Policy Points:
The policy context of direct‐to‐consumer personal genomic testing (DTC‐PGT) has been evolving over the last decade, with little empirical data available about consumers’ perspectives.
A majority of consumers of DTC‐PGT supported expanded access to services and their integration into the medical context and opposed more governmental regulation.
Consumers’ attitudes about access to services and regulation did not vary based on the specific genetic risk information they received from companies, but may vary based on whether consumers perceived their DTC experience negatively.
Context
While policymakers have been considering the appropriateness of direct‐to‐consumer personal genomic testing (DTC‐PGT) for more than a decade, there is little empirical data on consumers’ views regarding the regulation of these products. No research has assessed whether consumers’ personal experience with testing is related to their views about access to and regulation of DTC tests.
Methods
Data were analyzed from the PGen Study, a longitudinal prospective cohort of DTC‐PGT customers of 23andMe (n = 564) and Pathway Genomics (n = 377; total N = 941). Consumers were sent an electronic survey before receiving genetic test results and again 6 months after receipt of results.
Findings
At the 6‐month follow‐up, more than 80% of participants believed that people have a right to access genetic information directly, that parents should be able to get DTC‐PGT testing for their children, and that genetic information should be kept private. Participants supported health insurance coverage of PGT (60%), wider availability of PGT (68%), and inclusion of genetic information in medical records (63%). Participants were less supportive of government regulation (28%) and restricting testing to clinical settings (14%). Conservative political ideology was associated with less support for government regulation (P < 0.001), as was feeling more confident in one's genetic knowledge (P < 0.05). Participants’ level of computed genetic risk for common diseases, as indicated by their actual test results received from companies, showed no relationship with attitudes. However, those who perceived that they had received elevated risk results expressed lower support for expanded availability and incorporation of PGT into health care (P < 0.01). Those who reported being upset by their genetic test results were less likely to endorse access to DTC products without a medical professional (P < 0.01).
Conclusions
PGT consumers supported expanded access to these services and opposed additional regulation. Users who had a negative personal experience with PGT testing were less supportive of expanded availability without a medical professional.
Whether and how to regulate access to direct‐to‐consumer personal genomic testing (DTC‐PGT)—commercial services that provide genetic risk information directly to consumers outside of the traditional clinical context—have been topics of scrutiny for more than 10 years.1, 2, 3 Scholars have examined the potential benefits of and limitations to making genetic test results directly available to consumers, arguing, for instance, that the potential impact of genetic risk information on consumers’ privacy, health, health care utilization, behavior changes, and well‐being warrant heightened attention and regulation.1, 2, 3, 4, 5, 6, 7 While early discussions about the potential consequences of PGT were largely speculative, recent studies of actual PGT consumers that examine the psychosocial risks of testing and its impact on the health care system provide important information for policymakers.8 Data on users’ attitudes and perspectives on access to PGT testing are also important since experience in other policy contexts shows that political pressures can shape the regulatory process.9 Moreover, informed interest groups with strong opinions about the desirability of government oversight (such as PGT consumers) may be invested in shaping regulatory processes, such as through public comment periods and other mechanisms.10
In this study, we examined longitudinal data on consumers’ experiences with PGT and the impact of those experiences on their views about the availability of PGT and of its regulation. We examined consumers’ actual genetic test results—and their experience with testing—as predictors of their subsequent attitudes about PGT services, which previous research has not done.
Background
Regulation of Consumer Access to Genetic Services
Prior to 2007, US federal oversight of PGT was extremely limited.1 Initial federal government scrutiny (such as the Secretary's Advisory Committee on Genetics, Health, and Society convened by the Department of Health and Human Services and the Government Accountability Office) focused on examining reliability, accuracy, and clinical utility concerns of these new services.11, 12, 13 However, it took a proposed rapid increase in consumer access to DTC genetic testing—specifically, Pathway Genomics’ 2010 proposal to begin selling its product at Walgreens across the country—to spur action by the US Food and Drug Administration (FDA).14 The FDA's first round of cautionary letters to almost all DTC‐PGT providers served to push most out of the DTC market or out of business entirely.15 23andMe Inc alone began filing for premarket authorization of its Personal Genome Service in July 2013, while continuing to sell its product.16 When 23andMe launched a new national advertising campaign later that year, the FDA once again took note of the abrupt potential shift in access and sent 23andMe a more dire warning letter ordering that it stop marketing its health‐related tests immediately.17 In response, 23andMe reduced its online offering initially to ancestry testing only, but recently re‐entered the market with 36 carrier screens in addition to nonmedical “wellness,” “trait,” and ancestry testing.18 In April 2017, the company obtained FDA approval to market 10 genetic health risk tests for conditions including Alzheimer's disease, Parkinson's disease, and hereditary hemochromatosis. While regulatory oversight of DTC‐PGT services has been evolving over the past 10 years, there has been very little research examining how consumers themselves think about restricting or expanding access to these services.
Public and Consumer Attitudes About Personal Genomic Testing
Given that the general public to date has relatively low exposure to PGT, most existing survey research of the general public has examined trends in awareness,19, 20, 21, 22 not more detailed assessment of what the public thinks should be done about it. In an exception, Almeling and Gadarian surveyed a national sample of Americans in 2011 and asked a single question regarding public attitudes about DTC genetic testing; they found that 65% of respondents agreed that clinicians should be involved in all genetic testing.23 A survey of social network users similarly found that most (67%) agreed that PGT companies should provide a medical expert to interpret results, and half (51%) supported federal regulation of PGT companies.24 Members of the general public, however, are likely to consider access to PGT quite differently from informed consumers of these services. While other studies of early users of PGT have examined consumers’ beliefs about testing and their motivations to pursue it,25, 26, 27, 28 only one has examined consumers’ perceptions of policy oversight in particular. Bollinger, Green, and Kaufman surveyed consumers of Navigenics, 23andMe, and deCODEme (N = 1,046) about their attitudes toward PGT services and their preferences related to regulation.29 They found that most PGT consumers (66%) agreed that it was important that DTC tests be available without government oversight.29
Social science research illuminates the factors that generally explain variation in the public's perceptions of policy matters. This body of research demonstrates that individual demographic characteristics (such as gender or socioeconomic status), attitudes about groups in society, and political characteristics shape individuals’ perspectives about social policy issues.30 Within the health context in particular, researchers have described the factors that predict Americans’ opinions of issues on a range of health policy topics, from health disparities to obesity prevention, with political liberals generally supporting more governmental action compared to political conservatives, and racial and ethnic minorities more supportive of government action than whites.31, 32
Less clear is the relationship between individuals’ “self‐interest” in an issue and their attitudes about policy action related to that issue.33 Studies find, for instance, that people's presumed “interest” in a policy arena (such as low‐income individuals’ interest in welfare reform, or smokers’ interest in tobacco regulation) is a less important factor shaping support for policies than one might expect.34 In fact, the association of self‐interest with policy opinion is variable and depends on the context, particularly how salient the risks and benefits of a particular policy are to that individual.35 Attitudes about PGT offer an illuminating context in which to explore these relationships between self‐interest, salience of risk, and opinion about policy. All consumers who have themselves sought access to DTC‐PGT are likely supportive of that access and have an interest in ensuring access to these services, as the Bollinger and colleagues study revealed.29 But a more nuanced investigation of consumers’ “interest” in regulating access should also consider consumers’ personal and varying experience with PGT. On the one hand, receipt of results suggesting elevated risk for a disease could lead consumers to be less supportive of expanded access to genetic services and more supportive of regulatory oversight. This might be the case if these consumers perceive that the elevated risk results could lead to negative psychosocial consequences and/or they could come to believe that having clinician involvement is important to navigate the implications of the test results. On the other hand, receiving elevated risk results could lead consumers to be more supportive of expanded access and less supportive of regulatory oversight, if, for example, they perceive the results as having important personal value or medical utility or if they value the experience of receiving such results without the discriminatory risk of having such information included in their medical record.
