Abstract
Background/Aim: We hypothesized that combined therapy using adipose-derived stem cells (ASCs) and vitamin C might improve tendon regeneration in tendonitis. To determine combined effects of ASC transplantation with vitamin C, we used senescence marker protein 30 (SMP30)-knockout (KO) mice that cannot biosynthesize vitamin C by themselves. Materials and Methods: SMP30-KO were divided into four groups: Control, vitamin C, ASCs, and vitamin C plus ASCs. Tendonitis was induced in the achilles tendons via injection of collagenase type I. After 1 week, ASCs were injected into the intratendonal region. After 30 days, all mice were sacrificed and Achilles tendons were isolated. Results: Gross and microscopic findings showed mice treated with combination of ASC transplantation and vitamin C showed better tendon regeneration than those in other groups. This combination led to higher serum vitamin C levels than use of vitamin C alone. This indicates that the vitamin C-treated group used more vitamin C as a precursor to collagen synthesis, whereas vitamin C was in excess in the combination group because of the added effect of ASCs on tendon healing. Conclusion: This study showed that vitamin C improved the effect of ASC transplantation on tendonitis by inducing a better stem cell niche.
Keywords: Tendonitis, vitamin C, adipose-derived stem cell, SMP30-KO mouse, stem cell therapy
Tendons are tough bands of dense and fibrous regular connective tissue that usually connect muscle to bone and are capable of withstanding tension (1). Tendons comprise of collagen bundles similar to those seen in ligaments (1).
Tendinopathy is the most frequent tendon disorder (2-4) and is usually described as an over-use of tendons (5). Tendinopathy can develop in any tendon, but is most frequently found in the following tendons: Achilles, patellar, shoulder rotator cuff, and forearm extensor tendons (6). Tendonitis, a type of tendinopathy, is an inflammatory process in the tendon (5). Tendonitis often occurs in daily life and through sports, but tendon repair requires a long period of time because tendons have a low blood supply compared with other organs (7).
Many challenging therapies, such as the use of non-steroidal anti-inflammatory medications, eccentric exercises, topical glyceryl trinitrate, and shock-wave therapy, are performed to treat tendinopathy (8-11). Cell therapy is a new type of therapy for tendinopathy that occurs after tendon injury (12,13). Recently, mesenchymal stem cell therapy, especially that using adipose-derived stem cells (ASCs), has emerged as a new therapeutic option for tendinopathy (14-16).
Vitamin C is known to be a potent antioxidant (17) and can increase the viability of transplanted cells by reducing oxidative and inflammatory stress. Previous studies have revealed that vitamin C promoted tendon healing by stimulating extracellular matrix remodeling as a precursor of collagen synthesis (18,19). In addition, vitamin C increased the therapeutic effects of combination stem cell therapy in a muscle laceration model (20). However, there has been no study as far as we are aware on the therapeutic effects of combination stem cell therapy and vitamin C administration on tendinopathy.
We hypothesized that combined therapy using ASCs and vitamin C might improve tendon regeneration in tendinopathy. To determine the combined effects of ASCs transplantation with vitamin C, we used senescence marker protein 30 (Smp30)-knockout mice that cannot biosynthesize vitamin C by themselves (21).
Materials and Methods
Animals. Ten-week-old male Smp30 gene-knockout mice were used in this study. The mice were maintained in a room at 22±2˚C with a relative humidity of 50±10% and a 12-h light-dark cycle. The mice were fed pellets and water ad libitum. The genotypes were identified by polymerase chain reaction using the primers TA4 (5’-CAAGTAACTCTAGGTATGGAC-3’), TS3 (5’-CTAGCCATGG TGGATGAAGAT-3’), and NEO (5’-TCGTGCTTTACGGTATCG CCGCTCCCGATT-3’) to detect Smp30 alleles (21). Animal experiments were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and with approval from the Institutional Animal Care AND Use Committee of Kyungpook National University (KNU 2014-0167).
