ABSTRACT
Otitis media (OM) is a common pediatric disease, and nontypeable Haemophilus influenzae (NTHI) is the predominant pathogen in chronic OM, recurrent OM, and OM associated with treatment failure. OM is also a polymicrobial disease, wherein an upper respiratory tract viral infection predisposes to ascension of NTHI from the nasopharynx, the site of colonization, to the normally sterile middle ear, resulting in disease. Using a clinically relevant viral-bacterial coinfection model of NTHI-induced OM, we performed transcutaneous immunization (TCI) via a band-aid delivery system to administer each of three promising NTHI vaccine candidates derived from bacterial adhesive proteins and biofilm mediators: recombinant soluble PilA (rsPilA), chimV4, and integration host factor. Each immunogen was admixed with the adjuvant LT(R192G/L211A), a double mutant of Escherichia coli heat-labile enterotoxin, and assessed for relative ability to prevent the onset of experimental OM. For each cohort, the presence of circulating immunogen-specific antibody-secreting cells and serum antibody was confirmed prior to intranasal NTHI challenge. After bacterial challenge, blinded video otoscopy and tympanometry revealed a significant reduction in the proportion of animals with signs of OM compared to levels in animals receiving adjuvant only, with an overall vaccine efficacy of 64 to 77%. These data are the first to demonstrate the efficacy afforded by TCI with a band-aid vaccine delivery system in a clinically relevant polymicrobial model of OM. The simplicity of TCI with a band-aid and the significant efficacy observed here hold great promise for reducing the global burden of OM in the pediatric population.
KEYWORDS: IHF, biofilms, chimV4, nontypeable Haemophilus influenzae, rsPilA
INTRODUCTION
Transcutaneous immunization (TCI) is a noninvasive strategy to induce an immune response by engagement of the numerous antigen-presenting cells within the dermis and epidermis (1, 2). This regime results in both systemic and mucosal immune responses, important outcomes as the mucosae serve as critical defensive barriers to antigenic insult and bacterial disease (3, 4). TCI is needle free, which is expected to aid in patient compliance, to limit risks associated with both administration and waste, and to reduce costs related to formulation and delivery (5). Thus, TCI promises to facilitate greater vaccine distribution.
Otitis media (OM) is a disease of the uppermost respiratory tract mucosa and is one of the most common bacterial diseases of childhood due to a multifactorial combination of anatomical, immunological, and environmental factors (6). OM is also a polymicrobial disease caused by one or more of several bacterial species that typically reside within the human nasopharynx. The ability of nontypeable Haemophilus influenzae (NTHI), Streptococcus pneumoniae, and Moraxella catarrhalis to ascend from the nasopharynx through the Eustachian tube and gain access to the normally sterile middle ear space is facilitated by perturbation of the physical and innate immune defenses of the upper airway, often induced by prior or concurrent upper respiratory tract viral infection (7). As such, OM involves a complex interplay between respiratory tract viruses and the nasopharyngeal bacterial flora (8, 9).
Typical treatment strategies for OM include antibiotic therapy and/or “watchful waiting” for acute OM and tympanostomy tube insertion for chronic and recurrent OM (10, 11), and while these strategies are often successful for managing the current episode, they are ineffective in preventing future incidences of disease. Immunization to prevent the onset of OM is the preferred goal, with a tremendous associated potential to diminish dependence on the aforementioned therapeutic strategies and their associated risks, such as development of antibiotic resistance, for which treatment of OM is considered a major driving force worldwide (12). Importantly, immunization against the first incidence of OM is projected to limit subsequent episodes and their associated sequelae (13).
As adherence to the respiratory mucosa and biofilm formation contribute significantly to the chronic and recurrent nature of OM due to NTHI, one interventional strategy is to target both adhesive proteins expressed by this bacterium as well as the proteins essential to the formation and structural stability of its biofilms. We have developed three immunogens that demonstrate efficacy against NTHI both in vitro and preclinically in animal models of disease. These include the following: an NTHI type IV pilus (Tfp)-targeted recombinant protein called rsPilA, for recombinant soluble PilA, designed to inhibit NTHI adherence, twitching motility, and biofilm formation (14); a chimeric immunogen that targets both NTHI outer membrane protein (OMP) P5 and Tfp, called chimV4, designed to block adherence and pathogenesis of NTHI as mediated by two important adhesive proteins/virulence determinants (14); and integration host factor (IHF), a DNABII protein family member that serves as a critical structural element to the extracellular DNA scaffold within the extracellular polymeric substance incorporated into biofilms formed by many bacterial species (15–17).
We have demonstrated in animal models using chinchillas challenged by direct inoculation of the middle ear with NTHI to induce experimental OM therapeutic and prophylactic protection when vaccine formulations were pipetted onto the pinnae (or outer ear) of the animals and therapeutic efficacy after TCI was performed via a simple small circular band-aid placed at the postauricular region (skin just behind the ear) (18, 19). The means for achieving efficacy are multifold: the anatomical location of band-aid placement, migration of dermal dendritic cells to the nasal-associated lymphoid tissue, activation of gamma interferon (IFN-γ)- and interleukin-17A (IL-17A)-producing CD4+ T cells, and production of immunogen-specific antibody (18–20). Our current work extends these findings to now examine the efficacy afforded by TCI with a band-aid to prevent the onset of OM using a polymicrobial model of disease. To do so, our lead candidates, chimV4, rsPilA, and IHF, were administered by band-aid either singly or in combination. Immunogens were admixed with the potent adjuvant LT(R192G/L211A) or dmLT, a double mutant of Escherichia coli heat-labile enterotoxin (21), to potentiate the immune response induced by the incorporated antigens, and efficacy was assessed in a polymicrobial model that more closely mimics the natural progression of disease in children in whom an upper respiratory tract viral infection predisposes to development of bacterial OM (22, 23). These data confirm the utility of TCI via simple band-aid delivery to induce an immune response that prevents the development of NTHI-induced OM and further reveal the kinetics of inhibition that were unique to each immunogen tested.
RESULTS
Detection of immunogen-specific antibody-secreting cells within blood after TCI.
