Abstract
The objective of the present study was to investigate the relationship of IQ in children with maternal blood mercury concentration during late pregnancy. The present study is a component of the Mothers and Children's Environmental Health (MOCEH) study, a multi-center birth cohort project in Korea that began in 2006. The study cohort consisted of 553 children whose mothers underwent testing for blood mercury during late pregnancy. The children were given the Korean language version of the Wechsler Preschool and Primary Scale of Intelligence, revised edition (WPPSI-R) at 60 months of age. Multivariate linear regression analysis, with adjustment for covariates, was used to assess the relationship between verbal, performance, and total IQ in children and blood mercury concentration of mothers during late pregnancy. The results of multivariate linear regression analysis indicated that a doubling of blood mercury was associated with the decrease in verbal and total IQ by 2.482 (95% confidence interval [CI], 0.749–4.214) and 2.402 (95% CI, 0.526–4.279), respectively, after adjustment. This inverse association remained after further adjustment for blood lead concentration. Fish intake is an effect modifier of child IQ. In conclusion, high maternal blood mercury level is associated with low verbal IQ in children.
Keywords: Blood Mercury, Blood Lead, Fish, IQ, WPPSI-R
Graphical Abstract
INTRODUCTION
Mercury is a well-known toxic metal, and exposure can be from natural sources or from the products or waste of industrial activities in medicine, agriculture, and manufacturing (1). Workers may be exposed by inhalation of elemental or inorganic mercury. Exposure may also arise through dental amalgams, thermometers, batteries, pesticides, mines, incineration plants, and other sources. Organic mercury from the diet, especially seafood, is the major source of exposure in the general population (1). Methyl mercury is a potent neurotoxin and its toxicity to the human fetus became evident after the discovery of congenital Minamata disease, which developed in the fetuses of mothers exposed to methyl mercury from wastewater (2). Other research reported that mercury-contaminated bread exposed an Iraqi population to high doses of methyl mercury, and had neurotoxic effects on human fetuses (3). However, the effect of low-level mercury exposure from fish consumption on child development remains uncertain. Prenatal exposure to mercury can affect long-term child neurodevelopment (4,5), but the results of epidemiologic studies of such exposures have been inconsistent (6,7,8,9,10). The present study investigated the effect of low-level blood mercury concentration of mothers during late pregnancy on the IQ levels of their children.
MATERIALS AND METHODS
Subjects
The present study is a component of the Mothers and Children's Environmental Health (MOCEH) study, a multi-center prospective birth cohort project of 1,751 pregnant women that was conducted in Korea from May 2006 to December 2010 (11). The study cohort consisted of 558 children whose mothers underwent testing for blood mercury during late pregnancy. Five babies with body weight under 2 kg were excluded. All children were tested using the Korean version of the Wechsler Preschool and Primary Scale of Intelligence, revised edition (WPPSI-R), at 60 months of age.
Trained personnel used a detailed questionnaire to interview the enrolled mothers to obtain information about demographics, socioeconomic status, characteristics of residence, medical and reproductive history, exposure to occupational hazards, alcohol consumption, nutritional habits, and exposure to secondhand smoke in the home. Each questionnaire was completed the same day that the blood sample was collected.
Laboratory testing
Heparinized venous blood samples were obtained from each mother and child after an overnight fast, with special care taken to avoid contamination of skin and equipment. Whole blood samples were frozen and stored at −20°C prior to analysis. For analysis, samples were allowed to attain room temperature, and were thoroughly vortexed after thawing. Each 0.1 mL sample was diluted with 1.8 mL of matrix modifier reagent (containing Triton™ X-100 [Dow Chemical Company, Midland, MI, USA] and ammonium phosphate). Blood mercury concentrations were measured using the gold-amalgam collection method with a DMA-80 (Milestone, Bergamo, Italy) at the Neodin Medical Institute, a laboratory certified by the Korean Ministry of Health and Welfare. Commercial reference materials were used for internal quality assurance and control (Lyphochek® Whole Blood Metals Control; Bio-Rad, Hercules, CA, USA). The coefficients of variation for blood mercury were within 1.59%–4.86% of the 3 reference samples. The Neodin Medical Institute passed the German External Quality Assessment Scheme of Friedrich-Alexander University in the occupational and environmental medical ranges, the Quality Assurance Program operated by the Korean Occupational Safety and Health Agency, and is certified by the Korean Ministry of Employment and Labor as a designated laboratory for analysis of specific chemicals, including heavy metals and certain organic chemicals. The detection method had a limit of 0.07 µg/L mercury. Only one sample had a mercury level below this limit, and we considered the level in that sample to be the detection limit divided by 2.
Korean version of the WPPSI-R (K-WPPSI)
The K-WPPSI (12) is commonly used to measure intelligence in children aged 3 years to 7 years and 3 months. It provides 12 subtest scores and 3 composite scores that represents verbal, performance, and full scale IQ. The verbal subtests cover information, comprehension, arithmetic, vocabulary, similarities, and sentences; the performance subtests cover object assembly, geometric design, block design, mazes, picture completion, and animal pegs. The WPPSI scores are standardized for Korean children compared with western countries (12). Experienced personnel performed all assessments at the hospital. The standardized mean WPPSI-R score was 100 ± 15, a score of 89–80 was classified as low average, 79–70 as borderline, and 69 and below as indicating intellectual deficiency (13).
