Abstract
The condensation of nucleic acids into well-defined particles is an integral part of several approaches to artificial cellular delivery. Improvements in the efficiency of nucleic acid delivery in vivo are important for the development of DNA- and RNA-based therapeutics. Presently, most efforts to improve the condensation and delivery of nucleic acids have focused on the synthesis of novel condensing agents. However, short oligonucleotides are not as easy to condense into well-defined particles as gene-length DNA polymers and present particular challenges for discrete particle formation. We describe a novel strategy for improving the condensation and packaging of oligonucleotides that is based on the self-organization of half-sliding complementary oligonucleotides into long duplexes (ca. 2 kb). These non-covalent assemblies possess single-stranded nicks or single-stranded gaps at regular intervals along the duplex backbones. The condensation behavior of nicked- and gapped-DNA duplexes was investigated using several cationic condensing agents. Transmission electron microscopy and light-scattering studies reveal that these DNA duplexes condense much more readily than short duplex oligonucleotides (i.e. 21 bp), and more easily than a 3 kb plasmid DNA. The polymeric condensing agents, poly-l-lysine and polyethylenimine, form condensates with nicked- and gapped-DNA that are significantly smaller than condensates formed by the 3 kb plasmid DNA. These results demonstrate the ability for DNA structure and topology to alter nucleic acid condensation and suggest the potential for the use of this form of DNA in the design of vectors for oligonucleotide and gene delivery. The results presented here also provide new insights into the role of DNA flexibility in condensate formation.
INTRODUCTION
During the past several years, major advances have been made in the development of oligonucleotides as therapeutic agents. Triplex-forming oligonucleotides (i.e. antigene), antisense oligonucleotides, aptamers and ribozymes (catalytic RNA) have shown promise in modulating gene expression (1–4). More recently, the discovery of RNA interference has revealed how duplex RNA oligonucleotides can be used for gene-silencing, a mechanism that is both useful for basic research and very promising for the treatment of acquired and genetic diseases (5–7). Effective implementation of oligonucleotide technology in biology and medicine depends on the efficient transfection of oligonucleotides. However, the cell membrane is a formidable barrier to the delivery of therapeutic nucleic acids (8–10). Most chemical-based (i.e. non-viral and non-mechanical) artificial DNA delivery systems involve charge-neutralization and condensation of DNA into small particles that facilitate DNA entry into cells by endocytosis and, in some cases, a mechanism for escape into the cytoplasm before endosomal degradation. Even the widely implemented method of cell transfection using cationic lipids can be enhanced several fold when DNA is pre-condensed by cationic polymers into nanometer-scale condensates (11).
Efforts to improve oligonucleotide delivery have driven the development of novel reagents for DNA condensation that include cationic liposomes (12,13), polycationic dendrimers (14), polyethylenimine (PEI) (15,16) and various cationic peptides (17). In contrast, relatively few studies have addressed the potential for alterations in nucleic acid structure to improve DNA packaging for delivery. Nevertheless, altering the DNA structure appears to be a promising approach to controlling DNA condensation. For example, the introduction of a particular G-rich sequence into plasmid DNA can decrease condensate particle size by 22%, and supercoiled DNA has been shown to condense into smaller particles than linear DNA (18,19). Our laboratory has also demonstrated that the size of DNA condensates produced by plasmid DNA is substantially reduced if static DNA loops are incorporated into the plasmid to act as nucleation sites for condensation (20,21). These studies suggest that the influence of DNA structure on condensation can be as great as the effects of condensing agent structure.
In this report, we describe the development of a new strategy for the compaction of short oligonucleotides into well-defined condensates. We have designed oligonucleotides with half-sliding complementary sequences (22) that self-assemble to produce duplexes with flexible sites at regular points along the double helix, in the form of single-stranded nicks and singled-stranded gaps. These nicked- and gapped-DNA duplexes were prepared by mixing equal amounts of two oligonucleotides that self-assemble into duplexes and reach lengths longer than 2 kb. The condensation behavior of nicked- and gapped-DNA are compared with the condensation of a 21mer duplex and a 3 kb plasmid DNA by transmission electron microscopy (TEM) and light scattering. Condensation studies were carried out using the trivalent cation hexammine cobalt(III), an arginine-rich peptide and two polymeric condensing agents. The peptide and polymeric condensing agents chosen for this study are each known for their ability to enhance artificial DNA delivery. The results presented here demonstrate that long nicked- and gapped-DNA duplexes generally condensed into smaller and more homogenous particles than short oligonucleotides duplexes. We also demonstrate that nicked- and gapped-DNA condenses more easily than continuous duplex DNA of comparable length. The substantial difference in the average size of nicked- and gapped-DNA condensates and those of continuous DNA duplexes demonstrate that the increased local flexibility of nicks and gaps provide both a kinetic and a thermodynamic advantage to DNA condensation. Because controlling the size of condensed DNA particles is a critical parameter for in vivo delivery (23), we propose that DNA with regular nicks or gaps represents a new class of nucleic acid structure that should prove useful in conjunction with a variety of non-viral mediated nucleic acid delivery systems.
MATERIALS AND METHODS
Plasmid DNA preparation
Bluescript II SK- (Stratagene, La Jolla, CA) (2961 bp), referred to as 3kbDNA in the text, was grown in the Escherichia coli cell line DH5α (Life Technologies, Carlsbad, CA) and isolated using the Qiagen Maxi-Prep kit (Valencia, CA). The circular plasmid DNA was linearized by incubation with the restriction enzyme HindIII (New England Biolabs, Beverly, MA). The linearized plasmid DNA was rinsed five times with 5 mM Bis-Tris and 50 μM EDTA (pH 7.0) using a Microcon YM-30 spin column (Millipore, Bedford, MA) to remove excess salt introduced during restriction digestion. The DNA was then eluted from the spin column membrane with 5 mM Bis-Tris, 50 μM EDTA (pH 7.0). DNA concentration was verified spectrophotometrically.
Nicked-DNA, gapped-DNA and short DNA duplex preparation
To produce nicked-DNA, two 42mer oligonucleotides were designed such that the 3′ half-sequence (i.e. 21 bases at the 3′ end) of one strand (N1) is complementary to the 3′ half-sequence of another strand (N2), with the same being true for the 5′ half-sequences of these two oligonucleotides.
