Abstract
Aims
Remote monitoring of implantable cardioverter-defibrillators may improve clinical outcome. A recent meta-analysis of three randomized controlled trials (TRUST, ECOST, IN-TIME) using a specific remote monitoring system with daily transmissions [Biotronik Home Monitoring (HM)] demonstrated improved survival. We performed a patient-level analysis to verify this result with appropriate time-to-event statistics and to investigate further clinical endpoints.
Methods and results
Individual data of the TRUST, ECOST, and IN-TIME patients were pooled to calculate absolute risks of endpoints at 1-year follow-up for HM vs. conventional follow-up. All-cause mortality analysis involved all three trials (2405 patients). Other endpoints involved two trials, ECOST and IN-TIME (1078 patients), in which an independent blinded endpoint committee adjudicated the underlying causes of hospitalizations and deaths. The absolute risk of death at 1 year was reduced by 1.9% in the HM group (95% CI: 0.1–3.8%; P = 0.037), equivalent to a risk ratio of 0.62. Also the combined endpoint of all-cause mortality or hospitalization for worsening heart failure (WHF) was significantly reduced (by 5.6%; P = 0.007; risk ratio 0.64). The composite endpoint of all-cause mortality or cardiovascular (CV) hospitalization tended to be reduced by a similar degree (4.1%; P = 0.13; risk ratio 0.85) but without statistical significance.
Conclusion
In a pooled analysis of the three trials, HM reduced all-cause mortality and the composite endpoint of all-cause mortality or WHF hospitalization. The similar magnitudes of absolute risk reductions for WHF and CV endpoints suggest that the benefit of HM is driven by the prevention of heart failure exacerbation.
Keywords: Remote monitoring, Implantable cardioverter-defibrillator, Meta-analysis, Death, Hospitalization , Heart failure
Introduction
The rapidly growing number of patients with implantable cardioverter-defibrillators (ICDs) translates into increasing demand for their regular follow-up.1,2 Remote ICD monitoring has been introduced to improve clinical efficiency by reducing the need for conventional in-office follow-up visits.2–9 Remote monitoring also affords the chance to adjust clinical care after detecting a change in the patient’s clinical condition or device malfunction, which may improve major clinical outcomes such as death and hospitalization. This is still an active area of research with inconsistent findings,2,7–14 as several non-randomized clinical studies observed a considerable outcome benefit of remote ICD monitoring, including all-cause mortality,10,14,15 and randomized trials mostly reported neutral results.3,5,8,9,12
A meta-analysis of nine randomized controlled trials published by Parthiban et al.13 in June 2015 suggested that remote ICD monitoring does not improve all-cause mortality, cardiovascular (CV) mortality, or overall hospitalization compared with conventional in-office follow-up.13 Only one trial (IN-TIME) showed a significant positive effect of remote monitoring on the primary clinical outcome (composite clinical score16) and on all-cause mortality.12,13 A significant mortality reduction was also seen in pooled data for all trials (TRUST, ECOST, IN-TIME) using a specific remote monitoring system with daily verification of transmission [Biotronik Home Monitoring (HM)].13 This observation warrants closer examination and corroborates the suggestion that not all telemonitoring approaches in heart failure are the same in practice and in their resulting outcomes.17 Hence, system specific analyses may be preferable to a global analysis combining systems with different operational characteristics.
In the present meta-analysis, we combined individual data for the TRUST,3 ECOST,5 and IN-TIME12 patients. The aim was to verify and deepen the Parthiban meta-analysis in the part related to the remote technology specialized for daily data transmission.13 We first verified the survival finding by using time-to-event models instead of the pooled odds ratio estimates,13 and subsequently investigated several clinical endpoints combining cause-specific deaths and hospitalizations in a way suitable to explore the mechanism of clinical benefit.
Methods
Trials included
We pooled patient-level data from the three trials identified by Parthiban et al.13 as the only randomized controlled trials using specific remote monitoring system with daily verification of transmission (Biotronik Home Monitoring, Biotronik SE & Co. KG, Berlin, Germany), subsequently referred to as ‘HM’. In chronological order, TRUST (Lumos-T safely reduces routine office device follow-up),3 ECOST (Effectiveness and cost of ICDs follow-up schedule with telecardiology),5 and IN-TIME (Influence of HM on mortality and morbidity in heart failure patients with impaired left ventricular function)12 study investigators reported clinical outcomes for HM vs. conventional in-office follow-up without HM in patients treated with ICDs or cardiac resynchronization therapy defibrillators (CRT-Ds). Table 1 compares trial characteristics.
Table1.