Prior to NTHI challenge, we collected blood to quantify the relative number of immunogen-specific antibody-secreting cells (ASCs) and further discriminated among cells that secreted IgM, IgG, or IgA. ASCs that produced immunogen-specific antibody were detected in all cohorts, and their presence confirmed the immunogenicity of rsPilA, chimV4, and IHF when they were administered by TCI and the ability of TCI to induce an immune response (Fig. 1). Moreover, there was no statistically significant difference in either the total number of ASCs among the cohorts or the relative quantity of cells that secreted a particular antibody isotype; thus, any differences among the cohorts in terms of observed protective efficacy against experimental OM could be attributed, at least in part, to the relative availability and/or accessibility of the respective targets. ASCs secreting immunogen-specific IgG and IgM predominated over those secreting IgA in each cohort, and the presence of comparable quantities of the two antibody isotypes indicated an immune response still in maturation, as expected since the cells were obtained after an abbreviated immunization regimen (only 7 days after receipt of two immunizing doses administered 1 week apart). For the cohort immunized with 5 μg of rsPilA plus 5 μg of IHF, while approximately half of the ASCs secreted rsPilA-specific antibody and half produced IHF-specific antibody, the total number of ASCs was comparable to that of the cohorts immunized with 10 μg of either immunogen individually. Collectively, TCI with chimV4, rsPilA, or IHF elicited the production of comparable numbers of immunogen-specific ASCs, which were present within the blood prior to bacterial challenge.
FIG 1.
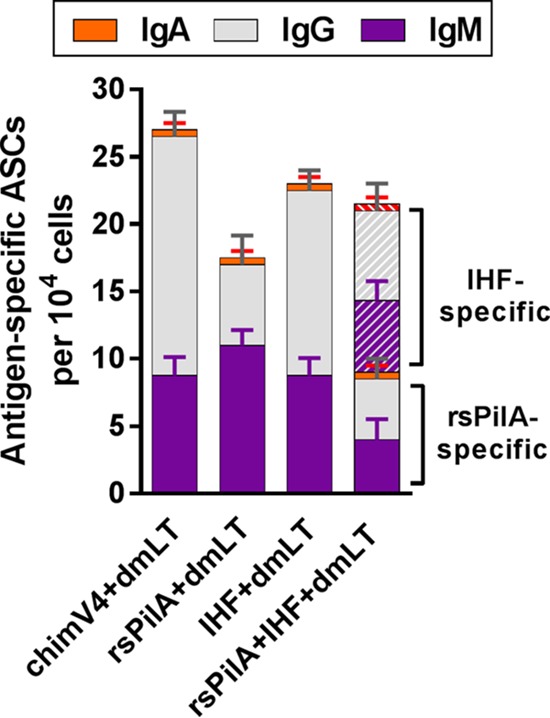
ASCs that secreted immunogen-specific antibody were present in blood recovered from chinchillas immunized by TCI with a band-aid. Blood was collected 9 days after receipt of the second of two immunizing doses delivered 1 week apart, prior to NTHI challenge. Antibody production by resiquimod- and IL-2-stimulated lymphocytes was assessed by ELISPOT assay. The total numbers of ASCs that produced immunogen-specific IgM, IgG, and IgA were comparable among the cohorts. The mean ± SEM for each antibody isotype within each cohort is presented.
Quantitation of immunogen-specific antibody isotypes in serum.
As a correlate to the observed number of ASCs in the blood of immunized chinchillas, we also examined the relative quantity of antigen-specific IgM, IgG, and IgA in serum by enzyme-linked immunosorbent assay (ELISA). As anticipated, immunogen-specific antibody was detected in serum recovered from all animals (Fig. 2), and there were no statistically significant differences in the total amounts of antibody detected among the four cohorts (P = 0.43). Within each cohort, antibody of the IgM and IgG isotypes predominated over the IgA isotype, and the relative quantities of immunogen-specific IgM and IgG were comparable. These data correlated with the determined number of ASCs (Fig. 1). In past studies wherein vaccine formulations were pipetted onto the skin of the pinnae (or outer ear) of chinchillas, an immunogen-specific IgG geometric mean titer (GMT) of ≥160 was found to be associated with enhanced resolution of experimental NTHI-induced OM (18). In the present study, wherein TCI was performed by application of a band-aid, three of the four cohorts exceeded this GMT value (GMT of chimV4 with dmLT, 185.1; GMT of IHF with dmLT, 200.9; GMT of rsPilA with IHF and dmLT, 185.1) when assayed versus their respective immunogens, whereas the GMT for the cohort administered rsPilA plus dmLT was close to the targeted value (GMT of 139.0). Therefore, these data indicated that animals immunized with chimV4, rsPilA, IHF, or a combination thereof were predicted to possess a quantity of immunogen-specific serum antibody that potentially could provide protection against development of experimental OM.
FIG 2.
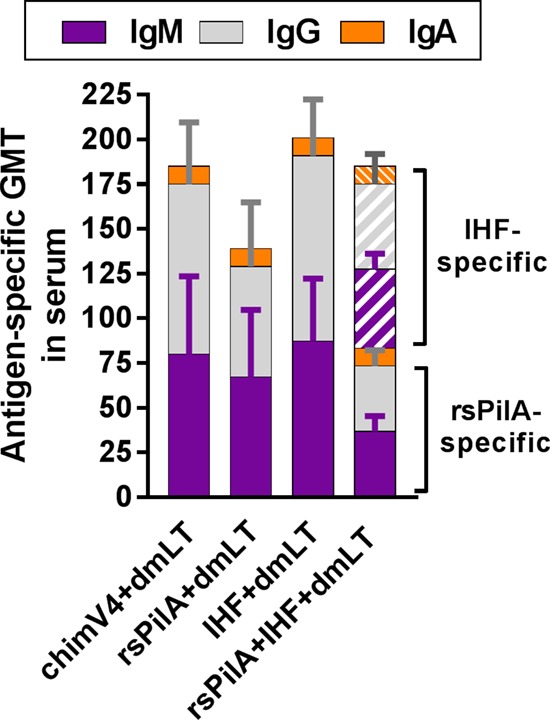
Immunogen-specific IgM, IgG, and IgA were detected in sera recovered from chinchillas after TCI. Blood was collected 9 days after receipt of the second of two immunizing doses delivered a week apart and prior to NTHI challenge. Serum was assessed for relative quantity of immunogen-specific IgM, IgG, and IgA by ELISA. There was no statistically significant difference in the total quantities of antibody induced by TCI via band-aid among the cohorts. The geometric mean ± SD for each antibody isotype is shown.
We have shown protection in previous nonpolymicrobial (e.g., no virus) models and that serum antibody and antibody present at mucosal surfaces are important for clearance of NTHI (18, 20). In our current work, we did not wish to disrupt the kinetics of NTHI ascension from the nasopharynx into the middle ear that might occur due to repeated lavage of the nasal cavity or middle ear space to collect sequential fluids for assessment. However, as viral challenge induces transudation of serum antibody onto mucosal surfaces, including the mucosa which lines the middle ear space (24), this phenomenon likely both supplemented the total antibody already present on the mucosae and contributed to the protection observed in this study.
