Abstract
With the aim to utilize human mesenchymal stem cells (hMSCs) grown in large scale for regenerative medicine, effects of agitation rate on aggregation during beads-to-beads subcultivation of microcarrier culture of hMSCs were studied. hMSCs could attach and grew on surface-type microcarriers of Cytodex 1, whereas almost no cell elongation and growth were observed on porous type microcarriers of Cytopores. The percentages of aggregated Cytodex 1 microcarriers at an agitation rate of 60 and 90 rpm were lower than that at 30 rpm, which was the lowest agitation rate necessary for the suspension of Cytodex 1 microcarriers, and the cells grew fastest at 60 rpm. hMSC could be subcultivated on Cytodex 1 by the beads-to-beads method at both 30 and 60 rpm without trypsinization. However, agitation at 60 rpm resulted in a markedly lower percentage of aggregated microcarriers not only before but also after subcultivation. The percentages of CD90- and CD166-positive cells among cells grown on Cytodex 1 at 60 rpm (91.5 and 87.6 %) were comparable to those of cells grown in the pre-culture on dishes. In conclusion, hMSCs could be subcultivated on Cytodex 1 by beads-to-beads method maintaining the expressions of the cell surface antigens CD90 and CD166, while adjusting agitation rate could decrease the microcarrier aggregation.
Keywords: Mesenchymal stem cells, Microcarrier, Agitation, Beads-to-beads method
Introduction
Human mesenchymal stem cells (hMSCs) are an attractive candidate for cell-based therapies of disorders such as cartilage impairments (Sato et al. 2013), graft-versus-host disease (GVHD) (Yamahara et al. 2014), and brain infarct (Chua et al. 2010), because they can be harvested by a minimally invasive procedure. These therapies with an allograft system might require a large number of hMSCs in a single lot, and thus large-scale growth cultivation. For such cultivation of adhesion-dependent mammalian cells, microcarriers are used in bioreactors to provide a large surface area per unit volume of bioreactors (Takagi et al. 1994; Nienow 2006). However, there has been no report about the cultivation of hMSCs on microcarriers, other than those on the cultivation of nonhuman MSCs on microcarriers (Malda et al. 2003; Frauenschuh et al. 2007; Schop et al. 2008).
There are two types (surface and porous types) of microcarrier. Because not only the outer surface but also the inner surface of porous-type microcarriers is available for cell adhesion, cells may be able to form three-dimensional tissue-like structure in porous-type microcarriers (Takagi et al. 1999) and cells attached onto the inner surface of porous-type microcarriers may little be affected by shear stress caused by agitation. However, cells can attach only onto the outer surface of surface-type microcarriers and there has been no report of studies comparing both types of microcarriers for the growth of MSCs.
Among several operational factors for cell cultivation, agitation rate is important in the culture of mammalian cells on microcarriers. Too low an agitation rate might cause microcarrier sedimentation (Takagi et al. 1994) and aggregation (Ferrari et al. 2012), which lead to depression of oxygen and nutrients inside the sediments and aggregates. On the other hand, a high shear rate due to a high agitation rate might inhibit cell adhesion to microcarriers and induce cell detachment from microcarriers (Borys and Papoutsakis 1992).
For the subcultivation of cells from a primary microcarrier culture to other cultures containing fresh microcarriers, which might be essential for the scale-up of microcarrier cultivation, the detachment of cells from the surface of microcarriers to the suspension is generally employed. However, the cell harvest yield from cells adhering to microcarriers is not necessarily high. Moreover, the treatment of cells adhering to microcarriers with trypsine might damage the cells. Thus, the subcultivation of cells from microcarriers to fresh microcarriers without trypsine was proposed, in which fresh microcarriers are added to a suspension of old microcarriers bearing cells, and cells on the old microcarriers automatically move onto the fresh microcarriers (Wang and Ouyang 1999; Frauenschuh et al. 2007). This method is called the beads-to-beads (B-to-B) method; the mechanism by which cells transfer from old microcarriers to fresh ones is not clear.
Therefore, the adhesion and growth of hMSCs on surface- and porous-type microcarriers were compared in this study. Then, the effects of agitation rate on microcarrier aggregation and cell growth during B-to-B subcultivation was investigated. Moreover, the expressions of MSC-specific surface antigens on cells grown on microcarriers were compared with those on cells grown on the dish surface.
