Abstract
Background.
Quantitative cytomegalovirus (CMV) DNA–specific polymerase chain reaction (PCR) analysis is widely used as a surveillance method for hematopoietic stem cell transplant (HCT) recipients. However, no CMV DNA threshold exists in bronchoalveolar lavage (BAL) to differentiate pneumonia from pulmonary shedding.
Methods.
We tested archived BAL fluid samples from 132 HCT recipients with CMV pneumonia and 139 controls (100 patients with non-CMV pneumonia, 18 with idiopathic pneumonia syndrome [IPS], and 21 who were asymptomatic) by quantitative CMV and β-globin DNA–specific PCR.
Results.
Patients with CMV pneumonia had higher median viral loads (3.9 log10 IU/mL; interquartile range [IQR], 2.6–6.0 log10 IU/mL) than controls (0 log10 IU/mL [IQR, 0–1.6 log10 IU/mL] for patients with non-CMV pneumonia, 0 log10 IU/mL [IQR, 0–1.6 log10 IU/mL] for patients with IPS, and 1.63 log10 IU/mL [IQR, 0–2.5 log10 IU/mL] for patients who were asymptomatic; P < .001 for all comparisons to patients with CMV pneumonia). Receiver operating characteristic curve analyses and predictive models identified a cutoff CMV DNA level of 500 IU/mL to differentiate between CMV pneumonia and pulmonary shedding, using current CMV pneumonia prevalence figures. However, different levels may be appropriate in settings of very high or low CMV pneumonia prevalence. The presence of pulmonary copathogens, radiographic presentation, or pulmonary hemorrhage did not alter predictive values.
Conclusion.
CMV DNA load in BAL can be used to differentiate CMV pneumonia from pulmonary shedding.
Keywords: cytomegalovirus, viral load, pneumonia.
The role of quantitative polymerase chain reaction (PCR) analysis in the diagnosis of cytomegalovirus (CMV) pneumonia after hematopoietic stem cell transplantation (HCT) remains undefined [1–3]. In 1988, CMV detection by rapid culture of bronchoalveolar lavage (BAL) fluid became the mainstay of diagnosis for CMV pneumonia [4]. However, while CMV DNA–specific PCR is widely used as a surveillance method for blood and serum specimens and because many laboratories have largely abandoned traditional virologic techniques, the usefulness of quantitative PCR of BAL as a diagnostic tool for CMV pneumonia is poorly defined [5–8], and the recently updated international definition guidelines provide only limited definitive evidence for using PCR as an acceptable test for disease diagnosis [9, 10]. The key reason for the dilemma is that the presence of CMV DNA may not constitute disease, owing to the well-described phenomenon of pulmonary CMV shedding [11, 12]. Studies in the early 1990s established that CMV can be detected by culture in BAL fluid from asymptomatic bone marrow transplant recipients and that pulmonary shedding is predictive for subsequent CMV pneumonia [11, 12]. Further complicating this picture is the uncertain role of CMV as either a pathogen or bystander, when detected in BAL specimens from patients with other confirmed pathogens [1, 9, 13–15]. Detection of CMV DNA may also be influenced by blood contamination in patients with pulmonary hemorrhage, and the viral load may differ between lung compartments. Studies of CMV DNA–specific PCR of BAL fluid from HCT recipients have found a high negative predictive value [16], and a small study showed differences in viral load between pneumonia and pulmonary shedding [17], but a threshold value with a high positive predictive value in current CMV pneumonia prevalence situations has remained elusive.
MATERIALS AND METHODS
Subjects
The study was approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board. We compared a cohort of subjects with CMV pneumonia after HCT diagnosed between March 1988 and December 2014 at the FHCRC to 3 separate control cohorts of HCT recipients with available BAL samples for PCR: (1) patients who had radiographically proven infectious pneumonia but were found to be negative for CMV standard virologic testing, (2) patients with a diagnosis of idiopathic pneumonia syndrome (IPS) without occult pathogens [18], and (3) a cohort of asymptomatic patients who underwent HCT between 1988 to 1989 and, according to standardized protocol, had BAL performed 35 days after transplantation as part of a prospective study (the latter cohort served as a comparison group for evaluating BAL-associated CMV load in asymptomatic pulmonary shedders) [12]. All control patients were at risk for CMV pneumonia (seropositive recipient or donor).
Patients with CMV pneumonia had symptoms or signs of pneumonia during the post-HCT period, were found to be positive for CMV on diagnostic BAL testing by standard virologic criteria (ie, positive results of shell vial or conventional culture or direct fluorescent antibody [DFA] testing) [9] and had stored BAL samples available for analysis. Patients with non-CMV infectious pneumonia had radiographic and clinical signs of pneumonia that led to a BAL, had negative results of all standard BAL tests for CMV at the time of their diagnosis, had another pathogen identified, and were known to be CMV-seropositive recipients. Patients with IPS underwent an extensive diagnostic panel and had negative results of standard CMV cultures and all other tests (results of CMV-specific PCR were analyzed as the variable of interest in this study) [18]. Patients in the asymptomatic cohort (normal chest radiographic findings and absence of cytopenias) agreed to undergo a standard BAL for research purposes between days 35 and 45 after HCT [12].
Transplantation and Supportive Care Procedures
Subjects were followed by means of standard center-based pretransplantation and posttransplantation regimens, but these varied by transplantation year [19, 20]. Strategies to prevent CMV infection have varied over time, with preemptive therapy first given on the basis of results of rapid cultures [21], followed by pp65 antigenemia assays [22] and PCR [23]. The duration of acyclovir/valacyclovir prophylaxis against infection due to herpes simplex virus types 1 and 2 and varicella zoster virus was 1 month before 1998 and at least 1 year thereafter [24]. All patients received standard prophylactic antimicrobial and antifungal agents during follow-up [19]. The presence of antiviral drugs with activity against CMV at the time of diagnosis of pneumonia was recorded. Any patient found to have CMV pneumonia based on results of standard virologic tests of BAL fluid received treatment with intravenous ganciclovir or foscarnet for a minimum of 2–3 weeks as induction therapy and 2–4 weeks as maintenance therapy; most patients also received pooled or CMV-specific intravenous immunoglobulin G [25].
