Abstract
Background and purpose
Transient ischemic attack (TIA) increases the risk for a subsequent stroke. Typical symptoms include motor weakness, gait disturbance, and loss of coordination. The association between the presence of motor impairments during a TIA and the chances of a subsequent stroke has not been examined. In the current meta-analysis, we examine whether the odds of a stroke are greater in TIA individuals who experience motor impairments as compared with those who do not experience motor impairments.
Methods
We conducted a systematic search of electronic databases as well as manual searches of the reference lists of retrieved articles. The meta-analysis included studies that reported an odds ratio relating motor impairments to a subsequent stroke, or the number of individuals with or without motor impairments who experienced a subsequent stroke. We examined these studies using rigorous meta-analysis techniques including random effects model, forest and funnel plots, I2, publication bias, and fail-safe analysis.
Results
Twenty-four studies with 15,129 participants from North America, Australia, Asia, and Europe qualified for inclusion. An odds ratio of 2.11 (95% CI, 1.67–2.65, p = 0.000) suggested that the chances of a subsequent stroke are increased by twofolds in individuals who experience motor impairments during a TIA compared with those individuals who have no motor impairments.
Conclusion
The presence of motor impairments during TIA is a significantly high-risk clinical characteristic for a subsequent stroke. The current evidence for motor impairments following TIA relies exclusively on the clinical reports of unilateral motor weakness. A comprehensive examination of motor impairments in TIA will enhance TIA prognosis and restoration of residual motor impairments.
Keywords: transient ischemic attack, motor impairments, stroke, odds ratio, meta-analysis
Introduction
Transient ischemic attack (TIA) is a brief neurological event caused by temporary ischemia without acute infarction (1). Clinical symptoms include motor and speech impairments (2). These focal neurological symptoms are assumed to be resolved within 24 hrs. leaving no permanent damage to the central nervous system (3). Despite this, the risk for stroke increases up to 20% following a TIA (4). Nevertheless, the association between the presence of motor impairments during a TIA and a subsequent stroke is not well understood.
Interestingly, clinicians intuitively believe that motor impairments during a TIA predispose individuals to a greater risk for stroke. However, this clinical proposition lacks empirical validation. To-date, no study has quantified the influence of motor impairments during a TIA on the odds of a subsequent stroke. Therefore, the purpose of the current meta-analysis is to examine whether the odds of a subsequent stroke are greater in individuals who experience motor impairments during a TIA compared with those who do not experience motor impairments.
Typical motor impairments associated with TIA include unilateral motor weakness, paralysis of limbs, gait disturbance, and loss of coordination (2). However, clinical reports on motor impairments during a TIA have focused primarily on decreased muscle strength or motor weakness. A primary reason for the clinical focus on motor weakness is that weakness is easily examined in clinics as reduced muscle force during manual motor testing (5). Further, the pathophysiological rationale for assessing motor weakness is that it reflects upper motor neuron dysfunction associated with TIA and is a common accompaniment of other motor impairments (6, 7). Therefore, in the current meta-analysis, we use motor weakness as the primary measure of motor impairment during a TIA.
Several prognostic scores have been used to predict the risk of stroke after TIA (8) These scores are based on clinical characteristics of the patient such as age, blood pressure, clinical symptoms of motor weakness or speech impairment, duration of symptom, and diabetes. More recently, diagnostic neuroimaging has increased the predictive power of the clinical scores (9). Undoubtedly, the cumulative scores based on clinical and imaging characteristics provide useful information for TIA management. However, the unique contribution of motor impairments to increased risk for stroke has not been determined.
We use rigorous meta-analytic techniques to determine the extent to which the presence of motor impairments influences the likelihood of a subsequent stroke. Data from 24 studies with 15,129 participants from North America, Australia, Asia, and Europe are extracted and submitted to a meta-analysis. The current meta-analysis aims to precisely quantify the influence of motor impairments during a TIA on the risk for a subsequent stroke and inform clinicians, policy makers, and public educators on the importance of identifying and recognizing motor impairments in TIA.
Materials and Methods
Study Inclusion and Exclusion Criteria
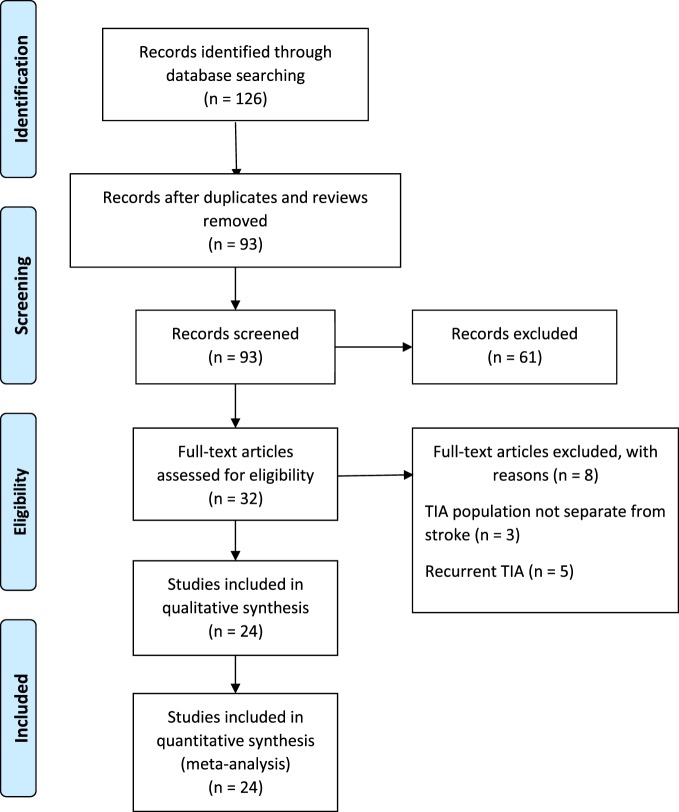
The PRISMA guidelines directed the search and reporting of this meta-analysis. We conducted an exhaustive search for TIA studies using four computerized databases: (a) MEDLINE, (b) ISI’s Web of Knowledge, (c) Cochrane Database of Systematic Reviews, and (d) PsycINFO from July 1989 to December 2016. Fourteen key words and phrases dictated the search: TIA, stroke recurrence, motor deficit, weakness, hemiparesis, limb function, unilateral weakness, coordination, impairments, walking, gait disturbance, ataxia, dysarthria, and physical limitations. Additional search strategy involved manual searches to examine the reference lists of retrieved articles. Our initial search identified 126 records that discussed motor impairments in TIA and a subsequent stroke. We excluded the systematic reviews and studies that used the same population as another included study. Ninety-three unique records remained for additional screening. Figure 1 describes the literature search and screening process.