Research Objectives
In this study, we leveraged data from a sample of 941 participants in a longitudinal study of consumers of 2 PGT companies (23andMe and Pathway) with the goal of examining the contribution of an “objective” measure of genetic risk (ie, level of disease risk as predicted by PGT) as well as consumers’ “subjective” responses to that genetic testing (eg, their perceptions of their results and whether they felt upset) and to consumers’ attitudes about access to personal genomic services. Specifically, our research objectives are twofold: (1) to describe consumers’ attitudes toward access to and regulation of PGT, and (2) to determine whether information about genetic risk and test experience (risk calculated by companies and consumers’ perceptions of their own genetic risk results) contribute to these attitudes about access to and regulation of PGT, after accounting for individuals’ demographic and political characteristics. The paper concludes with a discussion of the role of consumer attitudes about regulation in light of the dynamic policy context around PGT services.
Methods
Data
Data for this study come from the Impact of Personal Genomics (PGen) Study, a collaboration between researchers at Brigham & Women's Hospital/Harvard Medical School and the University of Michigan School of Public Health; scientists at 23andMe (Mountain View, California; www.23andme.com) and Pathway (San Diego, California; www.pathway.com), and survey research experts at a private, third‐party research firm, SoundRocket (previously Survey Sciences Group; Ann Arbor, Michigan). The Partners Human Research Committee and the University of Michigan Health Sciences and Behavioral Sciences Institutional Review Board approved the study. Complete details of the academic‐industry partnership and study design, including participant recruitment, survey design, and response rates, are detailed elsewhere.36, 37
Consumers were recruited between March and July 2012 (prior to the FDA action against 23andMe described in the introduction) through invitation emails sent to 3,900 23andMe customers who purchased DTC‐PGT during this period and participated in the company's informed consent process for general research studies. In contrast, Pathway customers were recruited during this same time period through 2 approaches: through banner advertisements on the company's website and through an email invitation to members of the health‐based social networking site PatientsLikeMe (www.patientslikeme.com). Participants recruited by way of this latter path were invited to order DTC‐PGT at a subsidized rate through Pathway. After ordering PGT, all study invitees received a link to the dedicated survey maintained by SoundRocket. Participants completed an online consent process, and after agreeing to have their de‐identified genetic data and survey responses shared with academic investigators, they were invited to complete a baseline (pre‐results) survey online. Participants who completed the baseline survey prior to receiving their DTC‐PGT results, and who subsequently received and opened their results reports, were eligible for invitation to the follow‐up surveys. Invitations to the follow‐up survey reported here were emailed at 6 months after PGT results were viewed. Both PGT companies provided regular updates to the survey firm regarding receipt and viewing of results for the purposes of timing follow‐up survey invitations. As noted elsewhere, a total of 1,085 23andMe customers (of 3,900 invited, or 28%) completed the baseline survey; because of the opt‐in recruitment, there is no comparable data to assess the response from Pathway.38
Measures
Outcome variables: attitudes about access to and regulation of PGT
Our main dependent variables were 8 survey items that were included in the 6‐month survey and measured on a 5‐point Likert scale (strongly disagree to strongly agree). The items included the following statements: “It is important to me that my genetic information is kept private”; “I think that health insurance should cover personal genomic testing”; “I think genetic information should be part of a standard medical record”; “Genetic tests should be available more widely (eg, test kits at drugstores)”; “I think the government should put more effort into regulating personal genomic testing”; “Tests like these should only be available to people through their doctor”; “I think people have a right to access their own genetic information without going through a medical professional”; and “I think that parents should be able to get results for their children if they want to.” These items were designed specifically for this study but were adapted from previous Likert‐scale questions used in other research on consumers of genetic testing.26, 27
Independent variables
We examined 2 sets of independent variables measuring genetic risk information. The first set of independent variables summarized our measures of company‐calculated genetic risk information. Each company provided individuals’ genetic test results for ancestry, nonmedical traits, carrier testing, disease risk, and pharmacogenomics. For this paper, we focused on the disease risk and pharmacogenetic risk results because they were more likely to prompt changes to health‐related policy attitudes (eg, regarding health insurance coverage) than nonmedical traits or ancestry information. Following other work using the PGen data, we created 2 measures: (1) proportion of total disease‐related test results indicating an elevated risk for the individual and (2) proportion of total pharmacogenomics‐related test results indicating atypical drug response.38 This proportion score is used since the 2 companies differed in the total number of results they offered consumers: 23andMe participants received disease‐risk estimates for 29 conditions, while Pathway participants received estimates for 25 conditions; male customers across both companies received 8 pharmacogenomic results and female customers received 9 (see van der Wouden et al.39 for more details). The threshold for elevated versus non‐elevated genetic risk was set at a relative risk (RR) ≥ 1.2.40 In sensitivity analyses examining whether the specific type of disease risk result matters (for conditions held in common across the 2 companies), we also constructed dichotomous measures of cancer‐related elevated risk (eg, prostate, melanoma, lung, colorectal, breast), neurological elevated risk result (eg, Parkinson's disease, Alzheimer's disease, multiple sclerosis), and cardiovascular elevated risk results (eg, coronary heart disease, atrial fibrillation).
The second set of independent variables measured perceptions of genetic risk information. We used 2 survey items measured at 6 months: whether the respondent perceived that they received high‐risk results (all or many vs few or none) and whether the respondent reported feeling upset about the results (yes vs no).
To examine whether genetic risk results were independently associated with attitudes about PGT access and regulation after accounting for demographic characteristics, we included in our models a set of variables measured at baseline: age (in years), gender (female vs male), race (white vs nonwhite), education (greater than college degree vs college degree or less), income categories (<$40,000/year; $40,000 to $69,999; $70,000 to $99,999; $100,000 to 199,999; and $200,000+), and health insurance status (insured vs uninsured). We included a measure of political ideology, based on political science literature examining the robust association of political predispositions on policy‐relevant attitudes,33 measured as liberal, moderate, or conservative. We also included a measure of baseline self‐rated health (1 item from the SF‐36 questionnaire41) to adjust for possible confounding of the genetic risk results with the respondents’ overall health. This was measured on a 5‐point ordinal scale: “Would you say that in general your health is excellent, very good, good, fair, or poor?” (coded 1 to 5).