Nasopharyngeal colonization.
Two days after intranasal challenge with NTHI, a single nasopharyngeal lavage (NPL) was performed to confirm the presence and relative abundance of NTHI that was now colonizing this anatomical niche. NPLs are not performed at later time points in this experimental model in order to facilitate the natural kinetics of NTHI ascension from the nasopharynx to the middle ear in contrast to what might be induced by reflux of NPL fluids into the middle ear space under the condition of repeated anesthesia. At this early time point relative to bacterial challenge, NTHI was detected within all lavage fluids (Fig. 3) and at concentrations that were both highly comparable among cohorts as well as to levels in published reports using this chinchilla superinfection model (mean for all animals, 3.7 × 103 ± 1 × 103 CFU of NTHI per ml of nasopharyngeal lavage fluid) (25–27). There was no statistically significant difference in relative bacterial loads among cohorts (P = 0.54); therefore, the potential to develop experimental OM was comparable among all animals, with the only discriminator being the specific vaccine formulation administered prior to NTHI challenge.
FIG 3.
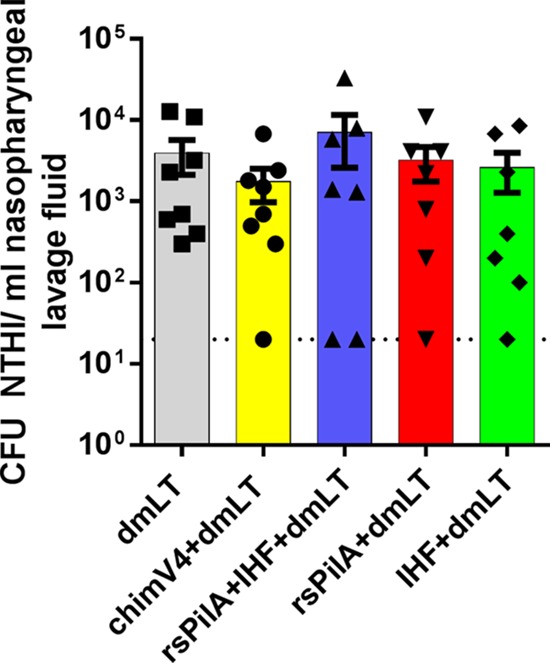
NTHI colonized the nasopharynges of chinchillas. Two days after intranasal inoculation, nasopharyngeal lavages were performed, and fluids were serially diluted and plated to quantitate the relative bacterial load within the nasopharynges of all animals. The relative bacterial loads of NTHI were comparable among the cohorts and thereby indicated an equivalent opportunity to develop ascending OM in adenovirus-compromised chinchillas. Symbols indicate the values for individual animals, and the filled bars indicate the values for each cohort ± SEM. The dotted line represents the limit of detection.
Incidence of experimental OM.
The incidence of experimental OM was determined by video otoscopy and tympanometry via use of an established metric to indicate relative signs of inflammation and presence of middle ear fluid behind the tympanic membrane (25). Four days after NTHI challenge, 31% of middle ears (5/16 ears) in the cohort immunized with dmLT alone had evidence of middle ear fluid, with a peak incidence of disease of 88% (14/16 ears) that occurred between days 12 and 15 (Fig. 4, gray circles). The observed progression and overall incidence of experimental OM in animals administered only the adjuvant were comparable to values in published reports and further confirmed the reproducible nature of disease induction in this outbred, mixed-sex experimental animal model of viral-bacterial coinfection (23, 25–27).
FIG 4.
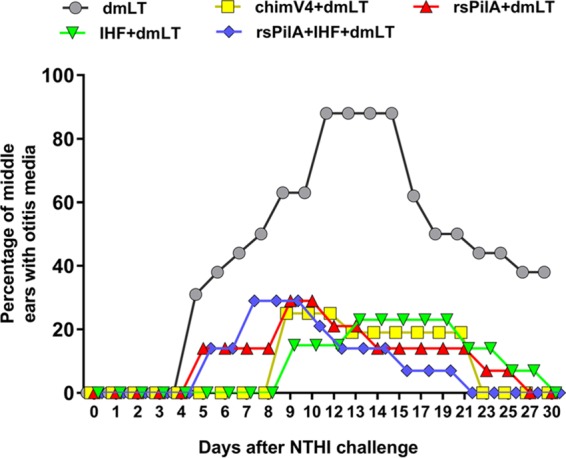
TCI with chimV4, rsPilA, IHF or a combination of rsPilA and IHF significantly reduced the incidence of experimental OM and also mediated more rapid resolution of disease in all cohorts, as determined by blinded video otoscopy and tympanometry. TCI with a band-aid also delayed the onset of disease in the cohorts that received chimV4 plus dmLT or IHF plus dmLT. The percentage of middle ears with OM for each cohort is shown.
Animals immunized with rsPilA plus dmLT or rsPilA plus IHF and dmLT began to show signs of experimental OM in a limited number of ears/animals within 5 days of NTHI challenge (Fig. 4, red triangles and blue diamonds, respectively). Whereas there was no significant delay in the time to first onset of disease in any animal in either of these two cohorts compared to time to onset in the cohort administered only dmLT, there was a significant reduction in the overall proportion of animals that developed OM compared to the proportion of animals administered dmLT (P ≤ 0.0001), with a maximal incidence of 29% (4/14 ears) at 9 and 7 days after NTHI challenge, respectively. Notably, the cohorts that received chimV4 plus dmLT (Fig. 4, yellow squares) or IHF plus dmLT (Fig. 4, green triangles) showed both a significant 9-day delay to first onset of OM compared to immunization with dmLT (P ≤ 0.0001) and also a significant reduction in the proportion of animals that developed OM (P ≤ 0.0001). Peak incidence of disease for the two cohorts administered chimV4 plus dmLT and IHF plus dmLT was 25% (4/16 ears) and 23% (3/13 ears), respectively.
Among the four cohorts immunized with antigen plus adjuvant, there was a delay to onset of OM among animals immunized with chimV4 plus dmLT or IHF plus dmLT compared to onset with rsPilA plus dmLT or rsPilA plus IHF and dmLT (P ≤ 0.05). However, there was no significant difference in the overall proportions of animals that developed experimental OM among any of these four cohorts (P = 0.24), and for the relatively few animals that did develop OM in each cohort, the rapidity with which signs of disease resolved was equivalent (P = 0.24). Moreover, vaccine efficacy among the immune cohorts ranged from 64% to 77% (Table 1). Therefore, whereas the immunogens administered, individually or in combination, were designed to target different NTHI adhesive proteins (OMP P5 and Tfp) or a DNABII protein (IHF), the overall observation was that a two-dose TCI regimen with any of these lead formulations induced significant protection against development of NTHI-induced OM in a viral-bacterial superinfection model of experimental disease.