Materials and methods
Isolation and cultivation of hMSCs
hMSCs were isolated from bone marrow aspirates obtained by routine iliac crest aspiration from a human donor (75-year-old male) as reported previously (Sato et al. 2013). The donor gave his informed consent in this study, which was approved by our institutional committee on human research, as required by the study protocol.
The isolated cells, whose population doubling level (PDL) was defined to be zero, were seeded at a concentration of 0.15 × 104 cells/cm2 on a dish (55 cm2; Corning, NY, USA) with Dulbecco’s modified Eagle’s medium–low glucose (DMEM-LG; Invitrogen, Carlsbad, CA, USA) supplemented with 10 % FCS (Invitrogen), 2500 U/l penicillin, and 2.5 mg/l streptomycin (Sigma-Aldrich, St. Louis, MO, USA). The cells were cultivated at 37 °C in a humidified atmosphere containing 5 % CO2. When the cell density reached near confluence, they were detached using trypsin–EDTA (Sigma) and inoculated onto dishes and microcarriers as described below.
Microcarrier cultivation
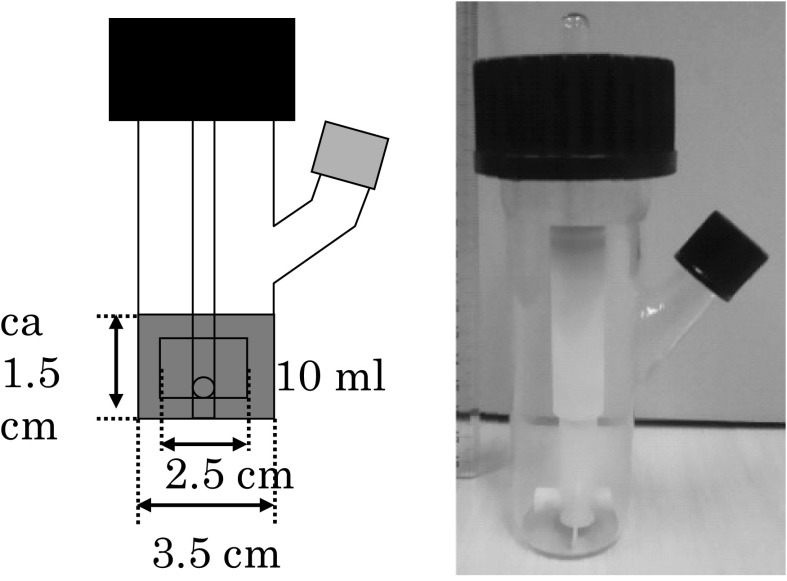
One kind of surface type microcarrier (Cytodex 1, DEAE-Sephadex, diameter; 131–220 μm, specific gravity; 1.03, charge density; 1.4–1.6 mequivalent/g) and two types of porous microcarriers (Cytopores 1 and 2, DEAE-cellulose, diameter; 200–280 μm, specific gravity; 1.03, charge density; 0.9–1.2, 1.65–1.95 mequivalent/g, mean diameter of pore; 30 μm) were purchased from GE Healthcare (Tokyo, Japan) and employed in this study. Three milliliters of the medium in a spinner bottle (Fig. 1, 100 ml, Shibata Hario Co., Japan) were inoculated with 1 × 106 cells that were harvested from the surface of culture dishes by trypsinization together with microcarrier beads (30 mg of Cytodex 1, 12 mg of Cytopores 1 and 2). The electric charge densities were 1.4–1.6 (Cytodex 1), 0.9–1.2 (Cytopore 1), and 1.65–1.95 meq/g (Cytopore 2). The spinner bottle used was tubular equipped with a two-blade turbine impeller. The diameters of the spinner bottle and impeller were 35 and 25 mm, respectively. The temperature of the culture was controlled at 37 °C in a water bath and the CO2 concentration in the gas phase was adjusted to 5 %. The culture was agitated intermittently at 30 rpm (10 s/15 min) for 2 h and then allowed to stand for 22 h. Then, the volume of the culture was made up to 10 ml and the medium was agitated continuously at 30 rpm. One-half of the culture medium was replaced with a fresh medium several times during the cultivation to avoid glucose depletion.
Fig. 1.
Schematic figure and photograph of a spinner bottle
In the subcultivation by the B-to-B method, excess culture was removed to retain 2.5 ml of a culture containing 3 g/l microcarriers (Cytodex 1) bearing cells, and a fresh medium and fresh microcarriers (Cytodex 1) were added to make a volume of 10 ml containing 3 g/l microcarriers (Cytodex 1). The culture was incubated in a water bath, and continuous agitation at 30 rpm was initiated immediately. All culture experiments were performed twice and the same trends were confirmed.