N1 5′-GCTGGTGAGACGACTATGAGTTCGAATGGCTTACTCGAATGGCTTACTGACACCG-3′
N2 5′-CTCATAGTCGTCTCACCAGCCGGTGTCAGTAAGCCATTCGAA-3′
DNA sequences were also designed to produce duplexes with short gaps at 21 bp intervals. These two 44mer sequences, G1 and G2, were created by inserting two thymine nucleotides (TT) into the middle of the 42mer oligonucleotide sequences.
G1 5′-GCTGGTGAGACGACTATGAGTTTTCGAATGGCTTACCGAATGGCTTACTGACACCG-3′
G2 5′-CTCATAGTCGTCTCACCAGCTTCGGTGTCAGTAAGCCATTCGAA-3′
Oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA) and separated from truncation products by denaturing PAGE. DNA was eluted from the gel matrix, precipitated with ethanol and then further purified by passage over a Sephadex G-15 column (Sigma). Purified oligonucleotides were lyophilized and resuspended in dH2O. Oligonucleotide concentrations were determined by UV-Vis spectroscopy using the following extinction coefficients: 42mer (N1), ε260 = 406 700 M−1 cm−1; 42mer (N2), ε260 = 410 500 M−1 cm−1; 44mer (G1), ε260 = 422 900 M−1 cm−1; and 44mer (G2), ε260 = 417 700 M−1 cm−1.
To create nicked-DNA, oligonucletoides N1 and N2 were mixed in a buffer of 10 mM Bis-Tris, 100 μM EDTA (pH 7.0), annealed at 85°C for 5 min and then slowly cooled to 4°C. The lengths of annealed products were determined by non-denaturing PAGE.
Gapped-DNA duplexes were prepared and analyzed in the same manner as nicked-DNA, except using the oligonucleotides G1 and G2. Nicked-gapped-DNAs, containing alternating nicks and gaps along the phosphate backbone, were produced by mixing and annealing equal amounts of oligonucleotides N1 and G2. The 21mer oligonucleotide duplex used in the present study had the nucleotide sequence 5′-TCGAATGGCTTACTGACACCG-3′ (complementary strand implied).
DNA condensate preparation
All solutions were filtered through Amicon Ultrafree-MC centrifugal filters with 0.22 μm pore diameter (Millipore) prior to use in condensation reactions. For light scattering and TEM experiments, condensates were prepared by mixing DNA (15 μM in bp) with an equal volume of the specified condensing agent in a buffer of 5 mM Bis-Tris, 50 μM EDTA (pH 7.0). Condensates were allowed to incubate for 5 min at room temperature before analysis by light scattering or TEM. The four condensing agents used in this study were hexammine cobalt chloride (Sigma), poly-l-lysine (PLL) [Mw (LALLS) 8.3 kDa; Sigma], PEI (Mw 750 kDa; Sigma) and TAT47–57 peptide (YGRKKRRQRRR) (Bachem). For all TEM studies with hexammine cobalt(III) as a condensing agent, 200 μM hexammine cobalt chloride was used. For condensate preparations involving the TAT peptide, PLL and PEI, the condensation reaction mixtures were prepared using appropriate concentrations of these condensing agents such that there were two positively charged nitrogens of the condensing agent for every phosphate group of DNA (at pH 7.0). Equivalents of condensing agents are defined as protonated nitrogen atoms of cationic groups of the condensing agents [for PEI, one out of the six amino nitrogen atoms (24)].
Light scattering
DNA condensation was monitored by measuring the average intensity of scattered light at a 90° scattering collection angle using a DynaPro MS/X dynamic light-scattering instrument (Proterion, Piscataway, NJ) with a laser of wavelength 824.8 nm. All light-scattering measurements were performed at room temperature. Each measurement reported is the average of thirty 10 s scattering intensity accumulations taken over the course of five minutes.
TEM
DNA condensate reaction mixtures were deposited on carbon-coated copper EM grids (Ted Pella, Redding, CA) and allowed to settle for 10 min. The solutions were negatively stained with aqueous (2% w/v) uranyl acetate (Ted Pella) for 1 min to enhance contrast. The grids were rinsed with 95% ethanol and subsequently air-dried. The TEM images of DNA condensates were collected using a JEOL-100C transmission electron microscope. Images were recorded on film at 100 000× magnification. Negatives were scanned into electronic format at 300 pixels/inch, and a computer graphics program was used to measure the size of DNA condensates. The average size of the DNA condensates in each sample was calculated by measuring the diameter of a minimum of 100 particles.
RESULTS
Assembly of duplex DNA with regularly spaced single-stranded nicks and/or gaps
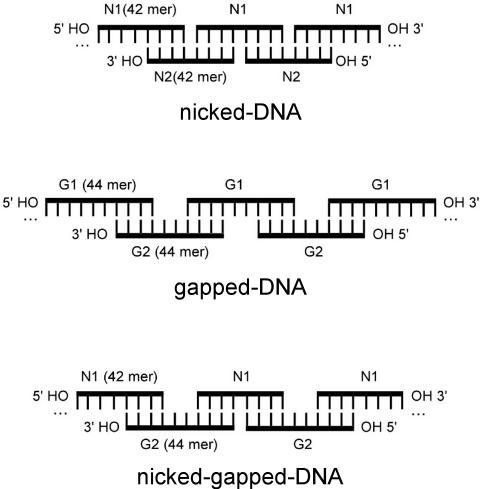
A pair of 42mer oliogonucleotides was designed such that each was a half-sliding Watson–Crick complement of the other (sequences N1 and N2, see Materials and Methods). The annealing of such oligonucleotides would be expected to produce long DNA duplexes (Scheme 1). The backbones of these duplexes would not be continuous along either strand, but would possess nicks at every 21 bp in alternate strands. Because the synthetic oligonucleotides are not phosphorylated at their 5′ ends, these nicks represent positions where a single phosphate group is missing from an otherwise continuous backbone. DNA of this composition will be referred to in the present study as ‘nicked-DNA’. Similarly, a pair of 44mer oligonucleotides was also generated, in which two thymine residues were inserted into the middle of the 42mer oligonucleotides (sequences G1 and G2, see Materials and Methods). The annealing of these 44mer oligonucleotides would be expected to produce duplex DNA with 2 nt gaps at 21 bp intervals along alternating strands (Scheme 1). DNA of this composition will be referred to as ‘gapped-DNA’. By mixing one of the 42mer oligonucleotides (i.e. N1) with its half-sliding complementary 44mer (i.e. G2), it is possible to generate DNA duplexes with alternating nicks and gaps (Scheme 1). DNA of this composition will be referred to as ‘nicked-gapped-DNA’. In general, our approach allows for the incorporation of flexible points, in the form of nicks and/or gaps, at regular intervals into duplex polymers of significant length. This mode of nucleotide assembly also provides a means to assemble many copies of two or more oligonucleotide sequences into a volume that is defined by the persistence length of the resulting nicked/gapped-DNA polymer.