TABLE 1.
Vaccine efficacy for each cohort
| Vaccine formulation | Vaccine efficacy (%) |
|---|---|
| chimV4+dmLT | 77 |
| rsPilA+dmLT | 64 |
| IHF+dmLT | 72 |
| rsPilA+IHF+dmLT | 66 |
DISCUSSION
Previous work showed that TCI performed by rubbing vaccine formulations onto the outer ears of chinchillas was prophylactic and therapeutic (18, 20). Additionally, a traditional small circular band-aid, a simple device with which to deliver a vaccine formulation onto the nonabraded skin located in the postauricular region, was also effective in inducing therapeutic resolution of existing experimental OM due to NTHI (19). Our current work was designed to test the ability of this TCI band-aid immunization regime to prevent the development of experimental OM due to NTHI in a polymicrobial model of disease. To achieve this goal, we used an experimental model that more closely mimics the polymicrobial nature and kinetics of disease reported for children, in whom an upper respiratory tract viral infection precedes the development of bacterial OM (22, 23). This animal model is robust and reproducible and is shown to be predictive of a pediatric clinical trial outcome (25, 28). Moreover, it has shown utility to test preclinically the robustness of various vaccine candidates in a polymicrobial disease model system (25–27, 29, 30). Therefore, we employed this viral-bacterial coinfection model to assess the efficacy afforded by TCI with three lead vaccine candidates under development by our lab: rsPilA, which targets the majority subunit of NTHI Tfp (14); chimV4, which is directed against both Tfp and a protective B-cell epitope of OMP P5 (14); and IHF, which is a DNABII protein family member that plays an important role in the structural integrity of bacterial biofilms, including those formed by NTHI (15–17).
To date, we have shown that parenteral immunization with rsPilA or chimV4 prevents experimental OM due to NTHI (14), likely due to inhibition of adherence and prevention of twitching motility by type IV pilus and OMP P5-specific antibody present at the respiratory mucosal surface. However, as TCI typically induces a lesser quantity of serum antibody than parenteral immunization, it was uncertain if we would see similar kinetics and comparable degrees of protection when we used this noninvasive immunization strategy. With regard to prior work with IHF as an immunogen, we have shown that the mechanism by which antibody against IHF (as induced by therapeutic TCI) effectively eradicates preformed biofilms within the chinchilla middle ear (15) is a result of sequestration of this protein from the eDNA matrix, which induces catastrophic collapse of the biofilm structure with release of the resident bacteria (17). However, prevention of biofilm formation following immunization with IHF has not yet been described. In addition, although OM is a polymicrobial disease (7, 8, 31), thus far the relative efficacy of TCI has not yet been described when it is used in a viral-bacterial coinfection challenge model that mimics the natural disease course in children. The present study was designed to address each of these remaining gaps in our understanding.
Our data revealed that TCI with chimV4, rsPilA, IHF, or a mixture of rsPilA and IHF, admixed with the adjuvant dmLT, gave rise to circulating antibody-secreting cells that produced antibody specific for the immunogen administered. Previously, we showed that an immunogen-specific IgG titer of ≥160 in serum or middle ear fluids correlated with bacterial clearance when TCI was performed by pipetting a 50-μl volume of vaccine formulation directly onto the pinnae (or outer ears) of chinchillas (18). Here, a cumulative immunogen-specific IgA, IgG, and IgM serum titer of ≥140 was associated with prevention of OM when TCI was performed via band-aid. We hypothesized that whereas the entire quantity of immunogen and adjuvant is available for sampling by cutaneous antigen-presenting cells when the immunogen is pipetted directly onto the chinchilla skin, a portion of the vaccine formulation may be retained within the adsorbent pad of the band-aid used as the delivery device and is therefore unavailable. This may explain the slight difference in relative IgG quantities induced by the two TCI regimens. Efforts are under way to quantitate the relative amount of antigen released from the band-aid.
The results of our vaccination approach clearly showed efficacy against OM in this stringent animal model. The primary readout for preclinical vaccine efficacy was blinded video otoscopy and tympanometry, procedures common in the clinic and the diagnostic techniques used to ascertain whether a child has OM. The onset, duration, and delayed resolution of disease by the cohort administered only dmLT were comparable to historical data for sham-immunized animals, in which OM initiates between 4 and 7 days after NTHI challenge, peaks after 10 to 14 days, and slowly, but not completely, declines over a 30- to 35-day period (14, 23, 25–27). In contrast, receipt of chimV4 plus dmLT, rsPilA plus dmLT, IHF plus dmLT, or rsPilA plus IHF and dmLT afforded significant protection against development of OM and confirmed the utility of each immunogen in a preventative immunization regime. Whereas signs of OM were detected in all four cohorts, the overall incidence was limited to ≤29%. Moreover, any lag in complete resolution of signs of disease was attributed to the same few (e.g., 2 to 4) middle ears per cohort, with complete resolution ultimately achieved by all four cohorts that received a vaccine candidate prior to completion of the study. As chinchillas are outbred, mixed sex, and not specific pathogen-free animals, some variation in the responses to immunization within a cohort is expected, which would also be anticipated to occur in children in clinical trials.
The mechanism for protection against OM is largely antibody mediated. The combined effect of TCI to induce production of immunogen-specific mucosal and systemic antibodies and of upper respiratory tract viral infection-induced inflammation to promote transudation of antibody onto mucosal surfaces, including the nasopharynx and middle ear, facilitated the significant 64 to 77% vaccine efficacy observed here. Moreover, targeting essential conserved bacterial adhesive proteins and biofilm mediators by immunization limited the translocation of NTHI from the nasopharynx, the site of benign colonization, into the middle ear, where active disease is induced. While the data presented herein demonstrated efficacy against a single clinical NTHI isolate, the highly conserved amino acid sequences (>86% identity for PilA and >76% identity for the OMP P5 epitope incorporated into chimV4) and/or the highly conserved structural conformation (for IHF) of each immunogen predicts that similar protection could be achieved upon challenge with other NTHI strains (14, 15, 32); this assumption is being tested.