Included trials
| TRUST3 | ECOST5 | IN-TIME12 | |
|---|---|---|---|
| No. of centres | 102 USA sites | 43 French sites | 26 German sites, 10 sites elsewherea |
| Patient eligibility | Class1 indication for ICD, not pacemaker dependent | Indication for ICD, not NYHA class IV | Indication for ICD or CRT-D, heart failure (≥ 3 months), NYHA class II or III, LVEF ≤ 35% |
| Primary objective | To evaluate safety and efficacy of extended IO intervals | To compare major CVAEs including all-cause death | To compare heart failure outcomes using composite (“Packer”) scoreb |
| Follow-up schedule | |||
| HM group | IO at 3M and 15M. HM replaced IO at 6M, 9M, and 12M | IO at 1-3M, 15M, and 27M. HM replaced IO at 9M and 21M | IO at 12M, and in-between according to hospital routine |
| Control group | IO every 3M | IO at 1-3M, then every 6M | Same as in the HM group |
| Blinded endpoint committee | No | Yes | Yes |
Denmark (three sites), Czech Republic (two), Israel (two), Australia (one), Austria (one), Latvia (one).
The score combines all-cause death, overnight hospitalization for heart failure, change in NYHA class, and change in patient global self-assessment.
CRT-D, cardiac resynchronization therapy defibrillator; CVAE, cardiovascular adverse event; ECOST, Effectiveness and cost of ICDs follow-up schedule with telecardiology; HM, Home Monitoring; ICD, implantable cardioverter-defibrillator; IN-TIME, Influence of HM on mortality and morbidity in heart failure patients with impaired left ventricular function; IO, in-office visit; LVEF, left ventricular ejection fraction; M, months; NYHA, New York Heart Association; TRUST, Lumos-T safely reduces routine office device follow-up.
The three trials had different primary objectives. TRUST (published in 2010) evaluated the safety of extended in-office follow-up intervals in the HM arm during 15 months after ICD implantation.3 ECOST (2013) compared major CV adverse events combined with all-cause death during 27 months after ICD implantation.5 IN-TIME (2014) focused on a composite clinical score for heart failure (‘Packer score’),16 combining all-cause death, overnight hospitalization for heart failure, change in New York Heart Association (NYHA) class, and change in patient global self-assessment. The number of patients with worsened composite clinical score was compared for HM vs. conventional follow-up at 12 months after ICD or CRT-D implantation.12,16
The regular in-office follow-up schedule was less intensive in the HM than control arm in TRUST and ECOST, but equally intensive in IN-TIME. In the HM arm, the implanted devices transmitted daily information about supraventricular and ventricular tachyarrhythmias including intracardiac electrograms and delivered ICD therapies; atrial tachyarrhythmia burden; mean heart rate; the incidence of ventricular extrasystoles; patient physical activity; technical parameters such as lead impedances, pacing threshold, or sensing parameters; low percentage of CRT in CRT-D devices; and alerts to data transmission failure during a predefined period (usually > 3 days).3,5,12
We appraised the quality of the included trials by the Cochrane Collaboration’s tool for assessing risk of bias in five key domains: (i) selection bias, based on the method of random sequence generation and allocation concealment; (ii) performance bias (blinding of the patients and personnel); (iii) detection bias (blinding of outcome assessment); (iv) attrition bias (incomplete outcome data, e.g. due to patients lost to follow-up); and (v) reporting bias caused by selective outcome reporting.18
Endpoints in the present analysis
The following seven outcome measures were defined prior to meta-analysis: (i) all-cause death, (ii) CV death, (iii) all-cause death or any hospitalization, (iv) all-cause death or CV hospitalization, (v) all-cause death or hospitalization for worsening heart failure (WHF), (vi) CV death or CV hospitalization, and (vii) WHF death or WHF hospitalization. Hospitalization was defined as at least one overnight admission.
All-cause death was the primary endpoint. Other endpoints served to gain insight into the relative contribution and implications of the mechanisms of clinical benefit of HM. The first five endpoints are frequently used in outcome studies with CV patients. The last two endpoints were added to ensure sufficient number of events by combining death and hospitalization while remaining specific for CV and WHF categories. To focus on the true CV morbidity caused by heart failure, ischaemia, arrhythmia, or thromboembolism, we excluded the device and procedure-related hospitalizations.
The all-cause mortality analysis involved all three trials. The other six endpoints involved only ECOST and IN-TIME, in which an independent, blinded endpoint committee adjudicated the underlying causes of hospitalizations and deaths.
Patients
The primary analysis populations of the TRUST, ECOST, and IN-TIME trials comprised 2436 patients.3,5,12 After exclusion of 31 patients with missing study termination date, we performed the all-cause mortality analysis on 2405 patients (Table 2). Other analyses involved 1078 (ECOST, IN-TIME) patients.