Statistical analyses
Student's t-test was used to identify significant differences in maternal mercury concentration and IQ in children according to categorical variables with 2 group. For categorical variables with 3 groups, 1-way analysis of variance was used. Maternal mercury concentrations were in right-skewed distribution, so we transformed maternal mercury concentrations into logarithm with base 2 to satisfy normal distribution. Log2-transformed mercury concentrations were determined to be in normal distribution by comparing a histogram of the maternal mercury data to a normal probability curve, and with a quantile-quantile plot (QQ plot) of the standardized data against the standard normal distribution. Performance, verbal, and total IQ were in normal distribution. Geometric means (GMs) and geometric standard deviations (GSDs) of maternal mercury concentrations were reported as results after performing the statistical testing on the log-transformed data.
Fish intake was classified into tertiles to show the relationship between fish intake and mercury concentrations. The cut-off value of the tertile in fish intake is in line with Canada's Food Guide recommending eating at least 2 servings (of 75 g each) of fish a week (14).
We used regression analyses to show the association of maternal mercury in late pregnancy with verbal, performance, and total IQ in 5 years old children after adjustment for sex and birth weight of the child, maternal age, maternal educational level, household income, and fish intake for 2 models. The model 1 was adjusted for covariates mentioned above, and model 2 was adjusted for confounding factors in model 1 and maternal blood lead in late pregnancy to show the association of maternal blood mercury with children's IQ, because lead may affect neurodevelopment in children. We assessed the fitness of regression model by the Durbin-Watson test, a test for autocorrelation in the residuals from a statistical regression analysis. A value ranged from 1.5 to 2.5 in the Durbin-Watson test statistic means the fitness of the regression model. Household income (USD/month) and maternal educational level (years) were categorized into < 2,000 and ≥ 2,000, and ≤ 12 years and > 12 years, respectively. Other confounding factors were adjusted as continuous variables in regression analyses.
We regressed verbal, performance, and total IQ against log2-transformed maternal blood mercury concentration (a continuous independent variable) to estimate adjusted mean differences in IQ associated with a doubling of blood mercury. We also performed multiple regression analysis (with adjustment for covariates) for blood mercury tertile and IQ to assess whether the association between blood mercury and IQ is observed in all concentration groups or relatively high mercury exposure group only within subjects. The tertiles of blood mercury concentrations in late pregnant women were transformed 2 dummy variables; dummy 1 has 1 for 2nd tertile, and 0 for 1st and 3rd tertile, and dummy 2 has 1 for 3rd tertile, and 0 for 1st and 2nd tertile.
We performed the regression analyses for the mercury and IQ by the tertile classification of fish intake because fish intake has benefit to neurodevelopment by supply of protein and polyunsaturated fatty acids. This regression analyses were done to show whether the association of maternal blood mercury with children's IQ is independent of fish intake.
Ethics statement
Prior to enrolment, all participating mothers provided written informed consent. The study protocol and consent form were approved by the Institutional Review Boards of Ewha Womans University (approval No. 12-07B-15), Dankook University Hospital (2011-09-0340), and Ulsan University Hospital (06-29).
RESULTS
Table 1 shows maternal blood mercury concentrations and verbal, performance, and total IQ of children by various categories. This univariate analysis indicates that higher maternal blood mercury concentration was significantly related to low paternal educational level and more fish intake. None of the variables were significantly related to performance IQ. However, female child, higher educational level of parents, higher household income, and low mercury tertile were associated with higher verbal IQ or total IQ.