Cell concentration analysis
The cell concentrations in the dish and microcarrier cultures were determined by trypan-blue dye exclusion and nuclei staining (Sanford et al. 1950) methods, respectively.
Microscopy observation of microcarrier culture
Scanning electron microscopy (SEM) was performed for cells attached to carriers after fixation with glutaraldehyde (2 % in PBS). A sample of culture containing microcarriers was placed on a glass slide and observed under an inverted phase contrast microscope, and the percentage of aggregated microcarriers was determined using Eq. (1).
| 1 |
Flow cytometric analysis
Cells were harvested from dish and microcarrier cultures by trypsinization and stained with a mouse IgG anti-human CD90 (CBL415, Chemicon, Tokyo, Japan) and a fluorescein isothiocyanate (FITC)-conjugated anti-murine goat IgG (AP124F, Chemicon) antibody. Then, the cells were stained with phycoerythrin (PE)-conjugated anti-human CD166 murine antibody (A22361, Beckman Coulter, Tokyo, Japan) and analyzed using a flow cytometer (EPICS XL, Beckman Coulter) equipped with an argon laser (488 nm).
Results and discussion
Comparison between surface- and porous-type microcarriers
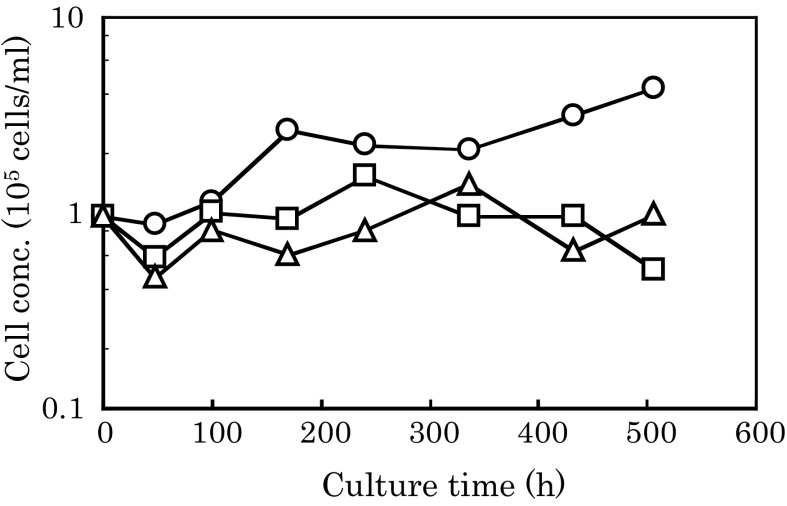
hMSCs were cultivated on several types of microcarriers (Cytodex 1 and Cytopores 1 and 2) (Fig. 2). The maximum cell concentrations with Cytopores 1 and 2 were 1.5 and 1.4 × 105 cells/ml, respectively, which were very close to the inoculum cell concentration (1 × 105 cells/ml). So, there might be no apparent cell growth with Cytopores 1 and 2. On the other hand, cells grew logarithmically and reached a concentration of 4.3 × 105 cells/ml on Cytodex 1 (Fig. 2). Almost no cell spreading and only globular cells were observed on Cytopores 1 and 2, whereas cells spread well on Cytodex 1 (Fig. 3).
Fig. 2.
Growth of hMSCs on several types of microcarrier. hMSCs were cultivated on the microcarriers Cytodex 1 (circles) and Cytopores 1 (triangles) and 2 (squares). The average of two parallel cultures is shown
Fig. 3.

Electron microscopy observations of hMSCs attached onto several types of microcarriers. Microcarriers Cytodex 1 (a) and Cytopores 1 (b), and 2 (c) bearing cells were observed under electron microscopy at the end of cultivation of hMSCs as shown in Fig. 2
The length of cells attached onto Cytodex 1 looked larger than the pore diameters of Cytopores 1 and 2 (Fig. 3). Thus, there might be no flat and wide space for the spread of hMSCs on not only the inner surfaces but also the outer surfaces of Cytopores 1 and 2. This may be the main reason for the absence of apparent growth on Cytopores 1 and 2. Larger pores in porous-type microcarriers may be necessary for the adhesion and growth of hMSCs. Cytodex 1 was employed in the subsequent experiments.