Figure 1.
Study selection. Literature search and screening process.
The inclusion/exclusion criteria were the following: (1) the study reported an odds ratio relating motor impairments to the chances of a subsequent stroke, or the number of individuals with and without motor impairments who experienced a subsequent stroke. Thirty-two of the original 93 studies met this criterion. (2) If studies evaluated multiple populations, such as minor stroke and TIA, then data for TIA population was reported separately. Three studies did not meet this criterion (10–12). (3) The recurrent ischemic event was required to be a stroke, not a recurrent TIA. Five papers failed this criterion (13–17). Twenty-four studies remained and were submitted to the meta-analysis (18–41). Two authors (Neha Lodha and Jane Harrell) independently coded and extracted data. A divergent evaluation was resolved in consultation with a third investigator (Evangelos A. Christou). All three investigators confirmed data extractions. All authors participated in the interpretation of the meta-analytic results. Table 1 describes the design and setting of the studies included in the meta-analysis.
Table 1.
Design and setting of studies included in the meta-analysis.
| Study name | Study period | Country | Clinical setting | Ascertainment method | Transient ischemic attack (TIA) diagnosed by | Follow-up |
|---|---|---|---|---|---|---|
| Al-Khaled and Eggers (18) | 2007–2010 | Germany | Multiple EDs | Prospective (consecutive referrals to stroke registry) | Neurologist, internist | Questionnaire |
| Ay et al. (19) | 2000–2006 | USA | Single ED | Retrospective (review of inpatient/outpatient reports) | Neurologist | Medical records |
| Bray et al. (20) | 2004 | Australia | Single ED | Retrospective (admissions to ED) | ED physician | Medical records and phone |
| Chandratheva et al. (21) | 2002–2007 | England | Multiple EDs and clinics | Prospective (multiple search methods) | Neurologist | In person |
| Chen et al. (22) | 2006–2009 | China | Multiple hospitals | Prospective (admissions to hospital) | Neurologist | In person |
| Coutts et al. (23) | Undetermined | Canada | Hospital stroke center | Prospective (referral to stroke team) | Stroke neurologist | In person |
| Dai et al. (24) | 2009–2013 | China | University hospital | Prospective (stroke registry) | Neurologist | In person or phone |
| Fujinami et al. (25) | 2008–2009 | Japan | Multiple stroke hospitals | Retrospective (review of medical records) | Attending physician | Medical records |
| Gon et al. (26) | 2006–2013 | Japan | Hospital stroke unit | Retrospective (review of medical records) | Not listed | Not listed |
| Hayashi et al. (27) | 2007–2010 | Japan | Single ED | Retrospective (review of medical records) | Neurologist | Medical records |
| Johnston et al. (28) | 1997–1998 | USA | Multiple EDs | Retrospective (review of medical records) | ED physician | Medical records |
| Jove et al. (29) | 2008–2012 | Spain | Single ED | Prospective (admissions to ED) | Neurologist | In person |
| Li et al. (30) | 2010–2011 | China | Single ED | Prospective (admissions to ED) | Neurologist | In person |
| Lim et al. (31) | 2010–2012 | Korea | Multiple EDs | Prospective (admissions to ED, Korean TIA Registry) | Neurologist | In person or phone |
| Nakajima et al. (32) | 2002–2004 | Japan | Specialist cardiovascular center | Prospective (admissions to cardiovascular center) | Neurologist | Medical records or Phone |
| Ohara et al. (33) | 2008–2013 | Japan | Hospital stroke center | Retrospective (review of stroke database records) | Stroke neurologist | In person |
| Ois et al. (34) | 2004–2007 | Spain | Single ED | Prospective (admissions to ED) | Neurologist | In person or Phone |
| Ong et al. (35) | 2005–2006 | Singapore | Single ED | Retrospective (ED database and medical records) | ED physician | Medical records |
| Perry et al. (36) | 2006–2011 | Canada | Multiple EDs | Prospective (admissions to ED) | ED, neurologists, residents | Phone |
| Purroy et al. (37) | 2002–2005 | Spain | Single ED | Prospective (admissions to ED) | Neurologist | In person |
| Purroy et al. (38) | 2006–2009 | Spain | Single ED | Prospective (admissions to ED) | Neurologist | In person |
| Purroy et al. (39) | 2008–2009 | Spain | Multiple stroke centers | Prospective (admissions to stroke center) | Stroke neurologist | In person |
| Tsivgoulis et al. (40) | 2008–2009 | Greece, Singapore | Multiple neurology departments | Prospective (admissions to ED) | Neurologist | In person |
| Zhao et al. (41) | 2008–2011 | China | Neurology department | Prospective (admissions to neurology) | Neurologist | Phone |
ED, Emergency Department.
Clinical Symptoms/Outcome Measures
We identified nine outcome measures related to motor impairment (a) motor lacunar symptom, (b) motor deficits, (c) motor weakness, (d) weakness, (e) unilateral weakness, (f) unilateral motor weakness, (g) focal weakness, (h) limb weakness, and (i) hemiparesis. The study authors defined these outcomes as motor weakness. We extracted data on all available motor outcome measures from each study. Only a few studies reported more than one outcomes (18, 27, 28, 32, 36, 38, 40). To prevent data biasing, we followed standard recommendations and selected one outcome measure per study that best represented motor weakness. Table 2 lists these outcome measures and other study characteristics.