Table2.
Patient baseline characteristics
| TRUST3 | ECOST5 | IN-TIME12 | |
|---|---|---|---|
| No. of patients we included | 1327 (99.1) | 414 (95.6) | 664 (100) |
| No. of patients we excludeda | 12 (0.9) | 19 (4.4) | 0 |
| Age (years) | 64 ± 13 | 62 ± 12 | 65 ± 9 |
| Male gender | 959 (72.3) | 367 (88.6) | 536 (80.7) |
| LVEF (%) | 29 ± 10 | 35 ± 13 | 26 ± 7 |
| NYHA classesb | |||
| I | 160 (12.2) | 108 (26.8) | 0 |
| II | 755 (57.4) | 256 (63.5) | 285 (43.0) |
| III | 393 (29.9) | 39 (9.7) | 378 (57.0) |
| IV | 8 (0.6) | 0 | 0 |
| History of atrial fibrillation | 208 (15.7) | 68 (16.4) | 168 (25.3) |
| Coronary artery disease | 890 (67.1) | 270 (65.2) | 458 (69.0) |
| Hypertension | 696 (52.4) | 138 (33.3) | 463 (69.7) |
| Diabetes | n.a. | 84 (20.3) | 266 (40.1) |
| Medication | |||
| Beta-blocker | 1046 (78.8) | 288 (69.6) | 608 (91.6) |
| ACEI or ARB | 682 (51.4) | 290 (70.0) | 593 (89.3) |
| Digitalis | 301 (22.7) | 13 (3.1) | 127 (19.1) |
| ICD related information | |||
| Primary prevention indication | 964 (72.8) | 219 (52.9) | 525 (79.1) |
| Single-chamber ICD | 562 (42.4) | 291 (70.3) | 0 |
| Dual-chamber ICD | 765 (57.6) | 123 (29.7) | 274 (41.3) |
| CRT-D | 0 | 0 | 390 (58.7) |
| Randomization group | |||
| HM | 901 (67.9) | 211 (51.0) | 333 (50.2) |
| Control group | 426 (32.1) | 203 (49.0) | 331 (49.8) |
Data are mean ± SD, or n (%).
Except for coronary artery disease, differences in any variable across trials were statistically significant (P < 0.001).
Patients with unknown date of study termination.
NYHA class was missing in 11/11/1 (TRUST/ECOST/IN-TIME) patients.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; n.a., not available; other abbreviations as in Table1.
Data management
The coordinating investigator of IN-TIME (G.H.) invited the coordinating investigators of TRUST (N.V.), and ECOST (S.K.) to participate in this collaborative analysis. Anonymized patient-level data from all three trials were compiled into a single dataset by the IN-TIME biostatistical team and forwarded to the Department of Public Health in Copenhagen (T.A.G.) and the Department of Clinical Epidemiology in Aalborg (C.T.-P.) for meta-analysis. No further ethics board review was sought for because each included study had already obtained it for the primary publication.
Statistical analysis
The absolute risk of all-cause mortality was calculated as a function of time during 12 months after randomization by the Kaplan-Meier method. For other endpoints, competing risks were accounted for by the Aalen-Johansen estimator.19 We generally preferred absolute risk differences over hazard ratios because (i) absolute risks have a direct interpretation for the patients, (ii) the proportional hazard assumption was violated in TRUST due to intersecting mortality curves,3 and (iii) comparison of absolute risk differences for different endpoints allows better understanding of relationships relevant for the interpretation of the mechanism of HM benefit. All absolute risk differences are reported for 12 months, oriented to the shortest-running study, IN-TIME. The corresponding 95% CIs were based on 5000 bootstrap samples per trial. To summarize results across trials, we used the inverse variance meta-analysis and assumed a fixed effect model. Absolute risk differences between the HM group and controls were statistically evaluated by the Z-test. Risk ratios at 12 months are provided for convenience, without statistical evaluation.
Baseline variables were compared across trials by the Chi-square test (categorical data) and ANOVA (continuous data). Continuous data are presented as mean ± SD. To assess whether any baseline variable modified the effect of HM on any endpoint, we used Cox regression stopped after 12 months, applied on pooled ECOST and IN-TIME data (TRUST data disallowed hazard ratio calculation). All models allowed the baseline hazard to differ between the two studies (stratified Cox regression). For each variable, we compared an additive model, including the variable and the treatment group variable, with an interaction model allowing the treatment effect to depend on the variable. P-values for the likelihood ratio tests were calculated using the Bonferroni-Holm correction for multiple comparisons.
