Abstract
Youth are particularly vulnerable to acquiring HIV, yet reaching them with HIV prevention interventions and engaging and retaining those infected in care and treatment remains a challenge. We sought to determine the incidence rate of loss to follow-up (LTFU) and explore socio-demographic and clinical characteristics associated with LTFU among HIV-positive youth aged 15–21 years accessing outpatient care and treatment clinics in Kisumu, Kenya. Between July 2007 and September 2010, youth were enrolled into two different HIV care and treatment clinics, one youth specific and the other family oriented. An individual was defined as LTFU when absent from the HIV treatment clinic for ≥ 4 months regardless of their antiretroviral treatment status. The incidence rate of LTFU was calculated and Cox regression analysis used to identify factors associated with LTFU. A total of 924 youth (79% female) were enrolled, with a median age of 20 years (IQR 18-21). Over half, (529 (57%)), were documented as LTFU, of whom 139 (26%) were LTFU immediately after enrolment. The overall incidence rate of LTFU was 52.9 per 100 person-years (p-y). Factors associated with LTFU were pregnancy during the study period (crude HR 0.68, 95% CI 0.53–0.89); CD4 cell count ≥350 (adjusted hazard ratios (AHR) 0.59, 95% CI 0.39–0.90); not being on antiretroviral therapy (AHR 4.0, 95% CI 2.70–5.88); and nondisclosure of HIV infection status (AHR 1.43, 95% CI 1.10–1.89). The clinic of enrolment, age, marital status, employment status, WHO clinical disease stage and education level were not associated with LTFU. Interventions to identify and enrol youth into care earlier, support disclosure, and initiate ART earlier may improve retention of youth and need further investigation. Further research is also needed to explore the reasons for LTFU from care among HIV-infected youth and the true outcomes of these patients.
Keywords: HIV, youth, resource-limited settings, Africa, loss to follow-up, antiretroviral therapy
Introduction
Adolescents and youth aged 15–24 years are among the populations most impacted by the global HIV epidemic, with an estimated 40% of all daily 6300 new infections occurring in this age group (Auld et al., 2014). Approximately two-thirds of the world's more than 10 million HIV-infected young people aged 15–24 years live in sub-Saharan Africa, accounting for 20% of all people living with HIV in this region (Lamb et al., 2014). HIV represents a continuing threat to the youth in Kenya, who comprise 38% of the national population but are believed to make up more than 60% of new HIV infections (Harper et al., 2014; National AIDS and STI Control Programme (NASCOP) & National AIDS Control Council, 2012; Nöstlinger et al., 2015).With such a large and growing burden of the disease among youth, and the life-long nature of HIV treatment, strategies that focus on their enrolment and retention into HIV care are paramount (Lamb et al., 2014).
Retention in care and adherence to antiretroviral therapy (ART) are critical for the optimal success of HIV treatment scale-up (Gwynn et al., 2015; Tweya et al., 2013). Adolescents and young adults living with HIV have complex medical and psychological needs and many disengage from health services (Hughes et al., 2013; Wolf et al., 2014). Due to factors such as poverty, marginalization, stigma, lack of social support structures and low self-perception of risk, youth may engage in high-risk health behaviour (e.g., defaulting from care) despite knowing the possible adverse consequences; they simply do not rationally weigh the relative risks and consequences of their actions (Shroufi et al., 2013).
There is paucity of data on the outcomes of youth enrolled in HIV care in sub-Saharan Africa (Lamb et al., 2014). We sought to determine the incidence rate of loss to follow-up (LTFU) and to explore socio-demographic factors and clinical characteristics associated with LTFU among HIV-infected youth accessing outpatient HIV care and treatment services in select facilities in Kisumu, Kenya.
Methods
Study design
This retrospective cohort analysis used data routinely collected from HIV-infected patients enrolled in care at Lumumba Health Centre and at Tuungane Youth Centre, both in Kisumu, Kenya. Patients aged 15–21 years who enrolled into care between July 2007 and September 2010 were included in the analysis. The protocol was reviewed and approved by the Kenya Medical Research Institute (KEMRI) ethical review committee and the University of California San Francisco (UCSF) Committee on Human Research prior to the abstraction of de-identified and delinked data from the medical records.
Programme description
Family AIDS Care & Education Services (FACES) is a family-centred HIV prevention, care and treatment programme funded by the US President's Emergency Plan for AIDS Relief (PEPFAR) through a cooperative agreement with the Centres for Disease Control and Prevention (CDC). It is a collaborative effort between KEMRI and UCSF. The FACES family model of care has been described elsewhere (Lewis Kulzer et al., 2012).
Tuungane Youth Centre is a youth-oriented HIV prevention, care and treatment clinic run by the Impact Research and Development Organization (IRDO) and is funded by PEPFAR through a cooperative agreement with the CDC.