We also included 2 attributes of individuals that are specific to the context of genetics: self‐assessed genetic knowledge and perceived self‐efficacy with genetic information. Genetic knowledge was measured using 9 true/false statements designed to reflect understanding of facts about genetics and genetic testing. Self‐efficacy, or confidence in one's ability to use genetic information, was measured as 5 items to which participants agreed or disagreed, such as “I am confident in my ability to understand information about genetics.” Details on these items and scoring are reported elsewhere.42 Finally, given the different recruitment approaches and sample composition coming from the 2 DTC companies, we included an indicator variable for whether the participant was a Pathway consumer as compared to a 23andMe consumer.
Analysis
Study participants who completed both the baseline and 6‐month surveys, and who had no missing data on the 8 outcome variables, the genetic risk results, and the covariates described above, were eligible for inclusion in the current analysis. The first step in the analysis was to estimate the frequencies of the 8 main attitude and opinion measures. Next, to reduce the number of outcomes examined, we conducted a principal component analysis on the 8 items (which revealed 4 distinct factors, described in Results, below) with varimax rotation. We conducted multivariable linear regression on the 4 outcomes, regressing the measures on demographics, political ideology, self‐rated health, genetic knowledge, self‐efficacy, calculated genetic risk information results, and perceived response to genetic risk. In initial specifications, we included the calculated genetic risk and perceived response to genetic risk in separate models (out of concern of collinearity), but because all model variance inflation factors were under 3, we report the model with all covariates included simultaneously. Models estimated with ordered logit regression for the 2 nonscalar measures were substantively identical. Finally, we performed a series of sensitivity analyses measuring the genetic risk results as disease‐specific risk results (eg, any cancer result, any cardiovascular result, any Alzheimer's high‐risk result, as described above). All analyses were performed using SAS 9.3 (SAS Institute, Cary, North Carolina).
Results
Of the 1,042 study participants who completed both baseline and 6‐month surveys, 941 had non‐missing data on all key outcomes and covariates and were included in the current analysis. Of these, 377 (40.1%) were Pathway customers, while 564 (59.9%) were 23andMe customers. Characteristics of the analytic sample are displayed in Table 1, for the full sample and separately by each company. Consistent with previous reports on this survey population,36 the sample was majority white and highly educated, with about half having more than a college degree; more than 60% had a household income of more than $70,000 a year. They were politically liberal and described their health as good, very good, or excellent. Pathway customers tended to have somewhat lower education and income and were in slightly poorer health than 23andMe customers.
Table 1.
Descriptive Characteristics of Study Sample of Consumers of Direct‐to‐Consumer Genetic Testing (N = 941)
| Characteristic | 23andMe (n = 564) | Pathway (n = 377) | Total (N = 941) |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 49.2 ± 16.2 | 42.5 ± 13.8 | 46.5 ± 15.6 |
| Range | 21‐94 | 19‐79 | 19‐94 |
| Gender | |||
| Female | 305 (54.1%) | 257 (68.2%) | 562 (59.7%) |
| Race/ethnicity | |||
| White | 485 (86.0%) | 320 (84.9%) | 805 (85.5%) |
| Nonwhite | 79 (14.0%) | 57 (15.1%) | 136 (14.5%) |
| Hispanic or Latino | 30 (5.3%) | 18 (4.8%) | 48 (5.1%) |
| Education | |||
| College or less | 263 (46.6%) | 221 (58.6%) | 484 (51.4%) |
| More than college | 301 (53.4%) | 156 (41.4%) | 457 (48.6%) |
| Household income, USD | |||
| <$40,000 | 69 (12.2%) | 97 (25.7%) | 166 (17.6%) |
| $40,000‐$69,999 | 107 (19.0%) | 67 (17.8%) | 174 (18.5%) |
| $70,000‐$99,000 | 117 (20.7%) | 80 (21.2%) | 197 (20.9%) |
| $100,000‐$199,999 | 173 (30.7%) | 111 (29.4%) | 284 (30.2%) |
| $200,000+ | 98 (17.4%) | 22 (5.8%) | 120 (12.8%) |
| Health insurance | |||
| Uninsured | 22 (3.9%) | 20 (5.3%) | 42 (4.5%) |
| Political ideology | |||
| Liberal | 372 (66.0%) | 220 (58.4%) | 592 (62.9%) |
| Moderate | 92 (16.3%) | 86 (22.8%) | 178 (18.9%) |
| Conservative | 100 (17.7%) | 71 (18.8%) | 171 (18.3%) |
| Self‐rated health | |||
| Excellent | 106 (18.8%) | 35 (9.3%) | 141 (15.0%) |
| Very good | 253 (44.9%) | 128 (34.0%) | 381 (40.5%) |
| Good | 158 (28.0%) | 119 (31.6%) | 277 (29.4%) |
| Fair | 40 (7.1%) | 64 (17.0%) | 104 (11.1%) |
| Poor | 7 (1.2%) | 31 (8.2%) | 38 (4.0%) |
| Self‐assessed genetic literacy | |||
| Mean ± SD | 8.2 ± 0.9 | 8.1 ± 1.0 | 8.2 ± 0.9 |
| Range | 4‐9 | 4‐9 | 4‐9 |
| Genetic self‐efficacy | |||
| Mean ± SD | 29.1 ± 5.5 | 29.2 ± 5.6 | 29.1 ± 5.6 |
| Range | 5‐35 | 5‐35 | 5‐35 |
Abbreviations: SD, standard deviation.
Figure 1 displays participants’ attitudes about PGT access and regulation. These results depict a sample of individuals who were generally very supportive of the practice of consumer access to genetic test results and wary of additional regulatory attention. Most participants agreed that people should have a right to access their genetic information without going through a doctor (89.9%) and that parents should be able to get results for their children (81.5%). Participants also wanted to maintain control of their information, with 83.2% agreeing overall and 60.4% strongly agreeing that it is important that genetic information is kept private. Participants’ views were less uniform about the routine incorporation of genetic information into health care, but a strong majority of participants agreed that health insurance should cover personal genomic testing (60.3% agreed overall) and that genetic information should be a standard part of the medical record (62.9% agreed overall). More than two‐thirds (68.3%) agreed that genetic tests should be available even more widely, such as in drug stores. Correspondingly, only 14.3% agreed that tests like these should only be available to people through their doctor. Despite these views supporting greater access to PGT, a small but sizable proportion (27.8%) agreed that the government should put more effort into regulating PGT. See the Appendix for the distribution of participants’ perceptions on these 8 items across all 5 response categories.