Isolation and culture of ASCs. ASCs were isolated from C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) that were younger than 1 year. Abdominal fat tissues were isolated from the inguinal fat pads of mice. Fat tissues were disinfected with 70% ethyl alcohol, washed with Dulbecco’s phosphate-buffered saline (PBS), and digested with 0.2% collagenase type I (Worthington Biochemical, Lakewood, NJ, USA) at 37˚C for 10 min. The digested tissues were filtered through a 70 μm nylon cell strainer to separate the dissociated cells and then centrifuged at 3,000 rpm for 3 min. The pellet in the bottom layer was collected and washed twice with PBS. The cells were plated onto a culture dish with medium consisting of low-glucose Dulbecco’s modified Eagle's medium (Gibco, Waltham, MA, USA), 10% fetal bovine serum (Gibco, Waltham, MA, USA), and 1% penicillin/streptomycin solution (WelGENE, Kyungsan, Korea). Cells were contained in a 37˚C incubator with 5% CO2, and ASCs were incubated at passage 5 before transplantation.
Establishment of mouse collagenase-induced tendonitis model. C57BL/6J mice (Jackson Laboratory,USA) were anesthetized with Zoletil (Virbac, Carros, France) and xylazine (Bayer, Leverkusen, Germany). Hair on the hind limbs of mice was shaved and then further removed with a hair remover lotion before collagenase injection. Collagenase type I (Sigma-Aldrich, St. Louis, MO, USA) (125 units/20 μl) was administered via intratendonous injection into the Achilles tendons using a 31G needle.
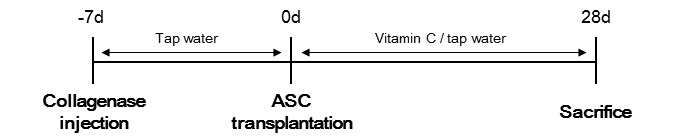
Experimental design. Smp30-knockout mice were divided into four groups: Negative control without any treatment (n=7), ASC transplantation without feeding vitamin C (n=7), vitamin C supplementation without cell therapy (n=7), and co-administration of ASC transplant and vitamin C supplementation (n=7). One week before experimentation, all groups were supplied with tap water and autoclaved pellet to equally induce vitamin C deficiency. In the groups treated with ASCs, ASCs (2×105 cells/30 μl) were transplanted by intratendonous injection into the Achilles tendons using a 31G needle during anesthesia with zoletil and xylazine. In the control group and group treated with ASCs, vitamin C deficiency was induced by feeding tap water and autoclaved pellet. In the groups treated with vitamin C, vitamin C (L-ascorbic acid, Sigma-Aldrich) was supplied in the drinking water (1.5 g/l). After 4 weeks, all mice were sacrificed, and their Achilles tendons and blood were collected. The experimental schedule are shown in Figure 1.
Figure 1. Experimental schedule. One week before experimentation, all groups were fed tap water. After adipose-derived stem cell (ASCs) transplantation, the control group and the group treated with ASCs were supplied with tap water, while the vitamin C-treated groups were supplied with water containing vitamin C (1.5 g/l) for 4 weeks. All mice were then sacrificed and specimens were collected.
Gross and histopathology. After sacrifice, the Achilles tendons were firstly exposed and evaluated by gross examination. For histopathological analysis, specimens were fixed in 10% neutral buffered formalin (DC Chemical Co., Seoul, Korea), processed by standard methods, and embedded in paraffin wax. Tissue sections were cut to a thickness of 4 μm; then hematoxylin and eosin (H&E) staining was performed. After staining, all areas of the tendon that were visible at the original magnification of 40-400× in the injured portions were captured with the Leica Application Suite program (Leica Biosystems, Wetzlar, Germany). Histopathological analysis of tendons was performed using a semi-quantitative pathological scoring system, the Bonar scoring system (0-12, in direct proportion to the injury of tendons), as previously described (22).