In general, we considered a P-value < 0.05 statistically significant. All analyses were performed in R version 3.2 (R Development Core Team, Vienna, Austria).
Results
The 2405 patients included in the meta-analysis were enrolled at 181 sites, mainly in the USA, France, and Germany (Table 1). The mean age of the patients varied from 62 to 65 years across trials (Table 2). The proportion of male patients ranged from 72 to 89%. The mean left ventricular ejection fraction (LVEF) varied from 26 to 35%, and the proportion of patients with NYHA class III/IV heart failure ranged widely from 10 to 57%. Symptomatic heart failure was diagnosed in 89% of all patients.
Baseline characteristics differed significantly between trials for nearly all variables listed in Table 2. The IN-TIME patients appear to have had the highest risk of worse clinical outcome as they were older and had a lower LVEF, more advanced heart failure symptoms, and a higher prevalence of atrial fibrillation and hypertension than TRUST and ECOST patients (Table 2).
Risk of bias
According to the Cochrane Collaboration’s tool, we judged all three trials to have appropriately randomized patients and adequately concealed allocation. Although it was not possible to blind investigators and patients in any trial, we judged the associated risk of bias to be low, given that all-cause death (primary endpoint in our meta-analysis) is not subject to modification by the investigator’s opinion and that causes of hospitalizations and deaths were adjudicated by blinded boards. The only notable difference between the trials was a higher attrition rate in TRUST (15.0% vs. 4.6% ECOST vs. 6.8% IN-TIME), which was also inhomogeneous (13.3% HM vs. 18.8% controls), suggesting better patient retention with the aid of HM.20 If the attrition concealed endpoints, the reported clinical benefit would be underestimated because of the larger attrition in the control group. The risk of selective reporting is low because the included three trials were taken from a high-ranked publication with a documented literature search strategy.13 To our best knowledge, no eligible studies were overseen there,13 while we did not consider any study published later. Overall, we judge the present meta-analysis to be at low risk of bias for all key domains.
All-cause mortality
The Kaplan-Meier curve for pooled all-cause mortality is shown in Figure 1. In the HM group, the absolute risk of death was reduced by 1.9% at 12 months (95% CI: 0.1–3.8%), which was statistically significant (P = 0.037) (Figure 2). The relative risk (RR) of death with HM was 0.62 (95% CI: 0.40 – 0.95). The number needed to treat (NNT) to prevent one death at 12 months was 52 patients.
Figure 1.
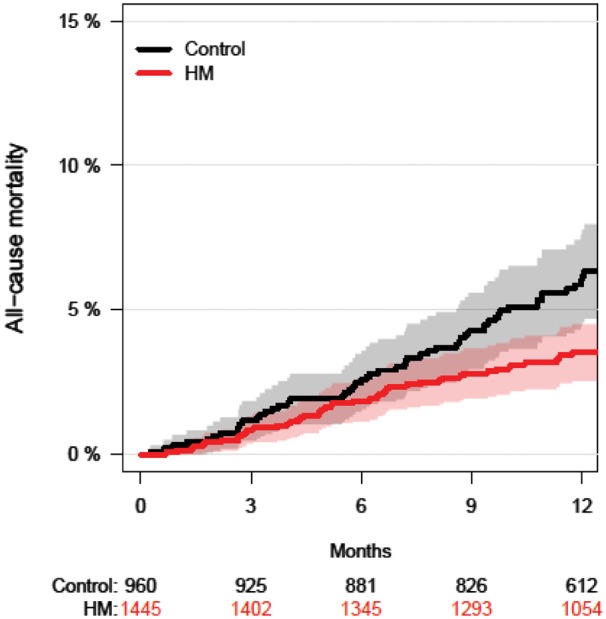
Time to all-cause death for pooled TRUST, ECOST, and IN-TIME patients. The shaded areas indicate the 95% CIs. The numbers below panel are patients at risk. The 1.9% reduction in the absolute risk of death in the HM group was statistically significant (95% CI: 0.1–3.8%; P = 0.037). Abbreviations: HM, Home Monitoring; ECOST/IN-TIME/TRUST as in Table 1.
Figure 2.