Figure 1.
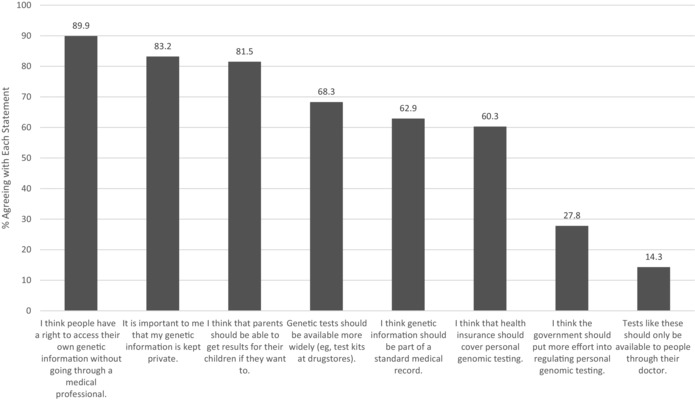
Consumers’ Overall Agreement With Statements About Regulation and the Ethics of Direct Access to Genetic Testing (N = 941)
Principal component factor analysis revealed 4 factors derived from these 8 items, which accounted for 69.5% of the overall variance. The first, which we refer to as “expanded PGT availability and inclusion in health care,” consisted of the average of 4 items: health insurance coverage of tests, genetic results as part of the medical record, tests available more widely, and parents getting test results for children. The second factor, which we call “access without a medical professional,” consists of the difference of 2 items: that people have the right to access the information without a medical professional and that these tests should only be available through the doctor. The last 2 items did not factor with either of these outcomes and we retained them as individual items: that government should regulate these services and the importance of keeping genetic information private.
Table 2 displays the associations of individual‐level characteristics with these 4 attitudinal outcomes. Few individual characteristics were significantly associated with attitudes. Women and people earning incomes between $100,000 and $200,000 were less enthusiastic about expanded PGT availability than men and those earning less than $40,000 a year. Liberals were more likely than political moderates, and conservatives less likely than political moderates, to support additional government regulation of these services; no other demographic characteristics were associated with support for regulation. The only characteristic that was associated with the importance of privacy (which as Figure 1 shows was important to nearly everyone) was education level, such that people with more than a college education were more supportive of the importance of protecting privacy than those with less education. These models reveal no statistically significant differences in attitudes by other individual demographic characteristics, such as race, income, or genetic literacy.
Table 2.
Factors Associated With Attitudes About Access to and Regulation of Personal Genomic Testing
| Demographic Characteristics | Expanded Availabilitye | Access Without Medical Professionalf | Government Should Regulate | Importance of Privacy |
|---|---|---|---|---|
| Age | −0.03 (0.02) | 0.01 (0.04) | −0.05 (0.03) | −0.03 (0.02) |
| Female | −0.17 (0.05)c | −0.22 (0.12) | −0.01 (0.09) | 0.08 (0.07) |
| White (vs nonwhite) | −0.04 (0.07) | 0.24 (0.16) | −0.09 (0.12) | 0.00 (0.10) |
| More than college education (vs less than college) | −0.05 (0.05) | 0.09 (0.11) | 0.05 (0.09) | 0.15 (0.07)a |
| $40k‐$69k (vs <$40k) | −0.08 (0.08) | −0.04 (0.19) | −0.03 (0.15) | −0.22 (0.12) |
| $70k‐$99k (vs ≤ $40k) | −0.14 (0.08) | 0.07 (0.19) | 0.11 (0.14) | 0.04 (0.11) |
| $100k‐$200k (vs ≤ $40k) | −0.14 (0.08) | 0.07 (0.18) | −0.04 (0.21) | 0.04 (0.11) |
| $200k+ (vs ≤ $40k) | −0.02 (0.10) | 0.01 (0.22) | 0.00 (0.17) | −0.06 (0.13) |
| Insured | 0.17 (0.12) | −0.20 (0.28) | −0.04 (0.21) | 0.11 (0.17) |
| Liberal (compared to moderate) | 0.01 (0.06) | 0.11 (0.15) | 0.26 (0.11)a | −0.01 (0.09) |
| Conservative (compared to moderate) | −0.15 (0.08) | −0.05 (0.18) | −0.49 (0.14)c | 0.14 (0.11) |
| Self‐rated healthd | −0.01 (0.03) | 0.01 (0.06) | 0.02 (0.04) | −0.01 (0.04) |
| Genetic literacy | −0.04 (0.03) | 0.07 (0.06) | −0.03 (0.05) | 0.02 (0.04) |
| Genetic self‐efficacy | 0.01 (0.02) | 0.11 (0.05)a | −0.09 (0.04)a | 0.02 (0.03) |
| Genetic risk results | ||||
| Disease risk score | −0.03 (0.02) | 0.05 (0.04) | −0.01 (0.03) | −0.02 (0.02) |
| Pharmacogenomic risk score | 0.01 (0.01) | 0.00 (0.02) | −0.01 (0.02) | 0.02 (0.01) |
| Perceived response | ||||
| Any high‐risk results | −0.24 (0.07)c | 0.04 (0.16) | −0.22 (0.12) | −0.10 (0.10) |
| Feeling upset | 0.00 (0.06) | −0.38 (0.12)b | 0.05 (0.09) | −0.01 (0.08) |
| Pathway consumer (vs 23andMe) | 0.28 (0.06)c | −0.50 (0.13)c | 0.36 (0.10)c | −0.08 (0.08) |
| R‐squared | 0.083 | 0.061 | 0.0786 | 0.0247 |
Entries are regression coefficients from linear regression; standard errors (SE) are in parentheses.
a P ≤ 0.05
b P ≤ 0.01
c P ≤ 0.001
dSelf‐rated health is a measure ranging from 1 to 5, where 5 is poor health.
eThis is a combination of 4 items: parents should be able to get results for children; tests should be available more widely; insurance coverage; inclusion of genetic information in the medical record.
fThis is a combination of 2 items: tests like these should only be available through a doctor and people have a right to access their genetic information without going through a medical professional.
Two other variables related to the genetic‐testing experience were associated with attitudes about access to and regulation of PGT. People with higher levels of perceived self‐efficacy with genetic information (that is, greater confidence in their ability to use and apply genetic information) were less likely to support regulation and more likely to support accessing genetic testing without a medical professional. Reflecting the very different samples recruited into the study, the test company itself also proved significant: Customers of Pathway were more likely to support expanded availability of, and routine medical incorporation of, PGT (eg, insurance coverage, allowing testing of children, including genetic information in the medical record), less likely to agree that people should access genetic testing without a medical professional, and more likely to support greater regulation than were customers of 23andMe.
