Abstract
Background: Psychosocial distress can be frequently observed in patients with sarcoma, depicting a relevant clinical problem. However, prospective data collection on psychosocial distress in patients with rare tumors is often time-consuming. In this context, social media such as Facebook can serve as a potential platform to expand research. The aim of this study was to assess the feasibility of psychosocial distress screening in patients with osteosarcoma and Ewing’s sarcoma via social media. Materials and Methods: For this study an online questionnaire including general information and self-assessment distress measurement tools for patients and parents was created. The link to the questionnaire was then posted on the main page of the two largest disease-specific Facebook communities on osteosarcoma and Ewing’s sarcoma. Results: Within 2 months, 28 patients and 58 parents of patients were enrolled. All patients with osteosarcoma and Ewing’s sarcoma, as well as the majority of parental caregivers of such patients, showed relevant psychosocial distress levels. Conclusion: Crowdsourcing via disease-specific patient communities on Facebook is feasible and provides great potential for acquisition of medical data of rare diseases.
Keywords: Psychosocial distress, psycho-oncology, crowdsourcing, Facebook, osteosarcoma, Ewing’s sarcoma
Psychosocial distress can be observed in 30-45% of all patients with cancer during the course of their disease, and is an important clinical problem (1-3). In particular, orthopedic patients with primary malignant bone tumors, such as osteosarcoma (OS) and Ewing’s sarcoma (ES), experience high levels of distress due to the malignancy of these tumors and often disabling surgical treatment (4-6). However, few patients with distress are detected early in the treatment process and offered adequate psychological support (7,8). This low detection rate may be explained by low correlations between the objective characteristics of the disease and the patient’s subjective perception (9,10). Furthermore, only 25% of patients discuss emotional concerns with their physician without being invited to do so (11).
International guidelines suggest routine psychosocial distress screening for all patients with cancer and provision of psychological support as needed (8,12). However, routine psychosocial distress screening is not an integral component of sarcoma treatment guidelines despite the high demand (6).
Most common screening tools are long questionnaires with multiple items, which are not generally accepted in clinical practice. Consequently, short forms of validated distress screening tools have been developed (2,7,8,13). A popular tool for self-assessment of psychosocial distress in daily life among patients with cancer is the Questionnaire on Stress in Cancer Patients (QSC-R23). This tool was originally developed in 1985 by Herschbach and colleagues, who demonstrated high reliability and validity in a sample of 1,645 patients with cancer (14,15). In order to increase clinical acceptance, Book et al. adapted a 10-item short form of the QSC-R23 (QSC-R10) (2).
Parents of these predominantly young patients also suffer from severe psychosocial distress, particularly fear of disease progression, as described by Schepper et al. (10) and Fidika et al. (16,17). These authors used the short form (12 items) of the Fear of Progression Questionnaire (FoP-Q-SF), another self-assessment tool, and modified it to capture the parental perspective (FoP-Q-SF/PR) (16-18).
A major problem in determining psychosocial distress in large cohorts of patients with OS or ES is their rarity, as they account for fewer than 1% of all cancers diagnosed in the United States per year (19). Prospective data collection on psychosocial distress in these patients is time-consuming and often limited to a single institution or single country in multicenter studies. In this context, social media such as Facebook are potential platforms to expand research (20). Specific topic-based groups bring together patients with rare diseases (e.g. sarcomas) from all over the world, providing unique potential for collecting medical data for large cohorts (crowdsourcing).
Therefore, we hypothesized that (i) social media platforms would facilitate data collection on rare diseases with inclusion of significantly more participants than those undergoing treatment in a single institution during the same time period, (ii) self-assessment questionnaires would be particularly suitable for crowdsourcing, and (iii) patients with OS or ES, as well as parents of such patients, would exhibit high levels of psychosocial distress.
Materials and Methods
Online questionnaire and social network. For this study, we developed an online questionnaire including general sociodemographic and medical information. The questionnaire was combined with previously published self-assessment distress measurement tools for patients and parents, as described below. The link to the questionnaire was posted along with detailed information about the purpose of the study and instructions for completing the questionnaire on the main page of the two largest specific topic-based Facebook communities. To evaluate patients with osteosarcoma and parental caregivers, we chose the Facebook group “Osteosarcoma–Survivors, Family, and Friends”, with 2649 current members. For Ewing’s sarcoma, we selected the group “Ewing’s Sarcoma Survivors,” with 1839 members. The survey was closed after 2 months (December 2014 and January 2015) and the collected data were analyzed.