In November 2005, FACES and IRDO began a collaboration to expand the HIV services for youth at Tuungane Youth Centre by adding HIV care and treatment to the prevention services already existing at the centre. In this way, young people could access a full spectrum of youth-focused HIV prevention, care and treatment services in a youth-friendly environment.
Care at both Lumumba Health Centre and Tuungane Youth Centre is offered with no fees charged. To facilitate high quality of care, patient encounters are recorded on standardized clinical forms, and staff members from FACES and IRDO hold joint weekly continuous medical education sessions and rotate staff between the two clinics.
At both sites, patients meeting the following criteria were initiated on ART, in accordance with the Kenyan national HIV treatment guidelines: WHO Stage I & II with a CD4 cell count of < 350 cells/mm3; WHO Stage III & IV regardless of CD4 cell count; TB/HIV co-infection; and TB/HIB co-infection with evidence of liver disease (NASCOP, 2011).
By September 2010, Lumumba Health Centre had 10,010 patients active in care, of whom 338 (3.4%) were aged 15–21 years, and Tuungane Youth Centre had 1424 HIV-positive patients active in care, of whom 431 (30.4%) were within that age range.
Missed appointments and defaulter tracing
To improve patient retention, FACES, through its Clinic and Community Health Assistants (CCHAs), runs an active defaulter tracing program. Upon enrolment, each patient's address and contact information are recorded. A patient missing his/her appointment is identified from the clinic daily attendance register and is actively sought three days after a missed appointment through the use of phone calls and/or a home visit. A similar cadre of staff in the outreach department at Tuungane Youth Centre implements a similar defaulter tracing mechanism. Due to staff and transportation constraints, the vast geographical coverage area, and inconsistent and sometime unreliable patient locator information, not all patients can be traced. Defaulter tracing is prioritized for patients who have recently initiated ART, expectant mothers and children.
Data collection
In April 2007, an open-source electronic medical records system (OpenMRS) was launched at Lumumba Health Centre and was extended to Tuungane Youth Centre in July 2007. Socio-demographic, clinical, laboratory and pharmacological data collected at each patient visit was recorded on standardized clinical visit forms then manually entered into OpenMRS on a daily basis.
Variables
The primary outcome for this analysis was LTFU, defined as a patient missing his/her last appointment by ≥ 4 months. The baseline socio-demographic and clinical characteristics examined for association with LTFU included: age, marital/civil status, clinic type, WHO clinical disease stage, disclosure of HIV-infection status, becoming pregnant during the study and highest education level. We also examined ART status at the time of LTFU for association with LTFU. Patients known to have transferred to a different care location, were deceased, or withdrew from care were not considered as LTFU.
Data analysis
The two-sample t-test was used to compare continuous variables and the Chi-square (χ2) test was used to compare categorical variables between patients enrolled at the youth-specific clinic and those at the family-oriented clinic during the study period. The incidence rate of LTFU is presented as events per 100 person-years (p-y), from date of enrolment. The event date of LTFU was defined as the date of the last clinic visit in the medical records. For patients determined to have been transferred out, withdrawn or deceased, data were censored at their date of last appointment or date of death. Data on patients still in active care at the end of the study period were censored at the date of their last clinic visit. The log-rank test was used to compare survival curves for LTFU. Cox regression was used to identify factors independently associated with LTFU and to estimate crude and adjusted hazard ratios (AHRs) for LTFU. AHRs and associated 95% confidence intervals (CI) were calculated from a single model that included all patient demographic and clinical characteristics under examination. All analyses were performed using STATA version 11/SE software (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics
Between 23 July 2007 and 26 September 2010, 924 patients were identified as eligible for inclusion in the data analysis: 584 (63%) at the youth-specific clinic and 340 (37%) at the family-oriented clinic. Patients had a median age of 20 years (IQR 18–21), 732 (79%) were female and only 55 (6%) were reported as having some form of employment. Two hundred and nine women were reported as having become pregnant during the study (Table 1).
Table 1.
Characteristics of youth accessing outpatient HIV care and treatment services in Kisumu, Kenya, from July 2007– September 2010.