Table 1. Maternal blood mercury concentrations and IQs in children by general characteristics.
| Variables | No. | Blood mercury, µg/L | Verbal IQ | Performance IQ | Total IQ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| GM ± GSD | P value | Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | |||
| Total | 553 | 3.14 ± 1.66 | - | 103.5 ± 14.3 | - | 102.0 ± 16.0 | - | 103.2 ± 15.0 | - | |
| Sex | Male | 294 | 3.14 ± 1.60 | 0.941 | 101.8 ± 13.9 | 0.004 | 101.0 ± 16.7 | 0.108 | 101.7 ± 15.3 | 0.014 |
| Female | 259 | 3.15 ± 1.72 | 105.3 ± 14.5 | 103.2 ± 15.3 | 104.9 ± 14.6 | |||||
| Educational level of father, yr | ≤ 12 | 128 | 3.41 ± 1.61 | 0.049 | 99.2 ± 13.1 | < 0.001 | 100.0 ± 15.5 | 0.105 | 99.5 ± 14.3 | 0.001 |
| > 12 | 393 | 3.08 ± 1.68 | 104.7 ± 14.3 | 102.7 ± 16.3 | 104.4 ± 15.1 | |||||
| Educational level of mother, yr | ≤ 12 | 132 | 3.21 ± 1.61 | 0.57 | 99.1 ± 13.2 | < 0.001 | 99.7 ± 16.7 | 0.058 | 99.2 ± 14.8 | 0.001 |
| > 12 | 404 | 3.12 ± 1.67 | 104.6 ± 14.2 | 102.8 ± 15.8 | 104.4 ± 14.8 | |||||
| Household income, USD/mon | < 2,000 | 139 | 3.11 ± 1.51 | 0.804 | 100.4 ± 13.4 | 0.006 | 101.6 ± 16.6 | 0.712 | 101.1 ± 15.0 | 0.059 |
| ≥ 2,000 | 395 | 3.15 ± 1.70 | 104.2 ± 14.2 | 102.2 ± 16.0 | 103.8 ± 14.9 | |||||
| Maternal age, yr | < 30 | 243 | 3.16 ± 1.56 | 0.919 | 102.7 ± 13.6 | 0.329 | 102.2 ± 15.7 | 0.614 | 103.0 ± 14.4 | 0.819 |
| ≥ 30 | 305 | 3.15 ± 1.74 | 103.9 ± 14.7 | 101.7 ± 16.4 | 103.3 ± 15.5 | |||||
| Birth weight, g | 1st tertile | 184 | 3.12 ± 1.80 | 0.783 | 102.4 ± 14.6 | 0.436 | 101.2 ± 16.2 | 0.401 | 102.1 ± 14.9 | 0.470 |
| 2nd tertile | 184 | 3.21 ± 1.61 | 104.3 ± 13.5 | 101.6 ± 15.7 | 103.5 ± 14.5 | |||||
| 3rd tertile | 185 | 3.11 ± 1.57 | 103.6 ± 14.7 | 103.3 ± 16.2 | 103.9 ± 15.6 | |||||
| Fish intake*, g | 1st tertile | 148 | 2.94 ± 1.64 | 0.045 | 103.4 ± 14.5 | 0.543 | 101.8 ± 14.5 | 0.932 | 103.0 ± 14.0 | 0.896 |
| 2nd tertile | 149 | 3.17 ± 1.59 | 102.0 ± 13.5 | 102.5 ± 18.4 | 102.6 ± 16.4 | |||||
| 3rd tertile | 149 | 3.41 ± 1.79 | 103.7 ± 14.5 | 102.3 ± 15.7 | 103.5 ± 15.2 | |||||
| Mercury†, µg/L | 1st tertile | 184 | 1.87 ± 1.47 | < 0.001 | 104.2 ± 15.3 | 0.027 | 102.0 ± 16.6 | 0.169 | 103.6 ± 16.2 | 0.024 |
| 2nd tertile | 184 | 3.18 ± 1.11 | 105.0 ± 14.8 | 103.6 ± 16.2 | 105.1 ± 15.1 | |||||
| 3rd tertile | 185 | 5.23 ± 1.33 | 101.2 ± 12.5 | 100.4 ± 15.2 | 100.9 ± 13.4 | |||||
GM = geometric mean, GSD = geometric standard deviation, SD = standard deviation.
*Fish intake: 1st tertile (≤ 20.5), 2nd tertile (20.5–75.0), 3rd tertile (> 75.0); †Blood mercury level: 1st tertile (≤ 2.622), 2nd tertile (2.622–3.829), 3rd tertile (> 3.829).
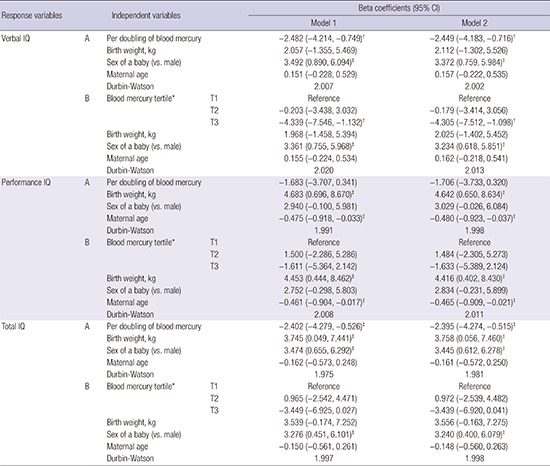
The results of multivariate linear regression analysis indicated that a doubling of blood mercury was associated with the decrease in verbal and total IQ by 2.482 (95% confidence interval [CI], 0.749–4.214) and 2.402 (95% CI, 0.526–4.279), respectively (Model 1, A). This inverse association remained after further adjustment for blood lead concentration (Table 2, Model 2). Lead is a potent toxin that alters neurodevelopment, but was not significantly associated with IQ (data not shown). Comparison of the highest and lowest tertiles also indicated the decrease in verbal IQ (4.339 and 4.305) in model without and with lead adjustment, respectively. However, prenatal blood mercury concentration was not associated with performance IQ. Female children had higher verbal and total IQ compared to male children. Maternal age was also inversely associated with performance IQ after adjustment for these covariates, whereas birth weight was also positively associated with performance and total IQ. These associations remained after further adjustment for blood lead concentration (Table 2, Model 2). Fish intake was not significantly associated with IQ (data not shown).