Table 2.
Characteristics of studies included in the meta-analysis.
| Study name | Age | Sex | Sample size | Clinical symptom/outcome measure | Symptom to evaluation | Clinical evaluation | Neuroimaging evaluation | Neuroimaging findings | Recurrence time |
|---|---|---|---|---|---|---|---|---|---|
| Al-Khaled and Eggers (18) | 70.6 ± 12.8 | M = 1,122, F = 1,078 | 2,200 | Unilateral Motor Weakness | 2 days | ABCD2 score | MRI with DWI | – | During hospitalization |
| Ay et al. (19) | 67.7 ± 14.7 | M = 231, F = 246 | 477 | Focal Weakness | 1 day | ABCD2 score | MRI with DWI | DWI +ve = 136; DWI −ve = 318 | 7 days |
| Bray et al. (20) | 73 ± 14.5 | M = 49, F = 49 | 98 | Unilateral Weakness | 2 days | ABCD score | – | – | 90 days |
| Chandratheva et al. (21) | 72.5 ± 12.7 | M = 219, F = 281 | 488 | Unilateral Weakness | 1 day | ABCD2 score | CT or MRI | – | 1 day |
| Chen et al. (22) | – | – | 199 | Limb Weakness | – | – | CT or MRI | – | 90 days |
| Coutts et al. (23) | Median 69 (27–99) | M = 293, F = 206 | 499 | Motor Weakness | 2 days | ABCD2 score | CT or MRI with DWI | CT +ve = 171; CT −ve = 328; DWI +ve = 243; DWI −ve = 256 | 7 days |
| Dai et al. (24) | 62 ± 12.5 | M = 486, F = 176 | 658 | Motor Weakness | 3 days | ABCD score | MRI with DWI | DWI +ve = 236; DWI −ve = 422 | 90 days |
| Fujinami et al. (25) | 69 ± 13 | M = 292, F = 172 | 464 | Hemiparesis | 7 days | ABCD2 score | MRI with DWI | DWI +ve = 96; DWI −ve = 368 | During hospitalization |
| Gon et al. (26) | 64 ± 15 | M = 88 F = 51 | 139 | Motor Weakness | 7 days | ABCD2 score | MRI with DWI | DWI +ve = 53; DWI −ve = 86 | 14 days |
| Hayashi et al. (27) | 66.6 ± 11.0 | M = 44, F = 30 | 74 | Hemiparesis | – | ABCD2 score | MRI with DWI | – | 2 years |
| Johnston et al. (28) | Mean 72 | M = 808, F = 899 | 1,707 | Weakness | – | – | – | – | 90 days |
| Jove et al. (29) | 71.7 ± 10.8 | – | 293 | Weakness | 1 day | ABCD2 score | MRI with DWI | 90 days | |
| Li et al. (30) | 67.5 ± 11.1 | M = 70, F = 36 | 106 | Motor Deficits | – | ABCD2 score | MRI with DWI | 7 days | |
| Lim et al. (31) | 64.4 ± 11.8 | M = 291, F = 209 | 500 | Motor Weakness | 2 days | ABCD2 score | MRI with DWI | DWI +ve = 140; DWI −ve = 335 | 90 days |
| Nakajima et al. (32) | 65 ± 12 | M = 81, F = 32 | 113 | Hemiparesis | 7 days | ABCD2 score | CT, MRI with DWI | DWI +ve = 39; DWI −ve = 74 | 90 days |
| Ohara et al. (33) | − | M = 263, F = 147 | 410 | Motor Lacunar Symptom | 2 days | ABCD2 score | MRI with DWI | DWI +ve = 119; DWI −ve = 291 | 7 days |
| Ois et al. (34) | − | − | 221 | Weakness | − | ABCD score | CT | 90 days | |
| Ong et al. (35) | 61 ± 13.2 | M = 293, F = 177 | 470 | Unilateral Weakness | − | ABCD2 score | CT or MRI | − | 90 days |
| Perry et al. (36) | 68.0 ± 14.4 | M = 1,930 F = 1,976 | 3,906 | Weakness | <7 days | − | CT | CT +ve = 1,101 | 7 days |
| Purroy et al. (37) | 70.8 ± 12 | M = 230, F = 158 | 388 | Weakness | 1 day | ABCD2 score | CT | – | 90 days |
| Purroy et al. (38) | 69.3 ± 11.8 | M = 156, F = 98 | 254 | Motor Weakness | 7 days | ABCD2 score | CT, MRI with DWI | DWI +ve = 117 DWI −ve = 137 | 90 days |
| Purroy et al. (39) | 68.6 ± 13.1 | M = 674, F = 463 | 1,137 | Motor Weakness | 2 days | ABCD2 score | CT, MRI with DWI | DWI +ve = 194 DWI −ve = 269 | 90 days |
| Tsivgoulis et al. (40) | 60 ± 14 | M = 82, F = 66 | 148 | Unilateral Weakness | – | ABCD2 score | CT or MRI | 90 days | |
| Zhao et al. (41) | – | M = 119, F = 61 | 180 | Weakness | – | – | – | – | 90 days |
M, males; F, females; ABCD/ABCD2, Stroke Risk Score; MRI, magnetic resonance imaging; DWI, diffusion weighted imaging; CT, computerized tomography; Recurrence time is time from TIA symptom onset to stroke.
