Abstract
Aims
Primary prevention implantable cardioverter defibrillators (ICDs) are established therapy for reducing mortality in patients with left ventricular systolic dysfunction and ischaemic heart disease (IHD). However, their efficacy in patients without IHD has been controversial. We undertook a meta-analysis of the totality of the evidence.
Methods and results
We systematically identified all RCTs comparing ICD vs. no ICD in primary prevention. Eligible RCTs were those that recruited patients with left ventricular dysfunction, reported all-cause mortality, and presented their results stratified by the presence of IHD (or recruited only those with or without). Our primary endpoint was all-cause mortality. We identified 11 studies enrolling 8567 participants with left ventricular dysfunction, including 3128 patients without IHD and 5439 patients with IHD. In patients without IHD, ICD therapy reduced mortality by 24% (HR 0.76, 95% CI 0.64 to 0.90, P = 0.001). In patients with IHD, ICD implantation (at a dedicated procedure), also reduced mortality by 24% (HR 0.76, 95% CI 0.60 to 0.96, P = 0.02).
Conclusions
Until now, it has never been explicitly stated that the patients without IHD in COMPANION showed significant survival benefit from adding ICD therapy (to a background of CRT). Even before DANISH, meta-analysis of patients without ischaemic heart disease already showed reduced mortality. DANISH is consistent with these data. With a significant 24% mortality reduction in both aetiologies, it may no longer be necessary to distinguish between them when deciding on primary prevention ICD implantation.
Keywords: Implantable cardiac defibrillators, Meta-analysis, Ischaemic heart disease, Cardiomyopathy, Non-ischaemic, Heart failure
Introduction
Implantable cardiac defibrillators (ICD) are established as preventing death in patients with left ventricular dysfunction and ischaemic heart disease (IHD).1 In patients without IHD, however, ICDs are already considered controversial,2 and recent trial data have been interpreted as indicating that they are not beneficial.3
We set out to analyse the totality of RCT data of ICD vs. no ICD therapy in primary prevention of mortality in patients with left ventricular dysfunction.
Methods
Eligibility and search strategy
We identified all reports of studies of the use of ICD therapy against no ICD therapy for primary prevention in patients with left ventricular systolic dysfunction, in which outcome data was available stratified by the presence of IHD, or recruited only one of these two groups. We included cardiac resynchronization therapy (CRT) RCTs that included a defibrillator arm (CRT-D) and a cardiac resynchronization pacing only arm (CRT-P). We did not include comparisons between CRT-D and no device.
Pubmed (1st January 1946 to 18th December 2016), EMBASE (1st January 1974 to 18th December 2016), and the Cochrane Central register for randomized controlled trials using the search strategy detailed in Supplementary material online, Appendix S1. Only articles in English were considered. Reference lists and relevant systematic reviews were hand-searched for additional publications. No published protocol exists for this systematic review and meta-analysis.
Data abstraction
Data was independently extracted by two authors (SZ, MJS), including year, participants, intervention, and outcomes. Disagreements were resolved by discussion with a third reviewer (DPF). The risk of bias was independently assessed by two authors (SZ, MJS). We sought data on the primary outcome measure of all-cause mortality. Secondary outcome measures included cardiovascular mortality and sudden cardiac death. We also collected data on specific ICD associated complications including inappropriate shocks and device-related infections. We abstracted reported hazard ratios with confidence intervals, and appropriately transformed them for meta-analysis. If hazard ratios or their confidence intervals were not available, but Kaplan-Meier plots were available, we extracted the underlying data using Digitizer4 and converted to hazard ratios and their standard errors.5 If a trial6 randomized patients to control, CRT-Defibrillator, and CRT-Pacemaker; and only presented data stratified by aetiology for the CRT-Defibrillator vs. control, and CRT-Pacemaker vs. control comparisons; the effect of the defibrillator component was determined by indirect comparison of the CRT-Defibrillator vs. the CRT-Pacemaker arms. The steps used to calculate the hazard ratio effect of the defibrillator component, and derive its confidence interval, for the groups with and without IHD separately, are shown in Supplementary material online, Appendix S2, and are based on formulae from Tierney et al.7
If hazard ratio data were unavailable8 we extracted risk ratios.