| Characteristic | Number (%) |
|---|---|
| Total number enrolled | 924 |
| Youth-specific clinic | 584 (63) |
| Family-oriented clinic | 340 (37) |
| Median age (years) | 20 (IQR: 18–21) |
| Gender (female) | 732 (79) |
| Became pregnant during study follow up | 209 (29) |
| Marital status | |
| Married/partnered | 295 (32) |
| Not married/partnered | 471 (51) |
| Status not recorded | 158 (17) |
| Currently working/employed | 55 (6) |
| Median CD4 count (cells/mm3) | 411 (IQR: 232–607) |
| CD4 cell count (cells/mm3) | |
| <250 | 219 (24) |
| 250–349 | 96 (10) |
| ≥350 | 480 (52) |
| CD4 cell count not documented | 129 (14) |
| WHO Clinical staging and disease classification | |
| Stage I/II | 737 (80) |
| Stage III/IV | 172 (19) |
| Staging not recorded | 15 (2) |
| Highest education level attained | |
| None | 14 (1) |
| Primary | 532 (58) |
| Secondary | 250 (27) |
| Tertiary | 38 (4) |
| Not recorded | 90 (10) |
| Disclosed HIV infection status | |
| Yes | 591 (64) |
| No | 268 (29) |
| Disclosure status not documented | 65 (7) |
| Ever initiated on ART | 364 (39) |
| Number discontinued from care | 60 (7) |
| Deceased | 9 (1) |
| Transferred to another clinic | 48 (5) |
| Withdrew from care | 3 (0.3) |
| Total number lost to follow-up | 527 (57) |
| Youth-specific clinic | 351 (60) |
| Family-oriented clinic | 176 (51) |
Patient characteristics by clinic type
Patients at the family-oriented clinic were younger (p < .001), more likely to be female (p < .001), married (p < .001), working/employed (p < .001), more likely to have become pregnant during the study (p < .001) and more likely to have disclosed their HIV status (p = .001). Patients at the youth-specific site had higher median CD4 cell counts (p = .001), had higher education level (p = .001) and were less likely to have ever initiated ART (p < .001) (Table 2).
Table 2.
Characteristics of youth accessing outpatient HIV care and treatment services in Kisumu, Kenya, by clinic type, July 2007–September 2010.
| Characteristic | Total (n = 924) | Youth-specific clinic (n = 584) | Family-oriented clinic (n = 340) | p-value |
|---|---|---|---|---|
| Median age (years) | 20 (IQR 19–21) | 19 (IQR 18–20) | <0.001 | |
| Gender (female) | 732 (79%) | 418 (72%) | 314 (92%) | <0.001 |
| Became pregnant during study | 209 (23%) | 64 (11%) | 145 (43%) | <0.001 |
| Marital statusa | ||||
| Married/partnered | 295 (32%) | 129 (22%) | 166 (49%) | <0.001 |
| Not married/partnered | 471 (51%) | 363 (62%) | 108 (32%) | |
| Currently working/employed | 55 (6%) | 13 (2%) | 42 (12%) | <0.001 |
| Median CD4 count (cells/mm3) | 446 (IQR 275–675) | 401 (IQR 217–576) | 0.001 | |
| CD4 cell count (cells/mm3)b | ||||
| <250 | 219 (24%) | 159 (27%) | 60 (18%) | 0.014 |
| 250–349 | 96 (10%) | 56 (9%) | 40 (12%) | |
| ≥350 | 480 (52%) | 300 (52%) | 179 (52%) | |
| WHO clinical disease stagec | ||||
| Stage I/II | 737 (80%) | 474 (81%) | 263 (77%) | 0.098 |
| Stage III/IV | 172 (19%) | 99 (17%) | 73 (21%) | |
| Highest education level attainedd | ||||
| None | 14 (2%) | 10 (2%) | 4 (1%) | 0.002 |
| Primary | 532 (58%) | 309 (53%) | 223 (65%) | |
| Secondary | 250 (27%) | 167 (29%) | 83 (24%) | |
| Tertiary | 38 (4%) | 32 (5%) | 6 (2%) | |
| Disclosed HIV infection status | 591 (64%) | 346 (59%) | 245 (75%) | 0.001 |
| Ever initiated on ART | 364 (39%) | 204 (35%) | 160 (47%) | <0.001 |
| Discontinued from care | 60 (7%) | 25 (4%) | 35 (10%) | |
| Deceased | 9 (1%) | 5 (1%) | 4 (1%) | 0.27 |
| Transferred out | 48 (5%) | 20 (3%) | 28 (8%) | |
| Withdrew from care | 3 (0.3%) | 0 | 3 (1%) | |
| Lost to follow-up | 529 (57%) | 354 (61%) | 175 (51%) | 0.007 |
Marital status was missing for 158 patients (17%).
CD4 cell count was missing for 129 patients (14%).
WHO staging was missing for 15 patients (2%).
Educational status was missing for 90 patients (10%).
Disclosure status was missing for 65 patients (7%).
Loss to follow-up
Of 924 eligible patients, 529 (57%) were LTFU by close of study, 418 (79%) of whom were female. Of patients who were LTFU, 354 (67%) were from the youth-specific clinic. Only 60 patients (7%) were identified as discontinued from care (9 deceased, 48 transferred to other clinics and 3 withdrew from care) and were thus not defined as LTFU.