Measurement of serum vitamin C. The vitamin C level in the serum was measured with high-performance liquid chromatography-electrochemical detection method as previously described (23). Blood samples from the sacrificed mice were centrifuged at 3,000 × g for 10 min to collect the serum. The serum was carefully separated and 100 μl of serum was mixed with 450 μl of 3% metaphosphoric acid. After centrifugation at 10,000 × g for 10 min, the supernatant was isolated and the serum vitamin C level was measured with a Shodex-5SIL-4E column (4.6×250 mm; Showa Denko, Tokyo).
Statistical analysis. All values are presented as the mean±S.E.M. Statistical analyses were performed using Student’s t-test. The value of statistical significance was set at p<0.01.
Results
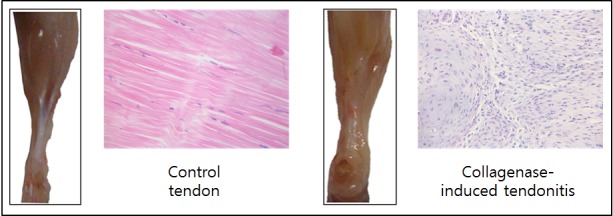
Establishment of mouse collagenase-induced tendonitis model. In gross morphology, collagenase-treated tendons showed swelling and an abundant secretion of mucus compared with the control tendons. In the microscopic lesions, regular collagen fibers, normal tenocytes (elongated spindle-shaped nuclei with scarce cytoplasm), and low cell density were observed in the control tendons. However, collagenase-treated tendons exhibited a complete loss of architecture, irregular fibers, infiltration of inflammatory cells, abnormal tenocytes (round nuclei with an abundant cytoplasm and lacuna formation), and neovascularization (Figure 2).
Figure 2. Establishment of mouse collagenase-induced tendonitis model. Control tendons exhibited histological features of normal tendons, but tendons in collagenase-induced tendonitis exhibited histopathological lesions of tendonitis (loss of architecture, irregular fibers, infiltration of inflammatory cells, abnormal tenocytes with round nuclei and an abundant cytoplasm, and lacuna formation) Gross view: Frontal; histopathology: Hematoxylin and eosin staining, magnification ×400.
Gross morphology of tendons from experimental groups. The gross morphological features of tendons are shown in Figure 3. The vitamin C-depletion groups showed more neovascularization, swelling, and mucus secretion than the vitamin C-treated groups Additionally, better tendon regeneration was observed in the group treated with vitamin C and ASCs than in the other groups.
Figure 3. Gross and microscopic features of tendons. Gross view: The vitamin C depletion groups exhibited more neovascularization, swelling, and mucus secretion than the groups supplied with vitamin C. Histopathology: The control group exhibited worsened lesions, indicated by separation of fibers with loss of bundle demarcation, high cell density, tenocytes with round nuclei, and infiltration of inflammatory cells. Tenocytes with round nuclei and immature collagen fibers were observed in the group treated with vitamin C, but demarcated bundles were maintained. The group treated with adipose-derived stem cells (ASCs) exhibited local tendon healing (upper part). The combination group exhibited nearly normal tendon features, such as well-demarcated collagen bundles, elongated tenocytes, mature collagen fibers, and low cell density. Gross view: Front and lateral views; histopathology: Hematoxylin and eosin staining, magnification ×100.
