Abstract
Little is known about platelet dynamics and the effect of antiplatelet therapy in Kawasaki disease (KD). This study sought to define platelet activation dynamics in KD patients by assaying platelet-derived microparticles (PDMPs). We measured plasma PDMPs levels in 46 patients with KD using an enzyme-linked immunosorbent assay (ELISA). Blood samples were collected before, at 2–5 days, and 9–15 days after intravenous immunoglobulin (IVIG) infusion, 2 months and 4–5 months after the onset of KD. We measured PDMP levels in 23 febrile and 10 afebrile control patients. In the acute phase of KD patients, PDMP levels increased significantly after IVIG treatment (12.04 ± 5.58 nmol before IVIG infusion vs. 19.81 ± 13.21 nmol at 2–5 days after IVIG infusion, P = 0.006). PDMP levels were negatively correlated with age and positively correlated with procalcitonin levels in the acute phase of KD. No significant difference was found in PDMP levels between KD patients with and without coronary artery lesion (CAL). Elevated PDMP levels after IVIG therapy significantly decreased below the pre-IVIG level in subacute phase (19.81 ± 13.21 nmol at 2–5 days after IVIG infusion vs. 8.33 ± 2.02 nmol at 9–15 days after IVIG infusion, P < 0.001), and PDMP levels stayed below the pre-IVIG level in the convalescent phase, during which antiplatelet therapy was given. However, PDMP levels rebounded after discontinuing aspirin in 17 patients. In conclusion, enhanced platelet activation was noted before treatment of KD and peaked immediately after IVIG treatment. Recurrent rising of PDMP levels was observed after discontinuing aspirin, although there were no significant differences between the PDMP levels at 2 months after the onset of KD and those at 4–5 months after the onset of the disease.
Keywords: Mucocutaneous Lymph Node Syndrome, Antiplatelet Therapy, Platelet-derived Microparticles
Graphical Abstract
INTRODUCTION
Kawasaki disease (KD) is an acute febrile, systemic vasculitic syndrome of unknown etiology that occurs primarily in children younger than 5 years of age. The standard treatment of acute KD is a combination of high-dose (2 g/kg) intravenous immunoglobulin (IVIG) and oral aspirin (50 mg/kg/day). The acute phase of KD involves platelet activation, and antiplatelet therapy is included in the treatment protocol for this phase (1). The finding of low levels of antithrombin III and depletion of the fibrinolytic system supports the concept that active intravascular clot formation and degradation occur in these patients and are most notable early in the acute phase of the disease (2). Thrombosis mainly occurs in the artery and is associated with platelet activation in KD (3). Following the cessation of fever for 2 to 3 days and improvement of all acute symptoms, the dosage of acetylsalicylic acid (ASA) can be reduced to the minimum level necessary to inhibit platelet aggregation.
Low-dose ASA has an antithrombotic effect through inhibiting thromboxane A2 (TxA2). Aspirin is continued for its antithrombotic effect until 6–8 weeks after KD onset, when the erythrocyte sedimentation rate (ESR) and thrombocytosis have normalized in patients that have not developed coronary artery lesion (CAL), as detected by echocardiography. However, the decision in the total duration of antiplatelet therapy in KD patients is dependent on a routine blood testing, including the level of platelets.
Microparticles, also referred to as microvesicles or more rarely ectosomes, are submicron fragments that are shed from the plasma membrane of stimulated or apoptotic cells (4). Platelet-derived microparticles (PDMPs) have recently been reported as a platelet activation marker. Therefore, the PDMP levels can be used as an index of platelet activation (5,6). PDMPs are a heterogeneous population of vesicles generated from the plasma membrane upon platelet activation by various stimuli. An increase in the total numbers of PDMPs is associated with disease states such as atherosclerosis, diabetes, cancer, sepsis, and pulmonary hypertension (7). However, little is known about these microparticles being linked to platelet dynamics in patients with KD. The purpose of this study was to prove that a state of platelet activation exists in patients with KD from the acute stage to the convalescent phase and to demonstrate the usefulness of the PDMP levels in evaluating the effect of antiplatelet therapy in KD.
MATERIALS AND METHODS
Patients and data collection
We enrolled 46 patients with KD and 33 age-matched control patients between June 2015 and August 2016. The diagnosis of KD was based on clinical features established by the Japanese Kawasaki Disease Research Committee (8). Complete KD is defined as having at least 5 of the principal clinical signs that the Japanese Kawasaki Disease Research Committee has validated. Low-dose ASA (3–5 mg/kg/day) was continued until 6–8 weeks after the onset of KD. Febrile control patients had acute tonsillitis, conjunctivitis, or acute cervical lymphadenitis. Afebrile control patients had acute gastroenteritis, acute urticaria, or viral pneumonia.