Table 2 also reveals that none of the calculated genetic risk results variables were significantly associated with attitudes about expanded availability, access without a medical professional, government regulation, or privacy. In follow‐up sensitivity analyses (available upon request from authors), we identified no statistically significant associations between measures of elevated risk for cancers, neurological conditions, cardiovascular disease, or Alzheimer's disease and PGT attitudes. Finally, these results offer some evidence of an association between perceptions of genetic risk results and attitudes. Specifically, respondents who reported that they had received many risk results indicating that they were at a higher than average risk for disease (compared to none or few such results) were less likely to agree with expanded availability of and incorporation of these services in medical care (eg, health insurance coverage, inclusion in a medical record, access for children). Respondents who reported feeling upset about their genetic risk results were less likely to support access to tests without a medical professional. Figure 2 illuminates the major participant factors that were associated with 3 of the 4 outcomes displayed in Table 2: test company (23andMe vs Pathway) and the 2 measures of perceptions of genetic risk—number of high‐risk results and feeling upset.
Figure 2.
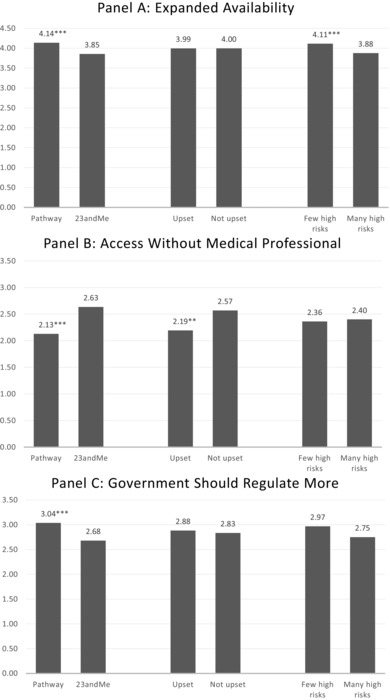
Factors Associated With Consumers’ Attitudes About Availability (Panel A), Access (Panel B), and Regulation (Panel C) of Personal Genomic Testing
Figures plot the predicted mean values (all measured from 0 to 5) for the first 3 outcomes reported in Table 2. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 indicates significant difference for the indicated group relative to the paired group (eg, Pathway vs 23andMe; upset vs not upset; and few high risks vs many high risks).
Discussion
These survey findings describe PGT consumers as enthusiastic about expanded access to genetic testing and negative about the prospects of heightened government regulation. Nearly all participants (89.9%) believed that people should have access to DTC genetic testing, but only 27.8% thought that the government should put more effort into regulation. In fact, a large majority (68.3%) thought that the test kits should be available more widely through outlets such as drug stores—the very proposal that focused the FDA on the DTC‐PGT industry in the first place. A large majority of participants (83.2%) believed in the importance of keeping genetic information private.
Study participants were considerably more enthusiastic about PGT compared to national population studies or to studies within specific patient populations. For instance, 2 national studies of Australians43 and Americans23 both reported majority discomfort with DTC delivery of genetic test results. In particular, the Australian study found heightened concerns about privacy, test accuracy, and general comfort levels for genetic test results delivered DTC compared to through the health care system.43 A US national study from 2011 found that 65% of respondents agreed that “medical professionals should be involved in explaining test results.”23 Reinforcing that generally healthy consumers are quite different from patients affected by genetic conditions, it is also notable that existing studies that focused on patient samples also found lower levels of support for these types of DTC genetics services. For instance, a study of cystic fibrosis patients and parents in Belgium demonstrated considerable skepticism toward commercial companies, with 41% of participants believing that the law should forbid genetic testing being offered directly to consumers.44 Similarly, among 86 women at high risk for breast and ovarian cancer in the United States (Connecticut), only 20% reported support for accessing genetic testing for breast cancer through online services.45
Considered collectively, these findings—support for more restricted access to PGT among the general public and specific populations of patients, and enthusiasm about DTC‐PGT access among generally healthy consumers of these services—suggest that individuals’ level of self‐interest is an important factor shaping attitudes. Aiming to unpack this “interest” with more nuance, our study design allowed us to examine how variation in the salience of genetic risk information shapes attitudes by examining whether people receiving higher‐risk genetic results had different attitudes than those receiving lower‐risk genetic results, holding all other characteristics constant. We did not find that the genetic risk results calculated by companies that consumers actually received were associated with their attitudes. (Note that we did not examine whether respondents’ own prior risk perceptions or family history moderated the impact of genetic test results on their attitudes, although other research does find that personal risk perceptions are important contributors to consumers’ interest in testing.46, 47)
In contrast, consumers’ personal perceptions of the testing experience did relate to their attitudes about test access and availability: Consumers who perceived that they received heightened genetic risk results from their DTC testing (compared to those who reported receiving mainly lower‐risk results) were less enthusiastic about expanded availability and routine incorporation of PGT into health insurance and the medical record. In addition, those who experienced negative emotional impact (feeling upset), compared to those who did not report such emotion, were less supportive about accessing these services without a medical professional. However, these perceptions of the testing experience were not associated with beliefs that the government should put more effort into regulating tests. Interestingly, participants who, at baseline, felt more confident in their ability to use and apply genetic information were both less likely to support regulation and more likely to support accessing genetic testing without a medical professional—regardless of what type of genetic risk results they ultimately received.
Attitudes about testing may vary both by the population (as described vis‐à‐vis disease groups above), as well as the type of genetic testing under consideration. Highly actionable tests or those with highly penetrant mutations may evoke different attitudes (and from a policy perspective, may require a different regulatory frameworks [see, eg, the FDA's discussion of the varieties of laboratory developed tests48]) than tests with more moderate risk and without clear clinical utility. However, we did not observe this in our data. People who received elevated risk results for Alzheimer's disease, for which the apolipoprotein E‐4 variant confers a relatively high risk of disease (∼20%‐35%), compared to other conditions covered by DTC‐PGT, were no more or less supportive of expanded access than those not receiving such a risk, but we were limited in the sample size of people who had received such results. More work is needed to ascertain the relationship between the type of genetic testing and regulatory approaches; since genetic information provided through PGT is extremely heterogeneous, a one‐size‐fits‐all approach when it comes to regulation does not make sense.
Overall, few demographic traits were significantly associated with attitudes about access to and availability of testing. About a quarter of the sample supported increased regulation, and political ideology (identifying as liberal) was, as expected, strongly associated with this view. Women were less supportive of expanded availability of PGT and of access without medical professionals; a previous study by Bloss and colleagues also indicated that women had more concerns about DTC testing,27 suggesting heightened caution about these services among female consumers compared to males. Interestingly, our findings also revealed important differences by type of consumer. Pathway, which offered disease risk and pharmacogenomics testing only, and which recruited from a social networking site for persons with medical conditions, attracted more medically oriented consumers than 23andMe, which also offered testing for more nonmedical traits and information. This difference likely explains why Pathway consumers were more supportive of the incorporation of tests into medical care, less supportive of access without medical professionals, and more supportive of regulation.