Risk of bias assessment
We used the Cochrane Risk of Bias Tool9 to assess all trials for bias across six domains (selection, performance, detection, attrition, reporting, and other).
Data analysis
Where appropriate, we quantitatively synthesised the extracted hazard ratios and risk ratios using a random-effects meta-analyses with the Restricted Maximum Likelihood (REML) estimator. We calculated the annualized mortality rate across for each aetiology by dividing the overall mortality rate in the control group by the mean follow-up time, and weighting by study size. The I2 statistic was used to measure heterogeneity of trial results.10 We carried out a sensitivity analysis for patients without IHD by omitting each of the trials in turn and repeating the meta-analysis. Publication bias was graphically assessed using Funnel plots, with Egger’s test for asymmetry.11 Data were analysed using “R”,12 and the package “metafor”.13 The PRISMA checklist is included as Supplementary Data.14
Results
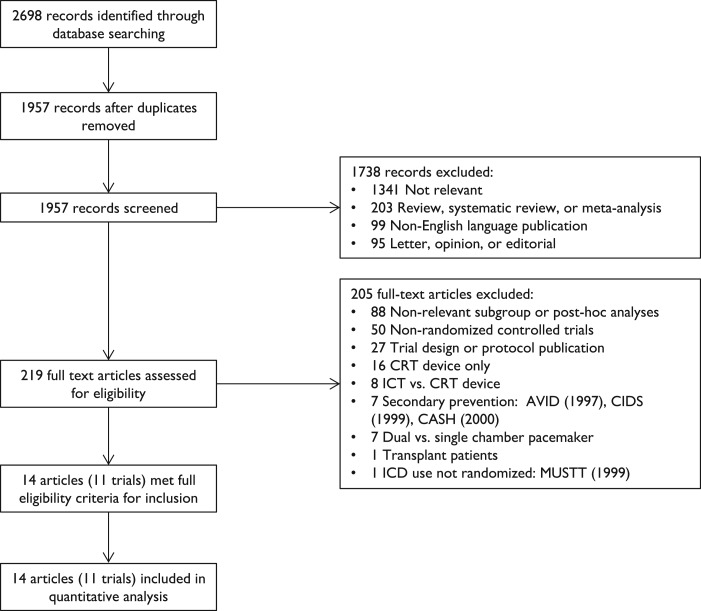
The primary search yielded 2698 records, which were processed as shown in the study flow chart (Figure 1). Full-text was independently reviewed for 219 articles and 11 trials of ICD therapy for primary prevention were included. Three additional articles reported secondary outcomes for included trials.15–17 Two trials enrolled patients with left ventricular dysfunction regardless of aetiology,6,18 four trials enrolled patients exclusively without IHD,8,19–21 three exclusively with chronic IHD,22–24 and two trials exclusively after an acute myocardial infarction.25,26 One trial8 used amiodarone as the comparator, all other trials continued prescribed therapy.
Figure 1.
Study flow chart.
Three trials27–29 were excluded as they recruited patients resuscitated from an arrhythmic cardiac arrest, with an ICD inserted as secondary prevention. One trial30 was excluded as, whilst it was a randomized controlled trial, allocation to insertion of an ICD was not randomized.
A total of 8567 participants were enrolled (4371 ICD therapy, 4196 control), 3128 without IHD and 5439 with IHD (Table 1, study characteristics).
Table 1.