Blood sampling
Two milliliters of blood were obtained and put in a tube containing 0.019 M (3.2%) citrate anticoagulant. The samples were centrifuged at 1,500–13,000 ×g to obtain platelet-poor plasma. All procedures were performed at room temperature. The samples were stored in a deep freezer (−80°C) until analysis. The PDMP level was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Zymuphen MP-activity; Medicago, Uppsala, Sweden) and expressed as phosphatidylserine equivalents (nmol). The assay performance and characteristics were as follows: 1) detection threshold, not more than 0.05 nmol; 2) intraassay coefficient of variation (CV), 3% to 8%; and 3) interassay CV, 5% to 10%.
Laboratory data were analyzed before and after IVIG treatment and the data included a complete blood count, ESR, and the levels of albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), sodium, N-terminal pro-brain natriuretic peptide (NT-proBNP), and C-reactive protein (CRP). Blood samples were collected from the patients with KD at the following times: 1) before IVIG infusion; 2) 2–5 days after IVIG infusion; 3) 9–15 days after IVIG infusion; 4) 2 months after the onset of disease; and 5) 4–5 months after the onset of disease. In control patients, blood samples for the PDMP levels were collected once upon admission or at the outpatient clinic.
Echocardiography
Echocardiography was performed twice during hospitalization (immediately prior to IVIG administration and before discharge) and twice during the follow-up observations (upon termination of low-dose ASA administration, and 4 months after the onset of KD). CAL was diagnosed based on Z scores of the left main coronary artery, proximal left anterior descending coronary artery, and proximal right coronary artery, and were defined as Z scores of 2.0 or more. The value of Z scores from a standardized coronary artery dimension was calculated using the body surface area (9) based on the Haycock's formula (10).
Statistical analysis
All data were expressed as the mean ± standard deviation. Comparisons of the frequencies between groups were analyzed using the χ2 test. Differences in continuous variables among groups were assessed using the 2-sample t-test and the analysis of variance (ANOVA). The significance of difference was calculated by the Scheffe's test. In addition, we performed the Pearson correlation analysis to identify the correlation between variables. The logistic regression analysis was used to determine the correlation of plasma PDMPs levels with other laboratory values, including NT-proBNP, ESR, and CRP. A P value less than 0.05 was considered as statistically significant.
Ethics statement
Informed consent was obtained from parents of all children, and the study protocol was approved by the Eulji University Hospital Institutional Review Board (IRB, No. 2015-03-014-002). Informed consent was confirmed by the IRB.
RESULTS
Baseline patient characteristics and laboratory findings
The KD group was composed of 26 boys and 20 girls, whose mean age at diagnosis was 33.78 ± 21.95 months (range, 6.0–84.0 months). A total of 33 control group was enrolled for the study (23 febrile patients and 10 afebrile patients). The mean age of the control group was 37.17 ± 19.79 months in the febrile patients and 30.00 ± 17.81 months in the afebrile patients. In the febrile control group, Epstein-Barr virus infection was confirmed in 2 patients and adenovirus was identified in 1 patient using a nasopharyngeal swab. In the afebrile group, parainfluenza virus, bocavirus, and coronavirus were identified in 3 patients using a nasopharyngeal swab.
Of the 46 patients with KD, 26 patients (56.5%) were diagnosed with complete KD and 20 patients (43.5%) with incomplete KD. The mean time until the start of IVIG treatment was 5.78 ± 1.88 fever days. Five patients did not respond to the initial IVIG infusion, but 2 patients responded to the second IVIG treatment without corticosteroid treatment. Three patients were crossed over methylprednisolone pulse therapy with a second IVIG treatment. The mean period of low-dose ASA usage in patients with KD was 53.27 ± 8.21 days (range, 41–75 days). In total, 4.3% (2/46) of patients experienced a recurrence of KD during the follow-up period.
Upon admission, the levels of white blood cell (WBC), neutrophil, ESR, CRP, and NT-proBNP were significantly higher in KD patients than in the control patients (Table 1). The level of hemoglobin was lower in KD patients compared with the afebrile control patients (P = 0.034).