General information. General information was subdivided by patients and parents. Items for patients included information on age, sex, tumor location, treatment, offered psychological support, and satisfaction with treatment and psychological support (if offered). For parents, only age and sex were collected.
QSC-R10. The QSC-R10 is a 10-item psycho_oncological screening instrument for self-assessment of distress, previously published by Book et al. (2). It is based on the QSC-R23 screening tool developed and validated by Herschbach and colleagues (15). Item selection for the QSC-R10 was based on intercorrelations and factor loadings.
Each item is scored from 0-5 points, with 0 points indicating no distress and 5 points indicating a high level of distress. The total score is calculated by summing the item scores. A value above 14 points indicates a relevant level of distress, possibly requiring psychooncological treatment (2).
FoP-Q-SF/PR. The FoP-Q-SF/PR comprises 12 items and is based on the FoP-Q-SF for partners, developed by Zimmermann et al. (18). The item content was appropriately modified to reflect the parents’ perspective.
Response categories range from 1 (never) to 5 (very often), resulting in a total score between 12 and 60 points. A value above 32 points indicates a relevant level of distress, potentially requiring psycho-oncological treatment.
Statistical analyses. Statistical analyses were performed with SPSS Statistics (IBM, Armonk, NY, USA). Descriptive statistics were calculated for sample characteristics in patients and parents. For each QSC-R10 and FoP-Q-SF/PR item, we calculated means and standard deviations as well as skewness and kurtosis. Internal consistency was assessed with Cronbach’s alpha. Results for OS and ES, patients and parents, were compared using a t-test for unpaired samples. Additionally, regression analysis was performed to evaluate the influence of age, gender or the presence of metastases on psychosocial distress levels. A p-value of 0.05 or less was considered statistically significant.
Results
Study sample. During the 2-month study period, data were collected for 28 patients with OS (n=14) and ES (n=14). We also collected data for 58 parents of patients (not necessarily parents of the included patients) (OS: 17; ES: 41), including 45 mothers and 13 fathers (Table I and Table II).
Table I. Sociodemographic and medical characteristics of the participants (patients with osteosarcoma and Ewing’s sarcoma) (n=28).
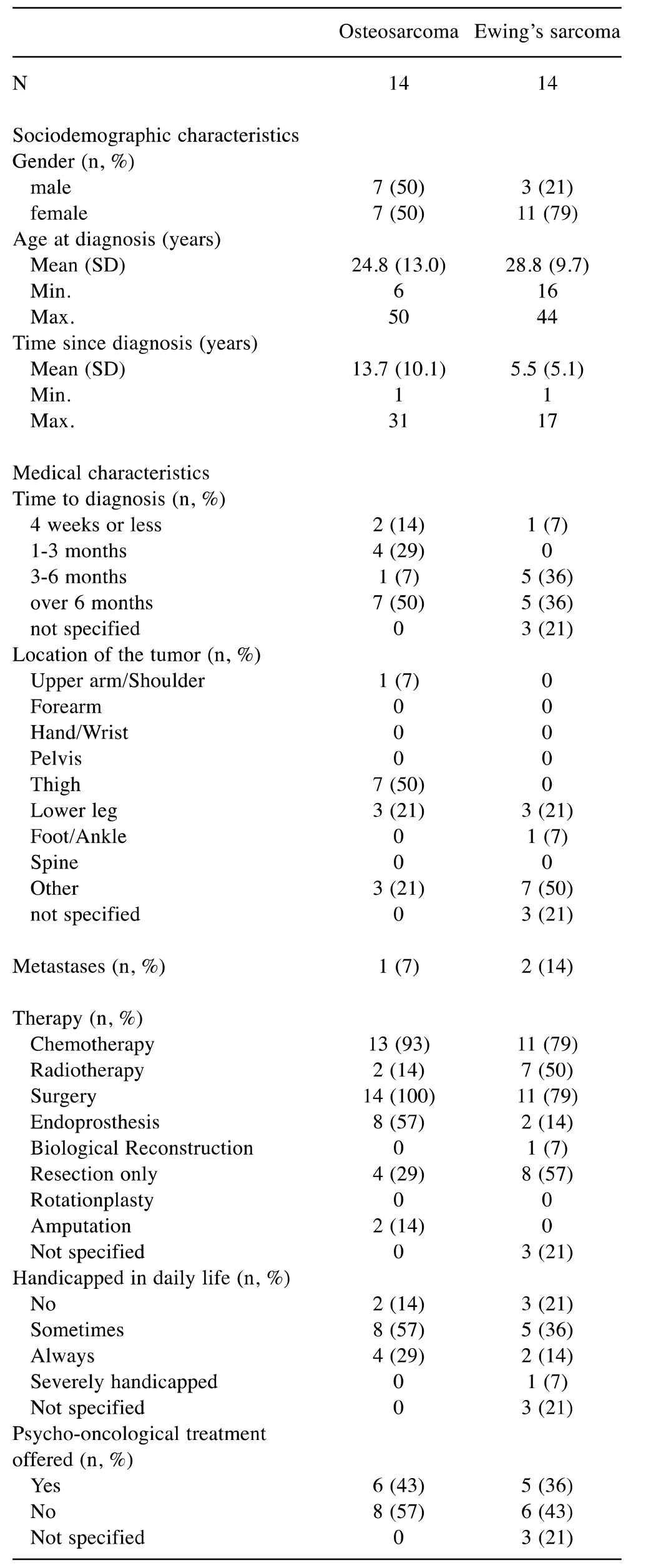
Table II. Sociodemographic characteristics of the participating parents of patients with osteosarcoma and Ewing’s sarcoma (n=58).