Scheme 1.
Schematic representations of DNA duplexes formed by oligonucleotides that contain nicks and/or gaps at regular intervals. (A) The pairing of the 42mer oligonucleotides N1 and N2 to produce a nicked-DNA duplex with nicks in the backbone at every 21 bp. (B) The formation of gapped-DNA, with 2 nt gaps at every 21 bp, by the pairing of the 44mer oligonucleotides G1 and G2. (C) The formation of nicked-gapped-DNA, with alternating nicks and 2 nt gaps at every 21 bp, by the pairing of the 42mer oligonucleotide N1 and the 44mer oligonucleotide G2. Oligonucleotide sequences are given in Materials and Methods.
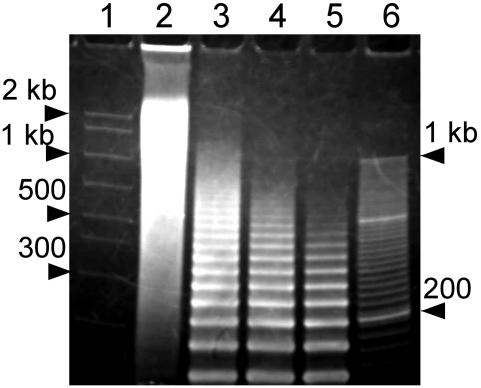
The length of nicked-DNA duplexes formed upon annealing of oligonucleotides N1 and N2 was examined by non-denaturing PAGE. A 1:1 mixture of oligonucleotides N1 and N2 produced DNA assemblies with a distribution of lengths between ∼200 bp and 2 kb, with the majority of these nicked-DNA duplexes being on the longer side of this range (Figure 1). The length of nicked-DNA duplexes was observed to vary directly with the stoichiometry of oligonucleotides N1 and N2. For N1:N2 ratios of 1:2, 1:3 and 1:4, where a significant excess of one strand was present, ladder-like bands appeared in the gel and the length of nicked-DNA duplexes decreased with the degree of deviation from a 1:1 ratio (Figure 1). The nicked-, gapped- and nicked-gapped-DNA assemblies formed from equal moles of monomer oligonucleotides, which in all cases yielded the longest assemblies (Supplementary Figure S1), were used in the condensation experiments described below.
Figure 1.
Characterization of nicked-DNA duplexes by non-denaturing PAGE. Lane 1, AmpliSize molecular ruler (Bio-Rad); lanes 2–5, nicked-DNA from oligonucleotides N1 and N2 with N1:N2 strand stoichiometries of 1:1, 1:2, 1:3, 1:4, respectively; lane 6, 20 bp molecular ruler (Bio-Rad). The concentration of each oligonucleotide was 150 μM per strand. Gel was 3.5% polyacrylamide with a running buffer of 1× TBE (pH 7.9).
Condensation of nicked- and gapped-DNA with hexammine cobalt chloride
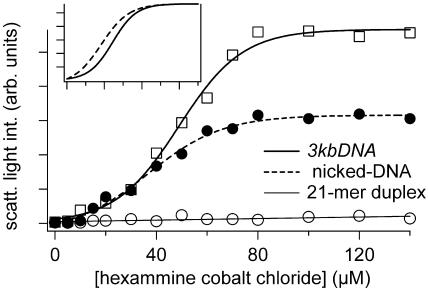
The condensation of nicked-DNA by hexammine cobalt chloride, a well-characterized DNA condensing agent (25), was compared with the condensation behavior of a short 21mer duplex and a linear 3 kb plasmid DNA (3kbDNA). For this study, condensation reactions were performed by mixing hexammine cobalt chloride solutions of increasing concentrations with equal volume solutions of DNA at a constant concentration (7.5 μM in base pair after mixing). The process of DNA condensation was monitored by measuring the average light-scattering intensity of the reaction mixture as a function of hexammine cobalt chloride concentration (Figure 2). When hexammine cobalt chloride was titrated into a solution of 3kbDNA, the average light-scattering intensity of this sample increased rapidly in the low concentration regime and then reached a plateau at ∼80 μM hexammine cobalt chloride (Figure 2). This increase in average light scattering can be attributed to the increased concentration of densely packed DNA particles (26–28). The midpoint of 3kbDNA condensation, under the conditions of our study, was at 49 μM hexammine cobalt chloride. This midpoint of condensation and the shape of the titration profile are consistent with earlier reports of DNA condensation under low salt conditions (29). A similar titration of a 21mer oligonucleotide duplex failed to produce a significant increase in light-scattering intensity within the titration range, which indicates no appreciable formation of DNA condensates. This observation is consistent with earlier reports that DNA duplexes even as long as 140 bp in length are more difficult to condense with hexammine cobalt chloride than DNA that is thousands of bp in length (25). In contrast, titration of nicked-DNA with hexammine cobalt chloride produced a light-scattering profile with a shape very similar to that of 3kbDNA. A sigmoidal fit of scattered light intensity as a function of hexammine cobalt chloride concentration demonstrates that the nicked-DNA condenses with a midpoint of 37 μM (Figure 2, inset). Thus, nicked-DNA actually has a greater propensity to condense than linear duplex DNA of comparable length (i.e. 3kbDNA), and dramatically different condensation properties when compared with the duplex 21mer oligonucleotides. We note that the maximum light-scattering intensity observed for condensed nicked-DNA is approximately half of that observed for 3kbDNA. This result does not necessarily indicate that the nicked-DNA is in a less condensed state, as particle number and particle morphology also contribute to light-scattering intensity. Hydrodynamic radius measurements based upon dynamic light scattering actually indicate that particles formed by nicked-DNA and hexammine cobalt chloride are larger than those formed by 3kbDNA (Supplementary Figure S2). Electron microscopy studies discussed below also confirm this to be the case. Light-scattering measurements clearly indicate that the condensation of nicked-DNA by hexammine cobalt chloride is more similar to 3kbDNA condensation than to the condensation of short duplex oligonucleotides.
Figure 2.