Table 1. Baseline characteristics of patients with KD and control patients.
| Parameters | KD (n = 46) | Febrile control (n = 23) | Afebrile control (n = 10) | P |
|---|---|---|---|---|
| Age, mon | 33.78 ± 21.95 | 37.17 ± 19.79 | 30.00 ± 17.81 | 0.643 |
| Gender (male/female) | 26/20 | 12/11 | 5/5 | 0.813 |
| Weight, kg | 13.94 ± 4.10 | 14.29 ± 4.09 | 13.66 ± 5.52 | 0.916 |
| BMI, kg/m2 | 16.51 ± 2.12 | 15.96 ± 1.50 | 16.63 ± 1.87 | 0.483 |
| Hemoglobin, g/dL | 11.58 ± 0.82 | 11.64 ± 1.21 | 12.47± 0.50 | 0.034 |
| WBC, 103/µL | 13.44 ± 4.24 | 8.58 ± 3.83 | 8.59 ± 2.85 | < 0.001 |
| Platelet count, 104/µL | 33.80 ± 11.84 | 22.80 ± 11.87 | 39.09 ± 16.82 | 0.088 |
| Neutrophil, % | 66.40 ± 12.95 | 49.57 ± 17.22 | 43.41 ± 20.23 | < 0.001 |
| Albumin, g/dL | 4.09 ± 0.26 | 4.10 ± 0.19 | 4.29 ± 0.16 | 0.066 |
| AST, U/L | 97.35 ± 136.06 | 43.52 ± 20.08 | 44.50 ± 18.99 | 0.091 |
| ALT, U/L | 102.91 ± 188.05 | 22.09 ± 21.28 | 22.50 ± 8.39 | 0.057 |
| ESR, mm/hr | 46.96 ± 27.18 | 24.15 ± 19.82 | 8.86 ± 5.84 | < 0.001 |
| CRP, mg/dL | 5.31 ± 3.59 | 1.74 ± 1.56 | 0.07 ± 0.02 | < 0.001 |
| NT-proBNP, pg/mL | 1,031.34 ± 1,152.60 | 136.94 ± 96.99 | 59.43 ± 36.71 | < 0.001 |
| Procalcitonin, ng/mL | 1.02 ± 2.06 | 0.46 ± 0.70 | 0.14 ± 0.04 | 0.662 |
| Total cholesterol, mg/dL | 134.57 ± 23.54 | 141.29 ± 24.05 | 142.67 ± 37.09 | 0.566 |
| HDL-C, mg/dL | 31.72 ± 8.81 | 33.36 ± 6.70 | 37.33 ± 12.61 | 0.323 |
| PDMP, nmol | 12.04 ± 5.58 | 9.27 ± 5.91 | 8.18 ± 2.88 | 0.059 |
KD = Kawasaki disease, BMI = body mass index, WBC = white blood cell, AST = aspartate aminotransferase, ALT = alanine aminotransferase, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, NT-proBNP = N-terminal pro-brain natriuretic peptide, HDL-C = high-density lipoprotein-cholesterol, PDMP = platelet-derived microparticle.
Levels of PDMPs in KD patients and control patients
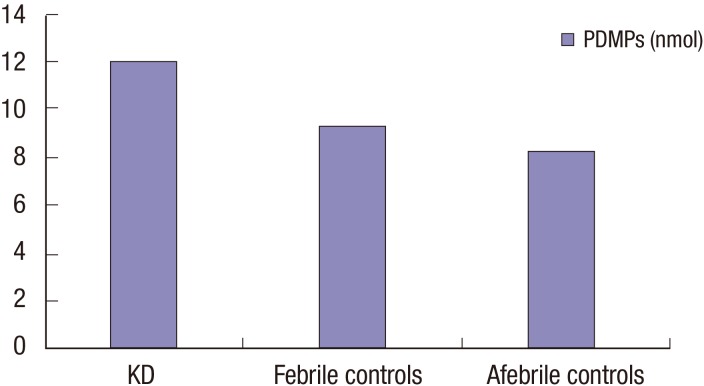
We measured the levels of PDMPs in the plasma of 23 febrile controls and 10 afebrile controls subjects. The mean plasma PDMP levels in the control groups were not significantly different (9.27 ± 5.91 nmol [2.99–33.92] in febrile controls vs. 8.18 ± 2.88 nmol [5.45–12.86] in afebrile controls, P = 0.872) (Fig. 1).
Fig. 1.
Comparison of initial PDMPs levels between KD patients and control patients.
PDMP = platelet-derived microparticle, KD = Kawasaki disease.
In patients with KD, the mean PDMP levels before IVIG treatment were 12.04 ± 5.58 nmol. The plasma PDMP levels at 2–5 days after IVIG infusion (19.81 ± 13.21 nmol) were significantly higher than those in febrile control patients (P = 0.034). KD patients with CALs showed a significantly elevated neutrophil and ESR levels compared to KD patients without CALs. There was no difference in the PDMP levels between the patients with refractory KD and the patients who responded the initial IVIG treatment. No difference was found in PDMPs, albumin, NT-proBNP, and CRP levels between KD patients with and without CALs (Table 2).