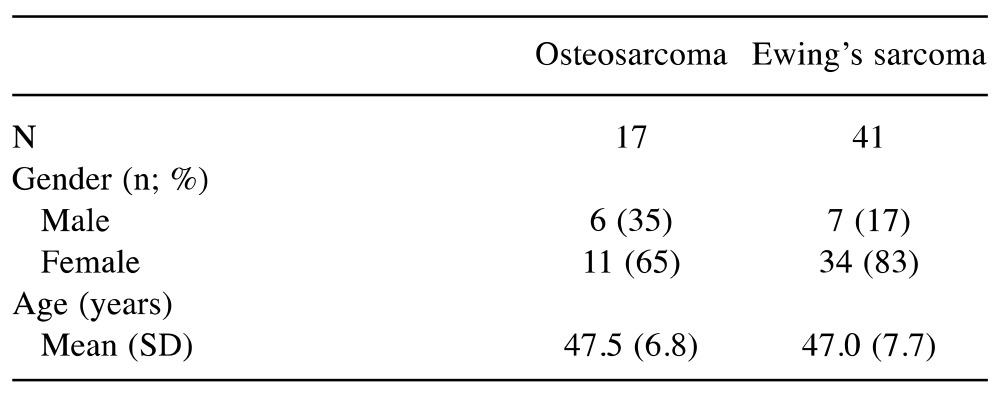
There was an equal sex distribution of patients with OS, whereas those with ES were mainly female (79%). The mean age at diagnosis was 24.8 (range=6-50; SD=13.0) years for patients with OS (median=21.5 years) and 28.8 (range=16-24; SD=9.7) years for those with ES (median=30 years). Time since initial diagnosis was 13.7 years for those with OS and 5.5 years for those with ES. In over 50% of all cases, the period from first symptoms to initial diagnosis was more than 3 months. In our sample, OS was predominately located around the thigh (50%) and lower leg (21%). ES mainly presented in the lower leg (21%) and in extraskeletal locations (50%). A majority of patients underwent chemotherapy and surgical tumor resection (OS=93%; ES=79%). Additional radiotherapy was applied in 50% of patients with ES. Occasional or permanent disability in daily life was indicated by 86% of patients with OS and 50% of those with ES. Psycho-oncological treatment was offered in less than 50% of all cases (OS=43%; ES=36%).
Parental caregivers of patients with OS had a mean age of 47.5 years and those of patients with ES had a mean age of 47.0 years. Parents were predominately mothers (OS=65%; ES=83%).
Internal consistency. Cronbach’s alpha was 0.77 (α=0.78 to 0.76 for OS and ES subsamples) for the QSC-R10 and 0.80 for the FoP-Q-SF/PR (α=0.77 to 0.82 for OS and ES subsamples) indicating a good reliability.