Condensation of 3kbDNA, nicked-DNA and a 21mer duplex by hexammine cobalt chloride, as monitored by light scattering. For each data point shown, DNA concentration was 7.5 μM in base pair (5 mM Bis-Tris, 50 μM EDTA, pH 7.0). Light-scattering intensities shown are averages from measurements taken over a 5 min period. Inset: 3kbDNA and nicked-DNA scattering intensities, as a function of hexammine cobalt chloride concentration, normalized to maximum observed intensity. Note: nicked-DNA condensation occurs at a lower hexammine cobalt chloride concentration than 3kbDNA.
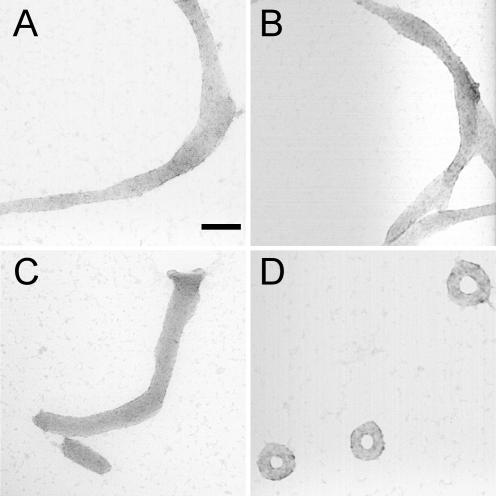
The size and morphology of particles formed by the condensation of nicked-, gapped- and nicked-gapped-DNA duplexes with hexammine cobalt chloride were directly analyzed using TEM and compared with those formed by 3kbDNA. Nicked-DNA condensed into ribbon-like aggregates that were ∼75 nm in width, but frequently over 1 μM in length (Figure 3A). Gapped- and nicked-gapped-DNA duplexes produced similar ribbon-like structures when condensed by hexammine cobalt chloride (Figure 3B and C). Spherical particles of a size close to that typical of DNA toroids were occasionally found among the elongated ribbon-like aggregates. Under the same solution conditions, the condensation of 3kbDNA by hexammine cobalt chloride produced toroids as the dominant morphology with a mean outer diameter of 85 nm (Figure 3D). This result for 3kbDNA is consistent with the previous reports of linear plasmid DNA condensation from a low-salt buffer (21).
Figure 3.
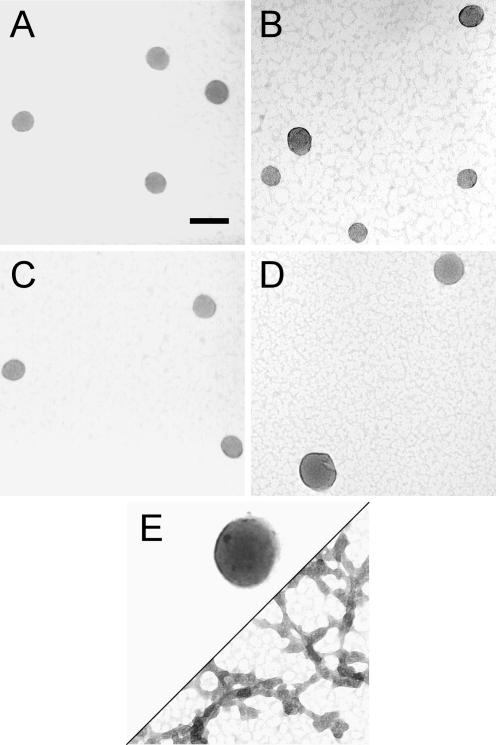
TEM images of particles formed by various DNA samples upon condensation with hexammine cobalt chloride. (A) Condensates formed by the nicked-DNA duplexes of oligonucleotides N1 and N2. (B) Condensates formed by the gapped-DNA duplexes of oligonucleotides G1 and G2. (C) Condensates formed by the nicked-gapped-DNA duplex of oligonucleotides N1 and G2. (D) Condensates formed by 3kbDNA. For all samples, DNA was 15 μM in base pair, and condensed by mixing with an equal volume of 200 μM hexammine cobalt chloride in 5 mM Bis-Tris, 50 μM EDTA (pH 7.0). Scale bar is 100 nm.
Condensation of nicked- and gapped-DNA by a cationic peptide
We also studied the condensation of DNA duplexes with multiple nicks and/or gaps by larger and more highly charged condensing agents. For these studies, condensing agents were chosen from molecules that had previously been shown to facilitate artificial gene delivery. Many cationic peptides have been reported as successful vectors for non-viral gene delivery (17,30). Some of these peptides include naturally derived sequences that facilitate the translocation of DNA across biological membranes in the absence of any specific transporter or receptor. For example, a short peptide derived from the nuclear localization sequence (NLS) of the Tat protein, residues 47–57 (YGRKKRRQRRR), has been shown to promote the entry of nucleic acids into several different cell types (31–34). Thus, we chose the Tat-NLS peptide as a model peptide to investigate whether nicked- and gapped-DNA are condensed by a cationic peptide into a form that would be suitable for delivery.
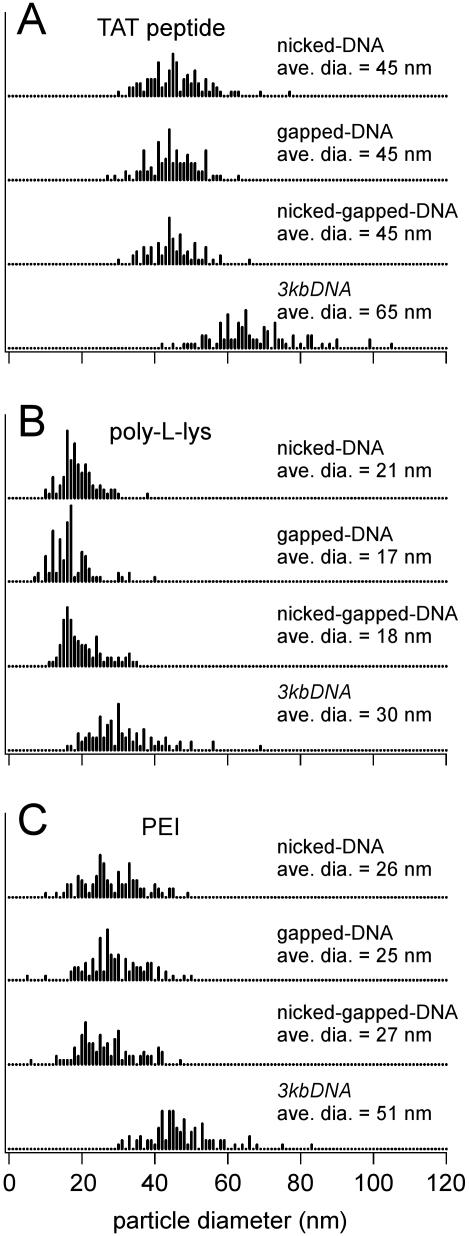
Changing the condensing agent from hexammine cobalt chloride to the Tat-NLS peptide caused a dramatic alteration in the size and morphology of condensates formed by DNA duplexes containing nicks and/or gaps. Nicked-, gapped- and nicked-gapped-DNA duplexes all condensed into spherical particles, each with an average diameter of 45 nm (Figure 4A–C). The continuous 3kbDNA was also condensed into spheres by the Tat-NLS peptide, but these particles were significantly larger with an average diameter of 65 nm (Figure 4D). Histograms of condensate particle diameters illustrate that the distribution of particle sizes was also very similar among the three types of nicked- and gapped-DNA, and narrower for each than the distribution observed for 3kbDNA (Figure 5A). We note that the 21mer oligonucleotide duplexes (which did not condense with hexammine cobalt chloride) were condensed by the Tat-NLS peptide, but much larger aggregates were observed compared with nicked- and/or gapped-DNA (Figure 4E). Thus, DNA with nicks and/or gaps can be condensed into smaller particles by the Tat-NLS peptide than either oligonucleotide duplexes or 3kbDNA. The difference observed between the size and morphology of nicked- and gapped-DNA condensates when the condensing agent is changed from a trivalent cation to an eight-charged cationic peptide also illustrates the fundamental interplay between condensing agent type and DNA structure on DNA condensation.