Data Synthesis and Analysis
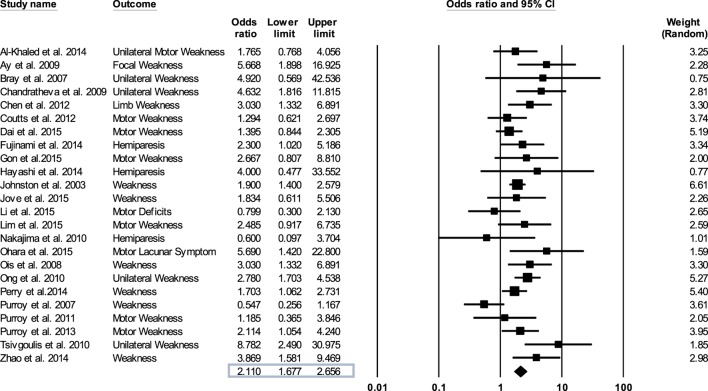
We evaluated the quality of the studies included in the meta-analysis by the Downs and Black method (42). The Comprehensive Meta-Analysis Program was used to synthesize and analyze the data extracted from the TIA studies. This procedure involved entering the odds ratios, lower and upper limits, and confidence levels relating motor weakness to subsequent stroke from each study and then determining an overall odds ratio (Figure 2). For those studies that did not report an odds ratio (18–21, 23, 24, 26, 27, 29–31, 36–41), we computed the odd ratios as OR = (A/B)/(C/D). Here, A is the number of individuals with weakness and a recurrent stroke, B is the number of individuals with weakness without a recurrent stroke, C is the number of individuals without weakness with a recurrent stroke, and D is the number of individuals without weakness and without a recurrent stroke. We conducted sensitivity analysis to determine the extent to which the odds ratio was influenced by a particular study.
Figure 2.
Forest plot derived from a random effects model. Each tick mark and line represents an individual odds ratio with a 95% confidence interval. The diamond shape at the bottom of the forest plot is the overall odds ratio (2.11) for all 24 studies.
Measuring Heterogeneity and Publication Bias
I2 was computed to determine the degree of variability across studies (43). We examined publication bias using (a) funnel plot asymmetry, (b) Duval and Tweedie’s trim and fill procedure that creates a funnel plot with imputed values inserted as close approximations to a completely unbiased distribution, and (c) fail-safe N analysis that uses the probability value of the cumulative odds ratio to determine the number of studies required to render the odds ratio insignificant.
Results
Characteristics of the Included Studies
Twenty-four studies with 15,129 participants qualified for inclusion in the meta-analysis. Figure 1 shows the step-by-step procedure of identifying studies that satisfied the criteria for inclusion in this meta-analysis. The studies were conducted between 1997 and 2013, in North America (N = 4, participants = 6,589), Australia (N = 1, participants = 98), Asia (N = 11, participants = 3,313), and Europe (N = 8, participants = 5,129). One study was conducted in two locations (40). TIA was diagnosed by a neurologist in the majority of the studies (N = 19). The duration between symptom onset to clinical evaluation ranged from 1 to 7 days. The clinical evaluation of motor impairment included ABCD or ABCD2 score (N = 20). The neuroimaging evaluations included magnetic resonance imaging (MRI; N = 18). The recurrence time from the onset of TIA symptom to occurrence of stroke varied from 1 day up to 2 years. Table 3 reports the study quality. All studies had a minimum quality score of 11 out of 17.
Table 3.
Quality scores using Downs and Black scale: checklist for measuring study quality.
| Study name | 1 | 2 | 3 | 5 | 6 | 7 | 9 | 10 | 11 | 12 | 13 | 16 | 18 | 20 | 25 | 26 | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Khaled and Eggers (18) | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 11 |
| Ay et al. (19) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 14 |
| Bray et al. (20) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Chandratheva et al. (21) | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 14 |
| Chen et al. (22) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Coutts et al. (23) | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Dai et al. (24) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 15 |
| Fujinami et al. (25) | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 16 |
| Gon et al. (26) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 11 |
| Hayashi et al. (27) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 11 |
| Johnston et al. (28) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 14 |
| Jove et al. (29) | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 14 |
| Li et al. (30) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 14 |
| Lim et al. (31) | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 16 |
| Nakajima et al. (32) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 |
| Ohara et al. (33) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 |
| Ois et al. (34) | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 14 |
| Ong et al. (35) | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 12 |
| Perry et al. (36) | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Purroy et al. (37) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 13 |
| Purroy et al. (38) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 13 |
| Purroy et al. (39) | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 16 |
| Tsivgoulis et al. (40) | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 |
| Zhao et al. (41) | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 16 |
Questions 4, 8, 14, 15, 17, 19, 21, 22, 23, 24, and 27 were not used because the studies included in the meta-analysis were not interventional. Questions 1, 2, 3, 5, 6, 7, 9, 10, 11, 12, 13, 16, 18, 20, 25, and 26 add to a maximum total possible score of 17.
Motor Impairments in TIA and Subsequent Stroke
Figure 2 shows forest plot of the odds ratio across individual studies. The odds ratio was computed using random effects model to determine the relation between the presence of motor impairments during a TIA and the chances of a subsequent stroke. The model revealed a pooled odds ratio of 2.11 (95% CI, 1.67–2.65; p = 0.000). Figure 2 shows the overall odds ratio to the right of the vertical line of no effect (1.00), indicating that the odds of a subsequent stroke are doubled in individuals who experience motor impairment during a TIA. The sensitivity analysis revealed that the odds of a subsequent stroke did not alter considerably with the exclusion of individual studies. Further, when studies with highest risk of bias (quality scores < 14) (18, 26, 27, 35, 37, 38) were excluded the overall odds ratio improved to 2.12 (95% CI, 1.58–2.84; p = 0.000).
Heterogeneity
Measurements of heterogeneity revealed an I2 of 45.85 (p = 0.008). Because of this relatively large proportion of dispersion in the TIA studies, we conducted a random effects meta-analysis. Plotting the log odds ratio as a function of standard error revealed a symmetrical funnel plot (Figure 3A). This symmetrical funnel plot represents an unbiased summary effect. The majority of studies congregating on the top half of the funnel indicate the large sample sizes of the studies and a more precise estimate of the odds ratio with a smaller standard error.
Figure 3.