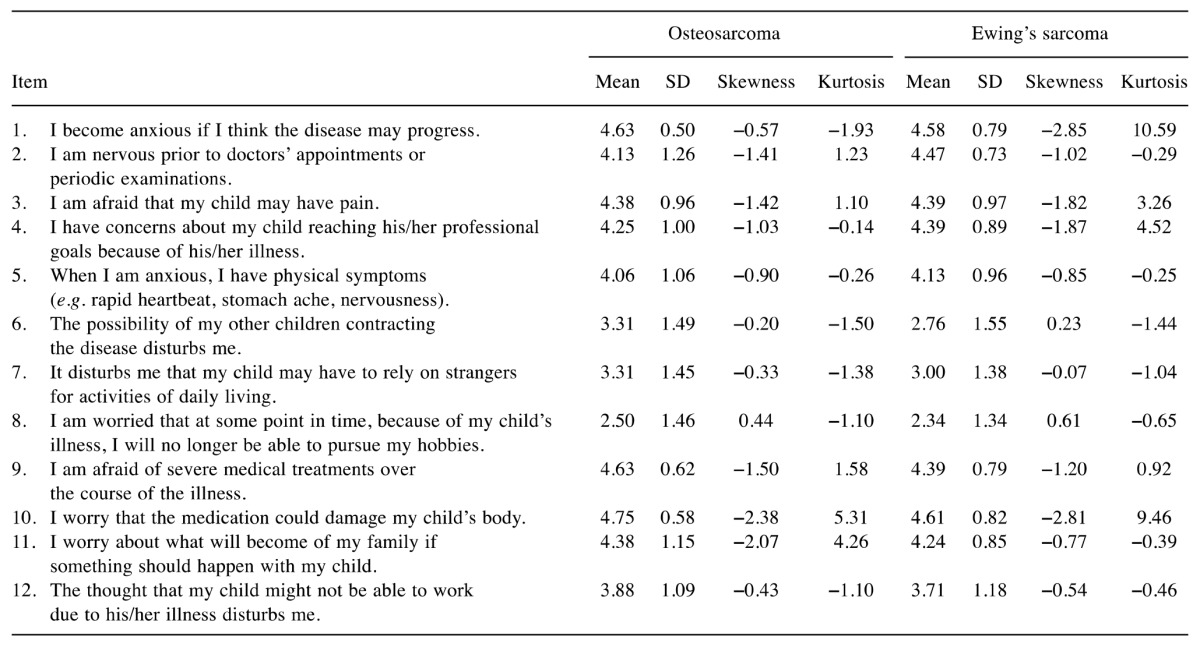
Item analyses. The item analysis of the QSC-R10 is presented in Table III. Mean scores for the items ranged from 1.54-4.69 in patients with OS and from 1.56-4.22 in patients with ES. The highest mean was found for item 8 “It is harder for me to take part in recreational activities (e.g. sports) now than it was before I became ill” and the lowest was for item 9 “I do not feel well informed about my disease/treatment” in both the OS and ES groups.
Table III. Item characteristics and component loading of the QSC-R10 questionnaire for osteosarcoma and Ewing’s sarcoma patients.

The item analysis of the FoP-Q-SF/PR is presented in Table IV. In the OS group, means for each item ranged from 2.50-4.75. The ES group showed values from 2.34-4.61. In both groups of parents, item 10 “I worry that the medication could damage my child’s body” had the highest mean, and item 8 “I am worried that at some point in time, because of my child’s illness, I will no longer be able to pursue my hobbies” had the lowest mean.
Table IV. Item characteristics and component loading of the FoP-Q-SF/parents questionnaire for parents of patients with osteosarcoma and Ewing’s sarcoma.