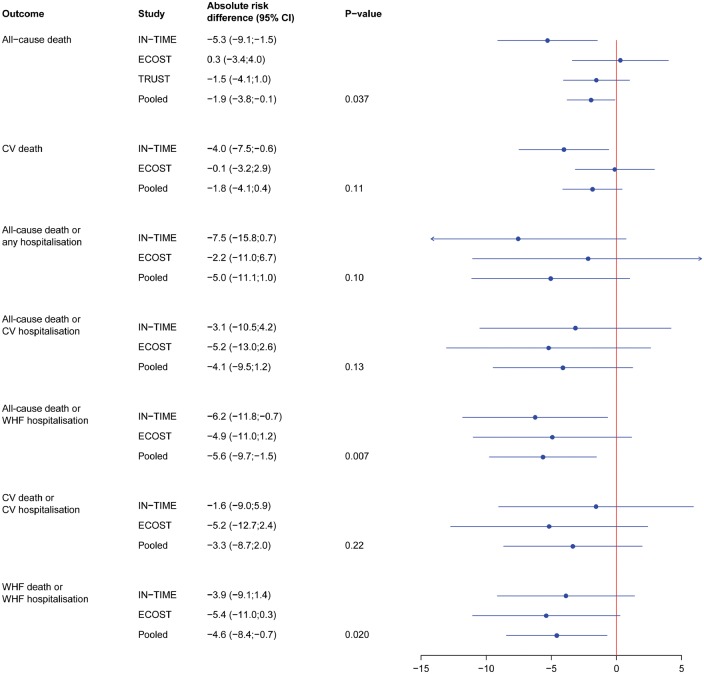
Forest plot of the absolute risk differences (in %) for all endpoints at 12 months. A negative value (reduction) is in favour of HM. Abbreviations: CI, confidence interval; CV, cardiovascular; hosp, hospitalization; WHF, worsening heart failure; ECOST/IN-TIME/TRUST as in Table 1.
Other endpoints
All other endpoints were assessed from 1078 patients of the combined ECOST and IN-TIME datasets, since TRUST had no event adjudication. As seen in Figure 2, the absolute risk of CV mortality was not significantly reduced at 12 months with HM [−1.8% (95% CI: −4.1 to +0.4%), P = 0.11; RR = 0.51]. Likewise, the absolute risk of all-cause mortality combined with either all-cause hospitalization [−5.0% (−11.1 to + 1.0%), P = 0.10; RR = 0.86] or CV hospitalization [−4.1% (−9.5% to + 1.2%), P = 0.13; RR = 0.85] was not reduced significantly (Figures 2 and 3). In contrast, the absolute risk reduction for all-cause mortality combined with WHF hospitalization was significant [−5.6% (−9.7 to −1.5%), P = 0.007; RR = 0.64; NNT = 18 patients] (Figures 2 and 3).
Figure 3.
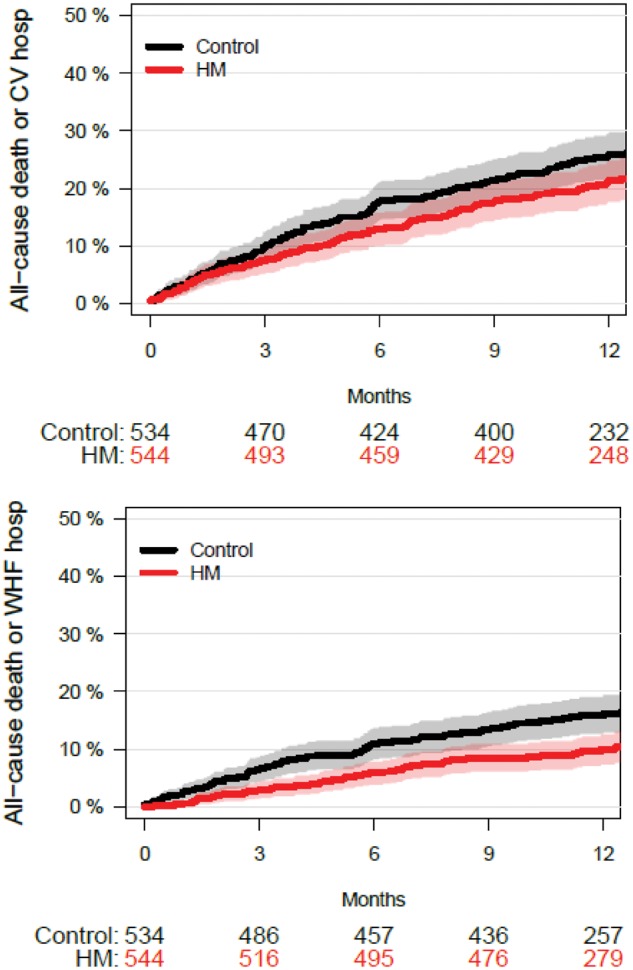
Time to occurrence of composite endpoints for pooled ECOST and IN-TIME patients. Upper panel: Composite of all-cause death and a CV hospitalization, excluding device related or procedure-related hospitalizations. The 4.1% absolute risk reduction in the HM group was not statistically significant (P = 0.13). Lower panel: Composite of all-cause death and a hospitalization due to WHF. The 5.6% absolute risk reduction in the HM group was statistically significant (P = 0.007). For confidence intervals, see Figure 2. Abbreviations as in Figures 1 and 2.