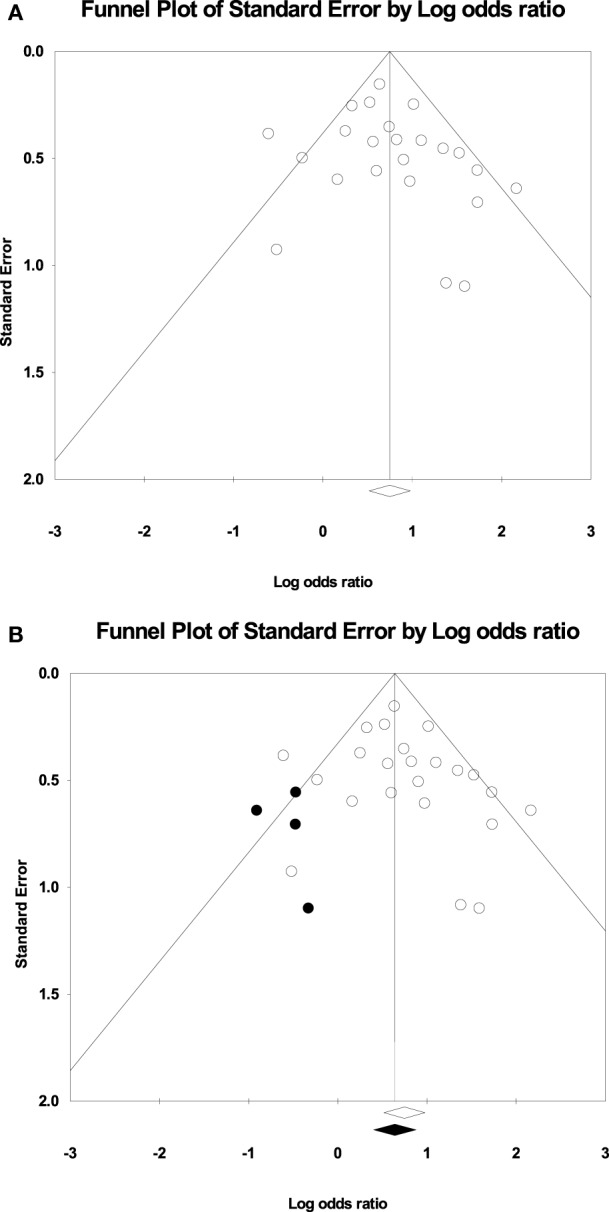
(A) Funnel plot of the studies for the random effects model: The x-axis is the log odds ratio, and the y-axis is the standard error associated with each study. (B) Funnel plot with imputed studies. Open circles represent the 24 original studies whereas black circles represent imputed studies.
Publication Bias
Figure 3B shows the funnel plot with imputed studies using Duval and Tweedie’s trim and fill technique (43). Only four studies were imputed on the left side of the plot to achieve symmetry, signifying an unbiased effect. The black diamond on the x-axis indicating the recalculated log odds ratio is highly similar to the original log odds ratio. The fail-safe analysis revealed that 462 null effect findings were necessary to lower the cumulative odds ratio of 2.11 to an insignificant level. Therefore, the odds ratio associating the presence of motor impairments in TIA with a recurrent stroke offers a robust finding.
Discussion
The purpose of the current meta-analysis was to examine whether the odds of a recurrent stroke are greater in TIA individuals who experience motor impairments compared with those who do not experience motor impairments. Our meta-analysis included 24 high-quality studies that examined motor impairments in TIA and a subsequent stroke in 15,129 individuals. The novel finding from this meta-analysis is that TIA individuals with motor impairments are twice as likely to experience a stroke as compared with those who have no motor impairments. Thus, motor impairments during TIA are a significantly high-risk clinical characteristic for a recurrent stroke.
Traditionally, stroke risk is determined using ABCD2 score in the clinical settings (40). ABCD2 score is a cumulative score derived from multiple TIA characteristics including motor and speech impairment (44). However, the evidence supporting ABCD2 score for predicting stroke risk remains inconclusive. While previous studies showed that TIA individuals with ABCD2 score of >3 were at a high early risk of stroke (44, 45), more recent studies have questioned the reliability of the score in distinguishing the low and high risk of stroke recurrence (46, 47). The current meta-analysis suggests that the odds of a recurrent stroke are doubled in individuals who experience motor impairments during a TIA. These findings complement the clinical ABCD2 score that ascribes twice the predictive weight to unilateral motor weakness than speech impairment. Further, our findings are consistent with a recent study in urgent care setting that reported greater risk of stroke in TIA individuals with unilateral motor weakness (48). Regardless of the predictive capacity of the cumulative score, emerging evidence clearly suggests that the presence of motor impairments during a TIA in itself is a compelling determinant of the increased odds of a subsequent stroke.
One question concerns why are the odds of a subsequent stroke increased when motor impairments are present during a TIA. Clinical reports indicate that motor impairments during a TIA are often concomitant with structural brain abnormalities (49). Additionally, the presence of acute brain lesions detected as positive diffusion-weighted imaging (DWI) significantly increases the probability of a subsequent stroke following TIA (50, 51). Thus, one possibility is that perhaps motor impairments during a TIA increase the odds of a subsequent stroke because of an underlying ischemic lesion. Future studies are needed to clearly identify the mechanisms underlying increased stroke risk in TIA individuals with motor impairments.
Current standard of diagnostic protocol for the evaluation of motor deficits in TIA focuses primarily on the assessment of motor weakness. However, individuals with TIA experience multiple motor impairments including reduced coordination, impaired motor control, gait disturbance, dysarthria, and ataxia (36, 52). Thus, the clinical diagnosis of motor weakness may be inadequate for identifying residual motor deficits following TIA (53, 54). Clearly then, the absence of comprehensive motor examination for TIA individuals potentially understates the extent to which motor impairments are prevalent following TIA.
Further, initial symptoms are considered to be resolved shortly after the onset of TIA. Contrary to this conventional view point, recent evidence suggests that individuals experience subtle problems in functional activities of daily living up to 6 months after TIA (55, 56). This is corroborated by a recent report indicating that TIA individuals are more likely to consult clinicians for residual impairments than age-matched controls (54). Furthermore, about 50% of the individuals who have a TIA require rehabilitation to resolve the residual impairments that affect everyday function (55, 57). Thus, the supposed transient nature of initial motor impairments may not be so transient, pointing to the pressing need for a systematic evaluation and restoration of motor abilities beyond the clinically determined weakness in individuals with TIA.