Effect of agitation rate on aggregation of microcarriers
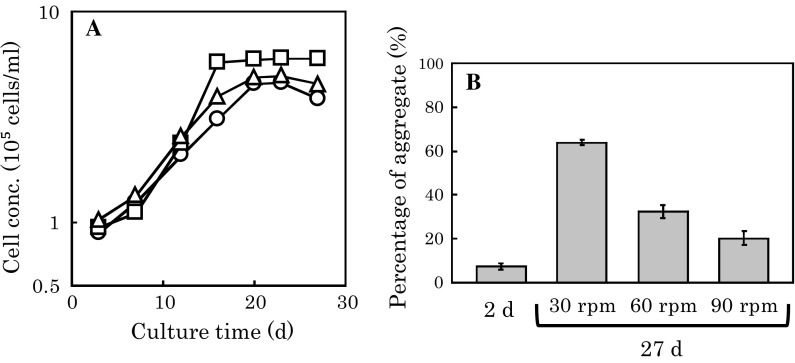
A suspension of 3 g/L microcarriers in the spinner bottle required an agitation rate of at least 30 rpm (data not shown). Two days after the inoculation of cells harvested from the dish surface by trypsinization to the microcarriers in the spinner bottles, the agitation rate was increased to 30, 60, and 90 rpm, and the stationary phase was confirmed on day 27. An agitation rate higher than 30 rpm resulted in a lower percentage of aggregated microcarriers on day 27 (Fig. 4b). However, the cell growth rate at 60 rpm was apparently higher than those at 30 and 90 rpm (Fig. 4a).
Fig. 4.
Effect of agitation rate on aggregation of microcarriers and growth of hMSCs on microcarriers. Two days after the inoculation of cells to microcarriers, the agitation rates were set to 30 (triangles), 60 (squares), and 90 (circles) rpm. Cell concentration (a) and the percentage of aggregated microcarriers (b) were determined. The average ±SD of triplicate cultures is shown
A higher agitation rate may tend to decrease the chance to form aggregates of microcarriers and disrupt aggregates. These may be the reasons for the lower percentage of aggregated microcarriers at an agitation rate higher than 30 rpm. On the other hand, a high agitation rate may lead to a high shear rate for the cell surface, which might inhibit cell growth. Thus, there might be an optimum agitation rate that can satisfy both a low aggregation rate and a high growth rate, which was found to be 60 rpm in this study.
Reynolds number (Re) and the shear stress of agitation were calculated as follows (Ferrari et al. 2012; Shiragami et al. 1993),
| 2 |
| 3 |
where N, di, d, ρ, and μ are the agitation rate (rpm), impeller diameter (25 × 10−3 m), microcarrier diameter (1 × 10−4 m), medium density (1 × 103 kg/m3), and medium viscosity (1 × 10−3 Pa s), respectively.
Using Eqs. (2) and (3), the Re values and shear stresses at 30, 60, and 90 rpm were calculated to be 310, 620, and 940, and 0.9, 1.8, and 2.7 Pa, respectively. Consequently, a Re of 620 and a shear stress of 1.8 Pa may be the candidate indices for the optimum agitation rate in the scale-up of cultivation using microcarriers.
Subcultivation by B-to-B method
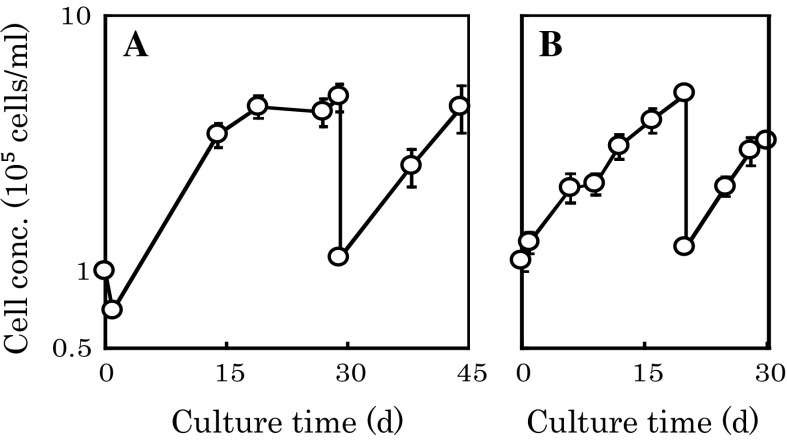
The subcultivation by the B-to-B method was performed at 30 rpm, as shown in Fig. 5a. After the stationary phase of the 1st step culture was confirmed on day 29, subcultivation by the B-to-B method (2nd step culture) was performed, and the log growth phase started immediately. The mean generation time from day 29 to day 44 was 183.3 h. The fold increases in cell concentration in 1st and 2nd step cultures were 4.82 (4.82/1.0) and 4.09 (4.91/1.20), respectively. However, the percentages of aggregated microcarriers reached 46.9 and 57.1 % at the end of the 1st (day 29) and 2nd cultures (day 44), respectively, whereas the percentage was only 9.5 % on day 2 (Table 1).