Limitations
Although the PGen Study has notable strengths (particularly, recruitment of actual customers, integration of genetic risk results, and a longitudinal design), these results should be considered in light of some limitations. First, as noted elsewhere, the PGen sample is subject to volunteer bias since participants have to both select PGT and also volunteer to be part of the study.36 Given that most of these individuals purchased a PGT product, it is not surprising that they would be supportive of direct access. However, participants are generally representative of the typical DTC genetic testing user about which this study aims to draw conclusions.36 Respondents to this study were broadly similar to the sampling frame of 23andMe consumers invited to participate, although our respondents were significantly more likely to be female. The concern about representation and selection bias is enhanced for the Pathway sample, however, since they were offered subsidized testing to participate. Second, survey research always faces the potential for response bias, with some study participants choosing not to respond to certain surveys. More than 70% of those surveyed at baseline responded to the survey at 6 months, and other evidence from the study population suggests that nonresponse bias is unlikely to be a major problem.36
Third, comparability of our items across other studies faces survey question wording issues. Our questions were not directly comparable to other studies that chose to word items about access to or regulation of PGT differently (such as variation in descriptions about the role of medical professionals across studies). Fourth, our measures of subjective response to testing may be vulnerable to recall bias since participants were asked at 6 months after testing to recall both their results and their emotional reaction to testing. Fifth, respondents may have interpreted the wording of the survey items with variation. In particular (and discussed in greater depth below), this study was mainly concerned with expanding or restricting access to PGT. However, access is only one component of federal policy attention and oversight. In fact, a great deal of regulatory attention to these services has concerned ascertaining the clinical importance, impact, and technical accuracy of test results. Research by Bollinger and colleagues,29 who did include more diverse measures of attitudes about regulation, suggests that consumers may have more supportive views toward a governmental role in regulation of test quality compared to restricting access completely. Our study did not ask consumers’ views about the value of these distinct regulatory objectives; consumers may have interpreted our single item about government regulation in different ways, which could explain why political ideology was most strongly associated with attitudes about regulation. Respondents likely used their attitudes about government as a shortcut to answering this question, rather than relying on a more informed understanding of genetic test regulation.
Conclusions and Policy Implications
Our results describe a population of PGT consumers that want to see expanded access to PGT services and who voiced moderate to strong opposition to federal regulation. An important question, however, concerns the extent to which these attitudes matter in the dynamic policymaking process over PGT. On the one hand, legislative priorities in a democratic political system are ideally meant to be accountable to public demands.49 On the other hand, after legislation has passed, public interest in a medical product typically does not play a major or formal role in agency regulatory decisions, such as that of the FDA. Historical exceptions exist, of course, such as the experience of HIV activists shaping FDA decisions around access to drugs.50 More recently, state legislative branches have compelled or restricted use of drugs against FDA approval or intent (eg, requiring drug access for emergency uses, mandating on‐label use of drugs that can cause abortions).51, 52 Regulatory agencies like the FDA are increasingly moving toward more patient and consumer involvement, such as including consumers on advisory committees and more involved deliberative processes to engage patients.53 For instance, the FDA convenes advisory committee meetings debating whether to recommend approval for a new drug application (however, their influence on the actual approval process is limited).54 Public interest can encourage the FDA to assign a product a higher “enforcement priority” and use its regulatory authority to compel quality compliance (as it did for DTC genetic testing in 2010 and 2013).15 The fact that the PGT users in this study would like to see expanded access could shape, at least in a limited way, regulatory actions moving forward, since greater consumer interest and utilization often leads to greater regulatory involvement. The fact that the majority of those surveyed did not believe that the government should put more effort into regulation reveals an interesting disconnect between consumer attitudes and the FDA's regulatory structure: Public interest can increase FDA enforcement priority in ensuring that a manufacturer has established the safety and efficacy of a device (as, indeed, the FDA confirmed for several genetic risk reports in April 2017). If it has not, the FDA's recourse is to restrict access.
Finally, while our survey focused mainly on access to services, it is important to reiterate that the bulk of governmental inquiry into the DTC genetic‐testing industry has focused on quality: the accuracy, reliability, and clinical utility of the information returned. After all, if the DTC genetic information is unreliable and inaccurate, access is worthless or harmful. Perhaps this quality concern is what drove the 27.8% of participants who stated an interest in more government regulation, but as noted above in limitations, we did not ask about specific regulatory objectives of the government. These data highlight the tension between consumer knowledge of and interest in a product and the mechanics of the regulatory approval process. They also underscore the need for future research to supplement these quantitative (and largely decontextualized) survey data with qualitative interviews and deliberative approaches55 that both educate and engage participants in the ongoing policy debate.
Funding/Support
The PGen Study is supported by the National Institutes of Health (NIH) National Human Genome Research Institute (R01‐HG005092). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, or the National Institutes of Health. Robert C. Green is also supported by U01‐HG006500 and U41‐HG006834. Amy L. McGuire is also supported by U01‐HG006500. Deanna Alexis Carere is funded by a Canadian Institutes of Health Research Postdoctoral Fellowship Award and by the Michael G. DeGroote Postdoctoral Fellowship from McMaster University. Barbara Koenig is also supported by P20 HG007243.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The research team partnered with 23andMe and Pathway Genomics in order to gain access to consumers of their services for implementation of the surveys described in our manuscript. However, these companies did not provide funding for the project, and this manuscript was independently conceived and completed. Outside of the submitted work, Amy McGuire provided consulting services to Human Longevity Inc, and Richard Sharp was a noncompensated adviser to 23andMe, which supported his travel to attend an in‐person meeting held near the company's headquarters.
Acknowledgments: Nonauthor members of the PGen Study at the time of publication are as follows: Joel B. Krier, Margaret H. Helm, Sarah S. Kalia, Kurt D. Christensen, Harvard Medical School and Brigham and Women's Hospital; Mack T. Ruffin IV, Lan Q. Le, Jenny Ostergren, University of Michigan School of Public Health; Wendy R. Uhlmann, Mick P. Couper, University of Michigan; Joanna L. Mountain, Amy K. Kiefer, 23andMe; Glenn Braunstein, Pathway Genomics; Scott D. Crawford, SoundRocket; L. Adrienne Cupples, Clara A. Chen, Catharine Wang, Boston University; Kimberly Kaphingst, University of Utah. The authors acknowledge helpful discussion on some of the ideas in this article with Patricia J. Zettler, JD.