Table 2. Beta coefficients and 95% CI of child IQ in multiple linear regression analysis in children after adjustment of covariates (n = 445).
| Response variables | Independent variables | Beta coefficients (95% CI) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| Verbal IQ | A | Per doubling of blood mercury | −2.482(−4.214, −0.749)† | −2.449 (−4.183, −0.716)† | |
| Birth weight, kg | 2.057 (−1.355, 5.469) | 2.112 (−1.302, 5.526) | |||
| Sex of a baby (vs. male) | 3.492 (0.890, 6.094)‡ | 3.372 (0.759, 5.984)‡ | |||
| Maternal age | 0.151 (−0.228, 0.529) | 0.157 (−0.222, 0.535) | |||
| Durbin-Watson | 2.007 | 2.002 | |||
| B | Blood mercury tertile* | T1 | Reference | Reference | |
| T2 | −0.203 (−3.438, 3.032) | −0.179 (−3.414, 3.056) | |||
| T3 | −4.339 (−7.546, −1.132)† | −4.305 (−7.512, −1.098)† | |||
| Birth weight, kg | 1.968 (−1.458, 5.394) | 2.025 (−1.402, 5.452) | |||
| Sex of a baby (vs. male) | 3.361 (0.755, 5.968)‡ | 3.234 (0.618, 5.851)‡ | |||
| Maternal age | 0.155 (−0.224, 0.534) | 0.162 (−0.218, 0.541) | |||
| Durbin-Watson | 2.02 | 2.013 | |||
| Performance IQ | A | Per doubling of blood mercury | −1.683 (−3.707, 0.341) | −1.706 (−3.733, 0.320) | |
| Birth weight, kg | 4.683 (0.696, 8.670)‡ | 4.642 (0.650, 8.634)‡ | |||
| Sex of a baby (vs. male) | 2.940 (−0.100, 5.981) | 3.029 (−0.026, 6.084) | |||
| Maternal age | −0.475 (−0.918, −0.033)‡ | −0.480 (−0.923, −0.037)‡ | |||
| Durbin-Watson | 1.991 | 1.998 | |||
| B | Blood mercury tertile* | T1 | Reference | Reference | |
| T2 | 1.500 (−2.286, 5.286) | 1.484 (−2.305, 5.273) | |||
| T3 | −1.611 (−5.364, 2.142) | −1.633 (−5.389, 2.124) | |||
| Birth weight, kg | 4.453 (0.444, 8.462)‡ | 4.416 (0.402, 8.430)‡ | |||
| Sex of a baby (vs. male) | 2.752 (−0.298, 5.803) | 2.834 (−0.231, 5.899) | |||
| Maternal age | −0.461 (−0.904, −0.017)‡ | −0.465 (−0.909, −0.021)‡ | |||
| Durbin-Watson | 2.008 | 2.011 | |||
| Total IQ | A | Per doubling of blood mercury | −2.402 (−4.279, −0.526)‡ | −2.395 (−4.274, −0.515)‡ | |
| Birth weight, kg | 3.745 (0.049, 7.441)‡ | 3.758 (0.056, 7.460)‡ | |||
| Sex of a baby (vs. male) | 3.474 (0.655, 6.292)‡ | 3.445 (0.612, 6.278)‡ | |||
| Maternal age | −0.162 (−0.573, 0.248) | −0.161 (−0.572, 0.250) | |||
| Durbin-Watson | 1.975 | 1.981 | |||
| B | Blood mercury tertile* | T1 | Reference | Reference | |
| T2 | 0.965 (−2.542, 4.471) | 0.972 (−2.539, 4.482) | |||
| T3 | −3.449 (−6.925, 0.027) | −3.439 (−6.920, 0.041) | |||
| Birth weight, kg | 3.539 (−0.174, 7.252) | 3.556 (−0.163, 7.275) | |||
| Sex of a baby (vs. male) | 3.276 (0.451, 6.101)‡ | 3.240 (0.400, 6.079)‡ | |||
| Maternal age | −0.150 (−0.561, 0.261) | −0.148 (−0.560, 0.263) | |||
| Durbin-Watson | 1.997 | 1.998 | |||
The covariates in Model 1 were: child birth weight and sex, maternal age, maternal educational level, household income, and fish intake. The covariates in Model 2: included all those from Model 1 plus maternal blood lead.
CI = confidence interval.
*Blood mercury level: 1st tertile (≤ 2.622), 2nd tertile (2.622–3.829), 3rd tertile (> 3.829); † P < 0.010; ‡ P < 0.050.
Following the stratification of fish intake, doubling of blood mercury was associated with the decrease in verbal and total IQ by 3.717 and 3.850 in the highest tertile of fish intake, which indicates higher mercury level, only (Table 3). Comparison of the highest and lowest tertiles of blood mercury also indicated the decrease in verbal IQ (5.926) in the highest tertile of fish intake only (Table 3).
Table 3. Beta coefficients and 95% CI of child IQ in multiple linear regression analysis in children according to stratification of fish intake (n = 445).
