean ± SEM for five samples. Values not sharing a common letter are significantly different at p < 0.05 by Student’s t test. b GLUT4 translocation to plasma membrane in cultured L6 myoblasts transfected with HaloTag®-glut4 vector. Immunocytochemistry was processed using anti-HaloTag and anti-caveolin-3 antibodies. Cav-3 caveolin-3, ENL enterolactone, GLUT4 glucose transporter 4
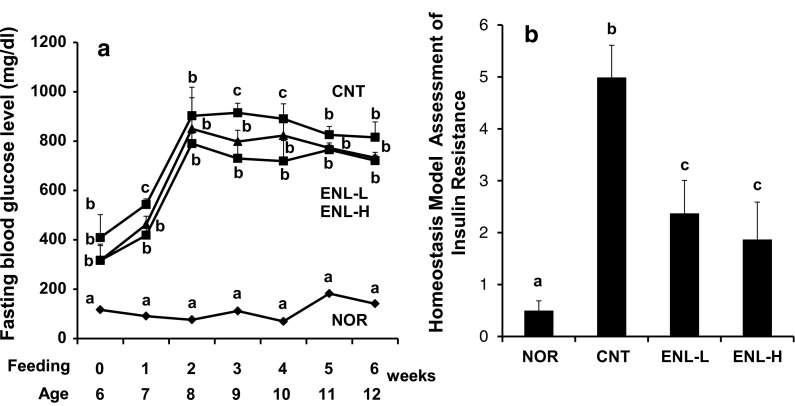
Effect of ENL on fasting blood glucose levels, insulin resistance, glucose intolerance and serum lipid levels in db/db mice
Based on these in vitro findings, we successively studied in vivo effect of ENL on glucose metabolism employing severe type 2 diabetic model db/db mice. Food intake and body weight gain for 6 weeks are shown in Table 1. Both of them were significantly higher in diabetic control mice than in normal mice (NOR vs. CNT). However, food intake and body weight gain were almost the same among three diabetic mice groups (CNT, ENL-L and ENL-H). The latter findings indicate that the action of ENL on glucose and lipid metabolism in mice described below is considered to be not due to reduced food consumption but due to biological effects of ENL. As shown in Fig. 3a, the FBG levels of diabetic control mice (CNT) increased up to the 2nd week of feeding and thereafter maintained the high FBG levels, while those of normal mice (NOR) were almost constant throughout the feeding period. Administration of ENL to db/db mice significantly suppressed at 1st, 3rd and 4th weeks after feeding and tended to suppress the FBG levels at 2nd, 5th and 6th weeks after feeding (CNT vs. ENL-L, CNT vs. ENL-H).
Table 1.
Effect of ENL on food intake and body weight gain in normal m/m and diabetic db/db mice
| Measurement | NOR m/m |
CNT db/db |
ENL-L db/db |
ENL-H db/db |
|---|---|---|---|---|
| Food intake (g/mouse/6 weeks) | 148.8 ± 1.5a | 291.3 ± 1.7b | 290.3 ± 2.3b | 287.7 ± 1.8b |
| Body weight gain (g/mouse/6 weeks) | 4.9 ± 0.2a | 7.2 ± 0.5b | 9.0 ± 0.6b | 8.3 ± 0.5b |
Each value represents the mean ± SEM for six mice. Values not sharing a common letter are significantly different at p < 0.05 by Tukey–Kramer multiple comparisons test
NOR normal m/m mice group, CNT control diabetic db/db mice group, ENL-L enterolactone-low dose (0.001 % in diet) group, ENL-H enterolactone-high dose (0.01 % in diet) group, ENL enterolactone
Fig. 3.