Consumers’ Attitudes About Regulation and the Ethics of Direct Access to Genetic Testing (N = 941)
| Overall Agree a | Strongly Disagree | Somewhat Disagree | Neither Agree nor Disagree | Somewhat Agree | Strongly Agree | |
|---|---|---|---|---|---|---|
| I think people have a right to access their own genetic information without going through a medical professional. | 845 (89.9%) | 13 (1.4%) | 15 (1.6%) | 67 (7.1%) | 219 (23.3%) | 626 (66.6%) |
| It is important to me that my genetic information is kept private. | 783 (83.2%) | 31 (3.3%) | 34 (3.6%) | 93 (9.9%) | 215 (22.8%) | 568 (60.4%) |
| I think that parents should be able to get results for their children if they want to. | 767 (81.5%) | 13 (1.4%) | 27 (2.9%) | 134 (14.2%) | 290 (30.8%) | 477 (50.7%) |
| Genetic tests should be available more widely (eg, test kits at drug stores). | 643 (68.3%) | 45 (4.8%) | 80 (8.5%) | 173 (18.4%) | 298 (31.7%) | 345 (36.7%) |
| I think genetic information should be part of a standard medical record. | 592 (62.9%) | 76 (8.1%) | 91 (9.7%) | 182 (19.3%) | 280 (29.8%) | 312 (33.2%) |
| I think that health insurance should cover personal genomic testing. | 567 (60.3%) | 70 (7.4%) | 84 (8.9%) | 220 (23.4%) | 259 (27.5%) | 308 (32.7%) |
| I think the government should put more effort into regulating personal genomic testing. | 261 (27.8%) | 242 (25.7%) | 131 (13.9%) | 306 (32.6%) | 157 (16.7%) | 104 (11.1%) |
| Tests like these should only be available to people through their doctor. | 135 (14.3%) | 484 (51.4%) | 191 (20.3%) | 131 (13.9%) | 62 (6.6%) | 73 (7.8%) |
aOverall agree is the sum of somewhat agree and strongly agree.
References
- 1. Hogarth S, Javitt G, Melzer D. The current landscape for direct‐to‐consumer genetic testing: legal, ethical, and policy issues. Annu Rev Genomics Hum Genet. 2008;9:161‐182. [DOI] [PubMed] [Google Scholar]
- 2. Evans JP, Green RC. Direct to consumer genetic testing: Avoiding a culture war. Genet Med. 2009;11(8):568‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lautenbach DM, Christensen KD, Sparks JA, Green RC. Communicating genetic risk information for common disorders in the era of genomic medicine. Annu Rev Genomics Hum Genet. 2013;14:491‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGuire AL, Burke W. An unwelcome side effect of direct‐to‐consumer personal genome testing: raiding the medical commons. JAMA. 2008;300(22):2669‐2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caulfield T, Ries NM, Ray PN, Shuman C, Wilson B. Direct‐to‐consumer genetic testing: good, bad or benign? Clin Genet. 2010;77(2):101‐105. [DOI] [PubMed] [Google Scholar]
- 6. Eng C, Sharp RR. Bioethical and clinical dilemmas of direct‐to‐consumer personal genomic testing: the problem of misattributed equivalence. Sci Transl Med. 2010;2(17):17cm15. [DOI] [PubMed] [Google Scholar]
- 7. Gollust SE, Wilfond BS, Hull SC. Direct‐to‐consumer sales of genetic services on the internet. Genet Med. 2003;5(4):332‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts JS, Ostergren J. Direct‐to‐consumer genetic testing and personal genomics services: a review of recent empirical studies. Curr Genet Med Rep. 2013;1(3):182‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carpenter D. Groups, the media, agency waiting costs, and FDA drug approval. Am J Polit Sci. 2002;46(3):490‐505. [Google Scholar]
- 10. Gollust SE, Apse K, Fuller BP, Miller PS, Biesecker BB. Community involvement in developing policies for genetic testing: assessing the interests and experiences of individuals affected by genetic conditions. Am J Public Health. 2005;95(1):35‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Secretary's Advisory Committee on Genetics, Health, and Society . US System of Oversight of Genetic Testing: A Response to the Charge of the Secretary of Health and Human Services. Washington, DC: US Department of Health & Human Services; 2008. osp.od.nih.gov/sites/default/files/SACGHS_oversight_report.pdf. Accessed March 6, 2017. [Google Scholar]
- 12. Secretary's Advisory Committee on Genetics, Health, and Society . Direct‐to‐Consumer Genetic Testing. Washington, DC: US Department of Health & Human Services; 2010. auth.osp.od.nih.gov/sites/default/files/SACGHS_DTC_Report_2010.pdf. Accessed March 6, 2017. [Google Scholar]
- 13. US Government Accountability Office (GAO) . Direct‐to‐Consumer Genetic Tests: Misleading Results Are Further Complicated by Deceptive Marketing and Other Questionable Practices. Washington, DC: GAO; 2010. www.gao.gov/assets/130/125079.pdf. Accessed March 6, 2017. [Google Scholar]
- 14. Pollack A. Walgreens delays selling personal genetic test kit. New York Times. May 12, 2010. [Google Scholar]
- 15. Spector‐Bagdady K, Pike E. Consuming genomics: regulating direct‐to‐consumer genetic and genomic information. Nebr Law Rev. 2014;92:677‐745. [Google Scholar]
- 16. 23andMe . 23andMe takes first step toward FDA clearance. 23andMeBlog blog.23andme.com/news/23andme‐takes‐first‐step‐toward‐fda‐clearance. Published July 30, 2012. Accessed March 6, 2017.
- 17. FDA warning letter from Alberto Gutierrez, Dir ., Office of In Vitro Diagnostics and Radiological Health, Ctr. for Devices & Radiological Health, Food & Drug Admin., US Dep't of Health & Human Services, to Ann[e] Wojcicki, CEO, 23andMe Inc. November 22, 2013. www.fda.gov/iceci/enforcementactions/warningletters/2013/ucm376296.htm. Accessed March 6, 2017. [Google Scholar]
- 18. 23andMe . A Note to Our Customers Regarding the FDA. 23andMeBlog blog.23andme.com/news/a‐note‐to‐our‐customers‐regarding‐the‐fda. Published February 19, 2015. Accessed March 6, 2017.