In terms of specific formulations, both chimV4 plus dmLT and IHF plus dmLT conferred the greatest delays to onset of experimental OM; however, overall there were no significant differences among the four cohorts that received immunogen plus adjuvant formulations by TCI. Whereas the combined formulation of rsPilA plus IHF and dmLT did not result in an enhanced immune response over that attributed to each antigen delivered singly, this was likely due to the fact that only half the concentration of each immunogen was administered compared to the amount given to the single-immunogen cohorts. Nonetheless, this cocktail was equally efficacious, and we fully anticipate that increasing the dose of each immunogen and/or implementation of an additional boosting dose could augment the efficacy observed. The additional benefit of multiple boosting doses and the resulting durability of the immune response induced by TCI via band-aid are under investigation.
Detection of antibody induced by TCI affirmed the immunogenicity of each vaccine candidate when it was administered by a regimen alternative to the already well-established and efficacious parenteral strategy. Moreover, protection against experimental OM afforded by immunization with rsPilA, chimV4, and IHF was consistent with our understanding of the functions of NTHI OMP P5, Tfp, and IHF in bacterial adherence, pathobiology, and biofilm formation. For example, NTHI Tfp engages ICAM-1, and OMP P5 engages both ICAM-1 and CEACAM-1, molecules whose expression levels on respiratory tract epithelial cells are increased in response to respiratory tract viral infection, including adenovirus (33–35). Moreover, Tfp and OMP P5 are essential for NTHI to adhere to the mucosal surface. Additionally, Tfp is required for NTHI to exhibit twitching motility, form biofilms, and persist within the chinchilla nasopharynx (34, 36, 37). Antibodies directed against an OMP P5-directed immunogen, called LB1, in addition to antibodies against PilA prevent adherence of NTHI to respiratory epithelial cells (37, 38). Moreover, antibodies against NTHI PilA inhibit biofilm formation in vitro and induce dispersal of NTHI from established biofilms via a LuxS-mediated quorum signaling event (37, 39). In vivo, parenteral immunization with LB1 or rsPilA, a soluble recombinant and N-terminally truncated PilA molecule, show significant protective efficacy in experimental models of NTHI-induced OM (14, 30, 39), and TCI with LB1 or rsPilA induces resolution of ongoing experimental OM (18–20).
To combine the observed efficacy afforded by OMP P5- and Tfp-targeted vaccine candidates individually and yet administer a single molecule, we also designed the chimeric immunogen chimV4. Data obtained by epitope mapping of NTHI OMP P5 and PilA guided the configuration of this chimeric protein such that the OMP P5 epitope is situated N-terminal to a modified rsPilA molecule in order to optimally expose the immunodominant and protective domains of each individual immunogen (14, 32, 38). Parenteral immunization with chimV4 thus induces the formation of antibody reactive to both OMP P5 and PilA epitopes and provides significant protection against experimental NTHI-induced OM (14). Whereas we have shown earlier that TCI with chimV4 admixed with the adjuvant dmLT results in an expected lower antibody titer than a parenteral strategy, placement of the vaccine formulation at the chinchilla postauricular region induces a local immune response that significantly and rapidly resolves existing OM due to NTHI (18–20). The mechanism for the observed therapeutic efficacy is attributed to inhibition of OMP P5- and Tfp-mediated adherence by NTHI and, for bacteria that form a biofilm, eradication of the biofilm structure due to antibody-induced bacterial dispersal.
As an additional biofilm-disruption strategy, we also developed an immunogen that targeted a DNABII protein family member (IHF) classically known for its role as an intracellular DNA-binding protein (40); however, its importance in the extracellular milieu, particularly as a critical structural component of biofilms formed by multiple bacterial species, is now appreciated. Within the extracellular polymeric substance of bacterial biofilms is an abundance of extracellular DNA (eDNA), organized in a mesh-like configuration, which serves as a support structure for the bacterial community (15, 41). Located at each vertex of crossed strands of eDNA are DNABII proteins, including IHF (15). In vitro, polyclonal and monoclonal antibodies directed against DNABII proteins induce catastrophic collapse of the biofilms formed by many bacterial pathogens (15–17, 45). The mechanism for biofilm disruption is the antibody-mediated sequestration of DNABII proteins from the extracellular milieu as the proteins rapidly cycle between eDNA-bound and -free states. The resulting equilibrium imbalance promotes dissociation of additional DNABII proteins from the eDNA matrix, destabilization of the eDNA lattice, and subsequent collapse of the biofilm structure (17). Earlier, we showed that therapeutic TCI with IHF promotes rapid clearance of preformed NTHI biofilms from the middle ears of chinchillas (39), as did infusion of the NTHI-challenged chinchilla middle ear with IgG-enriched polyclonal serum against IHF or monoclonal antibodies directed against the DNA-binding tips of IHF (16). It is expected that NTHI also establish biofilms rapidly in vivo, and as indicated by the data presented herein, the presence of IHF-specific antibody early in this process likely hindered the development of a robust biofilm structure, exposed NTHI to clearance by host immune mediators, and prevented the onset of OM.
While the current study was not designed to address whether TCI with the targeted immunogens induced clearance of NTHI from the nasopharynx, our previous work shows that TCI with rsPilA or chimV4 admixed with dmLT does result in eradication of NTHI from the nasopharynges of chinchillas challenged both intranasally and transbullarly within 10 to 14 days (18). Therefore, we would anticipate a similar if not more rapid outcome had subsequent nasopharyngeal sampling been done here. In this work, at least one nasopharyngeal lavage sample recovered from animals in each of the four immune cohorts was at the limit of detection, which indicated that vaccine-induced clearance was in effect at this early time point. One limitation of the nasopharyngeal lavage is that only planktonic bacteria are retrievable and that quantitation of NTHI adherent to the mucosa is not possible. Nonetheless, this procedure is the least invasive method to ensure that all animals enter the study colonized with NTHI. In the clinical setting, however, complete eradication of NTHI from the nasopharynx is not necessarily a desired outcome as it could provide the opportunity for replacement by a more pathogenic organism, as is of concern following licensure and broad use of current pneumococcal capsular conjugate vaccines (13, 42). Therefore, reduction of the nasopharyngeal NTHI burden to a colonizing level that is tolerated by the host immune system is more desirable.
To our knowledge, these data are the first to assess TCI as a strategy to prevent experimental OM and to do so in a polymicrobial experimental model wherein an upper respiratory tract viral infection predisposes to ascending bacterial superinfection. Moreover, whereas there are multiple cutaneous immunization delivery systems under investigation, including jet injectors, microneedles, and electroporators, our strategy of using a traditional small circular band-aid placed directly onto the intact skin just behind the ear as a delivery device may provide the opportunity to expand the reach of vaccines against NTHI-induced diseases.
MATERIALS AND METHODS
Animals.