| Response variables | Independent variables | Beta coefficients (95% CI) | |||
|---|---|---|---|---|---|
| 1st tertile of fish intake* | 2nd tertile of fish intake* | 3rd tertile of fish intake* | |||
| Verbal IQ | Per doubling of blood mercury | −0.642 (−3.839, 2.555) | −2.543 (−5.852, 0.766) | −3.717 (−6.415, −1.018)‡ | |
| Durbin-Watson | 2.143 | 2.051 | 1.923 | ||
| Blood mercury tertile† | T1 | Reference | Reference | Reference | |
| T2 | 0.565 (−5.058, 6.188) | −2.013 (−7.604, 3.578) | 1.104 (−5.105, 7.313) | ||
| T3 | −3.844 (−9.307, 1.619) | −2.082 (−7.798, 3.633) | −5.926 (−11.776-, −0.077)§ | ||
| Durbin-Watson | 2.006 | 1.925 | 1.956 | ||
| Performance IQ | Per doubling of blood mercury | 0.119 (−3.162, 3.400) | −2.204 (−6.696, 2.289) | −2.959 (−5.975, −0.056) | |
| Durbin-Watson | 2.173 | 2.062 | 1.922 | ||
| Blood mercury tertile† | T1 | Reference | Reference | Reference | |
| T2 | 1.194 (−4.623, 7.010) | −0.961 (−8.520, 6.598) | 3.209 (−3.712, 10.130) | ||
| T3 | 0.503 (−5.184, 6.153) | −2.689 (−10.416, 5.038) | −3.483 (−10.004, 3.308) | ||
| Durbin-Watson | 1.864 | 1.98 | 2.176 | ||
| Total IQ | Per doubling of blood mercury | −0.278 (−3.463, 2.906) | −2.747 (−6.780, 1.286) | −3.850 (−6.688, −1.012)‡ | |
| Durbin-Watson | 2.212 | 2.063 | 1.875 | ||
| Blood mercury tertile† | T1 | Reference | Reference | Reference | |
| T2 | 1.324 (−4.300, 6.948) | −1.960 (−8.764, 4.843) | 3.326 (−3.164, 9.817) | ||
| T3 | −2.063 (−7.527, 3.401) | −2.723 (−9.678, 4.231) | −5.205 (−11.320, 0.910) | ||
| Durbin-Watson | 2.227 | 2.048 | 1.947 | ||
The covariates in Model 1 were: child birth weight and sex, maternal age, maternal educational level, and household income.
CI = confidence interval.
*Fish intake: 1st tertile (≤ 20.5 g), 2nd tertile (20.5–75.0 g), 3rd tertile (> 75.0 g); †Blood mercury level: 1st tertile (≤ 2.622), 2nd tertile (2.622–3.829), 3rd tertile (> 3.829); ‡P < 0.010; §P < 0.050.
DISCUSSION
The mean blood mercury concentration in the 553 Korean mothers in the present study was 3.14 µg/L. This is lower than that of Korean women from the general population aged 20 years or more (3.84 µg/L) (15), but much higher than females in a National Health and Nutrition Examination Survey (NHANES) sample from the United States (0.845 µg/L) (16). Mercury is ubiquitous in the global environment. In aquatic environments, bacteria transform mercury to methyl mercury, and biological magnification leads to higher levels in predatory fish and sea mammals (1). The large difference in total blood mercury concentration in Korea and the United States may be explained by the greater consumption of seafood in Korea, because seafood is the primary source of mercury exposure in humans (17).
Both mercury and methyl mercury are neurotoxins, and children and developing fetuses are most susceptible to these toxins (1,2,3). Low-level prenatal exposure to mercury can affect long-term neurodevelopment of children (4,5). However, the results of epidemiologic studies of low-level exposure have been inconsistent (6,7,8,9,10). A large cohort study started in the mid-1980s in the Faroe Islands reported an inverse association between prenatal methyl mercury exposure and scores on tests of verbal memory, attention, language, and visuospatial perception in 7 years old children born to mothers with a GM methyl mercury concentration of 4,270 ng/g in the hair (4). Moreover, a nested case-control study within this cohort, which compared children with high prenatal exposure (maternal hair mercury: 10–20 µg/g) to those with less exposure (maternal hair mercury < 3 µg/g), showed somewhat lower test scores in the first group, especially in the domains of motor function, language, and memory (18). These neuropsychological deficits remained when the children were retested at 14 years of age (5).
These results contrast with those of the Seychelles Child Development Study (SCDS), a large cohort study in the Republic of Seychelles where the mean prenatal methyl mercury level was 6,900 ng/g due to abundant consumption of seafood. This study reported no consistent pattern of associations between prenatal exposure to methyl mercury and cognitive ability of children aged 107 months. This study reported a positive association of blood mercury level with hyperactivity as measured by the Conners' Teacher Rating Scale, but the authors attributed these results to chance (7). In addition, longitudinal data from the SCDS found no consistent pattern of adverse effects from prenatal exposure to methyl mercury on child development (10). Another large cohort study in northeastern Italy reported no association between neurodevelopment of children and maternal hair mercury level in mothers whose mean hair mercury level was 1.375 ppm (19). In particular, children whose mothers had mercury levels of at least 2 ppm had full scale, verbal, and performance IQs that were 4–5 points lower than those of children whose mothers had lower mercury levels, but these differences were not statistically significant (19). A New Zealand cohort study found no significant association between maternal hair mercury concentration during pregnancy and child performance on a psychological test (20). In the last decade, there have also been large cohort studies such as the Seychelles study's new nutrition 2 cohort (21), an update of the British Avon Longitudinal Study of Parents and Children (ALSPAC) cohort (22), the Spanish Infancia y Medio Ambiente (INMA, Environment and Childhood) cohort (23), and a Mediterranean cohort (24), all of which do not find consistent evidence of associations between prenatal mercury exposure and children neurodevelopment.