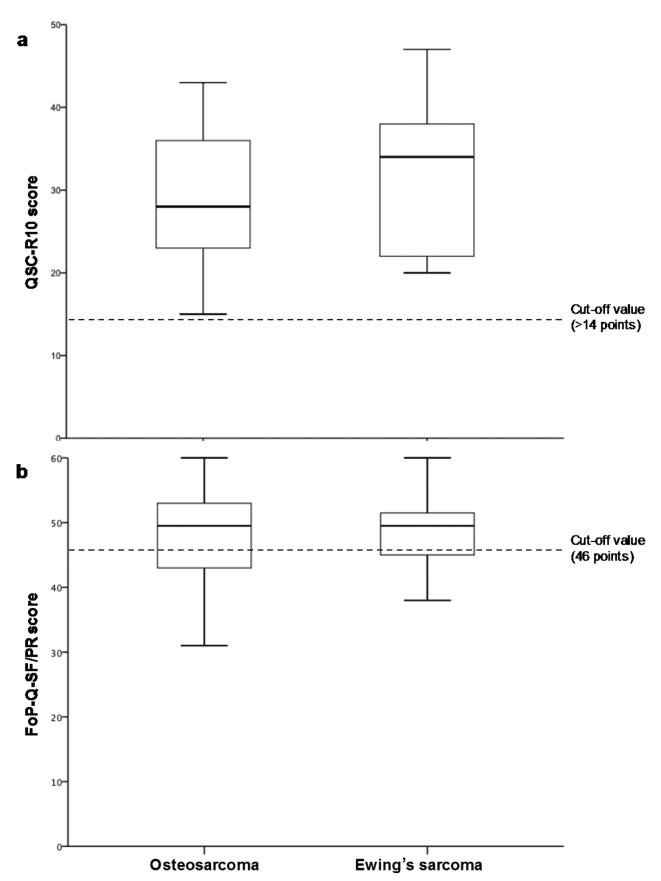
Psychosocial distress level. The QSC-R10 for the OS patient group had an overall mean of 29.46±9.41 (range=15-43). The ES group had a mean of 32.00±9.25 (range=21-47). All results for both groups were above the cut-off value of ≤14 points (Figure 1a). Psycho-oncological treatment was offered in 43% of OS cases and 36% of ES cases. No difference in distress level was identified between patients with OS and ES (p=0.54) nor between male and female patients with OS (p=0.20) or ES (p=0.53).
Figure 1. Psychosocial distress levels in patients with osteosarcoma (n=14) and Ewing’s sarcoma (n=14) according to the questionnaire on distress in cancer patients – short form (QSC-R10) (a) and in parents of patients with osteosarcoma (n=14) and Ewing’s sarcoma (n=14) according to the fear of progression questionnaire – short form (FoP-Q-SF/PR) (b).
Overall results for the FoP-Q-SF/PR showed a mean of 48.19±7.71 for the OS parent group and of 47.03±6.71 for the ES parent group. The lowest individual result for the OS parent group was 31 and for the ES parent group was 32, and a maximum of 60 was identified in both groups (cut-off value 34 points) (Figure 1b). In total, 93.8% of the OS parent group and 97.4% of the ES parent group were classified as having high fear of disease progression. Again, no difference in distress level was found between parents of patients with OS and those with ES (p=0.60), nor between mothers and fathers of patients with OS (p=0.78) or ES (p=0.35) patients.
No significant influence of age, gender or presence of metastases on psychosocial distress levels was found in the OS (r=−0.31, p=0.30; r=0.41, p=0.16; r=−0.46, p=0.11, respectively) and ES (r=0.38, p=0.32; r=−0.28, p=0.47; r=0.09 p=0.82, respectively) patient group. Similarly, no significant correlation between distress level and age or gender was identified for parents of patients with OS (r=0.43, p=0.09; r=0.07, p=0.81, respectively) and ES (r=−0.19, p=0.25; r=−0.14, p=0.39, respectively).
Discussion
In the present study, we assessed psychosocial distress in patients with a history of OS or ES and in parents of patients with OS or ES. Our survey used validated psycho-oncological screening tools and was conducted online via specific topic-based groups in the Facebook social network. We observed that (i) a relatively high number of participants were enrolled in the 2-month study period in relation to the low prevalence of OS and ES, and (ii) that all participating patients and a high percentage of parental caregivers suffered from relevant psychosocial distress and potentially required psycho-oncological treatment.
OS and ES represent the predominant musculoskeletal tumor entities, especially during childhood and adolescence, but account for only 1% of all cancer diagnosed in the United States each year (19). Despite a lack of fixed criteria for the definition of rare cancer, Gatta et al. proposed an incidence of fewer than six cases per 100,000 people per year (21). With only five cases per 1,000,000 children aged 19 years and younger per year, OS is an extremely rare disease, and it is difficult and time-consuming to conduct clinical studies with cohorts of sufficient sample size (22). Therefore, in a recent methodology article, Billingham and Steven suggested the creation of national and international networks, as well as maximization of trial duration, to overcome poor patient recruitment in clinical studies of rare cancer (23).
Social networks with specific topic-based groups bring together affected people (patients and relatives) and provide unique potential to collect medical data (crowdsourcing) from large cohorts with rare diseases in a relatively short time period. Substantiating this hypothesis in the field of orthopedic oncology, van der Heijden et al. investigated outcomes and quality of life in patients with pigmented villonodular synovitis via Facebook, using patient-reported outcome measures (24). That study enrolled 293 patients within a 16-month study period. A large study cohort was generated despite the low percentage of participants (26.3%) compared with 1112 group members. In contrast, recent studies using conventional recruitment strategies showed significantly smaller cohorts (25,26). Similarly, in the present study, we enrolled 28 patients with OS or ES and 58 parents of patients with OS or ES in only 2 months. In contrast, only four patients underwent treatment at our Institution in the same time period.