Patients contributed a total of 734 p-y of follow-up, with a mean individual contribution of 0.9 years. Of 529 patients who were LTFU, 139 (26%) were lost immediately after enrolment, and the median time to LTFU was 1.4 years (95% CI 1.1–1.6). Overall the incidence rate for LTFU was 52.9(95% CI 47.9–58.4) patients per 100 p-y. The incidence rate for LTFU was significantly greater among patients who never started ART compared to those who had ever initiated ART (Log rank p < .001) (Figure 1), and among those who had not disclosed their status compared to those who had (Log-rank test, p = .001) (Figure 2).
Figure 1.
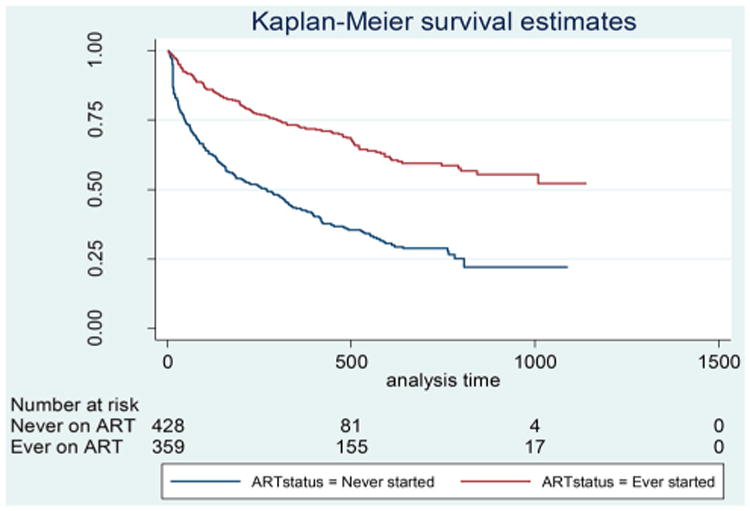
Kaplan–Meier survival curves for being lost to follow-up, by ART status, among youth accessing outpatient HIV care and treatment services in Kisumu, Kenya, July 2007–September 2010.
Figure 2.
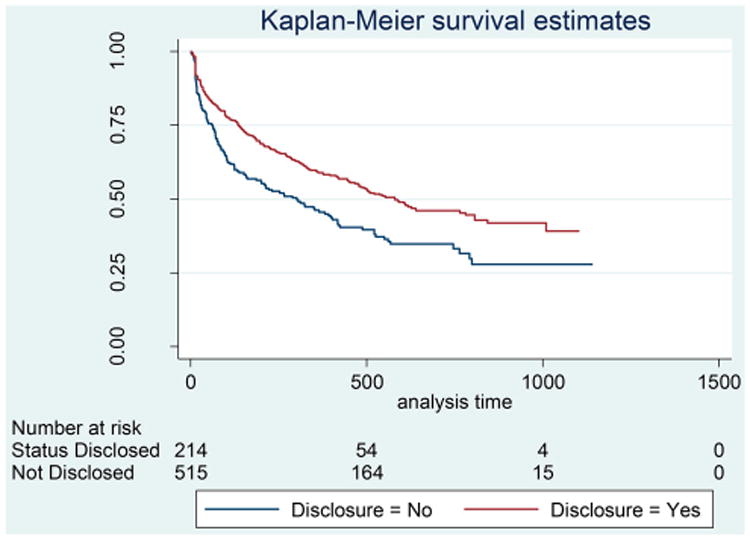
Kaplan–Meier survival curves for being lost to follow-up, by HIV infection disclosure status, among youth accessing outpatient HIV care and treatment services in Kisumu, Kenya, July 2007–September 2010.
In the univariate Cox regression analysis, compared to female patients who did not become pregnant at any time during the study period, those who did had 32% lower hazards of being LTFU (crude HR 0.68, 95% CI 0.53–0.89). Male patients were no more likely to become LTFU when compared to non-pregnant females (crude HR 0.97, 95% CI 0.54–1.24). Patients who had never initiated on ART at the time of being LTFU had a 52% higher hazard of being LTFU compared to those who had ever started ART (crude HR 1.52, 95% CI 1.22–1.89). Clinic of enrolment, age, marital status, occupational status, WHO HIV clinical disease stage, education level and disclosure status were not associated with LTFU in the univariate analysis (Table 3).
Table 3.