Fish intake may make the basis for the apparent conflict of the findings of the Faroe Islands and Seychelles Islands studies. A woman's consumption of seafood may expose her fetus to nutrients that promote optimal development such as omega-3 fatty acids, as well as methylmercury, and thus, a failure to consider this in the data analysis can result in an underestimate of the adverse impact of mercury. The existence of such negative confounding is illustrated by analyses of a birth cohort in Boston (25) and by a re-analysis of the Faroe Islands data (26). In a new Seychellois cohort, in which children's seafood intake was considered, inverse associations were found between prenatal methylmercury exposure and infant development (27). The present study showed that low-level maternal blood mercury concentration during late pregnancy was inversely associated with verbal IQ of children after adjustment for covariates including fish intake, whereas fish intake was not associated with IQ of children (Table 2). In addition, following stratification of the fish intake, the inverse association between prenatal mercury concentration and IQ of children was observed in the highest tertile of fish intake, which indicates higher mercury level (Table 3). Thus, fish intake acted as an effect modifier of child IQ in the present study.
Many previous studies of methyl mercury examined populations with relatively high levels of exposure due to dietary habits, such as frequent consumption of contaminated fish (4,5,18), so these results may not be generalizable to women with lower exposures. The present study showed that low-level maternal blood mercury concentration during late pregnancy was inversely associated with verbal IQ of children after adjustment for covariates (Table 2). However, maternal blood mercury concentration during late pregnancy was not associated with performance IQ. The inverse association between maternal blood mercury and child verbal IQ remained after statistical adjustment for the concentration of blood lead, another potent neurotoxin.
The blood mercury concentration of mothers in the present study is lower than reported in other studies, although few studies used maternal blood mercury concentration as an exposure marker. For example, Valent et al. (24) found no association between neurodevelopment in 18-month-old children and methyl mercury concentration in mothers' blood during pregnancy (mean blood mercury concentration: 3.14 ng/g; mean hair concentration: 1.06 ppm). This blood concentration of mercury is similar to that of the present study (3.15 µg/L), although children were tested at 18 months in the previous study, but at 5 years in the present study. Mothers' hair in the Faroese birth cohort had a higher methyl mercury concentration during pregnancy (GM: 4.3 ppm) (4,5,18). Orenstein et al. (28) recently found an adverse effect of low-level prenatal methyl mercury exposure on childhood memory and learning, particularly visual memory, in mothers whose hair had a mean concentration of 0.6 ppm. However, this cohort was also exposed to polychlorinated biphenyls and methyl mercury. Taken together, the inconsistencies among these numerous studies may be due to use of different biological samples (umbilical cord blood, maternal blood, or maternal hair), different levels of exposure, exposure to multiple neurotoxins, measurement of different outcomes at different times, and the presence of different confounders/effect modifier including fish intake (21,22,23,24).
Methyl mercury bioaccumulates and crosses the placenta (1). There have been several mass poisoning incidents of pregnant mothers, in which their children developed psychomotor and cognitive delays even though the mothers remained relatively unaffected (2,3). The domains most affected are memory, attention, reaction time (psychomotor speed), and language (4). Verbal IQ, but not performance IQ, was affected in the present study, although other studies reported psychomotor and cognitive deficits. The present results suggest that cognitive function may be more sensitive to low-level mercury, although this requires further study.
We also found that maternal age was inversely associated with performance IQ after adjustment for covariates. Previous studies showed that older maternal age was associated with increased risk for educational disabilities (29). In particular, babies conceived by women at old age have higher risk for several adverse pregnancy outcomes, including fetal death, preterm birth, low birth weight, certain types of birth defects, and infant mortality (30). In addition, surviving children of very young or very old mothers may have an elevated risk for poor cognitive function (31). Birth weight was also positively associated with performance and total IQ in the present study. This finding was compatible with previous studies (32,33). The present finding that female children had higher verbal and total IQ compared to male children was in agreement with those of previous studies (34,35).
This is the first study to document an association between low-level prenatal mercury exposure and neurodevelopment of children in an East Asian country with considering fish intake. These results are particularly relevant for countries that have significant fish consumption, such as Korea and Japan. A Japanese study reported no significant correlation between child neurodevelopment at 42 months and cord blood methyl mercury concentration (median concentration: 10.1 ng/g) (36). The present study contributes to the limited body of literature that has examined the effect of prenatal low level mercury exposure on neurodevelopment of children according to the stratification of fish intake. If future studies support our finding of an effect of low level mercury in mothers on neurodevelopment in children, this will have important public health implications.
The present study had several important strengths. First, a large and experienced research team performed the experiments and this enabled good quality-control of neurodevelopmental measurements. Moreover, WPPSI scores have been standardized for Korean children when compared with western countries. Second, assessment of the effect of prenatal exposure to a toxin requires recruitment of children prenatally and following them for several years. The birth cohort in the present study was prospective, and this allowed us to assess the correlation between prenatal exposure and neurodevelopment. In addition, we attempted to rule out the possibilities of exposure misclassification and selection bias, because parents more concerned about the behavior of their children may have been more likely to volunteer for this study. Third, exposure to low levels of numerous toxins is inevitable, and adverse effects of methyl mercury and lead on neurodevelopment are likely (4,5). Thus, the present study assessed lead and mercury simultaneously, and found that verbal IQ was inversely associated with mercury, but not lead. Finally, we showed that fish intake is not a risk factor, but an effect modifier of child IQ.