For combined CV death or CV hospitalization, the absolute risk reduction was not significant [−3.3% (−8.7 to + 2.0%), P = 0.22; RR = 0.88], but for combined WHF death or WHF hospitalization, it was significant [−4.6% (−8.4 to − 0.7%), P = 0.02; RR = 0.67; NNT = 22 patients] (Figure 2).
Patient baseline characteristics did not modify the effect of HM for any endpoint studied (P > 0.9 for all interactions, after correction for multiple testing).
Discussion
The main findings of the present study are that HM reduced the risk of all-cause mortality and the risk of all-cause mortality combined with WHF hospitalization. A less pronounced trend towards reduction in CV endpoints, such as CV hospitalization combined with all-cause or CV mortality, appears to be driven in full by the reduction in WHF endpoints.
The observed 1.9% absolute risk reduction for all-cause mortality after one year (P = 0.037) is equivalent to a risk ratio of 0.62 (95% CI: 0.40–0.95), which closely matches the odds ratio of 0.65 (95% CI: 0.45–0.94) reported for the same three trials by Parthiban et al.13 The confirmation of this earlier result is important, because the Kaplan-Meier method that we were able to use treats the inhomogeneous durations of the pooled studies (12–27 months) and cases of early study termination correctly.13 In an attempt to explain why a significant survival benefit was observed in the IN-TIME trial,12 and not in TRUST3 and ECOST5 (as illustrated by the first item in Figure 2), we compared patient baseline characteristics between the trials, but the baseline differences did not seem to be the underlying reason. It should be noted, however, that none of the individual trials was designed or powered to show a survival advantage. The 95% CI for the risk of all-cause mortality was therefore wide in each trial, requiring a pooled analysis to increase the statistical power.
The survival benefit in the present meta-analysis is supported by a significant reduction of the combined endpoint of all-cause mortality and WHF hospitalization (P = 0.007; RR 0.64). This endpoint has become the most widely used outcome measure in heart failure trials due to an increasing difficulty to prove all-cause mortality benefit alone in the setting of generally declining heart failure mortality. The population of our study is primarily defined as an ICD population. Yet, in conditions when 89% of patients had symptomatic heart failure, it may be expected that the population’s morbidity would be mostly related to heart failure so that heart failure-specific endpoints may be appropriate study outcomes. Conversely, the endpoint combining all-cause mortality and hospitalization, and the endpoints combining all-cause or CV mortality and CV hospitalization, were not significantly reduced.
We also found that the composite endpoint of WHF death or WHF hospitalization was significantly reduced in the HM group, yielding a RR of 0.67 (95% CI: 0.46–0.97), which is very close to the odds ratio of 0.63 (95% CI: 0.4 – 0.90) for worsened composite clinical score (primary endpoint) in the IN-TIME study.12 Furthermore, the absolute risk reduction for WHF death or WHF hospitalization was similar in ECOST (5.4% at 1 year) and IN-TIME (3.9%) and, in the same time, slightly higher than the absolute risk reduction for CV death or CV hospitalization (5.2% ECOST, 1.6% IN-TIME). As WHF events represent a subset of CV events, this implies that HM does not influence CV endpoints other than WHF.
Although our data are not sufficient to definitively rule out a clinically meaningful effect of HM on the prevention of arrhythmic, ischaemic, or thromboembolic events, the assumption that its major clinical benefit is driven by the prevention of WHF appears plausible for two reasons: (i) WHF decompensation requires several days or even weeks to develop, which offers time for automatic alerting and pre-emptive intervention;11,12 and (ii) ICDs are capable of detecting and remotely alerting to several WHF prodromal symptoms and upstream factors predisposing to WHF, such as the onset of atrial or ventricular tachyarrhythmia, increased ectopic activity, low percentage of biventricular pacing, and change in patient activity.11,12,21 In contrast to these slower developments, thromboembolic events and arrhythmias typically have a sudden onset without warning prodromes, whereas ST-segment elevation or depression, a potential marker of cardiac ischaemia, was not measured by the devices used in the trials included in this meta-analysis.