Table 2. Relationship between clinical parameters and development of CALs in patients with KD.
| Parameters | CAL (+) (n = 10) | CAL (−) (n = 36) | P |
|---|---|---|---|
| Age, mon | 50.30 ± 23.90 | 29.19 ± 19.31 | 0.006 |
| Gender (male/female) | 7/3 | 19/17 | 0.412 |
| Weight, kg | 17.32 ± 4.19 | 13.01 ± 3.59 | 0.002 |
| BMI, kg/m2 | 15.53 ± 1.35 | 16.78 ± 2.23 | 0.100 |
| Total fever duration, day | 7.50 ± 2.01 | 7.22 ± 1.83 | 0.681 |
| Hemoglobin, g/dL | 11.34 ± 0.76 | 11.65 ± 0.83 | 0.282 |
| WBC, 103/µL | 13.44 ± 3.53 | 13.44 ± 4.47 | 0.998 |
| Neutrophil, % | 75.81 ± 8.24 | 63.79 ± 12.88 | 0.008 |
| Platelet count, 104/µL | 32.76 ± 12.33 | 34.09 ± 11.87 | 0.757 |
| ESR, mm/hr | 62.70 ± 22.15 | 42.58 ± 27.08 | 0.037 |
| CRP, mg/dL | 5.84 ± 5.60 | 5.16 ± 2.91 | 0.604 |
| Albumin, g/dL | 4.01 ± 0.34 | 4.11 ± 0.23 | 0.249 |
| NT-proBNP, pg/mL | 1,090.66 ± 1,141.63 | 1,014.86 ± 1,171.16 | 0.856 |
| Procalcitonin, ng/mL | 1.61 ± 3.30 | 0.76 ± 1.33 | 0.409 |
| IgG, mg/dL | 982.00 ± 666.36 | 718.26 ± 229.12 | 0.054 |
| PDMP, nmol | 11.56 ± 3.78 | 11.94 ± 5.61 | 0.844 |
CAL = coronary artery lesion, KD = Kawasaki disease, BMI = body mass index, WBC = white blood cell, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, NT-proBNP = N-terminal pro-brain natriuretic peptide, IgG = immunoglobulin G, PDMP = platelet-derived microparticle.
Serial changes of PDMP levels in patients with KD
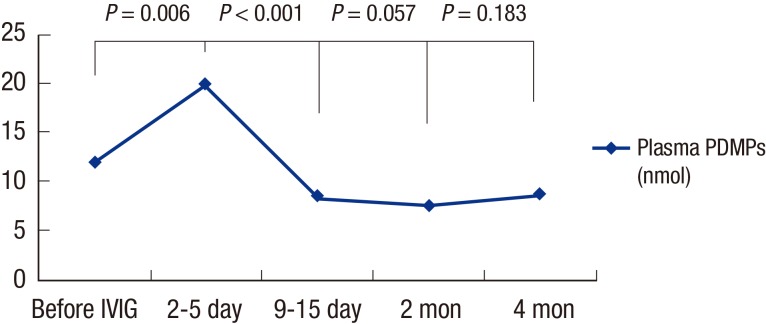
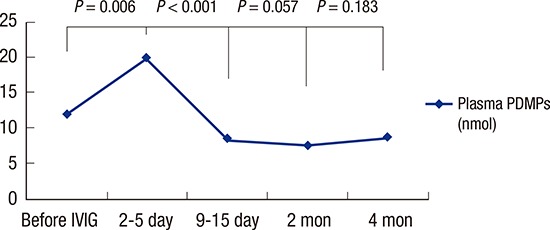
Fig. 2 shows the change in PDMP levels between the acute, the subacute (9–15 days after IVIG infusion), and the convalescent phase. After IVIG therapy, plasma PDMP levels significantly increased (12.04 ± 5.58 nmol before IVIG infusion vs. 19.81 ± 13.21 nmol at 2–5 days after IVIG infusion, P = 0.006). The PDMP levels at 9–15 days after IVIG infusion (8.33 ± 2.02 nmol) were significantly lower than the pre-IVIG level (P = 0.001). In addition, the PDMP levels at 2 months after the onset of KD (7.39 ± 2.08 nmol) was significantly lower than the pre-IVIG level (P < 0.001). However, 17 patients (36.9%) had a recurrent rising PDMP levels after 67.41 ± 11.83 days (range, 47–95 days), when ASA was discontinued. The mean PDMP levels at 4–5 months after the onset of KD (8.65 ± 4.31 nmol) were higher than the PDMP levels at 2 months after the onset of KD, although it did not reach statistical significance (P = 0.183). Table 3 shows the comparison between the patients who did and did not show rebounded PDMP levels. The PDMP levels of patients who had recurrent rising was significantly higher than those in the patients who did not show rebounded PDMP levels at 4–5 months after the onset of KD (P < 0.001).
Fig. 2.
Serial changes of PDMPs levels in KD patients.