This study had several limitations. First, many genetic and environmental factors can affect neurodevelopment in children. Factors associated with poor IQ include low socioeconomic status, poverty, lack of stimulation in the home (including lack of maternal warmth and poor maternal education) (37,38) and iron deficiency (39). Thus, in our study, it was necessary to consider many parameters, including maternal age, child birth weight, maternal educational level, and household income. The complexity of neurodevelopment in children is a multifactorial phenomenon, and there were undoubtedly genetic, social, and environmental factors that caused residual confounding in our analysis. Further studies with adjustment for these additional factors are needed to clarify the causal relationship between maternal low-level mercury concentration and IQ deficits in children. A second limitation is a lack of species analysis of mercury. Methyl mercury data were not available in the present study. Methyl mercury is the organic form of mercury that bioaccumulates in fish and enters the human body through the food chain. Methyl mercury is a more appropriate marker for study of a population that has a diet with abundant seafood, rather than occupational or environmental exposure to inorganic mercury. We assumed that blood mercury would be a reasonable proxy for methyl mercury.
In conclusion, the present study showed that verbal IQ of children from a population in Korea was negatively associated with blood mercury concentration of their mothers during late pregnancy.
Footnotes
Funding: This study was supported by the National Institute of Environmental Research, Korea and the Dongguk University Research Fund of 2016.
DISCLOSURE: The authors have no potential conflicts of interest to disclosure.
AUTHOR CONTRIBUTION: Conceptualization: Jeong KS, Park H, Ha E, Shin J, Hong YC, Ha M, Kim BN. Data curation: Park H, Ha E, Ha M, Lee B, Lee SJ, Lee KY, Kim JH. Writing - original draft: Jeong KS. Writing - review & editing: Kim Y.
References
- 1.Agency for Toxic Substances and Disease Registry (US) Toxicological Profile for Mercury (Update) Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 1997. [Google Scholar]
- 2.Harada M. Congenital Minamata disease: intrauterine methylmercury poisoning. Teratology. 1978;18:285–288. doi: 10.1002/tera.1420180216. [DOI] [PubMed] [Google Scholar]
- 3.Amin-Zaki L, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood M. Intra-uterine methylmercury poisoning in Iraq. Pediatrics. 1974;54:587–595. [PubMed] [Google Scholar]
- 4.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sørensen N, Dahl R, Jørgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 5.Debes F, Budtz-Jørgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Myers GJ, Davidson PW, Shamlaye CF, Axtell CD, Cernichiari E, Choisy O, Choi A, Cox C, Clarkson TW. Effects of prenatal methylmercury exposure from a high fish diet on developmental milestones in the Seychelles Child Development Study. Neurotoxicology. 1997;18:819–829. [PubMed] [Google Scholar]
- 7.Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang LS, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles Child Development Study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- 8.Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- 9.Davidson PW, Palumbo D, Myers GJ, Cox C, Shamlaye CF, Sloane-Reeves J, Cernichiari E, Wilding GE, Clarkson TW. Neurodevelopmental outcomes of Seychellois children from the pilot cohort at 108 months following prenatal exposure to methylmercury from a maternal fish diet. Environ Res. 2000;84:1–11. doi: 10.1006/enrs.2000.4084. [DOI] [PubMed] [Google Scholar]
- 10.Davidson PW. Jean-Sloane-Reeves, Myers GJ, Hansen ON, Huang LS, Georger LA, Cox C, Thurston SW, Shamlaye CF, Clarkson TW. Association between prenatal exposure to methylmercury and visuospatial ability at 10.7 years in the Seychelles Child Development Study. Neurotoxicology. 2008;29:453–459. doi: 10.1016/j.neuro.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BM, Ha M, Park HS, Lee BE, Kim YJ, Hong YC, Kim Y, Chang N, Roh YM, Kim BN, et al. The mothers and children’s environmental health (MOCEH) study. Eur J Epidemiol. 2009;24:573–583. doi: 10.1007/s10654-009-9370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HW, Kwak KJ, Park KB. Korean-Wechsler Preschool and Primary Scale of Intelligence. Seoul: Kidspop; 1996. [Google Scholar]
- 13.Bhang SY, Ha E, Park H, Ha M, Hong YC, Kim BN, Lee SJ, Lee KY, Kim JH, Jeong J, et al. Maternal stress and depressive symptoms and infant development at six months: the mothers and children’s environmental health (MOCEH) prospective study. J Korean Med Sci. 2016;31:843–851. doi: 10.3346/jkms.2016.31.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health Canada. Eating well with Canada's food guide [Internet] [accessed on 7 March 2017]. Available at www.healthcanada.gc.ca/foodguide.