Thirty-seven juvenile chinchillas (Chinchilla lanigera, 525 ± 66 g) without evidence of middle ear infection or serum antibody reactivity to outer membrane proteins of NTHI strain 86-028NP were procured from Rauscher's Chinchilla Ranch, LLC (LaRue, Ohio). Chinchillas were randomly divided into five cohorts of 7 to 8 animals each based on weight (mean cohort weight, 525 g) prior to viral challenge. All animal work was performed under a protocol approved by the Institutional Animal Care and Use Committee at The Research Institute at Nationwide Children's Hospital.
Viral and bacterial isolates.
Adenovirus serotype 1 is a pediatric clinical isolate that has been used in chinchilla models (25–27, 43). NTHI 86-028NP is a minimally passaged clinical isolate collected from the nasopharynx of a child with chronic OM and has been characterized in vitro and in animal models of OM (25–27, 43, 44).
Vaccine formulations.
Cohorts were administered vaccine formulations consisting of rsPilA, chimV4, or IHF admixed with the adjuvant LT(R192G/L211A). rsPilA (for recombinant soluble PilA) is the N-terminally truncated form of PilA, which is the majority subunit of NTHI Tfp (14, 36). chimV4, is a recombinant, chimeric protein designed to target two critical NTHI adhesive proteins/virulence determinants, OMP P5 and Tfp, utilizing PilA as both an immunogen and carrier for a 24-mer protective B-cell epitope identified within NTHI OMP P5 (14). IHF (integration host factor), is a member of the DNABII family of DNA-binding proteins and was purified from E. coli (15). The systemic and mucosal adjuvant LT(R192G/L211A), or dmLT, is a double mutant of E. coli heat-labile enterotoxin (21). Formulations consisted of the following: (i) 10 μg of rsPilA admixed with 10 μg dmLT, (ii) 10 μg of IHF admixed with 10 μg of dmLT, (iii) 5 μg of IHF plus 5 μg of rsPilA (for 10 μg of antigen in total) admixed with 10 μg of dmLT, (iv) 10 μg of chimV4 admixed with 10 μg of dmLT, and (v) 10 μg of dmLT alone.
Transcutaneous immunization.
The time course for TCI and viral and bacterial challenge is described in Table 2. Two days prior to receipt of the first immunizing dose, the fur directly caudal to each pinna (or external ear; e.g., the postauricular region) was plucked to permit resolution of any nonspecific inflammation that might be induced by hair removal prior to immunization. The procedure for TCI with a band-aid is described in Novotny et al. (19). Briefly, vaccine formulations in a 50-μl volume were applied to the gauze pads of small circular band-aids (CVS brand) and affixed to the nonabraded skin at each postauricular region. Band-aids were removed 24 h later. Two doses were delivered 1 week apart.
TABLE 2.
Schedule for immunizations, adenovirus inoculation, and NTHI challenge
| Study daya | Procedure(s) |
|---|---|
| −16 | TCI via band-aid |
| −9 | TCI via band-aid |
| −7 | Adenovirus serotype 1 challenge (intranasal) |
| 0 | Collection of blood; NTHI 86-028NP challenge (intranasal) |
| 2 | Nasopharyngeal lavage |
Relative to day of NTHI challenge.
Viral and bacterial challenge.
Two days after receipt of the second immunizing dose by band-aid, when the maximal immune response following TCI is observed for this immunization regimen (likely an outcome due to the administration of both immunizing band-aids within a short time frame) (20), all chinchillas were inoculated with 1.9 × 107 50% tissue culture infective doses (TCID50) of adenovirus serotype 1 by passive inhalation into the nares of droplets that contained the virus (Table 2). Seven days later, when adenovirus-induced compromise of the airway was established (43), all animals were challenged with 1 × 108 CFU of NTHI 86-028NP administered by passive inhalation into the nares. To confirm NTHI colonization and quantitate the relative bacterial load within the nasopharynx, 2 days after bacterial challenge, nasopharyngeal lavage was performed on all animals, and the fluids were serially diluted and plated onto chocolate agar formulated with 15 μg of ampicillin per milliliter of medium. Antibiotic within the medium served to limit growth of normal chinchilla nasopharyngeal flora. Plates were incubated for 24 h at 37°C in a humidified atmosphere prior to enumeration of NTHI colonies.
Video otoscopy and tympanometry.
Video otoscopy using a 0°, 3-inch probe connected to a digital camera system (MedRx, Largo, FL) was utilized to monitor signs of OM (e.g., tympanic membrane inflammation and/or presence of fluid in the middle ear space). Tympanometry was performed with a Madsen Otoflex tympanometer, and data were analyzed with OTOsuite software (Otometrics, Schaumburg, IL). Overall signs of OM were blindly rated on an established scale of 0 to 4+, and middle ears with a score of ≥2.0 were considered positive for OM as middle ear fluid (MEF) was visible behind the tympanic membrane (25). If the tympanic membrane could not be visualized due to an obstruction within the ear canal (i.e., due to cerumen accumulation), that ear was excluded from the day's count. Per established protocol, each middle ear was considered independently, and for each cohort, the percentage of middle ears with OM was calculated. To calculate vaccine efficacy, the number of observations of OM during the 30-day study period was first determined and converted to a percentage relative to the total number of observations for each cohort. This value was then subtracted from the percentage computed for the cohort administered dmLT only.
Enumeration of antigen-specific antibody-secreting cells by enzyme-linked immunospot (ELISPOT) assay.
To enumerate circulating antibody-secreting cells within fully immunized chinchillas prior to adenovirus or NTHI challenge (Table 2), blood was collected from anesthetized animals by cardiac puncture and mixed with 5 U of heparin per ml. An equal volume of Dulbecco's phosphate-buffered saline (DPBS) was added, and the mixture was layered onto Ficoll-Paque Plus (GE Healthcare, Pittsburg, PA) prior to centrifugation at 500 × g for 30 min at 18°C. Lymphocytes were collected at the interface and washed twice with culture medium (RPMI 1640 medium, 2 mM l-glutamine, 100 U penicillin-streptomycin, 10% heat-inactivated fetal bovine serum [FBS], 50 μM β-mercaptoethanol) prior to seeding at a density of 1 × 106 cells in a 24-well tissue culture plate. Cells were stimulated with 1 μg of resiquimod (Sigma-Aldrich, St. Louis, MO) per ml and 10 ng of recombinant human IL-2 (R&D Systems, Inc., Minneapolis, MN) per ml of medium and incubated for 5 days at 37°C and 5% CO2 in a humidified atmosphere. As chinchillas are outbred, cells were processed and assessed by individual animal.
