Abstract
Aim: To evaluate the accuracy of multiparametric magnetic resonance imaging apparent diffusion coefficient (mpMRI ADC) in the diagnosis of clinically significant prostate cancer (PCa). Patients and Methods: From January 2016 to December 2016, 44 patients who underwent radical prostatectomy for PCa and mpMRI lesions suggestive of cancer were retrospectively evaluated at definitive specimen. The accuracy of suspicious mpMRI prostate imaging reporting and data system (PI-RADS ≥3) vs. ADC values in the diagnosis of Gleason score ≥7 was evaluated. Results: Receiver operating characteristics (ROC) curve analysis gave back an ADC threshold of 0.747×10–3 mm2/s to separate between Gleason Score 6 and ≥7. The diagnostic accuracy of ADC value (cut-off 0.747×10–3 mm2/s) vs. PI-RADS score ≥3 in diagnosing PCa with Gleason score ≥7 was equal to 84% vs. 63.6% with an area under the curve (AUC) ROC of 0.81 vs. 0.71, respectively. Conclusion: ADC evaluation could support clinicians in decision making of patients with PI-RADS score <3 at risk for PCa.
Keywords: Multiparametric MRI and prostate cancer, apparent diffusion coefficient (ADC), diffusion-weighted imaging (DWI)
The advent of multiparametric magnetic resonance imaging (mpMRI) scanners combined with the international score Prostate Imaging Reporting and Data System (PI-RADS) (1) has improved the diagnosis of clinically significant prostate cancer (PCa). Maps of the apparent diffusion coefficient (ADC), computed from diffusion-weighted imaging (DWI), provide a quantitative parameter to evaluate prostate regions with suspicion of PCa. In neoplastic tissue, ADC values decrease from the one measured in normal prostatic tissue and some studies have demonstrated a possible differentiation in PCa grading, according to Gleason score (GS) classification (2-9). Currently, there are no defined thresholds that have been accepted by the radiological scientific community to discriminate healthy and diseased patients as well as to differentiate PCa grading. Thus, the purpose of our study is to analyze DWI and the corresponding ADC maps in predicting definitive GS in men submitted to radical prostatectomy for PCa.
Patients and Methods
From January 2016 to December 2016, 44 men with median prostate-specific antigen (PSA) of 7.3 ng/ml (range=4.2-18), clinical stage T1c and median GS equal to 6.8 (range=6-9) underwent radical retropubic prostatectomy (RRP) for biopsy of clinically significant PCa (GS ≥6 and/or more than 2 positive cores and/or a greatest percentage of cancer for each core >50%) (10); 16/44 (36.6%) patients previously enrolled in Active Surveillance (AS) program were upgraded at confirmatory biopsy and 28/44 (63.4%) men underwent repeat saturation biopsy (median=30 cores; range=28-34 cores) for persistent suspicious cancer. Previously, all patients about 10 days before prostate biopsy underwent pelvic mpMRI and two radiologists blinded to pre-imaging clinical parameters evaluated the mpMRI data separately and independently. In the presence of mpMRI lesions suggestive of cancer (PI-RADS score ≥3) (11) mpMRI/transrectal ultrasonography (TRUS) transperineal fusion guided-biopsies (12) were added to transperineal saturation biopsy using a Hitachi 70 Arietta ecograph (Hitachi Medico, Chiba, Japan) (13). All analyzed mpMRI images were acquired using a 3.0 Tesla Achieva Philips MRI scanner (Philips Medical Systems, Eindhoven, the Netherlands); the scanner was characterized by gradients of amplitude of 80 mT/m and a maximum slew rate of 200 mT/m/s. For image acquisition, a pelvic coil was used; model SENSE XL Torso 16, phased-array with 16 elements. A quality assurance protocol ensured the scanner performance, including specific quality controls focused on DWI images. For each patient, the examination protocol included: T2-weighted images, dynamic contrast-enhanced (DCE) perfusion, DWI sequences and a multi-voxel magnetic resonance spectroscopic imaging (MRSI) of the prostate (14). Two radiologists (AG, GP), without any knowledge about biopsy results, retrospectively analyzed the DWI images and the ADC maps. Radiologists considered positive DWI at least one lesion found; a more specific ADC evaluation was performed subdividing PCa per Gleason score grading. Finally, the accuracy of PI-RADS score vs. ADC value in diagnosing clinically significant PCa was compared. Prostatectomy specimens were processed as follows: after inking the specimen, the apical and basal parts were removed by a transversal cut at 4-mm from the distal and proximal margins, respectively. The apical and proximal parts were sectioned parasagittally at 4-mm intervals and perpendicularly to the inked surface. The specimen was step-sectioned at 4-mm intervals perpendicularly to the apical-basal axis of the gland. The volume of cancer was reported as the percentage of cancer in the entire specimen according to Bostwick et al. (15); each case was analyzed independently by two dedicated pathologists (FF, AG).