Effect of ENL on FBG levels (a) and HOMA-IR (b) in Type 2 diabetic model db/db mice. a Fasting blood glucose level. During 6 weeks of ENL feeding, fasting blood glucose levels were measured once a week, after mice were fasted for 3 h before blood collection. b HOMA-IR calculated from concentrations of serum glucose and insulin after 6 weeks of feeding. Each value represents the mean ± SEM for six mice. Values not sharing a common letter are significantly different at p < 0.05 by Tukey–Kramer multiple comparisons test. NOR normal m/m mice group, CNT control diabetic db/db mice group, ENL-L enterolactone-low dose (0.001 % in diet) group, ENL-H enterolactone-high dose (0.01 % in diet) group, ENL enterolactone, FBG fasting blood glucose, HOMA-IR homeostasis model assessment of insulin resistance
As depicted in Fig. 3b, HOMA-IR, an index of insulin resistance, was strikingly increased in diabetic mice as compared to that of normal mice (CNT vs. NOR). This rise in HOMA-IR was significantly suppressed by ENL administration (CNT vs. ENL-L, CNT vs. ENL-H).
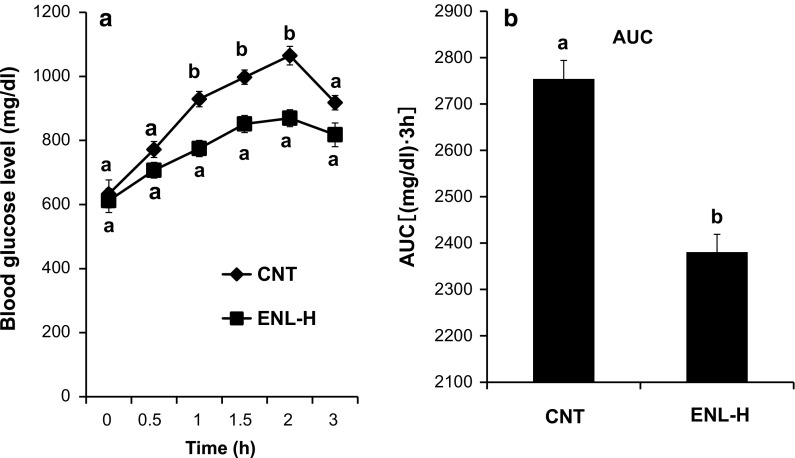
Figure 4 shows the results of IPGTT using mice of CNT and ENL-H groups. The blood glucose levels in diabetic control mice approximately linearly increased up to 120 min after intraperitoneal glucose administration, while ENL treatment significantly suppressed this rise 60, 90 and 120 min after glucose administration (Fig. 4a). The area under the curve (AUC) was also significantly lower in the ENL-treated group than in the vehicle-treated CNT group (Fig. 4b).
Fig. 4.
Effect of ENL on glucose intolerance (a) and AUC (b) in type 2 diabetic model db/db mice. a IPGTT was conducted using control and ENL-H groups after feeding for 5 weeks. b AUC was calculated from a. Each value represents the mean ± SEM for six mice. Values not sharing a common letter are significantly different at p < 0.05 by Student’s t test. AUC area under the curve, CNT control diabetic db/db mice group, ENL-H enterolactone-high dose (0.01 % in diet) group, ENL enterolactone, IPGTT intraperitoneal glucose tolerance test
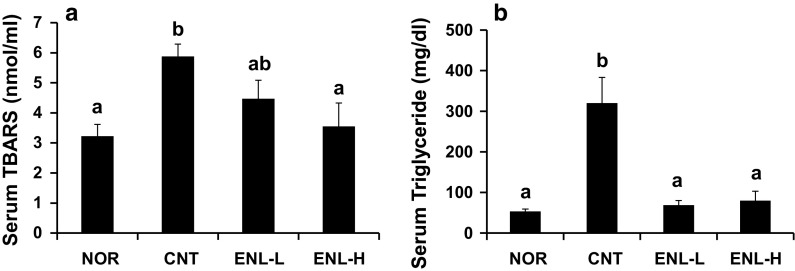
Serum TBARS and TG levels were significantly higher in mice of CNT group than in those of NOR group. These rises were suppressed dose-dependently (TBARS, Fig. 5a) or significantly (TG, Fig. 5b) by the treatment of ENL.
Fig. 5.