Figure 4.
TEM images of particles formed by various DNA samples upon condensation with the Tat-NLS peptide. (A) Condensates formed by the nicked-DNA duplexes of oligonucleotides N1 and N2. (B) Condensates formed by the gapped-DNA duplexes of oligonucleotides G1 and G2. (C) Condensates formed by the nicked-gapped-DNA duplex of oligonucleotides N1 and G2. (D) Condensates formed by 3kbDNA. (E) Condensates formed by 21mer duplex. For all samples, DNA was 15 μM in base pair, and was condensed by mixing with the Tat-NLS peptide at a charge ratio of 1:2 (DNA phosphate:cationic charged group of the peptide) in 5 mM Bis-Tris, 50 μM EDTA (pH 7.0). Scale bar is 100 nm.
Figure 5.
Histograms of DNA condensate diameters. Measurements were taken from TEM images similar to those shown in Figure 4. The DNA molecules associated with each histogram are given to the right of each. The condensing agents for the three sets of histograms are (A) Tat-NLS peptide (B) PLL and (C) PEI. The average particle diameters given in the figure are based upon measurements from at least 100 particles for each value reported. Experimental conditions for condensate formation are given in Materials and Methods.
Condensation of nicked- and gapped-DNA by PLL
We have also studied the condensation of DNA with multiple nicks and/or gaps by the much larger cationic peptide PLL. PLL is of interest as a DNA condensing agent because it is known to enhance the cellular uptake of DNA and protect DNA from nuclease digestion (17). All three types of nicked- and gapped-DNA duplexes were found to uniformly condense into small spherical particles with PLL (Supplementary Figure S3). The average diameter of nicked-, gapped- and nicked-gapped-DNA-PLL condensates was essentially the same at 21, 17 and 18 nm, respectively. However, 3kbDNA under the same conditions produced appreciably larger spheres with an average diameter of 30 nm and with a somewhat greater size distribution (Figure 5B). Based upon an average length for our nicked- and gapped-DNA of 2 kb, the volume of a sphere with a 20 nm diameter and the previously determined packing density of condensed DNA (35), the spherical nicked- and gapped-DNA-PLL particles are the result of a single DNA polymer collapse. On the other hand, 30 nm PLL-3kbDNA particles are estimated to contain 6 kb, or two strands of 3kbDNA. The short 21mer duplex was also condensed by PLL into well-defined spheres, but with a much greater degree of aggregation as compared with DNA with nicks and/or gaps (Supplementary Figure S3).
Condensation of nicked- and gapped-DNA by PEI
Among the various polymeric DNA transfection agents described in the literature, PEI has proven particularly efficient in a variety of in vitro and in vivo transfection studies (15,16). Apart from DNA condensation and protection from nucleases, endosomal release of DNA is known to be essential for efficient transfection (36). Among the different DNA carriers, PEI has been shown to effectively promote early release of DNA from the endosomal pathway (15). Furthermore, additional chemical modifications can easily be introduced to the PEI molecule to enhance target specificity (37). Thus, we have also investigated the condensation of DNA duplexes with multiple nicks and/or gaps by PEI. PEI condensed these DNA duplexes into spherical condensates with average diameters of 26, 25 and 27 nm for nicked-, gapped- and nicked-gapped-DNA duplexes, respectively (Figure 5C). Thus, PEI condensates of nicked- and gapped-DNA are substantially smaller than condensates formed with hexammine cobalt chloride or the Tat-NLS peptide, but larger than those formed with PLL. PEI also condensed 3kbDNA duplexes into spherical particles, however, the average diameter of the PEI-3kbDNA spheroids was 51 nm and with increased particle size distribution as compared with all three types of DNA duplexes with nicks and/or gaps (Figure 5C). Unlike PLL, PEI did not condense the duplex 21mer oligonucleotides into discrete particles with a regular morphology (Supplementary Figure S4).
DISCUSSION
Our motivation to study the condensation of nicked- and gapped-DNA is two-fold: to acquire a more fundamental understanding of DNA condensation and to develop methods for the packaging of therapeutic oligonucleotides into discrete particles. In the present study, we have focused on characterizing the condensation of nicked- and gapped-DNA by various condensing agents. Here, we discuss what our results reveal about condensation of DNA duplexes with multiple nicks or gaps and about DNA condensation in general. As noted above, efficient condensation of DNA into nanometer-scale particles correlates strongly with enhanced DNA delivery to living cells (11,38,39). Thus, based on this criterion, the small particles produced by the self-assembly of oligonucleotides into long DNA duplexes with regularly spaced nicks or gaps represent promising vectors for oligonucleotide delivery. Nevertheless, we acknowledge that the utility of our approach for cellular delivery must still be proven by actual delivery studies.