Fig. 5.
Effect of agitation rate on subcultivation of hMSCs on microcarriers. hMSCs were subcultivated by the B-to-B method at 30 (a) and 60 (b) rpm. The average of duplicate cultures is shown
Table 1.
Effect of agitation rate on percentage of aggregated microcarriers
| Agitation rate (rpm) | 30 | 60 | ||||
|---|---|---|---|---|---|---|
| Culture time (d) | 2 | 29 | 44 | 2 | 20 | 30 |
| Percentage of aggregated microcarriers (%) | 9.5 | 46.9 | 57.1 | 3.8 | 20.8 | 23.9 |
In order to study subcultivation by the B-to-B method at a higher agitation rate of 60 rpm, microcarriers were subcultivated by the B-to-B method at 60 rpm except for the initial 2 days, as shown in Fig. 5b. After the subcultivation on day 20, the log growth phase started immediately and the mean generation time from day 20 to day 30 was 172.6 h, which was slightly shorter than that at 30 rpm (183.3 h, Fig. 5a). The fold increases in cell concentration of 1st and 2nd step cultures were 4.95 (4.95/1.0) and 2.64 (3.25/1.23), respectively. The percentages of aggregated microcarriers were 20.8 and 23.9 % at the end of the 1st (20 days) and 2nd step cultures (30 days), respectively, which were less than one-half of those obtained during the culture at 30 rpm (46.9 and 57.1 %) (Table 1).
Surface antigen expression by cells grown on microcarriers
Cells grown on dishes (pre culture) and harvested by trypsinization were inoculated to both the main culture with microcarriers and that on dishes, respectively. Cells in the main culture on dishes grew faster (mean generation time = 125 h) than those in the main culture with microcarriers (mean generation time = 165 h) (data not shown). Therefore, the cells in the main culture on dishes were subcultivated onto dishes once again, in order to harvest and analyze cells from both the main cultures on dishes and that with microcarriers at the same time. The population doubling levels (PDLs) of these three types of cells, namely, cells harvested from the pre culture (dishes) and cells from the main cultures on dishes and microcarriers, were determined to be 4.0, 7.6, and 6.0, respectively (Table 2) and there was no marked difference between their “age”. The percentage of CD90-positive cells among cells grown on microcarriers (91.5 %) was apparently higher than those among pre-cultured cells (74.3 %) and cells subcultivated on dishes (58.1 %). The percentages of CD166-positive cells among these three types of cells were all approximately 90 %. The percentage of double-CD90- and CD166-positive cells among cells grown on microcarriers (85.5 %) was apparently higher than those among pre-cultured cells (67.4 %) and cells subcultivated on dishes (65.0 %).
Table 2.
Expressions of cell surface antigens on cells grown on microcarriers
| Percentage of antigen-positive cells (%) | ||||
|---|---|---|---|---|
| PDL | CD90 | CD166 | CD90 and CD166 | |
| Pre culture | ||||
| Dish | 4.0 | 74.3 | 92.5 | 67.4 |
| Main culture | ||||
| Dish | 7.6 | 58.1 | 96.6 | 65.0 |
| Microcarrier | 6.0 | 91.5 | 87.6 | 85.5 |
Cells grown on dishes (pre-culture) and harvested by trypsinization were inoculated to main cultures on microcarriers and dishes. These three types of cells harvested from the pre-culture (dishes) and main cultures on dishes and microcarriers were analyzed using flow cytometry
It is not necessarily the case that cells used in this study were homogeneous like usual primary cells. So, the quality of cells should be checked employing the markers of MSCs such as CD90 and CD166. Here, the percentages of CD90-positive cells, CD166-positive cells, and double-CD90- and CD166-positive cells among cells grown on microcarriers (91.5, 87.6, and 85.5 %) were all approximately 90 % and not significantly lower than those among pre-cultured cells (74.3, 92.5, and 67.4 %) and cells subcultivated on dishes (58.1, 96.6, and 65.0 %). Consequently, the purity of MSCs in cell population grown on microcarriers was not lower compared with that of cells grown on dishes.