Factors associated with LTFU among youth accessing outpatient HIV care and treatment services in Kisumu, Kenya, July 2007-September 2010.
| Covariate | Univariate analysis | Adjusted/Multivariable analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | p | 95% CI | AHR | p | 95% CI | |
| Youth-focused site | ||||||
| No* | 1 | 1 | ||||
| Yes | 1.03 | 0.82 | [0.83–1.27] | 1.09 | 0.56 | [0.80–1.50] |
| Age at enrolment (years) | ||||||
| <20* | 1 | 1 | ||||
| ≥20 | 1.07 | 0.51 | [0.88–1.31] | 0.94 | 0.62 | [0.72–1.22] |
| Became pregnant during the study | 1 | |||||
| No* | 1 | |||||
| Yes | 0.68 | 0.005 | [0.53–0.89] | 1.10 | 0.63 | [0.75–1.61] |
| Males | 0.97 | 0.78 | [0.54–1.24] | 1.08 | 0.63 | [0.80–1.46] |
| Marital status at enrolmenta | ||||||
| Not married* | 1 | 1 | ||||
| Married/partnered | 1.15 | 0.25 | [0.91–1.45] | 0.84 | 0.23 | [0.62–1.12] |
| Currently working at enrolment | ||||||
| No* | 1 | 1 | ||||
| Yes | 1.22 | 0.39 | [0.78–1.89] | 0.99 | 0.99 | [0.59–1.67] |
| Baseline CD4 count (cells/mm3)b | ||||||
| <250* | 1 | 1 | ||||
| 250–349 | 0.84 | 0.40 | [0.57–1.25] | 0.63 | 0.071 | [0.39–1.04] |
| ≥350 | 0.84 | 0.19 | [0.66–1.09] | 0.59 | 0.015 | [0.39–0.90] |
| Baseline WHO stagec | ||||||
| Stage I/II* | 1 | 1 | ||||
| Stage III/IV | 1.04 | 0.77 | [0.81–1.33] | 1.20 | 0.29 | [0.86–1.67] |
| Education level at enrolmentd | ||||||
| None* | 1 | 1 | ||||
| Primary | 0.97 | 0.95 | [0.66–2.61] | 1.44 | 0.47 | [0.53–3.93] |
| Secondary | 1.00 | 1.00 | [0.37–2.72] | 1.06 | 0.90 | [0.38–2.95] |
| Tertiary | 0.94 | 0.92 | [0.31–2.87] | 0.66 | 0.50 | [0.19–2.22] |
| ART status at LTFU | ||||||
| Ever started* | 1 | 1 | ||||
| Never started | 1.52 | <0.001 | [1.22–1.89] | 4.0 | <0.001 | [2.70–5.88] |
| Disclosed HIV infection statuse | ||||||
| Yes* | 1 | 1 | ||||
| No | 1.22 | 0.074 | [0.98–1.52] | 1.43 | 0.008 | [1.10–1.89] |
Reference category.
Marital status was missing for 158 patients (17%).
CD4 cell count was missing for 132 patients (14%).
WHO staging was missing for 15 patients (2%).
Educational status was missing for 90 patients (10%).
Disclosure status was missing for 65 patients (7%).
In the multivariable Cox regression analysis, compared to patients who had CD4 cell counts <250 cells/mm3, patients who had CD4 cell counts ≥ 350 cells/mm3 had a 41% lower hazard of being LTFU (AHR 0.59, 95% CI 0.39–0.90). Patients who had never started ART had four times higher hazards of becoming LTFU compared to those who had ever initiated ART (AHR 4.0, 95% CI 2.70–5.88). Compared to those who had disclosed their status to anyone (family/friends), those who had never disclosed had a 43% higher hazard of becoming LTFU (AHR 1.43, 95% CI 1.10–1.89). Pregnancy had no significant association with LTFU in the multivariable analysis, suggesting confounding. Type of clinic, age, marital status, employment status, WHO HIV clinical disease stage and education level were not associated with LTFU in the multivariable analysis (Table 3).
Discussion
LTFU was very high in this vulnerable population in both the youth-specific and family-oriented clinics, with more than half of the patients enrolled into care being documented as LTFU. The LTFU incidence of 52.9 per 100 p-y among this population is up to twice that seen among adults in HIV care and treatment programmes, whose LTFU rates range from 9% to 26%, with an annual incidence rate of 14–27 per 100 p-y (Charurat et al., 2010; Ochieng-Ooko et al., 2010).While some studies have shown that HIV-infected youth in adult-oriented clinics are less likely to start treatment, achieve viral suppression and stay on treatment compared to adults (Auld et al., 2014; Evans et al., 2013; Lamb et al., 2014); others have observed rates of LTFU twice as high in adults than in adolescents (Shroufi et al., 2013).
Identifying modifiable clinic-level factors associated with patient retention may suggest feasible points of intervention (Lamb, El-Sadr, Geng, & Nash, 2012). In our study, patients who had never been on ART had a higher LTFU rate, a finding consistent with studies done elsewhere among adult populations (Clouse et al., 2013). In a setting where patients often present for care in advanced stages of disease, this may mean that sicker patients, on ART, are more likely to remain in care since they are on treatment. On the other hand, it also suggests that less immunocompromised, healthier patients are less likely to remain in care (Gwynn et al., 2015). The association between ART and LTFU could also be a result of survivor bias, since only patients who remain in care long enough can be initiated on ART. We did not assess the time to ART initiation and its association with LTFU in this study.