BAL Evaluation
HCT recipients who present with radiographic and clinical signs of pneumonia routinely undergo evaluation by bronchoscopy, using standard procedures based on international consensus guidelines [26, 27]. If a patient underwent multiple BALs over time during a pneumonia event, only the first BAL specimen was evaluated in this study. The presence of pulmonary hemorrhage at the time of the BAL was recorded, based on the pathology report. Some patients had distinctly different radiographic findings and may have undergone 2 separate BALs with separate virologic and microbiologic evaluation during the same procedure; these samples were evaluated separately. Corresponding CMV DNA plasma viremia was determined in archived samples obtained close (within 7 days) to the bronchoscopy.
All BAL samples underwent CMV testing by 24-hour and 48-hour shell vial cultures and conventional cultures (rhesus monkey kidney, human foreskin fibroblasts, and A-549 cells); DFA and cytopathologic evaluation (for the presence of inclusion bodies) was done in a subset of patients [9]. The presence of other pathogens was evaluated by a broad diagnostic panel, as described elsewhere [18]; multiplex respiratory virus PCR was done on a subset of BAL specimens [28].
Archived BAL aliquots from study subjects in all cohorts (stored as temperatures ranging from −20°C to −80°C) were tested by quantitative CMV DNA–specific PCR, using gB/pp65 primers [29] adapted for BAL fluid: each 30 μL of PCR mixture contained 15 μL of 2× QuantiTect Multiplex PCR master mix (Qiagen, Valencia, CA), 415 nM of each primer, 100 nM of each probe, Exo internal control, and 10 μL of extracted DNA. Results were expressed as IU per milliliter. The PCR cycling steps were as follows: 1 cycle at 50°C for 2 minutes, 1 cycle at 95°C for 15 minutes, and 45 cycles of 94°C for 1 minute and 60°C for 1 minute. The lower limit of reproducible quantitation of this assay was 83.3 IU/mL; unquantifiable positive results below this threshold were assigned a level of 41.65 IU/mL. To confirm BAL cellularity and the quality of DNA extraction [30, 31], as well as for quantitation relative to cellular content, we amplified the β-globin gene from BAL samples as previously described [32]. If a patient had multiple BAL specimens from different locations during the same bronchoscopy procedure, CMV DNA–specific PCR results were averaged, unless otherwise indicated. BAL samples from the group with CMV pneumonia that did not amplify were tested by alternate primers.
Statistical Analysis
Characteristics of study subjects were summarized, and transplantation and other demographic factors were compared using either the Fisher exact, for categorical variables, or the Student t test, for continuous variables. Median CMV loads (log10) between groups were compared using the Mann-Whitney test. Sensitivity and specificity were estimated on the basis of different viral loads, and the best CMV load cutoff for CMV pneumonia was determined using a receiver operating characteristic (ROC) curve. For purposes of the ROC curve, we included all patients in the CMV pneumonia group with a BAL specimen that tested positive; all subjects in the comparison cohorts tested negative by standard CMV tests (ie, viral culture, shell vial, DFA, and cytology). To address the potential effects of anti-CMV therapy on BAL viral load, ROC curves were generated using only those patients who were not receiving active therapy at the time of BAL. Predictive models were developed by calculating the positive and negative predictive values of different viral load thresholds for a range of prevalence scenarios of CMV pneumonia among patients undergoing BAL evaluation. We also compared CMV DNA log10 viral load in BAL specimens from patients who tested positive by the different standard tests for CMV pneumonia.
RESULTS
Study Subjects
A total of 271 subjects met eligibility criteria for inclusion in this study: 132 symptomatic subjects with CMV pneumonia, 118 symptomatic subjects with non-CMV pneumonia, and 21 asymptomatic research subjects who had either been tested or had specimens available for PCR testing (Table 1). As expected on the basis of the cohort definitions and period of transplantation, differences in characteristics were seen for graft source and intensity of the conditioning regimen. Among cases, 21 patients had multiple pulmonary locations lavaged on the same day. Of these, samples from both locations were available for PCR testing in 16 cases.
Table 1.