A receiver operating characteristic (ROC) curve was calculated to evaluate the diagnostic performance of ADC values in the differentiation of prostate cancer with a GS of 6 vs. GS ≥7, as well as to determine the ADC cut-off level that provided the highest diagnostic performance.
Results
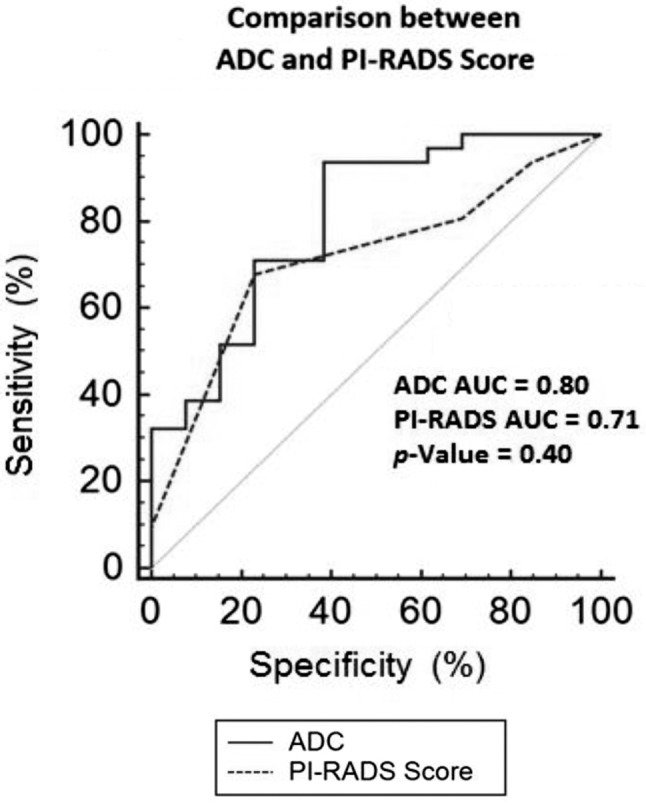
All patients had a clinically significant PCa (15) at definitive specimen (GS >7 and/or cancer volume >0.5 ml): 21 pT2CN0, 19 pT3aN0, 2 pT3bN0 and 2 pT3bN1, respectively; moreover, the GS (median=7; range=6-9) was upgraded in 3/44 (6.8%) cases and 10/44 (22.4%) men had positive surgical margins. Multiparametric MRI showed a PI-RADS score <3 vs. 3 vs. >3 in 13 (29.5%) vs. 9 (20.5%) vs. 22 (50%) cases, respectively (Table I); mean ADC value was equal to 0.639±0.146×10–3 mm2/s (range=0.367-1.032) and significantly decreased in the presence of GS >8 (Table I). The presence of GS equal to 7 (3+4) was correlated with an ADC value of 0.747×10–3 mm2/s, with the threshold being obtained from ROC curve analysis (Figure 1); the diagnostic accuracy sensitivity, specificity, positive predictive value and negative predictive value of ADC (cut-off=0.747) vs. PI-RADS score ≥3 in the diagnosis of GS ≥7 were equal to 84 vs. 63.6%, 93.5 vs. 77.4%, 61.5 vs. 30.7%, 85.2 vs. 77.5%, 80.0 vs. 36.3%, respectively. The AUC ROC of ADC (cut-off=0.747×10–3) vs. PI-RADS score ≥3 in diagnosing GS ≥7 PCa is reported in Figure 1.