Conclusions
Not the porous- (Cytopore) but the surface-type (Cytodex 1) microcarriers were appropriate for the adhesion and growth of bone-marrow-derived hMSCs. The percentage of aggregated microcarriers (Cytodex 1) could be decreased by increasing agitation rate. hMSCs could be subcultivated from microcarriers to microcarriers by the B-to-B method without trypsinization. The percentages of CD90- and CD166-positive cells among cells grown on microcarriers were both approximately 90 % and not significantly different from those among pre-cultured cells and cells subcultivated on dishes. Consequently, hMSCs might be grown on surface-type microcarriers (Cytodex 1) at large scale with the expression of surface antigens maintained and the percentage of aggregates decreased by adjusting agitation rate.
References
- Borys MC, Papoutsakis ET. Formation of bridges and large cellular clumps in CHO-cell microcarrier cultures: effects of agitation, dimethyl sulfoxide and calf serum. Cytotechnology. 1992;8:237–248. doi: 10.1007/BF02522041. [DOI] [PubMed] [Google Scholar]
- Chua S, Yuen CM, Leu S, Lin YC, Sun CK, Yip HK. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. Eur Heart J. 2010;31:80. doi: 10.1186/1479-5876-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C, Balandras F, Guedon E, Olmos E, Chevalot I. Limiting cell aggregation during mesenchymal stem cell expansion on microcarriers. Biotechnol Prog. 2012;28:780–787. doi: 10.1002/btpr.1527. [DOI] [PubMed] [Google Scholar]
- Frauenschuh S, Reichmann E, Ibold Y, Goetz PM, Sittinger M, Ringe J. A microcarrier-based cultivation system for expansion of primary mesenchymal stem cells. Biotechnol Prog. 2007;23:187–193. doi: 10.1021/bp060155w. [DOI] [PubMed] [Google Scholar]
- Malda J, Van Blitterswijk CA, Grojec M, Martens DE, Tramper J, Riesle J. Expansion of bovine chondrocytes on microcarriers enhances redifferentiation. Tissue Eng. 2003;9:939–948. doi: 10.1089/107632703322495583. [DOI] [PubMed] [Google Scholar]
- Nienow AW. Reactor engineering in large scale animal cell culture. Cytotechnology. 2006;50:9–33. doi: 10.1007/s10616-006-9005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford KK, Earle WR, Evans VJ, Waltz HK, Shannon JE. The measurement of proliferation in tissue cultures by enumeration of cell nuclei. Anat Rec. 1950;106:243. [PubMed] [Google Scholar]
- Sato Y, Wakitani S, Takagi M. Xeno-free and shrinkage-free preparation of scaffold-free cartilage-like disc-shaped cell sheet using human bone marrow mesenchymal stem cells. J Biosci Bioeng. 2013;116:734–739. doi: 10.1016/j.jbiosc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Schop D, Janssen FW, de Bruijn JD, Van Dijkhuizen-Radersma R. Expansion of mesenchymal stem cells using a microcarrier-based cultivation system: growth and metabolism. J Tissue Eng Regen Med. 2008;2:126–135. doi: 10.1002/term.73. [DOI] [PubMed] [Google Scholar]
- Shiragami N, Honda H, Unno H. Anchorage-dependent animal cell culture by using a porous microcarrier. Bioprocess Eng. 1993;8:295–299. doi: 10.1007/BF00369844. [DOI] [Google Scholar]
- Takagi M, Okumura H, Okada T, Kobayashi N, Kiyota T, Ueda K. An oxygen supply strategy for the large-scale production of tissue plasminogen activator by microcarrier cell culture. J Ferment Bioeng. 1994;77:301–306. doi: 10.1016/0922-338X(94)90238-0. [DOI] [Google Scholar]
- Takagi M, Sasaki T, Yoshida T. Spatial development of the cultivation of a bone marrow stromal cell line in porous carriers. Cytotechnology. 1999;31:225–231. doi: 10.1023/A:1008098313067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ouyang F. Bead-to-bead transfer of Vero cells in microcarrier culture. Cytotechnology. 1999;31:221–224. doi: 10.1023/A:1008079013375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahara K, Taguchi A, Soma T, Ogawa H, Ikeda T, Yoshimatsu J. Clinical application of human amnion-derived mesenchymal stem cells for the treatment of acute GVHD. Placenta. 2014;35:A6. doi: 10.1016/j.placenta.2014.08.022. [DOI] [Google Scholar]