Characteristics of Study Subjects
| Characteristic | Patients With CMV Pneumonia (n = 132) |
Asymptomatic CMV Shedders (n = 21) |
Patients With IPS (n = 18) |
Patients With Non-CMV Pneumonia (n = 100) |
|---|---|---|---|---|
| Male sex | 70 (53.0) | 13 (61.9) | 9 (44.4) | 59 (59.0) |
| Age, y, median (IQR) | 44.0 (32.5–54.2) | 35.4 (33.8–45.1) | 44.1 (36.1–48.1) | 50.9 (41.5–56.7) |
| Underlying disease | ||||
| AML | 31 (23.5) | 8 (38.1) | 3 (16.7) | 22 (22.0) |
| ALL | 15 (11.4) | 1 (4.8) | 1 (5.6) | 5 (5.0) |
| CML | 32 (24.2) | 10 (47.6) | 7 (38.9) | 13 (13.0) |
| MDS | 19 (14.4) | 0 | 5 (27.8) | 20 (20.0) |
| NHL | 14 (10.6) | 2 (9.5) | 0 | 9 (9.0) |
| MM | 7 (5.3) | 0 | 1 (5.6) | 7 (7.0) |
| Other | 14 (10.6) | 0 | 1 (5.6) | 24 (24.0) |
| HLA donor status | ||||
| Autologous | 9 (6.8) | 0 | 0 | 14 (14.0) |
| Matched related | 47 (35.6) | 11 (52.4) | 6 (33.3) | 30 (30.0) |
| Mismatched related | 12 (9.1) | 2 (9.5) | 3 (16.7) | 3 (3.0) |
| Unrelated | 64 (48.5) | 8 (38.1) | 9 (50.0) | 53 (53.0) |
| Graft source | ||||
| BM | 69 (52.3) | 21 (100.0) | 15 (83.3) | 35 (35.0) |
| PBSCs | 58 (43.9) | 0 | 3 (16.7) | 60 (60.0) |
| BM and PBSCs | 1 (0.8) | 0 | 0 | 1 (1.0) |
| Cord blood | 4 (3.0) | 0 | 0 | 4 (4.0) |
| Conditioning regimen | ||||
| Myeloablative | ||||
| With TBI (>1000 cGy) | 71 (53.8) | 18 (85.7) | 13 (72.2) | 31 (31.0) |
| Without TBI | 37 (28.0) | 3 (14.3) | 5 (27.8) | 47 (47.0) |
| Reduced intensity (with and without low-dose TBI) | 24 (18.2) | 0 | 0 | 22 (22.0) |
| Acute GVHDa | ||||
| Grade 2–4 | 107 (87.0) | 14 (66.7) | 13 (72.2) | 61 (70.9) |
| Grade 3–4 | 41 (33.3) | 8 (38.1) | 12 (66.7) | 32 (37.2) |
| CMV serostatus | ||||
| D+R+ | 69 (52.3) | 6 (28.6) | 6 (33.3) | 56 (56.0) |
| D−R+ | 51 (38.6) | 11 (52.4) | 7 (38.9) | 40 (40.0) |
| D+R− | 5 (3.8) | 4 (19.0) | 5 (27.8) | 4 (4.0) |
| D−R− | 7 (5.3) | 0 | 0 | 0 |
Data are no. (%) of patients, unless otherwise indicated. Percentages may not equal 100%, owing to rounding.
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BM, bone marrow; CML, chronic myeloid leukemia; CMV, cytomegalovirus; D, donor; GVHD, graft-versus-host disease; IPS, idiopathic pneumonia syndrome; IQR, interquartile range; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; PBSC, peripheral blood stem cell; R, recipient; TBI, total body irradiation; −, negative; +, positive.
aData are limited to allogeneic transplant recipients; no GVHD data were available for 7 patients with CMV pneumonia and 13 with non-CMV pneumonia.
CMV DNA Viral Load in BAL
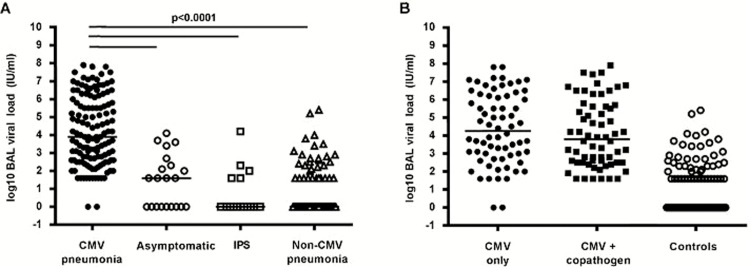
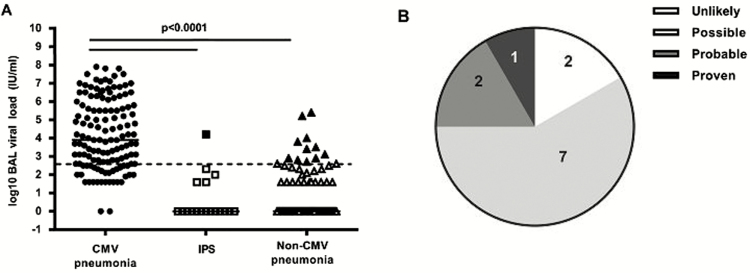
BAL was performed at a median duration of 45 days (interquartile range [IQR], 22.5–94.5 days) after transplantation for the entire cohort. The median time to BAL was different between the 3 control groups (42 days [IQR, 40–43 days] for the asymptomatic group, 22.5 days [IQR, 17.3–60 days] for the IPS group, and 25.5 days [IQR, 15–65.5 days] for the non-CMV pneumonia cohort) and the CMV pneumonia group (68.5 days; IQR, 42–157.5 days). Nearly all patients (130 of 132 [99%]) who had CMV pneumonia detected by standard testing had CMV detected by PCR in their BAL fluid. Patients in all 3 control groups (12 of 21 [57%] in the asymptomatic group, 5 of 18 with IPS [28%], and 32 of 100 [32%] with non-CMV pneumonia) had CMV DNA detected. The median quantitative viral load in individuals with CMV detected by BAL PCR was also significantly different between the CMV pneumonia group (3.9 log10 IU/mL; IQR, 2.6–6.0 log10 IU/mL) and the 3 control groups (1.63 log10 IU/mL [IQR, 0–2.5 log10 IU/mL] in the asymptomatic group, 0 log10 IU/mL [IQR, 0–1.6 log10 IU/mL] in the IPS group, and 0 log10 IU/mL [IQR, 0–1.6 log10 IU/mL] in the non-CMV pneumonia group; Figure 1A). Two patients in the CMV pneumonia cohort had a negative CMV DNA–specific PCR result; both tested positive by the shell vial assay but no virus was isolated from the cultures, both had negative cytology findings, neither was tested by DFA, and both were negative by PCR using different primers.
Figure 1.