Effect of ENL on serum TBARS (a) and TG (b) levels in type 2 diabetic model db/db mice. Each value represents the mean ± SEM for six mice. Values not sharing a common letter are significantly different at p < 0.05 by Tukey–Kramer multiple comparisons test. TBARS thiobarbituric acid-reactive substances, TG triglyceride. As to NOR, CNT, ENL-L and ENL-H, see abbreviations of Fig. 3
Discussion
The skeletal muscle cells are one of the main sites of glucose uptake through GLUT4 in response to insulin. In muscle cells, AMPK is known as another GLUT4 translocation promoter. Natural compounds that activate AMPK have a possibility to overcome insulin resistance in the diabetic state (Yagasaki 2014). Indeed, using cultured L6 myocytes, we have found food components and natural resources that activate AMPK, promote GLUT4 translocation to plasma membrane of myocytes and possess antidiabetic effects in T2D model animals; such as food components including aspalathin (Kawano et al. 2009; Son et al. 2013), resveratrol (Minakawa et al. 2011), piceatannol (Minakawa et al. 2012), genistein (Ha et al. 2012), daidzein (Cheong et al. 2014a), equol (Cheong et al. 2014b), nepodin (Ha et al. 2014), gingerol (Son et al. 2015) and green rooibos extract (Kamakura et al. 2015). Recently, urinary concentrations of lignan metabolites, especially ENL, are reported to be significantly associated with a lower risk of T2D in humans (Sun et al. 2014). In the present study, we studied the effect of ENL on glucose metabolism and its modes of action in cultured L6 myocytes and T2D model db/db mice.
In L6 myotubes, ENL dose-dependently and significantly promoted glucose uptake. This promotion by ENL was entirely eliminated by co-existing of compound C, an inhibitor of AMPK. This strongly suggested an involvement of AMPK in the promotive effect of ENL on glucose uptake. As was expected, AMPK was in fact activated (=phosphorylated) by ENL treatment. Activation of AMPK has emerged as the critical event that upregulates GLUT4 translocation to the plasma membrane independently of insulin (Krook et al. 2004). Thus, we tried to confirm whether or not ENL would promote GLUT4 translocation to plasma membrane of L6 myocytes by two procedures, Western blotting and immunocytochemistry. In L6 myotubes, ENL proved to promote GLUT4 translocation to plasma membrane by Western blotting. Furthermore, ENL treatment strengthened co-localization of GLUT4 protein and caveolin-3 in the plasma membrane region of L6 myoblasts transfected with HaloTag®-glut4 vector. These results visually verified that ENL did promote GLUT4 translocation to plasma membrane in L6 myoblasts, as previously demonstrated for the first time in piceatannol-treated L6 myoblasts as well as L6 myotubes (Minakawa et al. 2012). Recently, resveratrol has been shown to directly inhibit cAMP-dependent phosphodiesterases (PDEs), leading to elevated cellular cAMP levels and resveratrol increases AMPK phosphorylation via involvement of Ca2+ through phospholipase C and the ryanodine receptor Ca2+-release channel (Park et al. 2012). Hence, it seems worthy from the aspect of direct target identification to know if ENL could inhibit PDEs. In severe T2D model db/db mice, ENL suppressed hyperglycemia and improved the impaired glucose tolerance. ENL also suppressed the increases in serum TBARS and TG concentrations. Thus, ENL proved to ameliorate abnormal lipid metabolism as well as glucose metabolism in vivo.
In the present study, we could find ENL in the assay system in vitro as a novel antidiabetic molecule in cultured L6 myocytes and demonstrate its antidiabetic effect in vivo employing T2D model db/db mice. The most interesting and characteristic point in the present study is that effective dose (0.01 % in diet) of ENL in vivo is very low as previously reported in the antidiabetic effect of equol which significantly reduced hyperglycemia at a dose of 0.05 % in diet (Cheong et al. 2014b). Equol has been known as a soybean isoflavone metabolite formed by intestinal bacteria from daidzin and daidzein like ENL from lignans (Rowland et al. 2000). After ingestion, daidzein is converted to equol by the gut microflora. Thus, ENL and equol are intracorporeally formed after ingestion of lignans and isoflavones, respectively. These phenomena suggest that ENL and equol are main chemical entities showing antidiabetic actions and explain, at least partly, the reason for very low effective doses in their antidiabetic actions in vivo. Lignans are contained in many kinds of ordinary foods such as strawberry (Giampieri et al. 2012, 2014) and cereals (Bach Knudsen et al. 2003) and are suggested to possess various health beneficial effects including antidiabetic actions. Thus, studies on further detailed mechanisms for antidiabetic and other actions are required.