Our observation that HM exerts less or no influence on CV events unrelated to heart failure may be the main reason why HM had a significant effect on the primary outcome in the IN-TIME trial only (on a composite clinical score specifically designed for patients with heart failure) but not in ECOST (on the incidence of major CV adverse events combined with all-cause death).5,12,16
In two of the three trials (TRUST, ECOST), patients had fewer regular follow-up visits in the HM arm than in the control arm. It has been generally shown that ICD patients adhere poorly to recommended follow-up schedules and that this non-adherence results in fewer regular follow-up visits and is correlated with worse outcome.22 The clinical benefit we report from the present meta-analysis is compelling also because it was found against a control group treatment that was likely better than standard treatment owing to good patient adherence.
Several non-randomized studies with other single or combined remote monitoring systems reported a larger improvement in clinical outcomes than our meta-analysis did.10,14,15 In the context of remote monitoring of heart failure patients, it has been generally advised that the exact properties of different remote monitoring systems must be considered, as clinical results are not necessarily similar for different systems.17 We suggest that the (extraordinary) benefits found in non-randomized studies should be confirmed in adequate randomized controlled trials. The value of prospective randomized controlled trials in this context was underlined by the OptiLinkHF results.23 The OptiLinkHF trial randomized 1002 ICD recipients with heart failure to remote automated pulmonary congestion alert ON (n = 505) or OFF (n = 497). After 18 months of follow-up, there was no significant difference between groups in the primary endpoint, a composite of all-cause death and CV hospitalization.23 Both technology platform and work flow seem suited to achieve measurable benefit.23 Our data suggest that multiparameter, automatic, daily remote monitoring may be superior to other platforms, but this remains speculative in the absence of a direct comparison of different remote monitoring systems.
Study limitations
Except for all-cause mortality and CV mortality, all other endpoints were composite events that were not studied in this form in the original trials but were constructed here to investigate the underlying mechanism of HM benefit. To keep this investigation as sensitive as possible, different endpoints were tested without correction for multiple testing. Since most clinical events contributed to more than one endpoint, the studied endpoints were not independent from each other.
We found that the trials were similar in quality, except for a higher and asymmetric attrition rate in TRUST, which is unlikely to cause a meaningful distortion of the main results. Eventually, the point estimate of the mortality effect in TRUST is close to the meta-analysis result. Furthermore, we assumed a similar effect size in all trials and therefore chose a fixed effect model. Although most study characteristics were similar between trials (devices, remote monitoring system, our endpoint assessment), study procedures differed slightly, which might have translated into certain differences in clinical effects. However, similar reductions of the combined endpoints in ECOST and IN-TIME support the assumption of a common effect.
The violation of the proportional hazard assumption by the TRUST mortality curve remains elusive. We have therefore chosen the cumulative incidence of endpoints at 12 months as primary result.
Conclusion
In our meta-analysis, HM with daily verification of transmission was associated with a consistent reduction of clinically relevant endpoints. In the IN-TIME publication,12 we promised further analysis of the mechanism of HM benefit. The present analysis indicates that the benefit is likely driven by prevention of heart failure exacerbation. It is open if the results can be transferred to other remote monitoring systems. Survival benefits observed in non-randomized registries are promising but require confirmation in randomized controlled trials.
Acknowledgements
The authors acknowledge the contribution of Sophie Fauquembergue, MS, (ECOST) and Justin Michalski, MS, (TRUST) to data transfer, Jürgen Schrader, PhD, to data compilation and scientific input, Bernd Brüsehaber, PhD, to statistical discussion, and Dejan Danilovic, PhD, to critical reading of the manuscript and editorial assistance.
Funding
The work was supported by Biotronik SE & Co. KG, Berlin, Germany.
Conflict of interest: G.H. received research grants from Biotronik, Boston Scientific, and St Jude Medical. N.V. reports grants and personal fees from Biotronik, Medtronic, Sorin, St. Jude Medical, and Boston Scientific. S.K. reports grants and personal fees from Medtronic and Biotronik, and grants from St Jude Medical, Sorin, and Boston Scientific. T.L. reports honoraria for lectures from Biotronik. P.S. reports grants and personal fees from Biotronik. J.P. is an employee of Biotronik. T.A.G. reports fees for statistical analysis from Biotronik during this study. S.D.A. reports personal fees from Biotronik and CardioMems, grants and personal fees from Vifor, and personal fees from Bayer, Respicardia, Sorin, Novartis, and MedicalSensible. C.T.-P. reports grants and personal fees from Cardiome, Merck, Sanofi, and Daiichi, and grants from BMS.