Quantitative cytomegalovirus (CMV) load in bronchoalveolar lavage (BAL) fluid. A, Viral load in BAL fluid was significantly higher in CMV pneumonia cases than in any of the control subgroups. B, Viral load in BAL fluid from CMV pneumonia cases did not differ according to the absence (CMV only) or presence of a copathogen (BAL specimens were examined by Gram, fungal, and acid-fast bacilli staining; cytologic examination; cultures for bacteria, mycobacteria, fungi, and viruses; shell vial centrifugation culture for respiratory syncytial virus [RSV]; direct fluorescent antibody testing for Legionella, Pneumocystis jiroveci, RSV, influenza virus, parainfluenza virus types 1–3, and adenovirus; and Aspergillus galactomannan (GM) enzyme-linked immunosorbent assay [performed on all archived samples]). The viral loads of control groups (asymptomatic patients, patients with idiopathic pneumonia syndrome [IPS], and patients with non-CMV infectious pneumonia) are shown for comparison.
A comparison between CMV DNA–specific PCR and standard diagnostic tests of BAL specimens showed that patients with CMV pneumonia tested positive most frequently by the shell vial assay (126 of 132 [96%]), followed by culture (106 of 132 [80%]), DFA (24 of 83 [29%]), and cytology (8 of 132 [6%]). The CMV DNA load was similar between samples positive by the shell vial assay and conventional cultures but significantly different between samples positive by culture methods and those positive by DFA and cytology (Supplementary Figure 1). Storage time did not appear to affect viral load (Supplementary Figure 2).
Analysis of Possible Effect Modifiers
Copathogens, Radiographic Presentation, and Multiple Locations
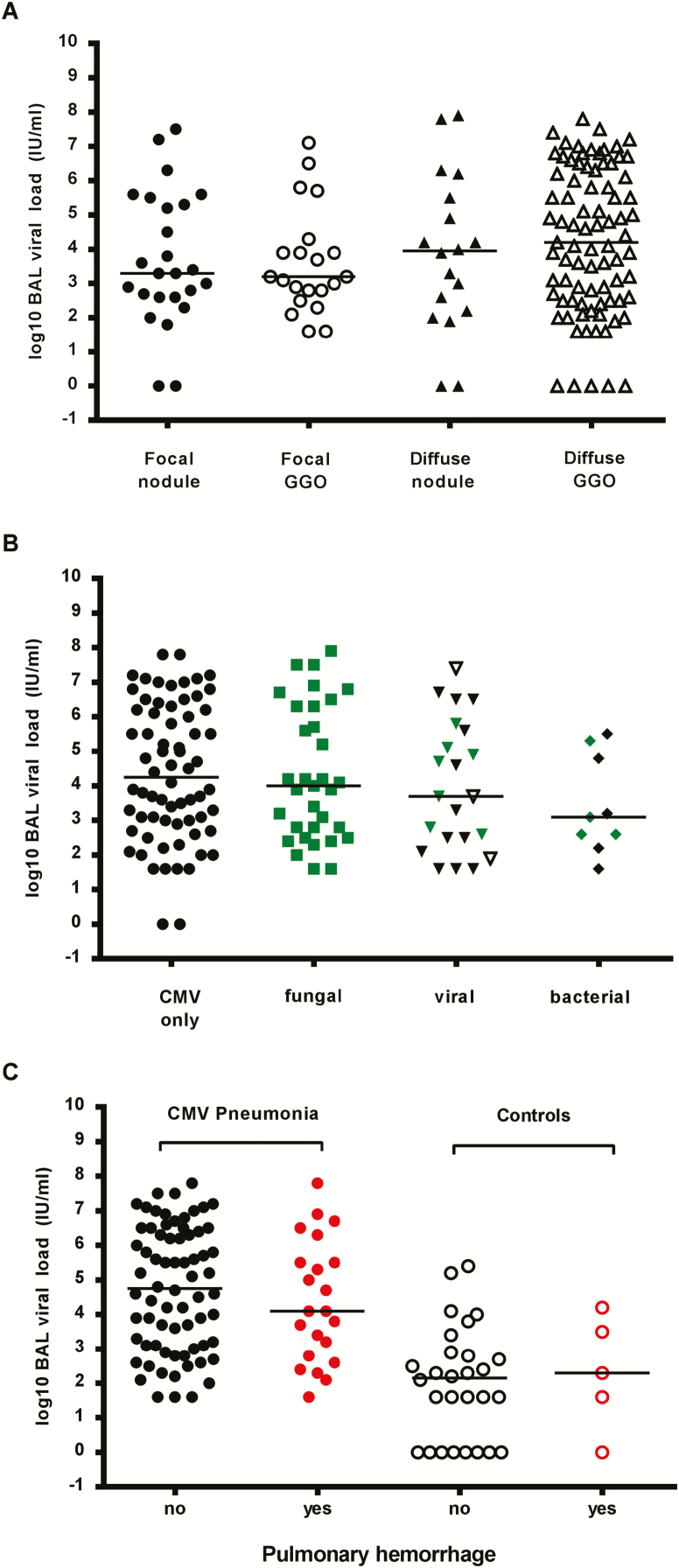
Among patients with CMV pneumonia, the median viral load was not significantly different between patients who did and those did not have copathogens detected (3.8 log10 IU/mL [IQR, 2.5–5.6 log10 IU/mL] and 4.3 log10 IU/mL [IQR, 3.0–6.2 log10 IU/mL], respectively; Figure 1B); there were also no statistically significant differences between autologous and allogeneic transplant recipients in these groups (Supplementary Figure 3). Further analysis of radiographic presentation (Figure 2A) and copathogens by type (fungal, viral, and bacterial; Figure 2B) also did not show significant differences. An analysis that combined radiographic presentation (focal nodular vs diffuse/ground glass) and the detection of fungal pathogens showed no significant differences among patients with probable pulmonary aspergillosis and those who tested negative for aspergillus galactomannan (Supplementary Figure 4).
Figure 2.