PDMP = platelet-derived microparticle, KD = Kawasaki disease, IVIG = intravenous immunoglobulin.
Table 3. Relationship between PDMP levels in patients with KD who had recurrent rising after discontinuation of ASA and who had no rebound of PDMPs level.
| Checking time | PDMPs, nmol | P | |
|---|---|---|---|
| Rebound after discontinuing ASA (n = 17) | No rebound after discontinuing ASA (n = 29) | ||
| At admission | 9.88 ± 3.99 | 13.05 ± 5.57 | 0.047 |
| Immediately after IVIG treatment | 18.07 ± 12.94 | 21.08 ± 13.07 | 0.465 |
| At 9–15 days after IVIG treatment | 7.64 ± 1.87 | 10.30 ± 4.32 | 0.026 |
| At 2 months after the onset of KD | 6.59 ± 1.54 | 7.97 ± 2.36 | 0.043 |
| At 4–5 months after the onset of KD | 11.66 ± 4.19 | 6.05 ± 1.87 | < 0.001 |
PDMP = platelet-derived microparticle, KD = Kawasaki disease, ASA = acetylsalicylic acid, IVIG = intravenous immunoglobulin.
Echocardiographic parameters
Ten of 46 KD patients (21.7%) showed mild dilation of coronary arteries in the acute stage. All of the KD patients with CALs upon admission exhibited normal coronary arteries at one month after discharge. None of KD patients developed coronary artery aneurysm as confirmed by serial echocardiography before and after IVIG therapy. There was no difference between KD patients and febrile control patients in the degree of left ventricular fractional shortening. The ratio of the mitral peak velocity in the early filling period to the early diastolic mitral annular velocity measured by tissue Doppler imaging (TDI) was increased in the acute stage of KD patients, compared with febrile control patients, although the difference was not statistically significant (Table 4).
Table 4. Echocardiographic findings in patients with KD and febrile control patients.
| Findings | KD patients (n = 46) | Febrile controls (n = 23) | P |
|---|---|---|---|
| LV fractional shortening, % | 31.28 ± 6.87 | 34.65 ± 4.33 | 0.100 |
| Base systolic velocity, cm/sec | 7.86 ± 1.35 | 7.85 ± 1.34 | 0.983 |
| Base E', cm/sec | 10.92 ± 2.49 | 12.23 ± 2.16 | 0.092 |
| Base A', cm/sec | 7.60 ± 2.48 | 6.85 ± 1.62 | 0.308 |
| E/A ratio | 1.40 ± 0.31 | 1.41 ± 0.26 | 0.916 |
| E/E' ratio | 11.39 ± 3.02 | 9.79 ± 2.49 | 0.087 |
| Deceleration time, msec | 163.06 ± 29.44 | 181.40 ± 33.82 | 0.061 |
| Tei index | 0.21 ± 0.06 | 0.23 ± 0.04 | 0.370 |
KD = Kawasaki disease, LV = left ventricular, E = base peak early diastolic E-wave velocity (cm/sec), A = base peak early diastolic A-wave velocity (cm/sec), E' = peak early diastolic myocardial velocity (cm/sec), A' = late diastolic myocardial velocity (cm/sec).
Correlations between PDMP levels and other parameters
In patients with KD, age was negatively correlated with initial PDMP levels (r = −0.312; P = 0.037) (Table 5). In addition, positive correlation was found between PDMPs and procalcitonin levels after IVIG treatment in the acute stage of KD (r = 0.695; P = 0.001). In febrile controls, PDMP levels were significantly correlated with elevated HDL levels (r = 0.706; P = 0.005).
Table 5. Correlation of initial PDMPs levels with clinical and laboratory variables in patients with KD.
| Parameters | PDMP | |
|---|---|---|
| r | P | |
| Age, mon | −0.312 | 0.037 |
| Weight, kg | −0.282 | 0.06 |
| BMI, kg/m2 | 0.294 | 0.05 |
| Hemoglobin, g/dL | −0.260 | 0.085 |
| WBC, 103/µL | 0.282 | 0.064 |
| CRP, mg/dL | −0.061 | 0.693 |
| HDL-C, mg/dL | −0.184 | 0.233 |
| Sodium, mEq/L | −0.048 | 0.757 |
| Albumin, g/dL | −0.143 | 0.355 |
| NT-proBNP, pg/mL | 0.131 | 0.395 |
PDMP = platelet-derived microparticle, KD = Kawasaki disease, BMI = body mass index, WBC = white blood cell, CRP = C-reactive protein, HDL-C = high-density lipoprotein-cholesterol, NT-proBNP = N-terminal pro-brain natriuretic peptide.