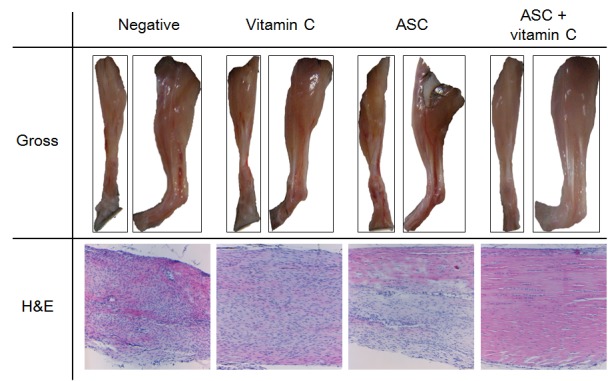
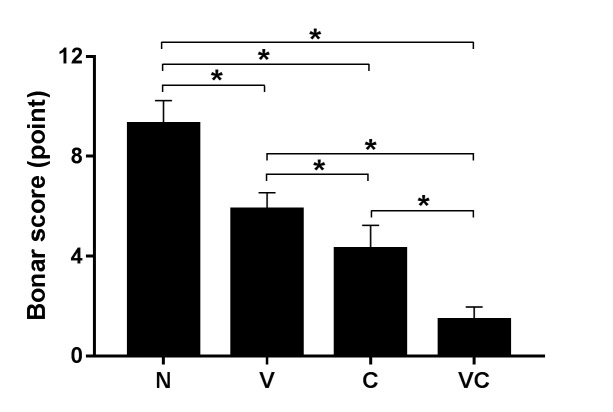
Microscopic features of tendons from experimental groups. The histopathological features of tendons are shown in Figure 3. The control group displayed a separation of fibers with a loss of bundle demarcation, high cell density, tenocytes with round nuclei, and infiltration of inflammatory cells. The group treated with vitamin C alone also exhibited tenocytes with round nuclei and immature collagen fibers, but demarcated bundles were maintained. The group treated with ASCs alone showed partial healing (upper part) of the tendons, and the group treated with ASCs and vitamin C exhibited normal tendon features (well-demarcated collagen bundles, elongated tenocytes, mature collagen fibers, and low cell density). The histopathological count scores by the Bonar scoring system are shown in Figure 4.
Figure 4. Bonar score: Semi-quantitative tendon pathological scoring system was used to assess tendons in all groups were evaluated for the degree of histopathological change using the Bonar scoring system: 0, normal state, to 12, worst state). N: Negative (control), V: vitamin C supplementation, C: adipose-derived stem cells (ASCs), and VC: vitamin C supplementation and ASCs transplantation. Data are shown as the mean±SD (*p<0.01).
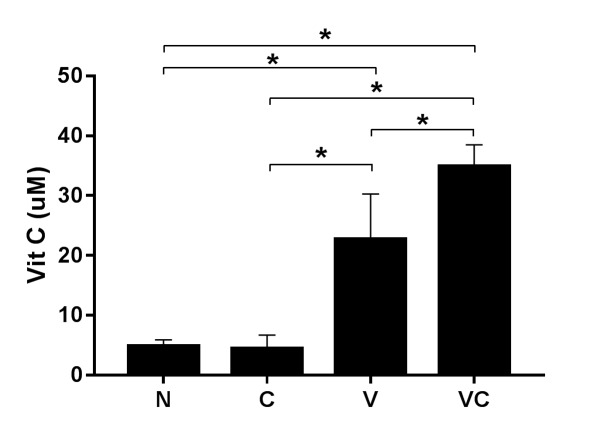
Serum vitamin C level. The vitamin C-treated groups had higher vitamin C levels in the serum than the vitamin C depletion groups (Control=4.76 μM, ASCS only=4.35 μM). The group treated with of combination vitamin C and ASCs had a higher serum level of vitamin C (34.85 μM) than the group treated with vitamin C alone (22.69 μM). A graph of the data is shown in Figure 5.
Figure 5. Vitamin C levels in serum. Groups supplied with vitamin C exhibited higher vitamin C levels in the serum than groups with limited vitamin C supply. Despite the equal supplementation of vitamin C, the combination group had higher vitamin C levels than did the group supplemented with vitamin C alone. N: Negative control, C: adiposederived stem cells (ASCs) transplantation, V: vitamin C supplementation, and VC: vitamin C supplementation and ASC transplantation. Data are shown as the mean±SD (*p<0.01).