Quantitative cytomegalovirus (CMV) load in bronchoalveolar lavage (BAL) fluid according to possible effect modifiers. A, Viral load in BAL fluid from CMV pneumonia cases did not differ with respect to the radiologic appearance of the lungs at the time of the BAL. Radiographic presentation was categorized by a pulmonologist blinded to the CMV results in the following categories: (1) focal nodule(s), (2) focal ground-glass opacities (GGOs), (3) diffuse nodules, (4) diffuse GGOs. Outcomes in subjects with non-CMV pneumonia with a high viral load were evaluated by chart review. If multiple locations were lavaged on the same day, each is represented separately. One CMV pneumonia case with multiple BALs on the same day was excluded because BAL polymerase chain reaction (PCR) results could not be assigned to a specific BAL location. A total of 147 BALs were performed on 131 patients. B, Quantitative CMV load in BAL fluid from CMV pneumonia cases did not differ according to the absence (CMV only) or presence of any specific copathogen subset. Detection of Aspergillus galactomannan in the BAL or blood counted as detection of a fungal copathogen. Co-occurrence of fungal copathogens with other copathogens is denoted by green markers. If bacteria were present in addition to a viral copathogen, the BAL finding is grouped with viral copathogens but is denoted with an open marker. A total of 68 subjects had CMV only detected, 33 had a fungal copathogen detected, 22 had a viral copathogen detected, and 9 had a bacterial copathogen detected. C, Viral loads in cases and controls were similar in the presence or absence of pulmonary hemorrhage in patients with CMV DNAemia within 7 days of BAL (data are for 92 patients with CMV pneumonia and 33 controls).
Pulmonary Hemorrhage
To determine whether the presence of pulmonary hemorrhage in patients with CMV DNAemia at the time of BAL affected the BAL viral load, we compared viral loads in plasma and BAL specimens from patients with and those without pulmonary hemorrhage. There was no difference in viral load relative to the presence of pulmonary hemorrhage both in patients with CMV pneumonia and those with non-CMV pneumonia (Figure 2C).
Viral Load in Lung Locations
Among 16 patients who underwent 2 separate BALs during the same session to evaluate 2 different pulmonary locations, the median difference in viral loads between locations did not differ, even if radiographic presentations differed significantly, but a few outliers were observed (Supplementary Figure 5).
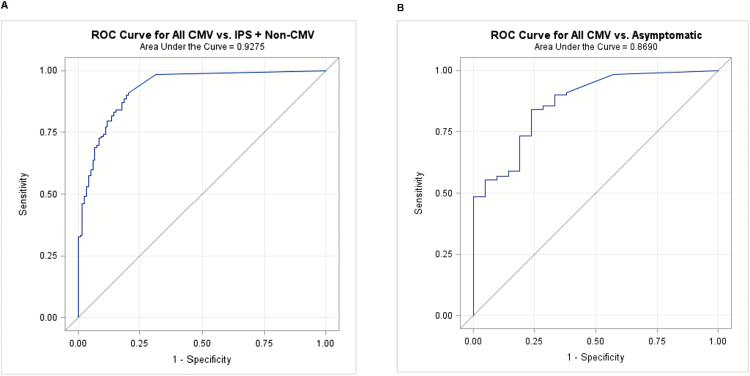
ROC Curve Analysis and Predictive Models
We constructed ROC curves of CMV DNA loads in BAL to determine thresholds that optimize both the sensitivity and specificity for CMV pneumonia. The ROC curves produced a high area under the curve (AUC) value. Figure 3A displays data for the CMV pneumonia group and the IPS and non-CMV pneumonia groups combined and Figure 3B compares patients with CMV pneumonia to asymptomatic control patients with cutoffs of 100 and 203 IU/mL, respectively. The exclusion of CMV pneumonia cases with copathogens did not improve the ROC curves or alter thresholds (data not shown).
Figure 3.
Receiver operating characteristic (ROC) curves and predictive models. A, Patients with cytomegalovirus (CMV) pneumonia (n = 132) versus controls with idiopathic pneumonia syndrome (IPS) or non-CMV pneumonia (n = 118). The optimal cutoff was 99.7 IU/mL, with a sensitivity of 90.2% and specificity of 80.5%. B, Patients with CMV pneumonia (n = 132) versus asymptomatic controls (n = 21). The optimal cutoff was 203.3 IU/mL, with a sensitivity of 84.1% and a specificity of 76.2%.
We also constructed ROC curves for CMV BAL PCR findings adjusted for sample cellularity as IU/106 copies of β- globin. β-globin results were available for 125 patients with CMV pneumonia and 96 patients with IPS or non-CMV pneumonia. Overall, the AUCs were somewhat lower than that for findings in IU per milliliter (Supplementary Figure 6).
We next analyzed whether antiviral treatment affected the viral load threshold. Antiviral agents (treatment duration, >3 days) were given either preemptively or presumptively during the diagnostic work-up for 15.2% of patients with CMV pneumonia and 30.5% with non-CMV pneumonia; an additional 8.5% and 3.4%, respectively, received treatment for up to 2 days. Supplementary Figure 7 shows an improved AUC when all antivirals were excluded (0.969 for findings in IU/mL and 0.956 for findings in IU/106 copies of β-globin). Results were similar when antivirals were used for up to 3 days (data not shown).