To determine the representativeness of the collected data and thus the feasibility of crowdsourcing via social media in medical research, demographic and medical results were compared with previous publications on OS and ES. In the present study, the mean age at primary diagnosis of OS was 24.8±13.0 years (range=6-50 years), confirming a bimodal age distribution with a first peak in the second decade of life and a second peak over age 40 years (27,28). Similarly, our finding that more than 70% of OS occurred in the distal femur and proximal tibia is consistent with previous studies (27-29). Surgical tumor resection (100%) and (neo)-adjuvant chemotherapy (93%) were identified as the main therapeutic strategies, substantiating the modern therapy regimen (30,31). The mean age of patients with ES was 28.8±9.7 years (range=16-44 years), and was higher than proposed by previous authors for skeletal manifestations (27,32). Baldini et al. found 49% extraosseous tumor manifestations in a study of ES in adults (33). Confirming their results, we also identified 50% extraosseous tumor manifestations, which might be associated with the higher age of our study cohort. Results for therapeutic modalities were similar to the OS patient group, with a high percentage of (neo-)adjuvant chemotherapy (79%) and surgical tumor resection (79%). In our study population, 50% of patients with ES underwent additional radiotherapy as suggested in modern multimodal treatment concepts (34).
Adjacent to oncological and surgical treatment, supplementary psycho-oncological therapy has often been underestimated in the past, although it is of paramount importance (6). In this context, Book et al. found that 34.9% of their cohort (1,850 patients with different cancer types) suffered from relevant psychosocial distress, based on the QSC-R10 (2). Compared with their results, we observed that all patients in the OS and ES groups in our cohort were above the cut-off value of 14 points. To some extent this might be due to the higher mortality of OS or ES compared to more common malignancies such as breast or prostate cancer. In contrast, potentially dissatisfied patients or patients with high psychosocial distress levels may be more likely to enroll in a patient community on Facebook and participate in an online survey regarding outcomes and quality of life (24). We recorded relevant psychosocial distress levels in most parents of patients with OS and ES (93.8% and 97.4%, respectively). In contrast, Schepper et al. identified psychosocial distress in only 36.4% of participants in a similar study involving parents of patients with childhood cancer (16). Similar to the patient group, our results might be explained by the higher mortality of sarcomas compared with other malignancies, and a potential selection bias in the topic-based online communities.
In this context, our study has some limitations. Firstly, the participants might have been subject to selection bias as indicated above. Secondly, the patients enrolled in our study were not uniformly treated in sarcoma centers but also underwent therapy in secondary and tertiary referral centers, possibly contributing to unfavorable outcomes and increased psycho-oncological distress levels (35). However, in our opinion, the present study cohort was a randomly selected group reflecting the current situation of sarcoma therapy worldwide, and the findings emphasize the need for international treatment guidelines including psycho-oncological support (6). Thirdly, the accuracy of medical data was subject to the understanding of each patient, possibly influencing study results. However, patient-reported outcome measures, as used in the present study, are easy-to-use tools for crowdsourcing studies via social media and for subjective patient outcomes, and are even more reliable than an evaluation by the treating physician (36,37).
In conclusion, crowdsourcing via specific topic-related patient communities on Facebook provides potential for expanding medical research in rare diseases. This novel approach is feasible for the acquisition of valid medical data and especially self-assessment quality of life measures with larger cohorts than may potentially be enrolled in a single institution, with little effort. Moreover, we identified high distress levels in both the patient and parent groups, indicating a high demand for psycho-oncological treatment. However, evaluation of subjective quality of life measures should be planned carefully due to potential selection bias, with dissatisfied or distressed patients being more likely to enroll in these communities in social media.
Acknowledgements
The Authors wish to explicitly thank the Wilhelm-Sander-Foundation, a charitable, non-profit foundation whose purpose is to promote cancer research, for funding this study.