Nicked- and gapped-DNA are more prone to condensation than continuous duplex DNA
The light-scattering studies reported here revealed that nicked-DNA condenses at a lower concentration of hexammine cobalt chloride than 3kbDNA (Figure 2). This observation is consistent with nicked-DNA being intrinsically more prone to condensation than continuous duplex DNA of comparable length. The greater tendency of nicked-DNA to condense is most probably due to the ability of nicked-DNA to fold upon itself every 21 bp. The energetic cost associated with the introduction of sharp bends (i.e. kinks) at nicked sites would be considerably less than that associated with the tight kinking of continuous duplex DNA. In other words, the greater propensity for nicked-DNA to condense is probably due to the reduced rigidity or persistence length of nicked-DNA in comparison with continuous duplex DNA (40).
The large ribbon-like condensates produced by mixing nicked- or gapped-DNA with hexammine cobalt chloride clearly contain more DNA than a single 2 kb nicked/gapped-DNA duplex. As an illustration, if we approximate the ribbon-like structure shown in Figure 3A as a cylinder and assume hexagonal DNA packing with an inter-helix spacing of 2.8 nm (35), then this particle contains ∼1 Mb of DNA. Given that the nicked-DNA duplexes used in this study are ∼2 kb in length, this structure contains on the order of 50 nicked-DNA duplex molecules. The actual mechanism for the assembly of nicked- and gapped-DNA into ribbon-like structures is not clear. It is possible that the greater flexibility of nicked-DNA may allow rearrangements to occur in the condensed state that lead to higher-order assemblies. In any case, this phenomenon is apparently particular to the condensation of nicked- and gapped-DNA when a trivalent cation, such as hexammine cobalt(III), is used as the condensing agent.
Nicked- and gapped-DNA favors small condensates by altering the kinetics of condensation
For all DNA samples of the current study, condensate size decreased as the net charge of the condensing agents increased from a trivalent to polyvalent cations. These results are consistent with the previous reports that DNA condensates tend to decrease in size as condensing agents increase in positive charge valency (39,41,42). The trend to smaller particle size with greater condensing agent charge valency can be understood by considering the fact that DNA condensation is a nucleation-growth phenomenon (43). Similar to crystal growth, if particle nucleation is rapid and the addition of molecules onto a growing particle is irreversible, then many small particles tend to form (44). In such cases, particle growth is said to be ‘kinetically limited’, because particle size is determined by the rate at which the bulk solution becomes depleted of free molecules from which the particles can grow. In contrast, if particle nucleation is slow and molecule addition is reversible, then a smaller number of particles grow to a much larger size. In terms of DNA condensation, condensing agents with greater charge valency are expected to more rapidly nucleate DNA condensation, and DNA condensed by such condensing agents is less likely to exchange back into solution than DNA condensed by a chemical agent of lower charge valency. Thus, like rapid crystal nucleation, condensing agents of greater charge valency can favor the formation of smaller condensates by kinetically limiting DNA particle growth.
We have demonstrated that the condensates formed by nicked- and gapped-DNA in the presence of TAT peptide, PLL and PEI are smaller than those formed by continuous duplex 3kbDNA. Our data also indicate that the spherical particles produced by nicked- and gapped-DNA in the presence of PLL result from the unimolecular collapse of nicked/gapped-DNA duplexes, whereas 3kbDNA does not appear to form unimolecular particles under the same conditions. Together, these observations indicate that DNA with multiple nicks or gaps has a greater propensity to nucleate condensation than continuous duplex DNA. Single-stranded nicks and gaps in duplex DNA are known to effectively reduce the overall persistence length of a DNA polymer in solution by increasing the conformational freedom of the polymer through localized dynamic kinks (40,45–48). These points of increased flexibility in nicked- and gapped-DNA would be expected to allow intra-polymer helix–helix associations that could act as nucleation sites for DNA condensate formation. As mentioned above, if DNA condensate nucleation is sufficiently rapid and essentially irreversible, then DNA condensate size can be limited by the kinetics of condensation. Since the condensates formed by nicked-, gapped- and nicked-gapped-DNA with the Tat-NLS peptide, PLL and PEI are smaller than the corresponding condensates of 3kbDNA, the condensate nucleation sites provided by the flexible sites of nicked- and gapped-DNA apparently provide a mode of condensate nucleation that is kinetically more accessible than the nucleation structures formed by continuous duplex DNA.
The similar condensation of nicked-, gapped- and nicked-gapped-DNA is somewhat surprising, as the persistence length of nicked- and gapped-DNA duplexes have been reported to be significantly different (45–47,49,50). Given the observation that these three types of DNA condense similarly, one might conclude that reduced persistence length does not fully account for the difference in the condensation of nicked- and gapped-DNA with respect to continuous DNA duplex. However, it is possible that the concept of DNA persistence length alone is not sufficient to describe what is observed and that the kinetics of nucleation must also be considered. Clearly, a common feature of our nicked-DNA and gapped-DNA is the propensity for sharp kinks to occur spontaneously along the helical axis (46–48). We propose that these kinks act as local nucleation sites for condensation and that at any given time a nicked-DNA duplex contains a sufficient number of these kinks to nucleate condensation. Gapped- and nicked-gapped-DNA may contain a larger number of these kinks, but because these sites principally act to nucleate condensation, having more kinks than nicked-DNA may not further alter DNA condensation.
CONCLUSIONS
We have described a new method for the efficient condensation of DNA oligonucleotides into discrete particles. By assembling oligonucleotides into long nicked or gapped duplexes, the condensation of short oligonucleotides into well-defined particles can be achieved with various condensing agents. In addition, we have provided one more illustration of the interplay that exists between DNA structure and condensing agent structure on the size and morphology of condensates. The introduction of dynamic kinks in the nucleic acid structure represents a simple way to introduce nucleation sites that kinetically favor the formation of small, uniform condensates. The results presented also provide additional support to earlier proposals that nucleation can be a defining step in the determination of condensate size (51).
The simple strategy presented here should be readily amenable to further studies that aim at using short oligonucleotides to block viral or cellular disease-causing genes. In addition to facilitating the condensation of oligonucleotides, appending nicked- or gapped-DNA onto one end of a continuous gene-length DNA could provide nucleation sites for condensation. This might be a way to promote the condensation of gene-length DNA into small spherical particles, as we have previously shown that the incorporation of static loops into a 3 kb long DNA kinetically favors condensation into toroids with reduced dimensions (20,21). Whether these improvements in DNA condensation result in enhanced gene/oligonucleotide transfer to target cells must ultimately be addressed directly by in vitro and in vivo gene delivery experiments.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Supplementary Material
Acknowledgments
We thank the Georgia Tech EM Center for use of their JEOL-100C, and Yolande Berta for her technical assistance. We gratefully acknowledge the NIH for the financial support of this research (GM62873). Funding to pay the open access publication charges for this article was provided by NIH grant GM62873.