Table I. PI-RADS score, ADC value and GS in 44 men submitted to retropubic radical prostatectomy for prostate cancer.
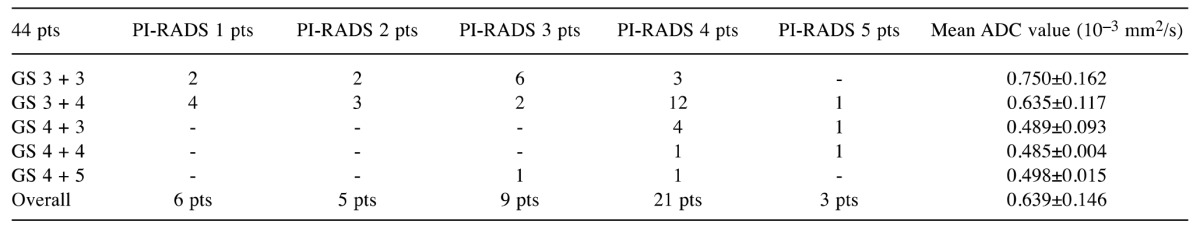
PI-RADS, Prostate imaging reporting and data system; pts, patients; GS, Gleason score; ADC, apparent diffusion coefficient
Figure 1. ROC curve of ADC (cut-off=0.747×10–3mm2/s) vs. PI-RADS score ≥3 in the diagnosis of Gleason score ≥7 in radical prostatectomy (definitive specimen). ADC, Apparent diffusion coefficient; PI-RADS, prostate imaging reporting and data system; AUC ROC, area under the curve receiver operating characteristics.
Discussion
The decrease in ADC value in high GS disease can be explained by the high level of tumor cellularity, which is representative of tumor aggressiveness; indeed, tumor cellularity is one of the major determinants for tumor grade of prostate cancer and an inverse relationship between tumor cellularity and ADC value has been previously reported (2-9). In detail, DWI is the only functional imaging technique that gives information about cellular microstructure and changes in neoplastic tissue that may be pointed out by DWI, reporting more restricted movement of water characterized by decreased ADC values. ADC could help clinicians to stratify patients throughout tumor aggressiveness becoming a biomarker in separating men with clinically insignificant PCa (i.e., men enrolled in AS protocols) from those with aggressiveness of PCa understaged by prostate biopsy. Hambrock et al. (3) evaluated prostate cancer aggressiveness using a 3.0 T MRI with DWI, reporting that ADC values had a high discriminatory performance in the differentiation of low- vs. intermediate vs. high-grade PCa. Wu et al. (8) showed that higher ADC values (0.830×10–3 mm2/s) were significantly associated with low-risk prostate cancer (GS 6 disease). Recently, Kim et al. (2) reported a mean ADC value for disease with a GS of 7 equal to 0.741±0.164×10–3 mm2/s; moreover, Salami et al. (16) showed that larger prostate cancers were associated with lower ADC values. Finally, Yoon et al. (17) and Kido et al. (18) demonstrated that ADC was significantly associated with biochemical-free recurrence and extracapsular PCa extension, respectively.