- 19. Finney Rutten LJ, Gollust SE, Naveed S, Moser RP. Increasing public awareness of direct‐to‐consumer genetic tests: health care access, internet use, and population density correlates. J Cancer Epidemiol. 2012;2012:309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goddard KA, Duquette D, Zlot A, et al. Public awareness and use of direct‐to‐consumer genetic tests: results from 3 state population‐based surveys, 2006. Am J Public Health. 2009;99(3):442‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goddard KA, Moore C, Ottman D, Szegda KL, Bradley L, Khoury MJ. Awareness and use of direct‐to‐consumer nutrigenomic tests, United States, 2006. Genet Med. 2007;9(8):510‐517. [DOI] [PubMed] [Google Scholar]
- 22. Kolor K, Liu T, St Pierre J, Khoury M. Health care provider and consumer awareness, perceptions, and use of direct‐to‐consumer personal genomic tests, United States, 2008. Genet Med. 2009;11(8):595. [DOI] [PubMed] [Google Scholar]
- 23. Almeling R, Gadarian SK. Public opinion on policy issues in genetics and genomics. Genet Med. 2014;16(6):491‐494. [DOI] [PubMed] [Google Scholar]
- 24. McGuire AL, Diaz CM, Wang T, Hilsenbeck SG. Social networkers’ attitudes toward direct‐to‐consumer personal genome testing. Am J Bioeth. 2009;9(6‐7):3‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McBride CM, Alford SH, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Characteristics of users of online personalized genomic risk assessments: implications for physician‐patient interactions. Genet Med. 2009;11(8):582‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gollust SE, Gordon ES, Zayac C, et al. Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics. 2012;15(1):22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bloss CS, Ornowski L, Silver E, et al. Consumer perceptions of direct‐to‐consumer personalized genomic risk assessments. Genet Med. 2010;12(9):556‐566. [DOI] [PubMed] [Google Scholar]
- 28. James KM, Cowl TC, Tilburt JC, et al. Impact of direct‐to‐consumer predictive genomic testing on risk perception and worry among patients receiving routine care in a preventive health clinic. Mayo Clin Proc. 2011;86(10):933‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bollinger JM, Green RC, Kaufman D. Attitudes about regulation among direct‐to‐consumer genetic testing customers. Genet Test Mol Biomarkers. 2013;17(5):424‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sniderman PM. The new look in public opinion research. In: Finifter A, ed. The State of the Discipline II. Washington, DC: American Political Science Association; 1993:219‐245. [Google Scholar]
- 31. Morain S, Mello MM. Survey finds public support for legal interventions directed at health behavior to fight noncommunicable disease. Health Aff (Millwood). 2013;32(3):486‐496. [DOI] [PubMed] [Google Scholar]
- 32. Lynch J, Gollust SE. Playing fair: fairness beliefs and health policy preferences in the United States. J Health Polit Policy Law. 2010;35(6):849‐887. [DOI] [PubMed] [Google Scholar]
- 33. Sears DO, Lau RR, Tyler TR, Allen HM Jr. Self‐interest vs symbolic politics in policy attitudes and presidential voting. Am Polit Sci Rev. 1980;74(3):670‐684. [Google Scholar]
- 34. Lau R, Heldman C. Self‐Interest, symbolic attitudes, and support for public policy: a multilevel analysis. Polit Psychol. 2009;30(4):513‐537. [Google Scholar]
- 35. Chong D, Citrin J, Conley P. When self‐interest matters. Polit Psychol. 2001;22(3):541‐570. [Google Scholar]
- 36. Carere DA, Couper MP, Crawford SD, et al. Design, methods, and participant characteristics of the Impact of Personal Genomics (PGen) Study, a prospective cohort study of direct‐to‐consumer personal genomic testing customers. Genome Med. 2014;6(12):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lehmann LS, Kaufman DJ, Sharp RR, et al. Navigating a research partnership between academia and industry to assess the impact of personalized genetic testing. Genet Med. 2012;14(2):268‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roberts JS, Gornick MC, Carere DA, et al. Direct‐to‐consumer genetic testing: user motivations, decision making, and perceived utility of results [published online ahead of print January 10, 2017]. Public Health Genomics https://doi.org/10.1159/000455006. [DOI] [PubMed]
- 39. van der Wouden C, Carere DA, Maitland‐van der Zee AH, et al. Consumer perceptions of interactions with primary care providers after direct‐to‐consumer personal genomic testing. Ann Intern Med. 2016;164(8):513‐522. [DOI] [PubMed] [Google Scholar]
- 40. Carere DA, VanderWeele T, Moreno TA, et al. The impact of direct‐to‐consumer personal genomic testing on perceived risk of breast, prostate, colorectal, and lung cancer: findings from the PGen study. BMC Med Genomics. 2015;8(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ware JE, Gandek B. Overview of the SF‐36 health survey and the international quality of life assessment (IQOLA) project. J Clin Epidemiol. 1998;51(11):903‐912. [DOI] [PubMed] [Google Scholar]
- 42. Carere DA, Kraft P, Kaphingst KA, Roberts JS, Green RC. Consumers report lower confidence in their genetics knowledge following direct‐to‐consumer personal genomic testing. Genet Med. 2016;18(1):65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Critchley C, Nicol D, Otlowski M, Chalmers D. Public reaction to direct‐to‐consumer online genetic tests: comparing attitudes, trust and intentions across commercial and conventional providers. Public Underst Sci. 2015;24(6):731‐750. [DOI] [PubMed] [Google Scholar]
- 44. Janssens S, Kalokairinou L, Chokoshvilli D, et al. Attitudes of cystic fibrosis patients and their parents towards direct‐to‐consumer genetic testing for carrier status. Personalized Med. 2015;12(2):99‐107. [DOI] [PubMed] [Google Scholar]
- 45. Perez GK, Cruess DG, Cruess S, et al. Attitudes toward direct‐to‐consumer advertisements and online genetic testing among high‐risk women participating in a hereditary cancer clinic. J Health Commun. 2011;16(6):607‐628. [DOI] [PubMed] [Google Scholar]
- 46. Hiraki S, Chen CA, Roberts JS, Cupples LA, and Green RC Perceptions of familial risk in those seeking a genetic risk assessment for Alzheimer's disease. J Genet Couns. 2009;18(2):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meisel SF, Carere DA, Wardle J, et al. Explaining, not just predicting, drives interest in personal genomics. Genome Med. 2015;7(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laboratory developed tests . US Food and Drug Administration website. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm407296.htm. Last updated January 13, 2017. Accessed March 6, 2017.
- 49. Burstein P. The impact of public opinion on public policy: a review and an agenda. Polit Res Q. 2003;56(1):29‐40. [Google Scholar]
- 50. France D. How to Survive a Plague: The Inside Story of How Citizens and Science Tamed AIDS. New York, NY: Alfred A. Knopf; 2016. [Google Scholar]
- 51. Brennan Z. California governor vetoes right‐to‐try bill, points to FDA compassionate use program. Regulatory Affairs Professionals Society website. www.raps.org/Regulatory-Focus/News/2015/10/12/23371/California-Governor-Vetoes-Right-to-Try-Bill-Points-to-FDA-Compassionate-Use-Program/#sthash.QgkztMfu.dpuf. Published October 12, 2015. Accessed March 6, 2017.
- 52. Tavernise S. New FDA guidelines ease access to abortion pill. New York Times. March 30, 2016. [Google Scholar]
- 53. Gusmano MK. FDA decisions and public deliberation: challenges and opportunities. Public Adm Rev. 2013;73(s1):S115‐S126. [Google Scholar]
- 54. Gortler D. FDA's drug approval process should be based on science, not public opinion. STAT. June 2, 2016. www.statnews.com/2016/06/02/fda-duchenne-muscular-dystrophy. Accessed March 6, 2017. [Google Scholar]
- 55. Gornick MC, Scherer AM, Sutton EJ, et al. Effect of public deliberation on attitudes toward return of secondary results in genomic sequencing. J Genet Couns. 2017;26(1):122‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]