REFERENCES
- 1.Stull R.A., Szoka F.C. Antigene, ribozyme and aptamer nucleic acid drugs: progress and prospects. Pharm. Res. 1995;12:465–483. doi: 10.1023/a:1016281324761. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal S. Antisense oligonucleotides: towards clinical trials. Trends Biotechnol. 1996;14:376–387. doi: 10.1016/0167-7799(96)10053-6. [DOI] [PubMed] [Google Scholar]
- 3.Pawlak W., Zolnierek J., Sarosiek T., Szczylik C. Antisense therapy in cancer. Cancer Treat. Rev. 2000;26:333–350. doi: 10.1053/ctrv.2000.0173. [DOI] [PubMed] [Google Scholar]
- 4.Thompson J.D. Applications of antisense and siRNAs during preclinical drug development. Drug Discov. Today. 2002;7:912–917. doi: 10.1016/s1359-6446(02)02410-8. [DOI] [PubMed] [Google Scholar]
- 5.Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 6.Hannon G.J. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 7.Song E.W., Lee S.K., Wang J., Ince N., Ouyang N., Min J., Chen J.S., Shankar P., Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nature Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 8.Zabner J., Fasbender A.J., Moninger T., Poellinger K.A., Welsh M.J. Cellular and molecular barriers to gene transfer by a cationic lipid. J. Biol. Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 9.LabatMoleur F., Steffan A.M., Brisson C., Perron H., Feugeas O., Furstenberger P., Oberling F., Brambilla E., Behr J.P. An electron microscopy study into the mechanism of gene transfer with lipopolyamines. Gene Ther. 1996;3:1010–1017. [PubMed] [Google Scholar]
- 10.Luo D., Saltzman W.M. Synthetic DNA delivery systems. Nat. Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 11.Gao X., Huang L. Potentiation of cationic liposome-mediated gene delivery by polycations. Biochemistry. 1996;35:1027–1036. doi: 10.1021/bi952436a. [DOI] [PubMed] [Google Scholar]
- 12.Capaccioli S., Dipasquale G., Mini E., Mazzei T., Quattrone A. Cationic lipids improve antisense oligonucleotide uptake and prevent degradation in cultured cells and in human serum. Biochem. Biophys. Res. Commun. 1993;197:818–825. doi: 10.1006/bbrc.1993.2552. [DOI] [PubMed] [Google Scholar]
- 13.Lewis J.G., Lin K.Y., Kothavale A., Flanagan W.M., Matteucci M.D., DePrince R.B., Mook R.A., Hendren R.W., Wagner R.W. A serum-resistant cytofectin for cellular delivery of antisense oligodeoxynucleotides and plasmid DNA. Proc. Natl Acad. Sci. USA. 1996;93:3176–3181. doi: 10.1073/pnas.93.8.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo H., Juliano R.L. Enhanced delivery of antisense oligonucleotides with fluorophore-conjugated PAMAM dendrimers. Nucleic Acids Res. 2000;28:4225–4231. doi: 10.1093/nar/28.21.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boussif O., Lezoualch F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B., Behr J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robaczewska M., Guerret S., Remy J.S., Chemin I., Offensperger W.B., Chevallier M., Behr J.P., Podhajska A.J., Blum H.E., Trepo C., et al. Inhibition of hepadnaviral replication by polyethylenimine-based intravenous delivery of antisense phosphodiester oligodeoxynucleotides to the liver. Gene Ther. 2001;8:874–881. doi: 10.1038/sj.gt.3301464. [DOI] [PubMed] [Google Scholar]
- 17.Lochmann D., Jauk E., Zimmer A. Drug delivery of oligonucleotides by peptides. Eur. J. Pharm. Biopharm. 2004;58:237–251. doi: 10.1016/j.ejpb.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Arscott P.G., Li A.Z., Bloomfield V.A. Condensation of DNA by trivalent Cations .1. Effects of DNA length and topology on the size and shape of condensed particles. Biopolymers. 1990;30:619–630. doi: 10.1002/bip.360300514. [DOI] [PubMed] [Google Scholar]
- 19.Schnell J.R., Berman J., Bloomfield V.A. Insertion of telomere repeat sequence decreases plasmid DNA condensation by cobalt(III) hexaammine. Biophys. J. 1998;74:1484–1491. doi: 10.1016/S0006-3495(98)77860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen M.R., Downing K.H., Balhorn R., Hud N.V. Nucleation of DNA condensation by static loops: formation of DNA toroids with reduced dimensions. J. Am. Chem. Soc. 2000;122:4833–4834. [Google Scholar]
- 21.Conwell C.C., Vilfan I.D., Hud N.V. Controlling the size of nanoscale toroidal DNA condensates with static curvature and ionic strength. Proc. Natl Acad. Sci. USA. 2003;100:9296–9301. doi: 10.1073/pnas.1533135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akasaka T., Matsuura K., Kobayashi K. Transformation from block-type to graft-type oligonucleotide–glycopolymer conjugates by self-organization with half-sliding complementary oligonucleotides and their lectin recognition. Bioconjug. Chem. 2001;12:776–785. doi: 10.1021/bc0100152. [DOI] [PubMed] [Google Scholar]
- 23.Dauty E., Behr J.P. Monomolecular condensation of DNA by cationic detergents. Polym. Int. 2003;52:459–464. [Google Scholar]
- 24.Dunlap D., Maggi A., Soria M., Monaco L. Nanoscopic structure of DNA condensed for gene delivery. Nucleic Acids Res. 1997;25:3095–3101. doi: 10.1093/nar/25.15.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widom J., Baldwin R.L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J. Mol. Biol. 1980;144:431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- 26.Ma C.L., Bloomfield V.A. Condensation of supercoiled DNA induced by MnCl2. Biophys. J. 1994;67:1678–1681. doi: 10.1016/S0006-3495(94)80641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloomfield V.A. Static and dynamic light scattering from aggregating particles. Biopolymers. 2000;54:168–172. doi: 10.1002/1097-0282(200009)54:3<168::AID-BIP20>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Zintchenko A., Rother G., Dautzenberg H. Transition highly aggregated complexes-soluble complexes via polyelectrolyte exchange reactions: kinetics, structural changes, and mechanism. Langmuir. 2003;19:2507–2513. [Google Scholar]