References
- 1.Singer S, Das-Munshi J, Brahler E. Prevalence of mental health conditions in cancer patients in acute care–a meta-analysis. Ann Oncol. 2010;21(5):925–930. doi: 10.1093/annonc/mdp515. [DOI] [PubMed] [Google Scholar]
- 2.Book K, Marten-Mittag B, Henrich G, Dinkel A, Scheddel P, Sehlen S, Haimerl W, Schulte T, Britzelmeir I, Herschbach P. Distress screening in oncology-evaluation of the questionnaire on distress in cancer patients-short form (QSC-R10) in a German sample. Psychooncology. 2011;20(3):287–293. doi: 10.1002/pon.1821. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, Meader N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 4.Varni JW, Setoguchi Y, Rappaport LR, Talbot D. Effects of stress, social support, and self-esteem on depression in children with limb deficiencies. Arch Phys Med Rehabil. 1991;72(13):1053–1058. [PubMed] [Google Scholar]
- 5.Herschbach P, Keller M, Knight L, Brandl T, Huber B, Henrich G, Marten-Mittag B. Psychological problems of cancer patients: A cancer distress screening with a cancer-specific questionnaire. Br J Cancer. 2004;91(3):504–511. doi: 10.1038/sj.bjc.6601986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenze U, Kirchhoff C, Book K, Pohlig F, Gradl G, Rechl H, Herschbach P, von Eisenhart-Rothe R. The role of psycho-oncology in orthopedic tumor centers. Orthopade. 2012;41(12):958–965. doi: 10.1007/s00132-012-1981-0. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell AJ. Pooled results from 38 analyses of the accuracy of distress thermometer and other ultra-short methods of detecting cancer-related mood disorders. J Clin Oncol. 2007;25(29):4670–4681. doi: 10.1200/JCO.2006.10.0438. [DOI] [PubMed] [Google Scholar]
- 8.Carlson LE, Bultz BD. Cancer distress screening. Needs, models, and methods. J Psychosom Res. 2003;55(5):403–409. doi: 10.1016/s0022-3999(03)00514-2. [DOI] [PubMed] [Google Scholar]
- 9.Siedentopf F, Marten-Mittag B, Utz-Billing I, Schoenegg W, Kentenich H, Dinkel A. Experiences with a specific screening instrument to identify psychosocial support needs in breast cancer patients. Eur J Obstet Gynecol Reprod Biol. 2010;148(2):166–171. doi: 10.1016/j.ejogrb.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, Passik SD. Distress screening in a multidisciplinary lung cancer clinic: Prevalence and predictors of clinically significant distress. Lung Cancer. 2007;55(2):215–224. doi: 10.1016/j.lungcan.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cape J, McCulloch Y. Patients’ reasons for not presenting emotional problems in general practice consultations. Br J Gen Pract. 1999;49(448):875–879. [PMC free article] [PubMed] [Google Scholar]
- 12.Network NCC. NCCN Clinical Practice Guidelines in Oncology. Network NCC. 2016;1 [Google Scholar]
- 13.Valderas JM, Kotzeva A, Espallargues M, Guyatt G, Ferrans CE, Halyard MY, Revicki DA, Symonds T, Parada A, Alonso J. The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Qual Life Res. 2008;17(2):179–193. doi: 10.1007/s11136-007-9295-0. [DOI] [PubMed] [Google Scholar]
- 14.Herschbach P, Marten-Mittag B, Henrich G. Revision und psychometrische Prüfung des Fragebogen zur Belastung von Krebskranken (FBK-R23) Zeitschrift für Medizinische Psychologie. 2003;12(2):69–76. [Google Scholar]
- 15.Herschbach P, Rosbund AM, Brengelmann JC. Problems of female cancer patients and their coping behavior. Onkologie. 1985;8(4):219–231. doi: 10.1159/000215661. [DOI] [PubMed] [Google Scholar]
- 16.Schepper F, Abel K, Herschbach P, Christiansen H, Mehnert A, Martini J. Fear of progression in parents of children with cancer: Adaptation of the fear of progression questionnaire and correlates] Klin Padiatr. 2015;227(3):151–156. doi: 10.1055/s-0035-1545352. [DOI] [PubMed] [Google Scholar]
- 17.Fidika A, Herle M, Herschbach P, Goldbeck L. Fear of disease progression questionnaire for parents: Psychometric properties based on a sample of caregivers of children and adolescents with cystic fibrosis. J Psychosom Res. 2015;79(1):49–54. doi: 10.1016/j.jpsychores.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann T, Herschbach P, Wessarges M, Heinrichs N. Fear of progression in partners of chronically ill patients. Behav Med. 2011;37(3):95–104. doi: 10.1080/08964289.2011.605399. [DOI] [PubMed] [Google Scholar]