In our study, we reported the relationships between DWI vs. prostate biopsy and definitive specimen in men submitted to RRP for PCa; an ADC value of 0.747×10–3 mm2/s distinguished between Gleason score 6 vs. ≥7 showing a significant correlation with aggressiveness of PCa (Table I); the ADC value of 0.747×10–3 mm2/s allowed to detect 7/44 (16%) PCa with GS of 7 (3 + 4) harbored using only the PI-RADS system (score <3). In addition, an ADC value of 0.747×10–3 mm2/s vs. PI-RADS ≥3 showed a diagnostic accuracy in the diagnosis of GS ≥7 equal to 84 vs. 63.6% with an AUC ROC of 0.81 vs. 0.71, respectively. Finally, ADC evaluation could help to reduce the false-negative rate of mpMRI (PI-RADS <3) for clinically significant PCa.
Regarding our preliminary results, some considerations should be made. First, a greater number of patients should be prospectively evaluated. Second, although mpMRI is strongly recommended in men candidates to repeat biopsy, still today, standard prostate biopsy (extended or saturation procedure) should be always combined with mMRI/TRUS fusion biopsy because of mpMRI’s false-negative rate (15-20% of PCa with low volume and Gleason score ≥7) (19,20). Finally, the reproducibility of ADC values should be evaluated performing multicentric studies and external validation using different mpMRI devices.
In conclusion, in our experience, an ADC value of 0.747×10–3mm2/s was significantly correlated with the presence of aggressive cancer (GS ≥7) diagnosing about 16% of clinically significant PCa with PI-RADS score <3.
References
- 1.Barentsz JO, Weinreb JC, Verma S, Thoeny HC, Tempany CM, Shtern F, Padhani AR, Margolis D, Macura KJ, Haider MA, Cornud F, Choyke PL. Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol. 2016;69:41–49. doi: 10.1016/j.eururo.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim TH, Kim CK, Park BK, Jeon HG, Jeong BC, Seo SI, Lee HM, Choi HY, Jeon SS. Relationship between Gleason score and apparent diffusion coefficients of diffusion-weighted magnetic resonance imaging in prostate cancer patients. Can Urol Assoc J. 2016;10(11-12):E377–E382. doi: 10.5489/cuaj.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, Scheenen T, Barentsz JO. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259:453–461. doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]
- 4.Nagarajan R, Margolis D, Raman S, Sheng K, King C, Reiter R, Thomas MA. Correlation of Gleason scores with diffusion-weighted imaging findings of prostate cancer. Adv Urol. 2012;2012:374805. doi: 10.1155/2012/374805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glazer DI, Hassanzadeh E, Fedorov A, Olubiyi OI, Goldberger SS, Penzkofer T, Flood TA, Masry P, Mulkern RV, Hirsch MS, Tempany CM, Fennessy FM. Diffusion-weighted endorectal MR imaging at 3T for prostate cancer: Correlation with tumor cell density and percentage Gleason pattern on whole mount pathology. Abdom Radiol. 2017;42(3):918–925. doi: 10.1007/s00261-016-0942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim TH, Jeong JY, Lee SW, Kim CK, Park BK, Sung HH, Jeon HG, Jeong BC, Seo SI, Lee HM, Choi HY, Jeon SS. Diffusion-weighted magnetic resonance imaging for prediction of insignificant prostate cancer in potential candidates for active surveillance. Eur Radiol. 2015;25:1786–1792. doi: 10.1007/s00330-014-3566-2. [DOI] [PubMed] [Google Scholar]