References
- 1. Mond HG, Proclemer A.. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009–a World Society of Arrhythmia’s project. Pacing Clin Electrophysiol 2011;34:1013–1027. [DOI] [PubMed] [Google Scholar]
- 2. Dubner S, Auricchio A, Steinberg JS, Vardas P, Stone P, Brugada J, Piotrowicz R, Hayes DL, Kirchhof P, Breithardt G, Zareba W, Schuger C, Aktas MK, Chudzik M, Mittal S, Varma N.. ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs). Europace 2012;14:278–293. [DOI] [PubMed] [Google Scholar]
- 3. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C.. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010;122:325–332. [DOI] [PubMed] [Google Scholar]
- 4. Mabo P, Victor F, Bazin P, Ahres S, Babuty D, DaCosta A, Binet D, Daubert JC.. A randomized trial of long-term remote monitoring of pacemaker recipients (The COMPAS trial). Eur Heart J 2012;33:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guedon-Moreau L, Lacroix D, Sadoul N, Clementy J, Kouakam C, Hermida JS, Aliot E, Boursier M, Bizeau O, Kacet S.. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J 2013;34:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hindricks G, Elsner C, Piorkowski C, Taborsky M, Geller JC, Schumacher B, Bytesnik J, Kottkamp H.. Quarterly vs. yearly clinical follow-up of remotely monitored recipients of prophylactic implantable cardioverter-defibrillators: results of the REFORM trial. Eur Heart J 2014;35:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burri H. Remote follow-up and continuous remote monitoring, distinguished. Europace 2013;15:i14–i16. [DOI] [PubMed] [Google Scholar]
- 8. Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH.. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol 2011;57:1181–1189. [DOI] [PubMed] [Google Scholar]
- 9. Landolina M, Perego GB, Lunati M, Curnis A, Guenzati G, Vicentini A, Parati G, Borghi G, Zanaboni P, Valsecchi S, Marzegalli M.. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012;125:2985–2992. [DOI] [PubMed] [Google Scholar]
- 10. Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, Meyer TE, Jones PW, Boehmer JP.. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359–2367. [DOI] [PubMed] [Google Scholar]
- 11. Varma N, Wilkoff B.. Device features for managing patients with heart failure. Heart Fail Clin 2011;7:215–225. [DOI] [PubMed] [Google Scholar]
- 12. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Sogaard P.. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583–590. [DOI] [PubMed] [Google Scholar]
- 13. Parthiban N, Esterman A, Mahajan R, Twomey DJ, Pathak RK, Lau DH, Roberts-Thomson KC, Young GD, Sanders P, Ganesan AN.. Remote monitoring of implantable cardioverter-defibrillators: A systematic review and meta-analysis of clinical outcomes. J Am Coll Cardiol 2015;65:2591–2600. [DOI] [PubMed] [Google Scholar]
- 14. Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S.. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol 2015;65:2601–2610. [DOI] [PubMed] [Google Scholar]
- 15. DeSimone A, Leoni L, Luzi M, Amellone C, Stabile G, LaRocca V, Capucci A, D'onofrio A, Ammendola E, Accardi F, Valsecchi S, Buja G.. Remote monitoring improves outcome after ICD implantation: the clinical efficacy in the management of heart failure (EFFECT) study. Europace 2015;17:1267–1275. [DOI] [PubMed] [Google Scholar]
- 16. Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001;7:176–182. [DOI] [PubMed] [Google Scholar]
- 17. Anker SD, Koehler F, Abraham WT.. Telemedicine and remote management of patients with heart failure. Lancet 2011;378:731–739. [DOI] [PubMed] [Google Scholar]
- 18. The Cochrane Collaboration’s tool for assessing risk of bias. http://handbook.cochrane.org/chapter_8/table_8_5_a_the_cochrane_collaborations_tool_for_assessing.htm (26 April 2016).
- 19. Aalen OO, Johansen S.. An empirical transition matrix for nonhomogeneous Markov chains based on censored observations. Scand J Stat 1978;5:141–150. [Google Scholar]
- 20. Varma N, Michalski J, Stambler B, Pavri BB.. Superiority of automatic remote monitoring compared with in-person evaluation for scheduled ICD follow-up in the TRUST trial–testing execution of the recommendations. Eur Heart J 2014;35:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brignole M, Auricchio A, Barón-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PP, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE.. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 22. Hess PL, Mi X, Curtis LH, Wilkoff BL, Hegland DD, Al-Khatib SM.. Follow-up of patients with new cardiovascular implantable electronic devices: is adherence to the experts’ recommendations associated with improved outcomes?. Heart Rhythm 2013;10:1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Böhm M, Drexler H, Oswald H, Rybak K, Bosch R, Butter C, Klein G, Gerritse B, Monteiro J, Israel C, Bimmel D, Käab S, Huegl B, Brachmann J.. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J 2016;37:3154–3163. [DOI] [PubMed] [Google Scholar]