Considerations and Future Directions
Few limitations of the meta-analysis findings require consideration. First, our findings may be modest in determining on the true impact of motor impairments on the odds of a recurrent stroke. This is because we extracted the data on most widely reported motor impairment, i.e., motor weakness, despite multiple motor symptoms associated with TIA. In addition, the relatively large variability in the time from symptom onset to evaluation at hospital admission contributes to the unrecognized diagnosis of motor impairments due to symptom recession or missed reporting by individuals who sought delayed medical attention. Further, the tissue-based definition for TIA diagnosis recommends the use of neuroimaging for distinguishing a minor stroke from a TIA. However, the majority of studies included in this meta-analysis based their diagnosis of TIA on the time-based definition. Finally, these findings do not understate the importance of careful clinical follow-up in TIA individuals without motor impairments. Rather, they point to the need for differential clinical care and rehabilitation of TIA individuals who experience motor impairments. In summary, the findings from the current meta-analysis offer strong evidence favoring significantly greater risk of a subsequent stroke in TIA individuals who experience motor impairments.
Given the findings from this meta-analysis, a comprehensive clinical evaluation of motor impairments is highly recommended. For example, examining motor output variability, accuracy, symmetry, and coordination deficits will provide more meaningful insights into the overall decline in motor ability following TIA. Our past work shows that motor control abilities are more predictive of everyday function in older adults than motor weakness (58). Thus, a comprehensive motor examination following TIA will further our understanding of the impact of motor impairments on functional tasks of daily living and improve the treatment and rehabilitation of residual motor impairments.
Conclusion
Transient ischemic attack individuals with motor impairments are at significantly greater risk for a recurrent stroke. The current evidence for motor impairments following TIA relies exclusively on the clinical reports of motor weakness. A comprehensive examination of motor impairments in TIA will enhance TIA prognosis and restoration of residual motor impairments.
Author Contributions
NL, JH, and EC were involved in conceptualization, design, data coding, extraction. NL, JH, SE, EC participated in the interpretation of the meta-analytic results. NL and JH wrote the first draft of the manuscript. NL, JH, SE, and EC revised and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Agostina Casamento-Moran for computational assistance.
Footnotes
Funding. American Heart Association (Scientist Development Award 14SDG20450151 to NL) and National Institutes of Health (R21NS096258 to NL and EC) provided the funding support for this work.
References
- 1.Easton D, Saver J, Albers G, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/ American Stroke Association stroke council; council on cardiovascular surgery and anesthesia; council on cardi. Stroke (2009) 40:2276–93. 10.1161/STROKEAHA.108.192218 [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics 2015 update: a report from the American Heart Association. Circulation (2015) 131:e29–39. 10.1161/CIR.0000000000000157 [DOI] [PubMed] [Google Scholar]
- 3.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA (2000) 284:2901–6. 10.1001/jama.284.22.2901 [DOI] [PubMed] [Google Scholar]
- 4.Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med (2007) 167:2417–22. 10.1001/archinte.167.22.2417 [DOI] [PubMed] [Google Scholar]
- 5.Simmons BB, Cirignano B, Gadegbeku AB. Transient ischemic attack: part I. Diagnosis and evaluation. Am Fam Physician (2012) 86(6):521–6. [PubMed] [Google Scholar]
- 6.Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain (1989) 112:749–63. 10.1093/brain/112.3.749 [DOI] [PubMed] [Google Scholar]
- 7.Patten C, Lexell J, Brown HE. Weakness and strength training in persons with poststroke hemiplegia: rationale, method, and efficacy. J Rehabil Res Dev (2004) 41:293–312. 10.1682/JRRD.2004.03.0293 [DOI] [PubMed] [Google Scholar]
- 8.Sorensen A, Ay H. Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am (2011) 21:303–13. 10.1016/j.nic.2011.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiyohara T, Kamouchi M, Kumai Y, Ninomiya T, Hata J, Yoshimura S, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke (2014) 45:418–25. 10.1161/STROKEAHA.113.003077 [DOI] [PubMed] [Google Scholar]
- 10.Camden M-C, Hill MD, Demchuk AM, Poppe AY, Shobha N, Barber PA, et al. Predicts progression in TIA/ minor stroke. Can J Neurol Sci (2014) 41:19–23. 10.1017/S0317167100016206 [DOI] [PubMed] [Google Scholar]
- 11.Coutts SB, Eliasziw M, Hill MD, Scott JN, Subramaniam S, Buchan AM, et al. An improved scoring system for identifying patients at high early risk of stroke and functional impairment after an acute transient ischemic attack or minor stroke. Int J Stroke (2008) 3:3–10. 10.1111/j.1747-4949.2008.00182.x [DOI] [PubMed] [Google Scholar]
- 12.Erdur H, Scheitz JF, Ebinger M, Rocco A, Grittner U, Meisel A, et al. In-hospital stroke recurrence and stroke after transient frequency and risk factors. Stroke (2015) 46:1031–7. 10.1161/STROKEAHA.114.006886 [DOI] [PubMed] [Google Scholar]