Correlating factors with the rebound of PDMP levels
The PDMP levels in 17 patients with KD rebounded at 2–3 months after discontinuing ASA. After multivariate logistic regression analysis, the serum NT-proBNP and CRP levels were the risk factors for rebounding PDMP levels. There was no correlation between other clinical variables and rebounding PDMP levels after discontinuation of ASA (Table 6).
Table 6. Effects of various laboratory and clinical factors to rebounding PDMPs level in 17 patients with KD in the convalescent phase.
| Factors | B | OR (95% CI) | P |
|---|---|---|---|
| Platelet | 0.000 | 1.000 (1.000–1.000) | 0.210 |
| NT-proBNP | −0.001 | 0.999 (0.997–1.000) | 0.040 |
| CRP | 0.422 | 1.525 (1.047–2.221) | 0.028 |
| Albumin | −0.448 | 0.639 (0.107–23.934) | 0.809 |
| Period of low dose ASA Tx | 0.023 | 1.024 (0.912–1.150) | 0.693 |
| CAL | 0.130 | 1.139 (0.107–1.150) | 0.914 |
PDMP = platelet-derived microparticle, KD = Kawasaki disease, B = nonstandard coefficient, OR = odds ratio, CI = confidence interval, NT-proBNP = N-terminal pro-brain natriuretic peptide, CRP = C-reactive protein, ASA = acetylsalicylic acid, Tx = treatment, CAL = coronary artery lesion.
DISCUSSION
This study was conducted to determine the role of PDMPs as a biomarker of antiplatelet agents in KD and to investigate the serial change of plasma PDMP levels per stage of KD. Our study showed that the PDMP levels were significantly elevated in patients with KD immediately after IVIG treatment, but the levels of PDMP decreased from the subacute stage to the convalescent phase during antiplatelet therapy.
Platelets are well-established components of the hemostatic system and are recognized as having a role in innate immunity (11). Recently, several additional functions of platelets have been demonstrated in the pathogenesis of various diseases in which inflammation is an important clinical sign. Activation of blood platelets is a typical finding in patients with systemic inflammation and sepsis. Under inflammatory conditions, platelets also show robust interactions with leukocytes (12). Activated platelets adhere to a damaged vessel wall and promote local recruitment of leukocytes.
Thrombocytosis is a universal finding in patients with subacute stage of KD, but it is not specific for KD. In previous epidemiological studies, platelet counts were found to be elevated in patients with chronic inflammatory diseases, malignancies, and myeloproliferative disorders (13). The degree of platelet activation was closely associated with the presence of coronary artery complications in the acute stage of KD (2). In the subacute stage of KD, the platelet count increases rapidly and severe stenosis or obstruction is reported to occur due to thrombi in the main coronary arteries with aneurysms. In the present study, the degree of thrombocytosis was not significantly different between groups of KD with and without CALs.
PDMPs are endoplasmic reticulum-derived vesicles ranging from 0.02 to 0.10 μm in size that are discharged from platelets upon activation by some sort of stimulus. PDMPs are the most abundant microparticles in the blood stream constituting approximately 70% to 90% of circulating microparticles (14). Detectable in the peripheral blood of normal individuals, microparticles are elevated in clinical situations where the thrombotic risk is increased. No significant difference in PDMP levels were observed between the KD patients with and without CALs in the present study. Because recent research has focused on microparticles in pathological conditions, the vesicles can easily be mistaken as purely detrimental. However, these microparticles can be beneficial and are present in healthy individuals. The present study showed that PDMP levels in control groups were not significantly different from those in the pre-IVIG KD group. It is probably because the control group in our study does not have healthy controls but disease controls.
Under most pathophysiologic conditions, PDMPs appear to be derived mostly from procoagulant circulating species with leukocytic or endothelial origins being less representative. Inflammation and coagulation are linked processes in many diseases such as septicemia and PDMPs are known to amplify the response by activating the endothelium (15). PDMPs themselves can initiate inflammation. Barry and coworkers demonstrated that PDMPs deliver arachidonic acid to endothelial cells, which results in the upregulation of CD54 (intercellular adhesion molecule-1 [ICAM-1]) and subsequent adhesion of monocytes (15). Therefore, these microparticles are not only a consequence of the disease but also have a casual role in the pathogenesis of inflammatory diseases.