Discussion
Stem cells can be used to repair tissues and treat diseases based on their self-renewal and multi-lineage cell differentiation abilities. There has been a wide range of reports on the application of adult stem cell therapy to various diseases (24-27), and many studies have been conducted on tendinopathy stem cell therapy in various species (e.g. horse, rabbit, rat) (28-30). However, there has been no study on co-administration of stem cell therapy with a particular compound (e.g. vitamin C or losartan) known to improve the stem cell niche in tendinopathy (20,31). The aim of this study was to determine the combined effects of stem cell therapy and vitamin C in the treatment of tendinopathy.
In this study, we induced a tendonitis model in Smp30-knockout mice and treated them with a combination of vitamin C and local transplantation of ASCs. We investigated the therapeutic effect of ASC transplantation for tendon healing, and we used these vitamin C-deficient mice to examine the effects of vitamin C in tendon healing in combination with stem cell transplantation.
Animal models for tendonitis have been developed for various species (e.g. horse, rabbit, rat) using different methods (e.g. mechanical overloading, chemical induction) (32). The mouse tendonitis model is rare because mice are too small and are not suitable for the tendonitis model. However, genetically modified models are required for studying the mechanism of tendonitis, causing an increased demand for the establishment of a mouse tendonitis model. In this study, we established a collagenase-induced mouse tendonitis model showing pathological features similar to those of tendonitis. The mouse tendonitis model might be presented as a basis for various studies on the mechanism of tendonitis.
Grossly, the groups supplied with vitamin C exhibited better morphological recovery than the groups that were subjected to vitamin C depletion). In particular, less neovascularization, swelling, and mucus secretion were observed. Vitamin C plays a crucial role in the hydroxylation of proline, which is a precursor of collagen synthesis (33). Collagen is a prominent structural protein found in connective tissues such as tendons, ligaments, and blood vessels (33) and is essential for the reconstruction of damaged tissues. The differences in gross morphology indicate that vitamin C plays a crucial role in tendon healing by aiding the reconstruction of damaged tissue.
Histopathologically, the group treated with ASC and vitamin C showed the best healing and the control group showed the worst. Comparison of the group treated with vitamin C with the control group showed that vitamin C accelerated the healing process in tendonitis. This result was also shown in a previous study (18). On comparing the group treated with ASCs alone with the N group, the former was found to have local healing in the upper region of the tendon, which might be a result of the high density fiber bundle in the tendon structure (34). Further studies on cell transplantation methodologies in local tendons would be required for wide application involving stem cells. Comparison of the combination group with the single therapy groups showed that the co-administration of ASC and vitamin C yielded more beneficial effects than the administration of each separately. The results indicated that vitamin C increased the effect of stem cells in tendon healing. In addition, a previous study reported that vitamin C supported cell transplantation by preventing oxidative stress (20).
Because Smp30-knockout mice cannot synthesize vitamin C naturally (21), the serum vitamin C level in the vitamin C-depleted groups was low, but groups supplied with vitamin C had higher vitamin C levels. Comparison of the combination group with the vitamin C-treated group showed that although both groups were equally supplied with vitamin C, the group treated with vitamin C had a lower vitamin C level than did the combination group. This result indicates that the vitamin C-treated group used more vitamin C as a precursor to collagen synthesis, whereas vitamin C was in excess in the combination group because of the added effect of ASCs on tendon healing. Thus, the co-administration of stem cell therapy and vitamin C is more effective in tendonitis than the administration of each separately.
In conclusion, this study shows that vitamin C improved the effect of ASC transplantation for the treatment of tendonitis by inducing a better stem cell niche. These results might provide a basis for clinical applications of such therapy.
Acknowledgements
This research was supported by the Bio-industry Technology Development Program, Korea Institute of Planning & Evaluation for Technology in Food, Agriculture, Forestry & Fisheries (312062-5).