- 13.Dolatabadi AA, Meisami A, Hatamabadi H, Mansori B, Shahrami A, Amini A, et al. Improving the prediction of stroke or death after transient ischemic attack (TIA) by adding diffusion-weighted imaging lesions and TIA etiology to the ABCD2 score. J Stroke Cerebrovasc Dis (2013) 22:e25–30. 10.1016/j.jstrokecerebrovasdis.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Engelter ST, Amort M, Jax F, Weisskopf F, Katan M, Burow A, et al. Optimizing the risk estimation after a transient ischaemic attack – the ABCDE score. Eur J Neurol (2012) 19:55–61. 10.1111/j.1468-1331.2011.03428.x [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Han SW, Heo JH. Predictive implications of recurrent transient ischemic attacks in large-artery atheroslerosis. Cerebrovasc Dis (2006) 22:240–4. 10.1159/000094010 [DOI] [PubMed] [Google Scholar]
- 16.Nah H-W, Kwon S, Kang D-W, Lee D-H, Kim J. Diagnostic and prognostic value of multimodal MRI in transient ischemic attack. Int J Stroke (2014) 9:895–901. 10.1111/ijs.12212 [DOI] [PubMed] [Google Scholar]
- 17.Park K, Youn YC, Chung C, Lee KH, Kim GDM, Chung PDM, et al. Large-artery stenosis predicts subsequent vascular events in patients with transient ischemic attack. J Clin Neurol (2007) 3:169–74. 10.3988/jcn.2007.3.4.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Khaled M, Eggers J. Early hospitalization of patients with TIA: a prospective, population-based study. J Stroke Cerebrovasc Dis (2014) 23:99–105. 10.1016/j.jstrokecerebrovasdis.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 19.Ay H, Arsava EM, Johnston SC, Vangel M, Schwamm LH, Furie KL, et al. Clinical- and imaging-based prediction of stroke risk after TIA: the CIP model. Stroke (2009) 40:181–6. 10.1161/STROKEAHA.108.521476 [DOI] [PubMed] [Google Scholar]
- 20.Bray JE, Coughlan K, Bladin C. Can the ABCD Score be dichotomised to identify high-risk patients with ischaemic attack in the emergency department? Emerg Med J (2007) 24:92–6. 10.1136/emj.2006.041624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandratheva A, Mehta Z, Geraghty OC, Marquardt L, Rothwell PM, Oxford Vascular Study . Population-based study of risk and predictors of stroke in the first few hours after a TIA. Neurology (2009) 72:1941–7. 10.1212/WNL.0b013e3181a826ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Zhao Y, Zhang J, Wang H, Wang X, Ma X, et al. Analysis of multiple risk factors for the recurrence of nondisabling stroke. J Natl Med Assoc (2012) 104:331–5. 10.1016/S0027-9684(15)30173-5 [DOI] [PubMed] [Google Scholar]
- 23.Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD, et al. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke results of the prospective CATCH study. Stroke (2012) 43:1013–7. 10.1161/STROKEAHA.111.637421 [DOI] [PubMed] [Google Scholar]
- 24.Dai Q, Sun W, Xiong Y, Hankey GJ, Xiao L, Zhu W, et al. From clinical to tissue-based dual TIA Validation and refinement of ABCD3-I score. Neurology (2015) 84:1426–32. 10.1212/WNL.0000000000001444 [DOI] [PubMed] [Google Scholar]
- 25.Fujinami J, Uehara T, Kimura K, Okada Y, Hasegawa Y, Tanahashi N, et al. Incidence and predictors of ischemic stroke events during hospitalization in patients with transient ischemic attack. Cerebrovasc Dis (2014) 37:330–5. 10.1159/000360757 [DOI] [PubMed] [Google Scholar]
- 26.Gon Y, Sakaguchi M, Okazaki S, Mochizuki H, Kitagawa K. Prevalence of positive diffusion-weighted imaging findings and ischemic stroke recurrence in transient ischemic attack. J Stroke Cerebrovasc Dis (2015) 24:1000–7. 10.1016/j.jstrokecerebrovasdis.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 27.Hayashi T, Kato Y, Nagoya H, Ohe Y, Deguchi I, Fukuoka T, et al. Prediction of ischemic stroke in patients with tissue-defined transient ischemic attack. J Stroke Cerebrovasc Dis (2014) 23:1368–73. 10.1016/j.jstrokecerebrovasdis.2013.11.020 [DOI] [PubMed] [Google Scholar]
- 28.Johnston SC, Sidney S, Bernstein AL, Gress DR. A comparison of risk factors for recurrent TIA and stroke in patients diagnosed with TIA. Neurology (2003) 60:280–5. 10.1212/01.WNL.0000042780.64786.EF [DOI] [PubMed] [Google Scholar]
- 29.Jové M, Mauri-capdevila G, Suarez I, Cambray S, Sanahuja J, Quilez A, et al. Metabolomics predicts stroke recurrence after transient ischemic attack. Neurology (2015) 84:36–45. 10.1212/WNL.0000000000001093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q, Zhu X, Feng C, Fang M, Liu X. Duration of symptom and ABCD2 score as predictors of risk of early recurrent events after transient ischemic attack: a hospital-based case series study. Med Sci Monit (2015) 21:262–7. 10.12659/MSM.892525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim J-S, Hong K-S, Kim G-M, Bang OY, Bae H-J, Kwon H-M, et al. Cerebral microbleeds and early recurrent stroke after transient ischemic attack. JAMA Neurol (2015) 72:301. 10.1001/jamaneurol.2014.3958 [DOI] [PubMed] [Google Scholar]
- 32.Nakajima M, Hirano T, Naritomi H, Minematsu K. Symptom progression or fluctuation in transient ischemic attack patients predicts subsequent stroke. Cerebrovasc Dis (2010) 29:221–7. 10.1159/000267844 [DOI] [PubMed] [Google Scholar]
- 33.Ohara T, Uehara T, Toyoda K, Suzuki R, Sato S, Nagatsuka K, et al. Early stroke risk after transient ischemic attack in patients without large-artery disease or atrial fibrillation. J Stroke Cerebrovasc Dis (2015) 24:1656–61. 10.1016/j.jstrokecerebrovasdis.2015.03.039 [DOI] [PubMed] [Google Scholar]
- 34.Ois A, Gomis M, Rodriguez-Campello A, Cuadrado-godia E, Jimenez-Conde J, Pont-Sunyer C, et al. Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke (2008) 39:1717–21. 10.1161/STROKEAHA.107.505438 [DOI] [PubMed] [Google Scholar]
- 35.Ong H, Huak Y, Ping W, Rn L. Validating the ABCD2 score for predicting stroke risk after transient ischemic attack in the ED. Am J Emerg Med (2010) 28:44–8. 10.1016/j.ajem.2008.09.027 [DOI] [PubMed] [Google Scholar]