To perform the ELISPOT assay, wells of 96-well filtration plates (Millipore, Billerica, MA) were coated with 5 μg of rsPilA, chimV4, or IHF overnight at 4°C and then blocked with phenol red-free RPMI 1640 medium plus 10% heat-inactivated FBS. Activated cells were seeded at 5 × 104 cells per well in triplicate and incubated for 5 h at 37°C in 5% CO2. After cells were washed with DPBS plus 0.25% Tween 20, goat anti-rat IgM, IgA, or IgG detection antibody conjugated to horseradish peroxidase (HRP; Bethyl Laboratories, Montgomery, TX) was applied, and cells were incubated for 2 h at 25°C. Plates were washed with distilled H2O (dH2O), and spots were revealed with a diaminobenzidine (DAB)-peroxidase substrate kit (Vector Laboratories, Burlingame, CA). Spots were detected and enumerated using an EliScan+ instrument (A.EL.VIS GmbH, Hannover, Germany). The mean ± standard error of the mean (SEM) was calculated for cells that secreted immunogen-specific IgM, IgG, and IgA for each cohort.
Quantitation of chimV4-specific immunoglobulins.
To quantitate antigen-specific antibody isotypes within serum collected from fully immunized chinchillas prior to NTHI challenge (Table 2), an enzyme immunosorbent assay (ELISA) was performed. Briefly, wells of C-bottom microtiter plates were coated with 0.2 μg of chimV4, rsPilA, or IHF for 1 h at 37°C prior to addition of serum samples serially diluted in 10 mM phosphate-buffered saline. Antibody was detected with horseradish peroxidase-conjugated goat anti-rat IgG, IgM, or IgA (Bethyl Laboratories, Montgomery, TX), and color was developed with 3,3′,5,5′-tetramethylbenzidine (Pierce Biotechnology, Rockford, IL). The reciprocal titer was defined as the inverse of the antibody dilution that yielded an optical density at 450 nm (OD450) of 0.1 above control wells incubated without serum samples. Serum was analyzed by individual animal three times on separate days, and the geometric mean ± standard deviation (SD) of the reciprocal titer for each antibody isotype per cohort was calculated.
Statistics.
Statistical significance was calculated using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). Statistical differences in nasopharyngeal colonization were assessed by Kruskal-Wallis one-way analysis of variance (ANOVA) and Dunn's method for multiple comparisons. Significant differences among cohorts in times to first incidence of OM, times to resolution of OM, and proportions of middle ears with OM were assessed by a Mantel-Cox log rank test where an event was classified as OM. Differences in numbers of ASCs and antibody titers were assessed by one-way ANOVA with Tukey's multiple-comparison test. For all analyses, a P value of ≤0.05 was considered significant.
ACKNOWLEDGMENTS
We thank Kenneth L. Brockman and Joseph A. Jurcisek for technical assistance and Jennifer Neelans for manuscript preparation.
This work was supported by NIDCD/NIH R01 003915 (L.O.B.) and R01 DC011818 (L.O.B. and S.D.G.).
J.D.C. and L.A.N. declare that they have no conflicts of interest. L.O.B. and S.D.G. are Scientific Advisors to and have equity in ProclaRx, LLC, to whom technology related to the DNABII proteins has been licensed. L.O.B. is an inventor of technology related to PilA-derived immunogens that is licensed to GlaxoSmithKline Biologicals.
REFERENCES
- 1.Giudice EL, Campbell JD. 2006. Needle-free vaccine delivery. Adv Drug Deliv Rev 58:68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Glenn GM, Kenney RT, Ellingsworth LR, Frech SA, Hammond SA, Zoeteweij JP. 2003. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev Vaccines 2:253–267. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- 3.Lawson LB, Clements JD, Freytag LC. 2012. Mucosal immune responses induced by transcutaneous vaccines. Curr Top Microbiol Immunol 354:19–37. [DOI] [PubMed] [Google Scholar]
- 4.Russell MW, Ogra PL. 2010. Mucosal decisions: tolerance and responsiveness at mucosal surfaces. Immunol Invest 39:297–302. doi: 10.3109/08820131003729927. [DOI] [PubMed] [Google Scholar]
- 5.Levine MM. 2011. “IDEAL” vaccines for resource poor settings. Vaccine 29(Suppl 4):D116–D125. doi: 10.1016/j.vaccine.2011.11.090. [DOI] [PubMed] [Google Scholar]
- 6.Schilder AG, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, Venekamp RP. 2016. Otitis media. Nat Rev Dis Primers 2:16063. doi: 10.1038/nrdp.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. 2008. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis 14:1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chonmaitree T. 2000. Viral and bacterial interaction in acute otitis media. Pediatr Infect Dis J 19:S24–30. doi: 10.1097/00006454-200005001-00005. [DOI] [PubMed] [Google Scholar]
- 9.Heikkinen T, Chonmaitree T. 2003. Importance of respiratory viruses in acute otitis media. Clin Microbiol Rev 16:230–241. doi: 10.1128/CMR.16.2.230-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Family P, American Academy of Otolaryngology-Head and Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media with Effusion. 2004. Otitis media with effusion. Pediatrics 113:1412–1429. doi: 10.1542/peds.113.5.1412. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. 2004. Diagnosis and management of acute otitis media. Pediatrics 113:1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 12.Tristram S, Jacobs MR, Appelbaum PC. 2007. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev 20:368–389. doi: 10.1128/CMR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagan R, Pelton S, Bakaletz L, Cohen R. 2016. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. Lancet Infect Dis 16:480–492. doi: 10.1016/S1473-3099(15)00549-6. [DOI] [PubMed] [Google Scholar]
- 14.Novotny LA, Adams LD, Kang DR, Wiet GJ, Cai X, Sethi S, Murphy TF, Bakaletz LO. 2009. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine 28:279–289. doi: 10.1016/j.vaccine.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman SD, Obergfell KP, Jurcisek JA, Novotny LA, Downey JS, Ayala EA, Tjokro N, Li B, Justice SS, Bakaletz LO. 2011. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol 4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- 16.Novotny LA, Jurcisek JA, Goodman SD, Bakaletz LO. 2016. Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine 10:33–44. doi: 10.1016/j.ebiom.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brockson ME, Novotny LA, Mokrzan EM, Malhotra S, Jurcisek JA, Akbar R, Devaraj A, Goodman SD, Bakaletz LO. 2014. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Mol Microbiol 93:1246–1258. doi: 10.1111/mmi.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novotny LA, Clements JD, Bakaletz LO. 2011. Transcutaneous immunization as preventative and therapeutic regimens to protect against experimental otitis media due to nontypeable Haemophilus influenzae. Mucosal Immunol 4:456–467. doi: 10.1038/mi.