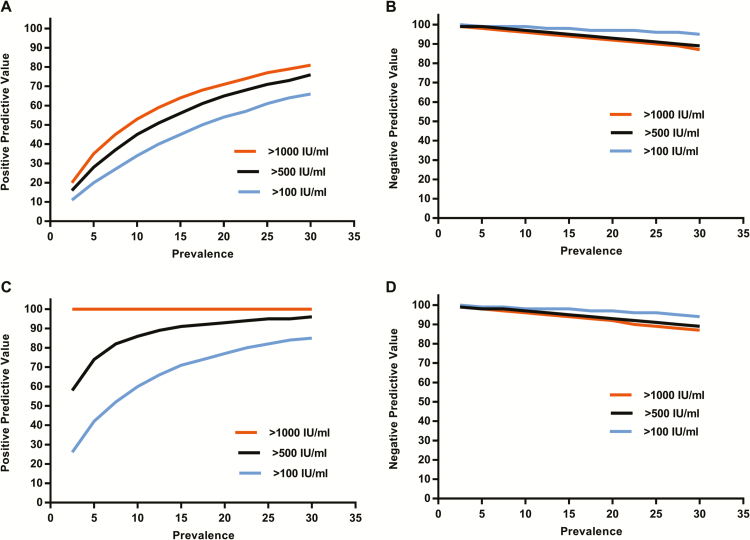
Using various CMV pneumonia prevalence estimates among patients undergoing BAL, we modeled the positive and negative predictive values for CMV pneumonia with different BAL DNA load thresholds (Figure 4). For instance, on the assumption of a CMV pneumonia prevalence of 10% among seropositive recipients undergoing BAL, the positive predictive value of a threshold of 500 IU/mL would be 45% (Figure 4A) and the negative predictive value would be >95% (Figure 4B). Predictive values were markedly improved among patients who did not receive any antiviral treatment at the time of PCR testing (Figures 4C and 4D), resulting in positive predictive values of ≥60% for the 500 IU/mL threshold in very low prevalence situations.
Figure 4.
Predictive models. Positive predictive values with thresholds of 100, 500, 1000 IU/mL across a range of cytomegalovirus (CMV) pneumonia prevalences in patients who underwent a bronchoalveolar lavage for evaluation of pulmonary infiltrates. A, Data for all patients (132 patients with CMV pneumonia and 118 controls with non-CMV pneumonia). C, Patients without antiviral treatment (99 patients with CMV pneumonia and 78 controls with non-CMV pneumonia). Corresponding negative predictive values are shown in panels B and D.
Clinical Outcomes Relative to Viral Load Thresholds
We performed chart reviews of both controls and cases to examine the clinical implications of using various DNA thresholds in BAL fluid. Among control patients with pneumonia, 12 of 118 (10.2%) had a CMV DNA level of >500 IU/mL and 21 had levels between 100 and 500 IU/mL (Figure 5A). By using a threshold of 500 IU/mL, the majority of patients (9 of 12) who would have been classified on the basis of the 500 IU/mL threshold as having CMV pneumonia had a clinical picture compatible with CMV pneumonia (Figure 5B). Conversely, use of the 500 IU/mL threshold would categorize 26% of patients (34 of 132) who received a diagnosis of CMV pneumonia as being CMV pulmonary shedders (2 tested negative, of whom neither had a copathogen detected; of the remaining 32 cases, 20 had a copathogen detected). Among the asymptomatic control subjects, 5 had levels of >500 IU/mL, of whom 2 developed subsequent CMV pneumonia 10 and 20 days after the BALs. Seventy-six percent of patients with a BAL viral load between 100 and 500 IU/mL had PCR-positive plasma specimens (Supplementary Materials).
Figure 5.
Clinical outcome in controls with cytomegalovirus (CMV)–specific polymerase chain reaction findings of >500 IU/mL in bronchoalveolar lavage (BAL) fluid (1 patient had idiopathic pneumonia syndrome [IPS], and 11 patients had non-CMV pneumonia). A, Viral loads of >500 IU/mL are shown by solid markers. The dotted line represents the threshold. B, The likelihood of subsequent development of CMV disease in subset. “Unlikely” denotes clinical presentation consistent with other causes, CMV-negative blood specimen; “possible” denotes CMV detected in blood specimen, treated with antiviral agents, other possible causes present; “probable” denotes CMV detected in blood specimen, no other causes of pneumonia in subsequent follow-up evaluations, clinical course consistent with viral pneumonia; and “proven” denotes subsequent CMV pneumonia diagnosed by standard criteria (eg, at autopsy).
Among the 132 cases of CMV pneumonia, overall mortality 90 days after diagnosis was 34.9%. The CMV load in BAL showed a poor correlation with overall and attributable mortality based on ROC analysis, and the exclusion of samples obtained from patients receiving antiviral agents did not significantly improve the AUC (Supplementary Figure 8).
DISCUSSION
The study showed that viral loads of >500 IU/mL in BAL fluid reliably differentiate disease in situations of CMV prevalence. In certain high-prevalence situations and among patients who are not receiving anti-CMV agents, thresholds as low as 100 or 200 IU/mL may be associated with high positive and negative predictive values for CMV pneumonia. Our study also showed that pulmonary hemorrhage, copathogens, and radiographic presentation do not seem to affect these thresholds.
The use of the CMV DNA load in BAL to diagnose CMV pneumonia has been difficult to establish because asymptomatic shedding of CMV, both as DNA and as replicating virus, is a well-established phenomenon [11], thereby complicating the interpretation of viral load results, especially in the context of copathogens. The present study is the first to establish diagnostic performance characteristics of different viral load thresholds in a cohort of patients who underwent a highly protocol-driven and unbiased diagnostic evaluation. Our data suggest that a threshold of 500 IU/mL of BAL fluid provides good positive and excellent negative predictive values for CMV pneumonia in most prevalence situations (Figure 4). It should be emphasized that the relevant prevalence figures should be based on the patients who require BAL evaluations for pulmonary infiltrates, rather than on all patients who are at risk on the basis of donor and recipient serostatus. Thus, a prevalence of 5%–20% maybe a reasonable estimate for most allogeneic transplant recipients at risk for CMV pneumonia. In particularly high-risk populations, such CMV-seropositive recipients of cord blood, those undergoing T-cell–depleted transplantation, or those for whom policies for performing BAL are more restrictive, lower thresholds may still provide positive predictive values of >50%, owing to the higher prevalence (Figure 4). A positive predictive value of approximately 50% is similar to that of BAL galactomannan positivity at the Food and Drug Administration–approved ≥0.5 level for invasive pulmonary aspergillosis with current prevalence figures of approximately 5%–10% [33].