- 29.Plum G.E., Arscott P.G., Bloomfield V.A. Condensation of DNA by trivalent cations. 2. Effects of cation structure. Biopolymers. 1990;30:631–643. doi: 10.1002/bip.360300515. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi M., Eguchi A., Akuta T., Nagoshi E., Fujita S., Okabe J., Senda T., Hasegawa M. Basic peptides as functional components of non-viral gene transfer vehicles. Curr. Protein Pept. Sci. 2003;4:141–150. doi: 10.2174/1389203033487234. [DOI] [PubMed] [Google Scholar]
- 31.Vives E., Brodin P., Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 32.Ignatovich I.A., Dizhe E.B., Pavlotskaya A.V., Akifiev B.N., Burov S.V., Orlov S.V., Perevozchikov A.P. Complexes of plasmid DNA with basic domain 47–57 of the HIV-1 Tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J. Biol. Chem. 2003;278:42625–42636. doi: 10.1074/jbc.M301431200. [DOI] [PubMed] [Google Scholar]
- 33.Torchilin V.P., Levchenko T.S., Rammohan R., Volodina N., Papahadjopoulos-Sternberg B., D'Souza G.G.M. Cell transfection in vitro and in vivo with nontoxic TAT peptide–liposome–DNA complexes. Proc. Natl Acad. Sci. USA. 2003;100:1972–1977. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu Y.L., Ali A., Chu C.Y., Cao H., Rana T.M. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem. Biol. 2004;11:1165–1175. doi: 10.1016/j.chembiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Hud N.V., Downing K.H. Cryoelectron microscopy of lambda phage DNA condensates in vitreous ice: the fine structure of DNA toroids. Proc. Natl Acad. Sci. USA. 2001;98:14925–14930. doi: 10.1073/pnas.261560398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ElOuahabi A., Thiry M., Pector V., Fuks R., Ruysschaert J.M., Vandenbranden M. The role of endosome destabilizing activity in the gene transfer process mediated by cationic lipids. FEBS Lett. 1997;414:187–192. doi: 10.1016/s0014-5793(97)00973-3. [DOI] [PubMed] [Google Scholar]
- 37.Kircheis R., Kichler A., Wallner G., Kursa M., Ogris M., Felzmann T., Buchberger M., Wagner E. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther. 1997;4:409–418. doi: 10.1038/sj.gt.3300418. [DOI] [PubMed] [Google Scholar]
- 38.Godbey W.T., Ku K.K., Hirasaki G.J., Mikos A.G. Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene Ther. 1999;6:1380–1388. doi: 10.1038/sj.gt.3300976. [DOI] [PubMed] [Google Scholar]
- 39.Wadhwa M.S., Collard W.T., Adami R.C., McKenzie D.L., Rice K.G. Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconjug. Chem. 1997;8:81–88. doi: 10.1021/bc960079q. [DOI] [PubMed] [Google Scholar]
- 40.Furrer P., Bednar J., Stasiak A.Z., Katritch V., Michoud D., Stasiak A., Dubochet J. Opposite effect of counterions on the persistence length of nicked and non-nicked DNA. J. Mol. Biol. 1997;266:711–721. doi: 10.1006/jmbi.1996.0825. [DOI] [PubMed] [Google Scholar]
- 41.McKenzie D.L., Collard W.T., Rice K.G. Comparative gene transfer efficiency of low molecular weight polylysine DNA-condensing peptides. J. Pept. Res. 1999;54:311–318. doi: 10.1034/j.1399-3011.1999.00104.x. [DOI] [PubMed] [Google Scholar]
- 42.Vijayanathan V., Thomas T., Antony T., Shirahata A., Thomas T.J. Formation of DNA nanoparticles in the presence of novel polyamine analogues: a laser light scattering and atomic force microscopic study. Nucleic Acids Res. 2004;32:127–134. doi: 10.1093/nar/gkg936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conwell C.C., Hud N.V. Evidence that both kinetic and thermodynamic factors govern DNA toroid dimensions: Effects of magnesium(II) on DNA condensation by hexammine cobalt(III) Biochemistry. 2004;43:5380–5387. doi: 10.1021/bi049872u. [DOI] [PubMed] [Google Scholar]
- 44.Lamer V.K., Dinegar R.H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950;72:4847–4854. [Google Scholar]
- 45.Mills J.B., Cooper J.P., Hagerman P.J. Electrophoretic evidence that single-stranded regions of one or more nucleotides dramatically increase the flexibility of DNA. Biochemistry. 1994;33:1797–1803. doi: 10.1021/bi00173a024. [DOI] [PubMed] [Google Scholar]
- 46.Roll C., Ketterle C., Faibis V., Fazakerley G.V., Boulard Y. Conformations of nicked and gapped DNA structures by NMR and molecular dynamic simulations in water. Biochemistry. 1998;37:4059–4070. doi: 10.1021/bi972377w. [DOI] [PubMed] [Google Scholar]
- 47.Protozanova E., Yakovchuk P., Frank-Kamenetskii M.D. Stacked-unstacked equilibrium at the nick site of DNA. J. Mol. Biol. 2004;342:775–785. doi: 10.1016/j.jmb.2004.07.075. [DOI] [PubMed] [Google Scholar]
- 48.Le Cam E., Fack F., Menissierdemurcia J., Cognet J.A.H., Barbin A., Sarantoglou V., Revet B., Delain E., de Murcia G. Conformational analysis of a 139 base-pair DNA fragment containing a single-stranded break and its interaction with human poly(ADP-ribose) polymerase. J. Mol. Biol. 1994;235:1062–1071. doi: 10.1006/jmbi.1994.1057. [DOI] [PubMed] [Google Scholar]
- 49.Cohen S.B., Cech T.R. A quantitative study of the flexibility contributed to RNA structures by nicks and single-stranded gaps. RNA. 1998;4:1179–1185. doi: 10.1017/s1355838298001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lane M.J., Paner T., Kashin I., Faldasz B.D., Li B., Gallo F.J., Benight A.S. The thermodynamic advantage of DNA oligonucleotide “stacking hybridization” reactions: energetics of a DNA nick. Nucleic Acids Res. 1997;25:611–616. doi: 10.1093/nar/25.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hud N.V., Vilfan I.D. Toroidal DNA condensates: unraveling the fine structure and the role of nucleation in determining size. Annu. Rev. Biophys. Biomol. Struct. 2005 doi: 10.1146/annurev.biophys.34.040204.144500. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.