Acknowledgments
This work was supported in part by the Regional Innovation Strategy Support Program, MEXT, Japan, in part by the Japan Society for the Promotion of Science, Japan, and in part by the Tojuro Iijima Foundation for Food Science and Technology, Japan.
Abbreviations
- AMPK
5′-Adenosine monophosphate-activated protein kinase
- ENL
Enterolactone
- FBG
Fasting blood glucose
- GLUT4
Glucose transporter 4
- HOMA-IR
Homeostasis model assessment of insulin resistance
- IPGTT
Intraperitoneal glucose tolerance test
- KHH buffer
Krebs–Henseleit–Hepes buffer
- TBARS
Thiobarbituric acid-reactive substances
- TG
Triglyceride
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
References
- Bach Knudsen KE, Serena A, Kjaer AK, Tetens I, Heinonen SM, Nurmi T, Adlercreutz H. Rye bread in the diet of pigs enhances the formation of enterolactone and increases its levels in plasma, urine and feces. J Nutr. 2003;133:1368–1375. doi: 10.1093/jn/133.5.1368. [DOI] [PubMed] [Google Scholar]
- Cheong SH, Furuhashi K, Ito K, Nagaoka M, Yonezawa T, Miura Y, Yagasaki K. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. J Nutr Biochem. 2014;25:136–143. doi: 10.1016/j.jnutbio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Cheong SH, Furuhashi K, Ito K, Nagaoka M, Yonezawa T, Miura Y, Yagasaki K. Antihyperglycemic effect of equol, a daidzein derivative, in cultured L6 myocytes and ob/ob mice. Mol Nutr Food Res. 2014;58:267–277. doi: 10.1002/mnfr.201300272. [DOI] [PubMed] [Google Scholar]
- Fecchi K, Volonte D, Hezel MP, Schmeck K, Galbiati F. Spatial and temporal regulation of GLUT4 translocation by flotillin-1 and caveolin-3 in skeletal muscle cells. FASEB J. 2006;20:705–707. doi: 10.1096/fj.05-4661fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampieri F, Tulipani S, Alvarez-Suarez JM, Quiles JL, Mezzetti B, Battino M. The strawberry: composition, nutritional quality, and impact on human health. Nutrition. 2012;28:9–19. doi: 10.1016/j.nut.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Giampieri F, Alvarez-Suarez JM, Battino M. Strawberry and human health: effects beyond antioxidant activity. J Agric Food Chem. 2014;62:3867–3876. doi: 10.1021/jf405455n. [DOI] [PubMed] [Google Scholar]
- Ha BG, Nagaoka M, Yonezawa T, Tanabe R, Woo JT, Kato H, Chung UI, Yagasaki K. Regulatory mechanism for the stimulatory action of genistein on glucose uptake in vitro and in vivo. J Nutr Biochem. 2012;23:501–509. doi: 10.1016/j.jnutbio.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Ha BG, Yonezawa T, Son MJ, Woo JT, Ohba S, Chung UI, Yagasaki K. Antidiabetic effect of nepodin, a component of Rumex roots, and its modes of action in vitro and in vivo. BioFactors. 2014;40:436–447. doi: 10.1002/biof.1165. [DOI] [PubMed] [Google Scholar]
- Kamakura R, Son MJ, de Beer D, Joubert E, Miura Y, Yagasaki K. Antidiabetic effect of green rooibos (Aspalathus linearis) extract in cultured cells and type 2 diabetic model KK-Ay mice. Cytotechnology. 2015;67:699–710. doi: 10.1007/s10616-014-9816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano A, Nakamura H, Hata S, Minakawa M, Miura Y, Yagasaki K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine. 2009;16:437–443. doi: 10.1016/j.phymed.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Krook A, Wallberg-Henriksson H, Zierath JR. Sending the signal: molecular mechanisms regulating glucose uptake. Med Sci Sports Exerc. 2004;36:1212–1217. doi: 10.1249/01.MSS.0000132387.25853.3B. [DOI] [PubMed] [Google Scholar]