- 7.Shaish H, Kang SK, Rosenkrantz AB. The utility of quantitative ADC values for differentiating high-risk from low-risk prostate cancer: A systematic review and meta-analysis. Abdom Radiol (NY) 2017;42:260–270. doi: 10.1007/s00261-016-0848-y. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Reinikainen P, Vanhanen A, Kapanen M, Vierikko T, Ryymin P, Hyödynmaa S, Kellokumpu-Lehtinen PL. Correlation between apparent diffusion coefficient value on diffusion-weighted MR imaging and Gleason score in prostate cancer. Diagnostic Intl Imaging. 2017;98:63–71. doi: 10.1016/j.diii.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Waseda Y, Yoshida S, Takahara T, Kwee TC, Matsuoka Y, Saito K, Kihara K, Fujii Y. Utility of computed diffusion-weighted MRI for predicting aggressiveness of prostate cancer. J Magn Reson Imaging. 2017;1 doi: 10.1002/jmri.25593. [DOI] [PubMed] [Google Scholar]
- 10.Valerio M, Anele C, Bott SR, Charman SC, van der Meulen J, El-Mahallawi H, Emara AM, Freeman A, Jameson C, Hindley RG, Montgomery BS, Singh PB, Ahmed HU, Emberton M. The prevalence of clinically significant prostate cancer according to commonly used histological thresholds in men undergoing template prostate mapping bopsies. J Urol. 2016;195:1403–1408. doi: 10.1016/j.juro.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Hassanzadeh E, Glazer DI, Dunne RM, Fennessy FM, Harisinghani MG, Tempany CM. Prostate imaging reporting and data system version 2 (PI-RADS v2): A pictorial review. Abdom Radiol (NY) 2017;42:278–289. doi: 10.1007/s00261-016-0871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepe P, Aragona F. Saturation prostate needle biopsy and prostate cancer detection at initial and repeat evaluation. Urology. 2007;70:1131–1135. doi: 10.1016/j.urology.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 13.Pepe P, Garufi A, Priolo G, Pennisi M. Transperineal versus transrectal MRI/TRUS fusion-targeted biopsy: Detection rate of clinically significant prostate cancer. Clin Genitourin Cancer. 2017;15(1):e33–e36. doi: 10.1016/j.clgc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Pepe P, Garufi A, Priolo G, Candiano G, Pietropaolo F, Pennisi M, Fraggetta F, Aragona F. Prostate cancer detection at repeat biopsy: Can pelvic phased-array multiparametric MRI replace saturation biopsy. Anticancer Res. 2013;33:1195–1199. [PubMed] [Google Scholar]
- 15.Bostwick DG, Grignon DJ, Hammond ME, Amin MB, Cohen M, Crawford D, Gospadarowicz M, Kaplan RS, Miller DS, Montironi R, Pajak TF, Pollack A, Srigley JR, Yarbro JW. Prognostic factors in prostate cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:995–1000. doi: 10.5858/2000-124-0995-PFIPC. [DOI] [PubMed] [Google Scholar]
- 16.Salami SS, Ben-Levi E, Yaskiv O, Turkbey B, Villani R, Rastinehad AR. Risk stratification of prostate cancer utilizing apparent diffusion coefficient value and lesion volume on multiparametric MRI. J Magn Reson Imaging. 2017;45:610–616. doi: 10.1002/jmri.25363. [DOI] [PubMed] [Google Scholar]
- 17.Yoon MY, Park J, Cho JY, Jeong CW, Ku JH, Kim HH, Kwak C. Predicting biochemical recurrence in patients with high-risk prostate cancer using the apparent diffusion coefficient of magnetic resonance imaging. Investig Clin Urol. 2017;58:12–19. doi: 10.4111/icu.2017.58.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kido A, Tamada T, Sone T, Kanomata N, Miyaji Y, Yamamoto A, Ito K. Incremental value of high b value diffusion-weighted magnetic resonance imaging at 3-T for prediction of extracapsular extension in patients with prostate cancer: Preliminary experience. Radiol Med. 2017;122:228–238. doi: 10.1007/s11547-016-0712-8. [DOI] [PubMed] [Google Scholar]
- 19.Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, Reiter RE, Marks LS. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer. 2016;122:884–892. doi: 10.1002/cncr.29874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepe P, Garufi A, Priolo G, Pennisi M. Can MRI/TRUS fusion-targeted biopsy replace saturation prostate biopsy in the re-evaluation of men in active surveillance. World J Urol. 2016;34:1249–1253. doi: 10.1007/s00345-015-1749-3. [DOI] [PubMed] [Google Scholar]