In our study, patients who had CD4 cell counts ≥ 350 cell/mm3were less likely to become LTFU. This could be suggestive of patients with lower CD4 cell counts being too sick to continue on care, transferring to clinics closer to them, or they may have died, unbeknown to their care providers (Tweya et al., 2013).
We also demonstrate that patients who had never disclosed their serostatus to anyone were more likely to become LTFU. Disclosure of HIV status has been shown to be associated with higher retention in care (Arrivé et al., 2012). Disclosure might have benefits for HIV-infected adolescents and youth, such as improved adherence to ART, and engagement of social support (Arrivé et al., 2012; Hill, Maman, Groves, & Moodley, 2015; Wolf et al., 2014). Therefore, screening of HIV-infected youth for social support needs and identification of sources for social support should be regularized as part of care. Material on disclosure should be readily availed for adaptation, evaluation and broader implementation in resource-limited settings (Vreeman, Gramelspacher, Gisore, Scanlon, & Nyandiko, 2013).
Though a majority of patients in this study were female, there was no association between gender and LTFU. Amir et al also observed a similar finding among adolescents (Shroufi et al., 2013). While others have found adult men to be at higher risk of LTFU, (Ahmed, Gugsa, Lemma, & Demissie, 2013; Mugisha et al., 2014), Gwynn et al observed a higher retention rate among men compared to women in a family-focused model of care. They also show pregnant women to be at higher risk of being LTFU compared to non-pregnant women (Gwynn et al., 2015). After controlling for other factors, we did not detect statistical association of pregnancy with LTFU, similar to findings from rural Nigeria (Aliyu et al., 2015).
A growing number of adolescents are now entering care in adult-oriented HIV clinics and criteria should be put in place to assist them or to guide clinic staff in how best to treat this vulnerable patient category within these settings. The adult-oriented HIV care model may not meet the specific needs of the youth as they face unique challenges in the management of HIV (Evans et al., 2013).Though family support may seem to be more important for HIV-infected youth than a youth-friendly environment in and of itself, adolescent-friendly services may improve access and retention in care (Shroufi et al., 2013). Further exploration of the reasons youth sought care at either clinic could help to ensure that services meet the expectations of HIV-infected youth with varied backgrounds and needs.
Implications of findings
The high LTFU immediately after enrolment calls for targeted interventions (Evans et al., 2013) and intensified patient education on the importance of adherence to clinic appointments during the first encounter. The availability of educational materials and adherence counselling services in ART clinics have been associated with lower rates of LTFU (Bygrave et al., 2012; Lamb et al., 2012).
The finding that never initiating ART was associated with higher chances of being LTFU call for strategies that enable earlier initiation of ART and promote retention in care (Clouse et al., 2013). The 2013 WHO HIV treatment guidelines, recently adopted in Kenya (Ministry of Health; National AIDS and STI Control Program, 2014), strongly recommend that ART be initiated in all HIV-infected adolescents and adults with CD4 count ≤ 500 cells/mm3, regardless of WHO clinical stage (WHO, 2013). There is also need for aggressive tracing of HIV-infected youth LTFU but are not yet on ART, since this population is more likely to have worse outcomes when they do eventually return to care (Lamb et al., 2014).
Study strengths and weaknesses
The follow-up period of three years and a sizeable population were relative strengths of this study, although the findings cannot be generalizable to the entire 15–21-year-old HIV-positive population of Kenya because it involved only two clinics in Kisumu. We did not ascertain the true outcomes of the patients who were documented as being LTFU. Studies tracing patients LTFU found high unascertained deaths and transfers, suggesting both contribute substantially to LTFU (Lamb et al., 2012; Tweya et al., 2013). Thorough tracing of a random sample of patients documented as LTFU would help determine site-specific reasons for and outcomes among these patients (Geng et al., 2010).
We neither ascertained the age at disclosure nor the strategies implemented in conducting the disclosure since this was a retrospective chart review. We also reported disclosure status as a dichotomous variable only at enrolment, yet it is a process that evolves over time (Arrivé et al., 2012; Siu, Bakeera-Kitaka, Kennedy, Dhabangi, & Kambugu, 2012).
Finally, though distance has been recognized as a barrier to accessing healthcare (Moneyham et al., 2010; Tweya et al., 2013) among HIV-infected patients, we did not have data to explore distance as a factor in becoming LTFU. In Kenya, since 2006, HIV services have largely been decentralized from secondary health facilities to an expanded network including primary health facilities. This strategy of expanding HIV services has been shown to be associated with reduced rates of pre-ART LTFU (Reidy et al., 2014).