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novotny LA, Clements JD, Bakaletz LO. 2015. Therapeutic transcutaneous immunization with a band-aid vaccine resolves experimental otitis media. Clin Vaccine Immunol 22:867–874. doi: 10.1128/CVI.00090-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novotny LA, Clements JD, Bakaletz LO. 2013. Kinetic analysis and evaluation of the mechanisms involved in the resolution of experimental nontypeable Haemophilus influenzae-induced otitis media after transcutaneous immunization. Vaccine 31:3417–3426. doi: 10.1016/j.vaccine.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norton EB, Lawson LB, Freytag LC, Clements JD. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol 18:546–551. doi: 10.1128/CVI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giebink GS. 1999. Otitis media: the chinchilla model. Microb Drug Resist 5:57–72. doi: 10.1089/mdr.1999.5.57. [DOI] [PubMed] [Google Scholar]
- 23.Bakaletz LO. 2009. Chinchilla as a robust, reproducible and polymicrobial model of otitis media and its prevention. Expert Rev Vaccines 8:1063–1082. doi: 10.1586/erv.09.63. [DOI] [PubMed] [Google Scholar]
- 24.Bakaletz LO, Holmes KA. 1997. Evidence for transudation of specific antibody into the middle ears of parenterally immunized chinchillas after an upper respiratory tract infection with adenovirus. Clin Diagn Lab Immunol 4:223–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novotny LA, Jurcisek JA, Godfroid F, Poolman JT, Denoel PA, Bakaletz LO. 2006. Passive immunization with human anti-protein D antibodies induced by polysaccharide protein D conjugates protects chinchillas against otitis media after intranasal challenge with Haemophilus influenzae. Vaccine 24:4804–4811. doi: 10.1016/j.vaccine.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Bakaletz LO, Kennedy BJ, Novotny LA, Duquesne G, Cohen J, Lobet Y. 1999. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect Immun 67:2746–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy BJ, Novotny LA, Jurcisek JA, Lobet Y, Bakaletz LO. 2000. Passive transfer of antiserum specific for immunogens derived from a nontypeable Haemophilus influenzae adhesin and lipoprotein D prevents otitis media after heterologous challenge. Infect Immun 68:2756–2765. doi: 10.1128/IAI.68.5.2756-2765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, Schuerman L. 2006. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 367:740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 29.Bakaletz LO. 1995. Viral potentiation of bacterial superinfection of the respiratory tract. Trends Microbiol 3:110–114. doi: 10.1016/S0966-842X(00)88892-7. [DOI] [PubMed] [Google Scholar]
- 30.Bakaletz LO, Leake ER, Billy JM, Kaumaya PT. 1997. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine 15:955–961. doi: 10.1016/S0264-410X(96)00298-8. [DOI] [PubMed] [Google Scholar]
- 31.Bakaletz LO. 2010. Immunopathogenesis of polymicrobial otitis media. J Leukoc Biol 87:213–222. doi: 10.1189/jlb.0709518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novotny LA, Bakaletz LO. 2003. The fourth surface-exposed region of the outer membrane protein P5-homologous adhesin of nontypeable Haemophilus influenzae is an immunodominant but nonprotective decoying epitope. J Immunol 171:1978–1983. doi: 10.4049/jimmunol.171.4.1978. [DOI] [PubMed] [Google Scholar]
- 33.Avadhanula V, Rodriguez CA, Ulett GC, Bakaletz LO, Adderson EE. 2006. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect Immun 74:830–838. doi: 10.1128/IAI.74.2.830-838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novotny LA, Bakaletz LO. 2016. Intercellular adhesion molecule 1 serves as a primary cognate receptor for the type IV pilus of nontypeable Haemophilus influenzae. Cell Microbiol 18:1043–1055. doi: 10.1111/cmi.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bookwalter JE, Jurcisek JA, Gray-Owen SD, Fernandez S, McGillivary G, Bakaletz LO. 2008. A carcinoembryonic antigen-related cell adhesion molecule 1 homologue plays a pivotal role in nontypeable Haemophilus influenzae colonization of the chinchilla nasopharynx via the outer membrane protein P5-homologous adhesin. Infect Immun 76:48–55. doi: 10.1128/IAI.00980-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakaletz LO, Baker BD, Jurcisek JA, Harrison A, Novotny LA, Bookwalter JE, Mungur R, Munson RS Jr. 2005. Demonstration of type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect Immun 73:1635–1643. doi: 10.1128/IAI.73.3.1635-1643.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jurcisek JA, Bookwalter JE, Baker BD, Fernandez S, Novotny LA, Munson RS Jr, Bakaletz LO. 2007. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol Microbiol 65:1288–1299. doi: 10.1111/j.1365-2958.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- 38.Novotny LA, Jurcisek JA, Pichichero ME, Bakaletz LO. 2000. Epitope mapping of the outer membrane protein P5-homologous fimbrin adhesin of nontypeable Haemophilus influenzae. Infect Immun 68:2119–2128. doi: 10.1128/IAI.68.4.2119-2128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novotny LA, Jurcisek JA, Ward MO Jr, Jordan ZB, Goodman SD, Bakaletz LO. 2015. Antibodies against the majority subunit of type IV Pili disperse nontypeable Haemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media. Mol Microbiol 96:276–292. doi: 10.1111/mmi.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swinger KK, Rice PA. 2004. IHF and HU: flexible architects of bent DNA. Curr Opin Struct Biol 14:28–35. doi: 10.1016/j.sbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Jurcisek JA, Bakaletz LO. 2007. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol 189:3868–3875. doi: 10.1128/JB.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dagan R. 2009. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect 15(Suppl 3):S16–S20. doi: 10.1111/j.1469-0691.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki K, Bakaletz LO. 1994. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect Immun 62:1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison A, Dyer DW, Gillaspy A, Ray WC, Mungur R, Carson MB, Zhong H, Gipson J, Gipson M, Johnson LS, Lewis L, Bakaletz LO, Munson RS Jr. 2005. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol 187:4627–4636. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novotny LA, Amer AO, Brockson ME, Goodman SD, Bakaletz LO. 2013. Structural stability of Burkholderia cenocepacia biofilms is reliant on eDNA structure and presence of a bacterial nucleic acid binding protein. PLoS One 8:e67629. doi: 10.1371/journal.pone.0067629. [DOI] [PMC free article] [PubMed] [Google Scholar]