- 36.Perry JJ, Sharma M, Sivilotti MLA, Sutherland J, Worster A, Émond M, et al. A prospective cohort study of patients with transient ischemic attack to identify high-risk clinical characteristics. Stroke (2014) 45:92–100. 10.1161/STROKEAHA.113.003085 [DOI] [PubMed] [Google Scholar]
- 37.Purroy F, Montaner J, Molina C, Delgao P, Ribo M, Alvarez-Sabin J. Patterns and predictors of early risk of recurrence after transient ischemic attack with respect to etiologic subtypes. Stroke (2007) 38:3225–9. 10.1161/STROKEAHA.107.488833 [DOI] [PubMed] [Google Scholar]
- 38.Purroy F, Begue R, Gil MI, Quilez A, Sanahuja J, Brieva L, et al. Patterns of diffusion-weighted magnetic resonance imaging associated with etiology improve the accuracy of prognosis after transient ischaemic attack. Eur J Neurol (2011) 18:121–8. 10.1111/j.1468-1331.2010.03080.x [DOI] [PubMed] [Google Scholar]
- 39.Purroy F, Jiménez Caballero P, Gorospe A, Torres MJ, Alvarez-Sabin J, Santamarina E, et al. Recurrent transient ischaemic attack and early risk of stroke: data from the PROMAPA study. J Neurol Neurosurg Psychiatry (2013) 84:596–603. 10.1136/jnnp-2012-304005 [DOI] [PubMed] [Google Scholar]
- 40.Tsivgoulis G, Stamboulis E, Sharma VK, Heliopoulos I, Voumvourakis K, Teoh HL, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology (2010) 74:1351–7. 10.1212/WNL.0b013e3181dad63e [DOI] [PubMed] [Google Scholar]
- 41.Zhao J, Zhou M, Guo J, Zhang J, Yang Y, Yu F, et al. Differences in the knowledge and compliance with secondary prevention of stroke between transient ischaemic attack patients with and without subsequent stroke. J Clin Nurs (2014) 23:2939–48. 10.1111/jocn.12530 [DOI] [PubMed] [Google Scholar]
- 42.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health (1998) 52:377–84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. 9th ed Chippenham, Wiltshire: John Wiley & Sons, Ltd; (2009). [Google Scholar]
- 44.Johnston SC, Rothwell PM, Nguyen-huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet (2007) 369:283–92. 10.1016/S0140-6736(07)60150-0 [DOI] [PubMed] [Google Scholar]
- 45.Fothergill A, Christianson TJH, Brown RD, Rabinstein AA. Validation and refinement of the ABCD2 score: a population-based analysis. Stroke (2009) 40:2669–73. 10.1161/STROKEAHA.109.553446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wardlaw JM, Brazzelli M, Chappell FM, Miranda H, Shuler K, Sandercock PAG, et al. ABCD2 score and secondary stroke prevention. Neurology (2015) 85:373–80. 10.1212/WNL.0000000000001780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perry J, Symington C, Worster A, Émond M, Stotts G, Jin AY, et al. Prospective validation of the ABCD2 score for patients in the emergency department with transient ischemic attack. Can Med Assoc J (2011) 183:1137–45. 10.1503/cmaj.101668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valls J, Peiro-Chamarro M, Cambray S, Molina-Seguin J, Benabdelhak I, Purroy F. A current estimation of the early risk of stroke after transient ischemic attack: a systematic review and meta-analysis of recent intervention studies. Cerebrovasc Dis (2017) 43:90–8. 10.1159/000452978 [DOI] [PubMed] [Google Scholar]
- 49.Redgrave J, Coutts S, Schulz U, Briley D, Rothwell P. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke (2007) 38:1482–8. 10.1161/STROKEAHA.106.477380 [DOI] [PubMed] [Google Scholar]
- 50.Giles MF, Albers GW, Amarenco P, Arsava EM, Asimos AW, Ay H, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology (2011) 77:1222–8. 10.1212/WNL.0b013e3182309f91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calvet D, Touzé E, Oppenheim C, Turc G, Meder J-F, Mas J-L. DWI lesions and TIA etiology improve the prediction of stroke after TIA. Stroke (2008) 40:187–92. 10.1161/STROKEAHA.108.515817 [DOI] [PubMed] [Google Scholar]
- 52.Batchelor FA, Williams SB, Wijeratne T, Said CM, Petty S. Balance and gait impairment in transient ischemic attack and minor stroke. J Stroke Cerebrovasc Dis (2015) 24:2291–7. 10.1016/j.jstrokecerebrovasdis.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 53.Moran GM, Calvert M, Feltham MG, Ryan R, Marshall T. A retrospective cohort study to investigate fatigue, psychological or cognitive impairment after TIA: protocol paper. BMJ Open (2015) 5:1–4. 10.1136/bmjopen-2015-008149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner GM, Calvert M, Feltham MG, Ryan R, Marshall T. Ongoing impairments following transient ischaemic attack: retrospective cohort study. Eur J Neurol (2016) 23:1642–50. 10.1111/ene.13088 [DOI] [PubMed] [Google Scholar]
- 55.Verbraak ME, Hoeksma AF, Lindeboom R, Kwa VIH. Subtle problems in activities of daily living after a transient ischemic attack or an apparently fully recovered non-disabling stroke. J Stroke Cerebrovasc Dis (2012) 21:124–30. 10.1016/j.jstrokecerebrovasdis.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 56.Strømmen AM, Christensen T, Jensen K. Quantitative measurement of physical activity in acute ischemic stroke and transient ischemic attack. Stroke (2014) 45:3649–55. 10.1161/STROKEAHA.114.006496 [DOI] [PubMed] [Google Scholar]
- 57.Strømmen AM, Christensen T, Jensen K. Intensive treadmill training in the acute phase after ischemic stroke. Int J Rehabil Res (2016) 39:145–52. 10.1097/MRR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 58.Lodha N, Moon H, Kim C, Onushko T, Christou EA. Motor output variability impairs driving ability in older adults. J Gerontol A Biol Sci Med Sci (2016) 71:1676–81. 10.1093/gerona/glw013 [DOI] [PubMed] [Google Scholar]