References
- 1.Benjamin M, Ralphs JR. Functional and developmental anatomy of tendons and ligaments. In: Repetitive Motion Disorders of the Upper Extremity. Gordon SL, Blair SJ and Fine LJ (eds.) Rosemont, . 1995 IL, American Academy of Orthopaedic Surgeons:pp. 185–203. [Google Scholar]
- 2.Ames PRJ, Longo UG, Denaro V, Maffulli N. Achilles tendon problems: not just an orthopaedic issue. Disabil Rehabil. 2008;30(20-22):1646–1650. doi: 10.1080/09638280701785882. [DOI] [PubMed] [Google Scholar]
- 3.Herring SA, Nilson KL. Introduction to overuse injuries. Clin Sports Med. 1987;6(2):225. [PubMed] [Google Scholar]
- 4.Longo UG, Rittweger J, Garau G, Radonic B, Gutwasser C, Gilliver SF, Kusy K, Zieliński J, Felsenberg D, Maffulli N. No influence of age, gender, weight, height, and impact profile in achilles tendinopathy in masters track and field athletes. Am J Sports Med. 2009;37(7):1400–1405. doi: 10.1177/0363546509332250. [DOI] [PubMed] [Google Scholar]
- 5.Maffulli N. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14(8):840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 6.Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006;45(5):508–521. doi: 10.1093/rheumatology/kel046. [DOI] [PubMed] [Google Scholar]
- 7.Magnan B, Bondi M, Pierantoni S, Samaila E. The pathogenesis of Achilles tendinopathy: a systematic review. Foot Ankle Surg. 2014;20(3):154–159. doi: 10.1016/j.fas.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Boudreault J, Desmeules F, Roy J, Dionne C, Frmont P, MacDermid JC. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46(4):294–306. doi: 10.2340/16501977-1800. [DOI] [PubMed] [Google Scholar]
- 9.Cumpston M, Johnston RV, Wengier L, Buchbinder R. Topical glyceryl trinitrate for rotator cuff disease. Cochrane Database Syst Rev. 2009;8:3. doi: 10.1002/14651858.CD006355.pub2. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge S, de Vos R, Van Schie HT, Verhaar JA, Weir A, Tol JL. One-year follow-up of a randomised controlled trial on added splinting to eccentric exercises in chronic midportion Achilles tendinopathy. Br J Sports Med. 2008;44:673–677. doi: 10.1136/bjsm.2008.052142. [DOI] [PubMed] [Google Scholar]
- 11.Seil R, Wilmes P, Nhrenbrger C. Extracorporeal shock wave therapy for tendinopathies. Expert Rev Med Devices. 2006;3(4):463–470. doi: 10.1586/17434440.3.4.463. [DOI] [PubMed] [Google Scholar]
- 12.Smith RK. Mesenchymal stem cell therapy for equine tendinopathy. Disabil Rehabil. 2008;30(20-22):1752–1758. doi: 10.1080/09638280701788241. [DOI] [PubMed] [Google Scholar]
- 13.Smith R, Webbon PM. Harnessing the stem cell for the treatment of tendon injuries: heralding a new dawn. Br J Sports Med. 2005;39(9):582–584. doi: 10.1136/bjsm.2005.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young M. Stem cell applications in tendon disorders: a clinical perspective. Stem Cells Int. 2012;2012:637836. doi: 10.1155/2012/637836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uysal AC, Mizuno H. Differentiation of adipose-derived stem cells for tendon repair. Methods Mol Bio. 2011;702:443–451. doi: 10.1007/978-1-61737-960-4_32. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu I, Wang JH, Iwasaki K, Shimizu T, Okano T. The effect of tendon stem/progenitor cell (TSC) sheet on the early tendon healing in a rat Achilles tendon injury model. Acta Biomater. 2016;42:136–146. doi: 10.1016/j.actbio.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee J, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 18.Ömeroğlu S, Peker T, Türközkan N, Ömeroğlu H. High-dose vitamin C supplementation accelerates the Achilles tendon healing in healthy rats. Arch Orthop Trauma Surg. 2009;129(2):281–286. doi: 10.1007/s00402-008-0603-0. [DOI] [PubMed] [Google Scholar]