Conclusion
LTFU was very high among HIV-infected youth enrolled in HIV care and treatment services at these two clinics. Innovative approaches to retain them, even at youth-specific clinics, are urgently required. Ongoing evaluation of LTFU among HIV-positive youth is required and care needs to be taken to disaggregate their numbers from the general LTFU data reported by most programmes. Interventions to identify and enrol youth into care earlier, support disclosure and initiate ART earlier may improve retention of youth and need further investigation. Further research is also needed to explore the reasons for LTFU from care among HIV-infected youth and the true outcomes of these patients.
Acknowledgments
Special thanks go to FACES staff and the patients at Lumumba Health Centre and Tuungane Youth Centre who made this study possible.
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the CDC and the Government of Kenya.
Funding: This research has been supported by PEPFAR through the CDC under the terms of Cooperative Agreement #PS001913.
Footnotes
Disclosure statement: No potential conflict of interest was reported by the authors.
References
- Ahmed I, Gugsa ST, Lemma S, Demissie M. Predictors of loss to follow-up before HIV treatment initiation in Northwest Ethiopia: a case control study. BMC Public Health. 2013;13867 doi: 10.1186/1471-2458-13-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliyu MH, Blevins M, Megazzini KM, Parrish DD, Audet CM, Chan N, et al. Vermund SH. Pregnant women with HIV in rural Nigeria have higher rates of antiretroviral treatment initiation, but similar loss to follow-up as non-pregnant women and men. International Health. 2015 doi: 10.1093/inthealth/ihv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrivé E, Dicko F, Amghar H, Aka AE, Dior H, Bouah B, et al. Leroy V. HIV status disclosure and retention in care in HIV-infected adolescents on antiretroviral therapy (ART) in West Africa. PloS One. 2012;7(3):e33690. doi: 10.1371/journal.pone.0033690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld AF, Agolory SG, Shiraishi RW, Wabwire-Mangen F, Kwesigabo G, Mulenga M, et al. Ellerbrock TV. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults–seven African countries, 2004–2013. Morbidity and Mortality Weekly Report. 2014;63(47):1097–1103. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25426651. [PMC free article] [PubMed] [Google Scholar]
- Bygrave H, Mtangirwa J, Ncube K, Ford N, Kranzer K, Munyaradzi D. Antiretroviral therapy outcomes among Adolescents and Youth in rural Zimbabwe. PLoS ONE. 2012;7(12):e52856. doi: 10.1371/journal.pone.0052856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charurat M, Oyegunle M, Benjamin R, Habib A, Eze E, Ele P, et al. Blattner W. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PloS One. 2010;5(5):e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse K, Pettifor A, Maskew M, Bassett J, Van Rie A, Gay C, et al. Fox MP. Initiating antiretroviral therapy when presenting with higher CD4 cell counts results in reduced loss to follow-up in a resource-limited setting. AIDS (London, England) 2013;27(4):645–650. doi: 10.1097/QAD.0b013e32835c12f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D, Menezes C, Mahomed K, Macdonald P, Untiedt S, Levin L, et al. Maskew M. Treatment outcomes of HIV-infected Adolescents attending public-sector HIV clinics across gauteng and mpumalanga, South Africa. AIDS Research and Human Retroviruses. 2013;29(6):892–900. doi: 10.1089/AID.2012.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, Yiannoutsos CT, et al. Martin JN. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. Journal of Acquired Immune Deficiency Syndromes. 2010;53(3):405–411. doi: 10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynn RC, Fawzy A, Viho I, Wu Y, Abrams EJ, Nash D. Risk factors for loss to follow-up prior to ART initiation among patients enrolling in HIV care with CD4+ cell count ≥200 cells/μL in the multi-country MTCT-Plus Initiative. BMC Health Services Research. 2015;15(1):247. doi: 10.1186/s12913-015-0898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper GW, Riplinger AJ, Neubauer LC, Murphy AG, Velcoff J, Bangi AK. Ecological factors influencing HIV sexual risk and resilience among young people in rural Kenya: Implications for prevention. Health Education Research. 2014;29(1):131–146. doi: 10.1093/her/cyt081. [DOI] [PubMed] [Google Scholar]
- Hill LM, Maman S, Groves AK, Moodley D. Social support among HIV-positive and HIV-negative adolescents in Umlazi, South Africa: Changes in family and partner relationships during pregnancy and the postpartum period. BMC Pregnancy and Childbirth. 2015;15(1):192. doi: 10.1186/s12884-015-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, Hope RL, Nwokolo N, Ward B, Jones R, Von Schweitzer M, Boag F. Meeting complex needs: Young people with HIV in London. HIV Medicine. 2013;14(3):145–152. doi: 10.1111/j.1468-1293.2012.01049.x. [DOI] [PubMed] [Google Scholar]