- 15.Kim Y, Lee BK. Associations of blood lead, cadmium, and mercury with estimated glomerular filtration rate in the Korean general population: analysis of 2008–2010 Korean National Health and Nutrition Examination Survey data. Environ Res. 2012;118:124–129. doi: 10.1016/j.envres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (US) Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 17.Balshaw S, Edwards J, Daughtry B, Ross K. Mercury in seafood: mechanisms of accumulation and consequences for consumer health. Rev Environ Health. 2007;22:91–113. doi: 10.1515/reveh.2007.22.2.91. [DOI] [PubMed] [Google Scholar]
- 18.Davidson PW, Myers GJ, Weiss B. Mercury exposure and child development outcomes. Pediatrics. 2004;113:1023–1029. [PubMed] [Google Scholar]
- 19.Deroma L, Parpinel M, Tognin V, Channoufi L, Tratnik J, Horvat M, Valent F, Barbone F. Neuropsychological assessment at school-age and prenatal low-level exposure to mercury through fish consumption in an Italian birth cohort living near a contaminated site. Int J Hyg Environ Health. 2013;216:486–493. doi: 10.1016/j.ijheh.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Crump KS, Kjellström T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–713. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- 21.Strain JJ, Yeates AJ, van Wijngaarden E, Thurston SW, Mulhern MS, McSorley EM, Watson GE, Love TM, Smith TH, Yost K, et al. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am J Clin Nutr. 2015;101:530–537. doi: 10.3945/ajcn.114.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golding J, Gregory S, Iles-Caven Y, Hibbeln J, Emond A, Taylor CM. Associations between prenatal mercury exposure and early child development in the ALSPAC study. Neurotoxicology. 2016;53:215–222. doi: 10.1016/j.neuro.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llop S, Guxens M, Murcia M, Lertxundi A, Ramon R, Riaño I, Rebagliato M, Ibarluzea J, Tardon A, Sunyer J, et al. Prenatal exposure to mercury and infant neurodevelopment in a multicenter cohort in Spain: study of potential modifiers. Am J Epidemiol. 2012;175:451–465. doi: 10.1093/aje/kwr328. [DOI] [PubMed] [Google Scholar]
- 24.Valent F, Mariuz M, Bin M, Little D, Mazej D, Tognin V, Tratnik J, McAfee AJ, Mulhern MS, Parpinel M, et al. Associations of prenatal mercury exposure from maternal fish consumption and polyunsaturated fatty acids with child neurodevelopment: a prospective cohort study in Italy. J Epidemiol. 2013;23:360–370. doi: 10.2188/jea.JE20120168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, Hu H, Gillman MW. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008;167:1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budtz-Jørgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environ Health Perspect. 2007;115:323–327. doi: 10.1289/ehp.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, Wallace JM, Robson PJ, Shamlaye CF, Georger LA, et al. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology. 2008;29:776–782. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orenstein ST, Thurston SW, Bellinger DC, Schwartz JD, Amarasiriwardena CJ, Altshul LM, Korrick SA. Prenatal organochlorine and methylmercury exposure and memory and learning in school-age children in communities near the New Bedford Harbor Superfund site, Massachusetts. Environ Health Perspect. 2014;122:1253–1259. doi: 10.1289/ehp.1307804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gueorguieva RV, Carter RL, Ariet M, Roth J, Mahan CS, Resnick MB. Effect of teenage pregnancy on educational disabilities in kindergarten. Am J Epidemiol. 2001;154:212–220. doi: 10.1093/aje/154.3.212. [DOI] [PubMed] [Google Scholar]
- 30.Fretts RC, Schmittdiel J, McLean FH, Usher RH, Goldman MB. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333:953–957. doi: 10.1056/NEJM199510123331501. [DOI] [PubMed] [Google Scholar]
- 31.Williams LO, Decouflé P. Is maternal age a risk factor for mental retardation among children? Am J Epidemiol. 1999;149:814–823. doi: 10.1093/oxfordjournals.aje.a009897. [DOI] [PubMed] [Google Scholar]
- 32.Lundgren EM, Cnattingius S, Jonsson B, Tuvemo T. Intellectual and psychological performance in males born small for gestational age with and without catch-up growth. Pediatr Res. 2001;50:91–96. doi: 10.1203/00006450-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Shenkin SD, Starr JM, Pattie A, Rush MA, Whalley LJ, Deary IJ. Birth weight and cognitive function at age 11 years: the Scottish Mental Survey 1932. Arch Dis Child. 2001;85:189–196. doi: 10.1136/adc.85.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallentin M. Putative sex differences in verbal abilities and language cortex: a critical review. Brain Lang. 2009;108:175–183. doi: 10.1016/j.bandl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Mullis IV, Martin MO, Gonzalez EJ, Kennedy AM. PIRLS 2001 International Report: IEA's Study of Reading Literacy Achievement in Primary School in 35 Countries. Chestnut Hill, MA: International Study Center, Lynch School of Education, Boston College; 2003. [Google Scholar]
- 36.Tatsuta N, Nakai K, Murata K, Suzuki K, Iwai-Shimada M, Kurokawa N, Hosokawa T, Satoh H. Impacts of prenatal exposures to polychlorinated biphenyls, methylmercury, and lead on intellectual ability of 42-month-old children in Japan. Environ Res. 2014;133:321–326. doi: 10.1016/j.envres.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitley E, Gale CR, Deary IJ, Kivimaki M, Batty GD. Association of maternal and paternal IQ with offspring conduct, emotional, and attention problem scores. Transgenerational evidence from the 1958 British Birth Cohort Study. Arch Gen Psychiatry. 2011;68:1032–1038. doi: 10.1001/archgenpsychiatry.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czajka-Narins DM, Haddy TB, Kallen DJ. Nutrition and social correlates in iron deficiency anemia. Am J Clin Nutr. 1978;31:955–960. doi: 10.1093/ajcn/31.6.955. [DOI] [PubMed] [Google Scholar]