- 19.Roach HI, Hillier K, Shearer JR. Ascorbic acid requirements for collagen synthesis (proline hydroxylation) during long-term culture of embryonic chick femurs. Biochim Biophys Acta. 1985;842(2-3):139–145. doi: 10.1016/0304-4165(85)90195-3. [DOI] [PubMed] [Google Scholar]
- 20.Kim A, Lee E, Lee E, Min C, Kang K, Park J, Hong I, Ishigami A, Tremblay J, Jeong K. Effects of vitamin C on cytotherapy-mediated muscle regeneration. Cell Transplant. 2013;22(10):1845–1858. doi: 10.3727/096368912X657846. [DOI] [PubMed] [Google Scholar]
- 21.Ishigami A, Fujita T, Handa S, Shirasawa T, Koseki H, Kitamura T, Enomoto N, Sato N, Shimosawa T, Maruyama N. Senescence marker protein-30 knockout mouse liver is highly susceptible to tumor necrosis factor-α-and Fas-mediated apoptosis. The Am J Pathol. 2002;161(4):1273–1281. doi: 10.1016/s0002-9440(10)64404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes' patellar tendons. J Orthop Res. 2004;22(2):334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Kondo Y, Sasaki T, Sato Y, Amano A, Aizawa S, Iwama M, Handa S, Shimada N, Fukuda M, Akita M, Lee J, Jeong K, Maruyama N, Ishigami A. Vitamin C depletion increases superoxide generation in brains of Smp30/Gnl knockout mice. Biochem Biophys Res Commun. 2008;377(1):291–296. doi: 10.1016/j.bbrc.2008.09.132. [DOI] [PubMed] [Google Scholar]
- 24.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116(8):1413–1430. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariano ED, Teixeira MJ, Marie SKN, Lepski G. Adult stem cells in neural repair: Current options, limitations and perspectives. World J Stem Cells. 2015;7(2):477–482. doi: 10.4252/wjsc.v7.i2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardo E, van der Poll T, DelaRosa O, Dalemans W. Mesenchymal stem cells as a therapeutic tool to treat sepsis. World J Stem Cells. 2015;7(2):368–379. doi: 10.4252/wjsc.v7.i2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zigdon-Giladi H, Rudich U, Michaeli Geller G, Evron A. Recent advances in bone regeneration using adult stem cells. World J Stem Cells. 2015;7(3):630–640. doi: 10.4252/wjsc.v7.i3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 2008;69(7):928–937. doi: 10.2460/ajvr.69.7.928. [DOI] [PubMed] [Google Scholar]
- 29.Nourissat G, Diop A, Maurel N, Salvat C, Dumont S, Pigenet A, Gosset M, Houard X, Berenbaum F. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PloS one. 2010;5(8):e12248. doi: 10.1371/journal.pone.0012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg. 2012;65(12):1712–1719. doi: 10.1016/j.bjps.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Lee E, Kim A, Lee E, Park J, Lee M, Hwang M, Kim C, Kim S, Jeong K. Therapeutic effects of mouse adipose-derived stem cells and losartan in the skeletal muscle of injured mdx mice. Cell Transplant. 2015;24(5):939–953. doi: 10.3727/096368914X678599. [DOI] [PubMed] [Google Scholar]
- 32.Lui P, Maffulli N, Rolf C, Smith R. What are the validated animal models for tendinopathy. Scand J Med Sci Sports. 2011;21(1):3–17. doi: 10.1111/j.1600-0838.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- 33.Parsons KK, Maeda N, Yamauchi M, Banes AJ, Koller BH. Ascorbic acid-independent synthesis of collagen in mice. Am J Physiol Endocrinol Metab. 2006;290(6):E1131–1139. doi: 10.1152/ajpendo.00339.2005. [DOI] [PubMed] [Google Scholar]
- 34.Andarawis-Puri N, Flatow EL, Soslowsky LJ. Tendon basic science: Development, repair, regeneration, and healing. J Orthop Res. 2015;33(6):780–784. doi: 10.1002/jor.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]