- 19.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lugo-Fagundo C, Johnson MB, Thomas RB, Johnson PT, Fishman EK. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol. 2016;13(3):316–319. doi: 10.1016/j.jacr.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, Otter R, Licitra L, Mallone S, Tavilla A, Trama A, Capocaccia R, RARECARE Working Group Rare cancers are not so rare: The rare cancer burden in europe. Eur J Cancer. 2011;47(17):2493–2511. doi: 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47(1):283–292. doi: 10.1016/j.ocl.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Billingham L, Malottki K, Steven N. Research methods to change clinical practice for patients with rare cancers. Lancet Oncol. 2016;17(2):e70–80. doi: 10.1016/S1470-2045(15)00396-4. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijden L, Piner SR, van de Sande MA. Pigmented villonodular synovitis: A crowdsourcing study of two hundred and seventy-two patients. Int Orthop. 2016;40:2459–2468. doi: 10.1007/s00264-016-3208-1. [DOI] [PubMed] [Google Scholar]
- 25.Auregan JC, Klouche S, Bohu Y, Lefevre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327–1341. doi: 10.1016/j.arthro.2014.04.101. [DOI] [PubMed] [Google Scholar]
- 26.Gelhorn HL, Tong S, McQuarrie K, Vernon C, Hanlon J, Maclaine G, Lenderking W, Ye X, Speck RM, Lackman RD, Bukata SV, Healey JH, Keedy VL, Anthony SP, Wagner AJ, Von Hoff DD, Singh AS, Becerra CR, Hsu HH, Lin PS, Tap WD. Patient-reported symptoms of tenosynovial giant cell tumors. Clin Ther. 2016;38(4):778–793. doi: 10.1016/j.clinthera.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Eisenhart-Rothe R, Toepfer A, Salzmann M, Schauwecker J, Gollwitzer H, Rechl H. Primary malignant bone tumors. Orthopade. 2011;40(12):1121–1142. doi: 10.1007/s00132-011-1866-7. [DOI] [PubMed] [Google Scholar]
- 28.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 29.Dorfman HD, Czerniak B. Bone cancers. Cancer. 1995;75(1 Suppl):203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::aid-cncr2820751308>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Bielack S, Carrle D, Casali PG, Group EGW. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):137–139. doi: 10.1093/annonc/mdp154. [DOI] [PubMed] [Google Scholar]
- 31.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Grier HE. The ewing family of tumors. Ewing’s sarcoma and primitive neuroectodermal tumors. Pediatr Clin North Am. 1997;44(4):991–1004. doi: 10.1016/s0031-3955(05)70541-1. [DOI] [PubMed] [Google Scholar]
- 33.Baldini EH, Demetri GD, Fletcher CD, Foran J, Marcus KC, Singer S. Adults with Ewing’s sarcoma/primitive neuroectodermal tumor: Adverse effect of older age and primary extraosseous disease on outcome. Ann Surg. 1999;230(1):79–86. doi: 10.1097/00000658-199907000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez JL, Cabrera P, Ordonez R, Marquez C, Ramirez GL, Praena-Fernandez JM, Ortiz MJ. Role of radiation therapy in the multidisciplinary management of ewing’s sarcoma of bone in pediatric patients: An effective treatment for local control. Rep Pract Oncol Radiother. 2011;16(3):103–109. doi: 10.1016/j.rpor.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogura K, Yasunaga H, Horiguchi H, Ohe K, Shinoda Y, Tanaka S, Kawano H. Impact of hospital volume on postoperative complications and in-hospital mortality after musculoskeletal tumor surgery: Analysis of a national administrative database. J Bone Joint Surg Am. 2013;95(18):1684–1691. doi: 10.2106/JBJS.L.00913. [DOI] [PubMed] [Google Scholar]
- 36.Slevin ML, Plant H, Lynch D, Drinkwater J, Gregory WM. Who should measure quality of life, the doctor or the patient. Br J Cancer. 1988;57(1):109–112. doi: 10.1038/bjc.1988.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gnanasakthy A, Mordin M, Clark M, DeMuro C, Fehnel S, Copley-Merriman C. A review of patient-reported outcome labels in the United States: 2006 to 2010. Value Health. 2012;15(3):437–442. doi: 10.1016/j.jval.2011.11.032. [DOI] [PubMed] [Google Scholar]