Although PDMP has recently received a great deal of attention as a marker of platelet activation in adults (5,6), there is little data on PDMP levels in pediatric patients. Taki et al. (16) reported that enhanced platelet aggregation was noted at a high frequency before treatment of KD by using the particle counting method. They suggested that platelet activation continues during the second stage (i.e., from the start of IVIG therapy to 20 days), but platelet activation is inhibited by antiplatelet agents. A recent study demonstrated that IVIG treatment significantly decreased PDMP levels in 18 patients with acute KD (17). They showed that the PDMP levels both immediately and 10–14 days after IVIG treatment were significantly lower than the pre-IVIG level. In contrast, we could find significant increase in PDMP level 2–5 days after IVIG treatment compared with the pre-IVIG level. We speculate that this difference was probably due to differences across ELISA kits used in this study and the timing of changes in ASA dosage. In the above-mentioned reports, they immediately changed the dose of ASA from an anti-inflammatory dose (30–50 mg/kg/day) to an anti-thrombotic dose (5 mg/kg/day) when the patients became afebrile. However, we maintained a high dose of ASA (50 mg/kg/day) until the patient had been afebrile for 2 to 3 days and all acute symptoms were improving. The ELISA test depends on the quality of reagents, usually antibodies. In addition, the possible interference of soluble antigens may result in underestimation of microparticle levels by antigenic capture.
In the present study, we used the Zymuphen MP-activity (Medicago) ELISA to measure prothrombinase activity with an ELISA reader (Bio-Rad, Hercules, CA, USA), measuring samples at a wavelength of 405 nm (18,19). The diluted assayed plasma sample, supplemented with calcium, factor Xa, and thrombin inhibitors were introduced into 1 of the microplate wells coated with streptavidin and biotinylated annexin V, and then incubated. The phospholipid concentration is the limiting factor in this ELISA test. Even if flow cytometry is considered the “gold standard” of microparticle detection, there are still technical limitations concerning detection of small microparticles (100 nm to 500 nm). Therefore, microparticle activity can be analyzed by prothrombinase assay (ELISA), which is easily performed and shows a significant correlation of results (20).
In the present study, the PDMP levels of 17 patients rebounded after aspirin was discontinued even though they had no CALs. These results suggest that vascular inflammation may persist after the convalescent phase even if the morphology of the coronary arteries appears normal onechocardiography. The causes and clinical implications of the persistent platelet activation after the acute stage in patients with KD were probably due to the persistence of subclinical low-grade inflammation in KD (21). A recent study reported that children with a previous episode of KD showed increased platelet activation when compared with healthy participants despite no apparent vascular abnormality at follow-up (22).
ASA was the first antiplatelet agent used for both the primary and secondary prevention of cardiovascular events. By irreversibly binding to cyclooxygenase-1, ASA inhibits TxA2, thus blocking TxA2-induced platelet aggregation and inducing vasoconstriction (23). In KD patients, low-dose ASA is continued for its antithrombotic effect until 6–8 weeks after KD onset. Moreover, recent evidence supports the fact that antiplatelet therapy affects host immunity. Several studies have shown that ASA and clopidogrel not only diminish the risk of atherothrombotic events, but also reduce markers of systemic inflammation, including CRP, and proinflammatory cytokines (24). Antiplatelet medications such as clopidogrel and ticagrelor were also associated with reduced mortality in patients with sepsis, without causing excessive bleeding in these patients (25,26).
In cardiovascular diseases, the inhibition of the shedding of microparticles account for the efficacy of various drugs (27). Abciximab, a platelet glycoprotein IIb–IIIa antagonist acts as a potent antiplatelet agent for KD patients with giant coronary artery aneurysm or thrombosis. A recent study showed that abciximab treatments have an important role in the management of severe complications of KD, although prospective randomized controlled studies are needed to fully evaluate their efficacy in preventing thrombotic complications and promoting vascular remodeling (28).
The current study has some limitations. First, we enrolled a relatively small number of patients. Therefore, we could not determine whether our results are also applicable to different geographic populations. Second, there were no coronary aneurysms, such as a sequela in the KD group. Lastly, we could not perform the platelet function tests such as adenosine diphosphate (ADP)-induced platelet aggregation test in patients with KD and could not check the follow-up PDMP levels in control patients because this would have required a large amount of additional blood to be drawn from these small infants and young children.
In conclusion, enhanced platelet activation is noted before treatment of KD until the convalescent phase of KD as long as 4 or 5 months after the onset of KD. These results indicate that the timing of ASA discontinuation should be evaluated in each individual patient with KD. Monitoring the biomarker of platelet activation is important because they can be used as sentinels for patients with a high risk for thrombosis such as those with intracardiac stents, complex congenital heart disease, and KD. Further studies with a larger sample size and longer follow-up period are required to clarify the clinical significance of the PDMPs in the pathogenesis and treatment of KD.
Footnotes
Funding: This research was supported by Chungnam National University Hospital Research Fund, 2013.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Kil HR. Data curation: Lim YJ. Investigation: Choi EH. Writing - original draft: Kim HJ.