The interpretation of viral DNA results in BAL is complicated by a number of potential result modifiers, including the presence of pulmonary hemorrhage, copathogens, radiographic presentation, prior antiviral treatment, and cellularity in the BAL fluid. Our results suggest that, with the exception of antiviral treatment, none of these factors significantly altered the proposed thresholds. Whether pulmonary hemorrhage in the presence of viremia (by culture or DNA detection) affects diagnostic precision has not been addressed by the international definition guidelines. We did not find higher BAL viral loads in patients with concomitant viremia (Figure 2C), suggesting that the viral loads in the lung and blood compartments are similar and primarily influenced by the level of immunosuppression. The analysis of patients with specific copathogens and/or radiographic presentation did not reveal different CMV levels from those in patients with CMV pneumonia without copathogens. Even patients with a focal nodular infiltrate and probable aspergillosis did not have significantly lower viral loads (Figure 2A). This clinical presentation would not be considered classic for CMV pneumonia; however, nodular presentation is a known manifestation of CMV pneumonia [34]. This finding suggests that other copathogens or inflammatory conditions in the lung may increase the pulmonary viral load through the inflammatory cytokine milieu throughout the lung and that the viral load does not have a high diagnostic precision to determine whether CMV is a bystander in this situation. Prior receipt of ganciclovir or foscarnet for up to 3 days as preemptive or empirical treatment affected the ROC curves and predictive values significantly (Supplementary Figure 7). In contrast, BAL cellularity and the assessment of viral load per cellular content [17] did not provide obvious benefits, although this approach can be used as an alternative way to quantify the virus.
Given the well-established risk of subsequent CMV pneumonia [12] among patients with pulmonary CMV shedding with active CMV replication (as determined by shell vial cultures) and the well-established benefit of preemptive therapy in this situation [11], we recommend a shorter course of antiviral treatment (eg, preemptive treatment with an induction dose for 2–3 weeks) for these shedding situations when both shell vial assay and PCR results are available. How to manage a patient with CMV DNA levels of <500 IU/mL in the absence of shell vial culture data (an increasingly common scenario) is more difficult to determine. Our data suggest that patients with extremely low levels (ie, <100 IU/mL) may not require treatment. However, patients with levels between 100 and 500 IU/mL (particularly in the absence of other pathogens), high-risk patients, or those who underwent BAL in the absence of antiviral treatment might benefit from antiviral treatment. Given the low prevalence of this condition, it will be difficult to definitively study this question.
The study has several strengths, including the large number of CMV pneumonia cases, the presence of appropriate controls (including uninfected HCT recipients) with a complete diagnostic work-up, a high and largely unbiased rate of BAL performance, and the detailed evaluation of possible effect modifiers. Limitations include our inability to account for minor differences in dilution (although it does not matter with respiratory viruses) [35–37] and perhaps the smaller number of cases in specific subgroups of copathogens and focal radiographic presentation.
In conclusion, our study provides strong evidence that CMV DNA quantitation in BAL fluid by PCR can be used to differentiate CMV pneumonia from asymptomatic pulmonary shedding in HCT recipients. A cutoff of 500 IU/mL appears to be reasonable with current prevalence figures. However, different levels may be used in specific risk settings. Thus, the CMV DNA load in BAL fluid can be used by investigators and clinicians to reliably diagnose CMV pneumonia in clinical trials and at the bedside.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Tracy Santo Hayes, Stacy Selke, Susan McArdle, and Anne Cent, for molecular testing; Kristen White, Heather Andrew, Laurel Joncas Schronce, and Elsa Garnace, for data and sample retrieval; Brad Edmison, for galactomannan testing; Craig Silva and Zach Stednick, for database services; and the Infectious Disease Sciences Biospecimen Repository, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, for samples.
M. B. and S. P. designed the study. T. S. A., H. X., W. L., and M. B. analyzed the data. M. H., L. C., and K. R. J. were responsible for assay development, molecular testing, and results interpretation. G. T., V. E., S. S., and L. K. collected clinical data. G. C. coded radiographic findings. M. B., T. S. A., G. T. and S. P. wrote the first manuscript draft. All authors critically reviewed the manuscript.
Financial support. This work was supported by the National Institutes of Health (grants K24HL093294 [to M. B.], K23HL096831 [to S. P.], CA18029 [to W. L., clinical database and biorepository], and CA15704 [to H. X.]), the Fred Hutchinson Cancer Research Center Vaccine and Infectious Disease Division (biorepository), and the Joel Meyers Endowment Scholarship (to V. E., S. P., and S. S.).