- Lee YS, Cha BY, Saito K, Yamakawa H, Choi SS, Yamaguchi K, Yonezawa T, Teruya T, Nagai K, Woo JT. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem Pharmacol. 2010;79:1674–1683. doi: 10.1016/j.bcp.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Mazur WM, Uehara M, Wähälä K, Adlercreutz H. Phyto-oestrogen content of berries, and plasma concentrations and urinary excretion of enterolactone after a single strawberry-meal in human subjects. Br J Nutr. 2000;83:381–387. [PubMed] [Google Scholar]
- Minakawa M, Kawano A, Miura Y, Yagasaki K. Hypoglycemic effect of resveratrol in type 2 diabetic model db/db mice and its actions in cultured L6 myotubes and RIN-5F pancreatic β-cells. J Clin Biochem Nutr. 2011;48:237–244. doi: 10.3164/jcbn.10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakawa M, Miura Y, Yagasaki K. Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem Biophys Res Commun. 2012;422:469–475. doi: 10.1016/j.bbrc.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Miura D, Saarinen NM, Miura Y, Santti R, Yagasaki K. Hydroxymatairesinol and its mammalian metabolite enterolactone reduce the growth and metastasis of subcutaneous AH109A hepatomas in rats. Nutr Cancer. 2007;58:49–59. doi: 10.1080/01635580701308133. [DOI] [PubMed] [Google Scholar]
- Nishiumi S, Ashida H. Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci Biotechnol Biochem. 2007;71:2343–2346. doi: 10.1271/bbb.70342. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- Saarinen NM, Wärri A, Airio M, Smeds A, Mäkelä S. Role of dietary lignans in the reduction of breast cancer risk. Mol Nutr Food Res. 2007;51:857–866. doi: 10.1002/mnfr.200600240. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Lawson AM, Borriello SP, Harkness R, Gordon H, Morgan DM, Kirk DN, Adlercreatz H, Anderson LC, Axelson M. Lignan formation in man–microbial involvement and possible roles in relation to cancer. Lancet. 1981;2:4–7. doi: 10.1016/S0140-6736(81)90250-6. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Brown NM, Zimmer-Nechemias L, Wolfe B, Jha P, Heubi JE. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014;5:491–501. doi: 10.1039/c3fo60402k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son MJ, Minakawa M, Miura Y, Yagasaki K. Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice. Eur J Nutr. 2013;52:1607–1619. doi: 10.1007/s00394-012-0466-6. [DOI] [PubMed] [Google Scholar]
- Son MJ, Miura Y, Yagasaki K. Mechanisms for antidiabetic effect of gingerol in cultured cells and obese diabetic model mice. Cytotechnology. 2015;67:641–652. doi: 10.1007/s10616-014-9730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Wedick NM, Pan A, Townsend MK, Cassidy A, Franke AA, Rimm EB, Hu FB, van Dam RM. Gut microbiota metabolites of dietary lignans and risk of type 2 diabetes: a prospective investigation in two cohorts of U.S. women. Diabetes Care. 2014;37:1287–1295. doi: 10.2337/dc13-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanharanta M, Voutilainen S, Lakka TA, van der Lee M, Adlercreutz H, Salonen JT. Risk of acute coronary events according to serum concentrations of enterolactone: a prospective population-based case-control study. Lancet. 1999;354:2112–2215. doi: 10.1016/S0140-6736(99)05031-X. [DOI] [PubMed] [Google Scholar]
- Yagasaki K. Anti-diabetic phytochemicals that promote GLUT4 translocation via AMPK signaling in muscle cells. Nutr Aging. 2014;2:35–44. [Google Scholar]
- Yagasaki K, Morisaki N, Kitahara Y, Miura A, Funabiki R. Involvement of protein kinase C activation in l-leucine-induced stimulation of protein synthesis in L6 myotubes. Cytotechnology. 2003;43:97–103. doi: 10.1023/B:CYTO.0000039898.44839.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]