- Lamb MR, El-Sadr WM, Geng E, Nash D. Association of adherence support and outreach services with total attrition, loss to follow-up, and death among ART patients in sub-Saharan Africa. PLoS ONE. 2012;7(6):e38443. doi: 10.1371/journal.pone.0038443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, Viola V, Mutabazi V, Alwar T, et al. Elul B. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS (London, England) 2014;28(4):559–568. doi: 10.1097/QAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis Kulzer J, Penner JA, Marima R, Oyaro P, Oyanga AO, Shade SB, et al. Cohen CR. Family model of HIV care and treatment: A retrospective study in Kenya. Journal of the International AIDS Society. 2012;15:8. doi: 10.1186/1758-2652-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health; National AIDS and STI Control Program. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: A rapid advice. Author; Nairobi, Kenya: 2014. [Google Scholar]
- Moneyham L, McLeod J, Boehme A, Wright L, Mugavero M, Seal P, et al. Kempf MC. Perceived barriers to HIV care among HIV-infected women in the Deep South. The Journal of the Association of Nurses in AIDS Care: JANAC. 2010;21(6):467–477. doi: 10.1016/j.jana.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Mugisha V, Teasdale CA, Wang C, Lahuerta M, Nuwagaba-Biribonwoha H, Tayebwa E, et al. Abrams EJ. Determinants of mortality and loss to follow-up among adults enrolled in HIV care services in Rwanda. PloS One. 2014;9(1):e85774. doi: 10.1371/journal.pone.0085774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National AIDS and STI Control Programme. Guidelines for antiretroviral therapy in Kenya. 4th. Author; Nairobi, Kenya: 2011. [Google Scholar]
- National AIDS and STI Control Programme & National AIDS Control Council. Kenya AIDS Epidemic Update 2011. Author; Nairobi, Kenya: 2012. [Google Scholar]
- Nöstlinger C, Jasna L, Sabrina BK, Obong'o C, Eric W, Buvé A. Translating primary into “positive” prevention for adolescents in Eastern Africa. Health Promotion International. 2015 doi: 10.1093/heapro/dav044. [DOI] [PubMed] [Google Scholar]
- Ochieng-Ooko V, Ochieng D, Sidle JE, Holdsworth M, Wools-Kaloustian K, Siika AM, et al. Braitstein P. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bulletin of the World Health Organization. 2010;88(9):681–688. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy WJ, Sheriff M, Wang C, Hawken M, Koech E, Elul B, et al. Abrams EJ. Decentralization of HIV care and treatment services in Central Province, Kenya. Journal of Acquired Immune Deficiency Syndromes (1999) 2014;67(1):e34–e40. doi: 10.1097/QAI.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroufi A, Gunguwo H, Dixon M, Nyathi M, Ndebele W, Saint-Sauveur JF, et al. Ferrand RA. HIV-infected adolescents in southern Africa can achieve good treatment outcomes: Results from a retrospective cohort study. AIDS (London, England) 2013;27(12):1971–1978. doi: 10.1097/QAD.0b013e32836149ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu GE, Bakeera-Kitaka S, Kennedy CE, Dhabangi A, Kambugu A. HIV serostatus disclosure and lived experiences of adolescents at the Transition Clinic of the Infectious Diseases Clinic in Kampala, Uganda: a qualitative study. AIDS Care. 2012;24(5):606–611. doi: 10.1080/09540121.2011.630346. [DOI] [PubMed] [Google Scholar]
- Tweya H, Feldacker C, Estill J, Jahn A, Ng'ambi W, Ben-Smith A, et al. Phiri S. Are they really lost? “true” status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in Urban Malawi. PloS One. 2013;8(9):e75761. doi: 10.1371/journal.pone.0075761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeman RC, Gramelspacher AM, Gisore PO, Scanlon ML, Nyandiko WM. Disclosure of HIV status to children in resource-limited settings: A systematic review. Journal of the International AIDS Society. 2013;16:18466. doi: 10.7448/IAS.16.1.18466. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3665848&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013 Retrieved from http://www.who.int/hiv/pub/guidelines/arv2013/en/ [PubMed]
- Wolf HT, Halpern-Felsher BL, Bukusi EA, Agot KE, Cohen CR, Auerswald CL. “It is all about the fear of being discriminated [against] … the person suffering from HIV will not be accepted”: A qualitative study exploring the reasons for loss to follow-up among HIV-positive youth in Kisumu, Kenya. BMC Public Health. 2014;14(1):1154. doi: 10.1186/1471-2458-14-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]