Potential conflicts of interest. M. B. and K. R. J. received research funding from Roche Molecular Systems for unrelated research. All other authors report no potential conflicts. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Erard V, Guthrie KA, Seo S, et al. Reduced mortality of cytomegalovirus pneumonia after hematopoietic cell transplantation due to antiviral therapy and changes in transplantation practices. Clin Infect Dis 2015; 61:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chemaly RF, Ullmann AJ, Stoelben S, et al. ; AIC246 Study Team. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med 2014; 370:1781–9. [DOI] [PubMed] [Google Scholar]
- 3. Marty FM, Winston DJ, Rowley SD, et al. ; CMX001-201 Clinical Study Group. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med 2013; 369:1227–36. [DOI] [PubMed] [Google Scholar]
- 4. Crawford SW, Bowden RA, Hackman RC, Gleaves CA, Meyers JD, Clark JG. Rapid detection of cytomegalovirus pulmonary infection by bronchoalveolar lavage and centrifugation culture. Ann Intern Med 1988; 108:180–5. [DOI] [PubMed] [Google Scholar]
- 5. Burgener EB, Waggoner J, Pinsky BA, Chen SF. Clinical characteristics and outcomes of pediatric patients with CMV DNA detection in bronchoalveolar lavage fluid. Ped Pulmonol 2017;. 52:112–8. [DOI] [PubMed] [Google Scholar]
- 6. Coussement J, Steensels D, Nollevaux MC, et al. When polymerase chain reaction does not help: cytomegalovirus pneumonitis associated with very low or undetectable viral load in both blood and bronchoalveolar lavage samples after lung transplantation. Transpl Infect Dis 2016; 18:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rozy A, Duk K, Szumna B, Skronska P, Gawryluk D, Chorostowska-Wynimko J. Effectiveness of PCR and immunofluorescence techniques for detecting human cytomegalovirus in blood and bronchoalveolar lavage fluid. Adv Exp Med Biol 2016;. 921:21–6. [DOI] [PubMed] [Google Scholar]
- 8. Tan SK, Burgener EB, Waggoner JJ, et al. Molecular and culture-based bronchoalveolar lavage fluid testing for the diagnosis of cytomegalovirus pneumonitis. Open Forum Infect Dis 2016; 3:ofv212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ljungman P, Griffiths P, Paya C. Definitions of Cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34:1094–7. [DOI] [PubMed] [Google Scholar]
- 10. Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of CMV infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017;. 64:87–91. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt GM, Horak DA, Niland JC, Duncan SR, Forman SJ, Zaia JA. A randomized, controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants; The city of hope-stanford-syntex CMV study group. N Engl J Med 1991; 324:1005–11. [DOI] [PubMed] [Google Scholar]
- 12. Slavin MA, Gooley TA, Bowden RA. Prediction of cytomegalovirus pneumonia after marrow transplantation from cellular characteristics and cytomegalovirus culture of bronchoalveolar lavage fluid. Transplantation 1994; 58:915–9. [DOI] [PubMed] [Google Scholar]
- 13. Crawford SW, Hackman RC, Clark JG. Open lung biopsy diagnosis of diffuse pulmonary infiltrates after marrow transplantation. Chest 1988; 94:949–53. [DOI] [PubMed] [Google Scholar]
- 14. Bissinger AL, Einsele H, Hamprecht K, et al. Infectious pulmonary complications after stem cell transplantation or chemotherapy: diagnostic yield of bronchoalveolar lavage. Diagn Microbiol Infect Dis 2005; 52:275–80. [DOI] [PubMed] [Google Scholar]
- 15. Gerna G, Vitulo P, Rovida F, et al. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol 2006; 78:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cathomas G, Morris P, Pekle K, Cunningham I, Emanuel D. Rapid diagnosis of cytomegalovirus pneumonia in marrow transplant recipients by bronchoalveolar lavage using the polymerase chain reaction, virus culture, and the direct immunostaining of alveolar cells. Blood 1993; 81:1909–14. [PubMed] [Google Scholar]
- 17. Boivin G, Olson CA, Quirk MR, Kringstad B, Hertz MI, Jordan MC. Quantitation of cytomegalovirus DNA and characterization of viral gene expression in bronchoalveolar cells of infected patients with and without pneumonitis. J Infect Dis 1996; 173:1304–12. [DOI] [PubMed] [Google Scholar]
- 18. Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015; 125:3789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakamae H, Kirby KA, Sandmaier BM, et al. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009; 15:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pergam SA, Xie H, Sandhu R, et al. Efficiency and risk factors for CMV transmission in seronegative hematopoietic stem cell recipients. Biol Blood Marrow Transplant 2012; 18:1391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodrich JM, Mori M, Gleaves CA, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med 1991; 325:1601–7. [DOI] [PubMed] [Google Scholar]
- 22. Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood 1996; 88:4063–71. [PubMed] [Google Scholar]
- 23. Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18:1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood 2007; 110:3071–7. [DOI] [PubMed] [Google Scholar]
- 25. Alexander BT, Hladnik LM, Augustin KM, et al. Use of cytomegalovirus intravenous immune globulin for the adjunctive treatment of cytomegalovirus in hematopoietic stem cell transplant recipients. Pharmacotherapy 2010; 30:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haslam PL, Baughman RP. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur Respir J 1999; 14:245–8. [DOI] [PubMed] [Google Scholar]
- 27. Harris B, Lowy FD, Stover DE, Arcasoy SM. Diagnostic bronchoscopy in solid-organ and hematopoietic stem cell transplantation. Ann Am Thorac Soc 2013; 10:39–49. [DOI] [PubMed] [Google Scholar]
- 28. Campbell AP, Guthrie KA, Englund JA, et al. Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clin Infect Dis 2015; 61:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol 2004; 42:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fréalle E, Decrucq K, Botterel F, et al. Diagnosis of invasive aspergillosis using bronchoalveolar lavage in haematology patients: influence of bronchoalveolar lavage human DNA content on real-time PCR performance. Eur J Clin Microbiol Infect Dis 2009; 28:223–32. [DOI] [PubMed] [Google Scholar]
- 31. Saiki RK, Gelfand DH, Stoffel S, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988; 239:487–91. [DOI] [PubMed] [Google Scholar]
- 32. Cone RW, Hackman RC, Huang ML, et al. Human herpesvirus 6 in lung tissue from patients with pneumonitis after bone marrow transplantation. N Engl J Med 1993; 329:156–61. [DOI] [PubMed] [Google Scholar]
- 33. D’Haese J, Theunissen K, Vermeulen E, et al. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. J Clin Microbiol 2012; 50:1258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meyers JD, Flournoy N, Thomas ED. Nonbacterial pneumonia after allogeneic marrow transplantation: a review of ten years’ experience. Rev Infect Dis 1982; 4:1119–32. [DOI] [PubMed] [Google Scholar]
- 35. Campbell AP, Chien JW, Kuypers J, et al. Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT. J Infect Dis 2010; 201:1404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Renaud C, Xie H, Seo S, et al. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant 2013; 19:1220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seo S, Xie H, Campbell AP, et al. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis 2014; 58:1357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.