References
- 1.Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 2.Burns JC, Glode MP, Clarke SH, Wiggins J, Jr, Hathaway WE. Coagulopathy and platelet activation in Kawasaki syndrome: identification of patients at high risk for development of coronary artery aneurysms. J Pediatr. 1984;105:206–211. doi: 10.1016/s0022-3476(84)80114-6. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura T. Study of activated platelets in Kawasaki disease. J Jpn Pediatr Soc. 1985;89:1845–1860. [Google Scholar]
- 4.VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277–287. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 5.Nomura S. Function and clinical significance of platelet-derived microparticles. Int J Hematol. 2001;74:397–404. doi: 10.1007/BF02982082. [DOI] [PubMed] [Google Scholar]
- 6.Italiano JE, Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackman N. On the trail of microparticles. Circ Res. 2009;104:925–927. doi: 10.1161/CIRCRESAHA.109.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, Ishii M, Harada K; Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition) Pediatr Int. 2005;47:232–234. doi: 10.1111/j.1442-200x.2005.02033.x. [DOI] [PubMed] [Google Scholar]
- 9.McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, Vetter VL, Gersony WM, Mitchell PD, Newburger JW, Pediatric Heart Network Investigators Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116:174–179. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 10.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 11.Cox D, Kerrigan SW, Watson SP. Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J Thromb Haemost. 2011;9:1097–1107. doi: 10.1111/j.1538-7836.2011.04264.x. [DOI] [PubMed] [Google Scholar]
- 12.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85:195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 13.Schafer AI. Thrombocytosis: too much of a good thing? Trans Am Clin Climatol Assoc. 2002;113:68–76. [PMC free article] [PubMed] [Google Scholar]
- 14.Horstman LL, Ahn YS. Platelet microparticles: a wide-angle perspective. Crit Rev Oncol Hematol. 1999;30:111–142. doi: 10.1016/s1040-8428(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 15.Barry OP, Praticò D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taki M, Kobayashi M, Ohi C, Shimizu H, Goto K, Aso K, Murano K. Spontaneous platelet aggregation in Kawasaki disease using the particle counting method. Pediatr Int. 2003;45:649–652. doi: 10.1111/j.1442-200x.2003.01810.x. [DOI] [PubMed] [Google Scholar]
- 17.Yahata T, Suzuki C, Yoshioka A, Hamaoka A, Ikeda K. Platelet activation dynamics evaluated using platelet-derived microparticles in Kawasaki disease. Circ J. 2014;78:188–193. doi: 10.1253/circj.cj-12-1037. [DOI] [PubMed] [Google Scholar]
- 18.Strasser EF, Happ S, Weiss DR, Pfeiffer A, Zimmermann R, Eckstein R. Microparticle detection in platelet products by three different methods. Transfusion. 2013;53:156–166. doi: 10.1111/j.1537-2995.2012.03720.x. [DOI] [PubMed] [Google Scholar]
- 19.Hugel B, Zobairi F, Freyssinet JM. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1846–1847. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 20.Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–164. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- 21.Projahn D, Koenen RR. Platelets: key players in vascular inflammation. J Leukoc Biol. 2012;92:1167–1175. doi: 10.1189/jlb.0312151. [DOI] [PubMed] [Google Scholar]
- 22.Laurito M, Stazi A, Delogu AB, Milo M, Battipaglia I, Scalone G, Infusino F, Villano A, Russo G, Iannotta R, et al. Endothelial and platelet function in children with previous Kawasaki disease. Angiology. 2014;65:716–722. doi: 10.1177/0003319713502392. [DOI] [PubMed] [Google Scholar]
- 23.Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:234S–264S. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- 24.Muhlestein JB. Effect of antiplatelet therapy on inflammatory markers in atherothrombotic patients. Thromb Haemost. 2010;103:71–82. doi: 10.1160/TH09-03-0177. [DOI] [PubMed] [Google Scholar]
- 25.Storey RF, James SK, Siegbahn A, Varenhorst C, Held C, Ycas J, Husted SE, Cannon CP, Becker RC, Steg PG, et al. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets. 2014;25:517–525. doi: 10.3109/09537104.2013.842965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akinosoglou K, Alexopoulos D. Use of antiplatelet agents in sepsis: a glimpse into the future. Thromb Res. 2014;133:131–138. doi: 10.1016/j.thromres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Goto S, Tamura N, Li M, Handa M, Ikeda Y, Handa S, Ruggeri ZM. Different effects of various anti-GPIIb-IIIa agents on shear-induced platelet activation and expression of procoagulant activity. J Thromb Haemost. 2003;1:2022–2030. doi: 10.1046/j.1538-7836.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 28.Bachlava E, Loukopoulou S, Karanasios E, Chrousos G, Michos A. Management of coronary artery aneurysms using abciximab in children with Kawasaki disease. Int J Cardiol. 2016;220:65–69. doi: 10.1016/j.ijcard.2016.06.062. [DOI] [PubMed] [Google Scholar]