Study characteristics
| Trial | CABG-Patch | MADIT I | MADIT II | CAT | AMIOVIRT | DEFINITE | DINAMIT | COMPANION | SCD-HeFT | IRIS | DANISH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 1996 | 1996 | 2002 | 2002 | 2003 | 2004 | 2004 | 2004 | 2005 | 2009 | 2016 |
| Author | Bigger | Moss | Moss | Bänsch | Strickberger | Kadish | Hohnloser | Bristow | Bardy | Steinbeck | Kober |
| Intervention | ICD | ICD | ICD | ICD | ICD | ICD | ICD | CRT-D | ICD | ICD | ICD |
| Control | SMT | SMT | SMT | SMT | Amiodarone | SMT | SMT | CRT-P | SMT | SMT | SMT |
| LVEF cut-off | <36% | ≤35% | ≤30% | ≤30% | ≤35% | <36% | ≤35% | ≤35% | ≤35% | ≤40% | ≤35% |
| Randomized (N) | 900 | 196 | 1232 | 104 | 103 | 458 | 674 | 1520 | 1676 | 898 | 1116 |
| Without IHD | – | – | – | 100% (n = 104) | 100% (n = 103) | 100% (n = 458) | – | 44% (n = 669) | 47% (n = 792) | – | 100% (n = 1116) |
| With IHD | 100% (n = 900) | 100% (n = 196) | 100% (n = 1232) | – | – | – | 100% (n = 674) | 56% (n = 851) | 53% (n = 884) | 100% (n = 898) | – |
| ICD group N | 446 | 95 | 742 | 50 | 51 | 229 | 332 | 595 | 829 | 445 | 556 |
| Follow-up (months) | 32 | 27 | 20 | 66 | 24 | 29 | 30 | 15.8 | 45.5 | 37 | 67.6 |
| Primary outcome | ACM | ACM | ACM | ACM | ACM | ACM | ACM | ACM and hospitalization | ACM | ACM | ACM |
| Inclusion criteria | Undergoing CABG, abnormal ECG | MI, NSVT, NYHA 1-3 | MI, NYHA 1–3 | Recent DCM diagnosis, NYHA 2-3 | NYHA 1-3, asymptomatic | Symptomatic DCM, ambient arrhythmias | Recent MI | NYHA 3–4, recent HF hospitalization | NYHA class 2–3, OMT | Recent MI | NYHA 2-4, raised NT- proBNP |
| Exclusion criteria | Sustained VT or VF | Cardiac arrest, syncopal VT, | MI within 1month | Valvular, HCM or restrictive, prior MI | Syncope | NYHA 4, familial cardiomyopathy | NYHA 4 | NYHA 4, ventricular arrhythmia before or ≥ 48 h after | |||
| EP inclusion criteria | QRS ≥ 114 or other signal averaged ECG abnormalities | NSVT (3–30 beats at rate >120) | VE | Excluded VT, VF, symptomatic brady | Asymptomatic NSVT (>3 beats, HR > 100, lasting <30s) | NSVT (3-15 beats, HR < 120) or < 10 PVC/h | None | QRS≥120 ms, PR ≥ 150 ms, SR | None | HR ≥ 90, or NSVT (≥3 beats, HR ≥ 150) | None |
| IHD definition | Undergoing CABG | Q wave or cardiac enzyme positive MI | Q wave, cardiac enzymes, fixed defect nuclear scan, akinesis ventriculography, CAD on angio | No stenosis > 70% at coronary angiography | Absent CAD or out of proportion to CAD | Clinically significant CAD on angio or negative stress imaging | Recent MI | Not specified | ≥75% narrowing of major artery, prior MI | STEMI or NSTEMI | No significant CAD on invasive or CT angiogram, or normal MPS. Allowed 2 stenosed coronaries if felt not significant. |
| Time after MI | – | >3 weeks | >1 month | NA | NA | NA | 6–40 days | – | – | 5–31 days | NA |
| ICD type | Epicardial | Epicardial 47% | Transvenous | Transvenous | Transvenous | Transvenous | Transvenous | Transvenous | Transvenous | Transvenous | Transvenous |
| Transvenous 53% | |||||||||||
| CRT implantation permitted | – | – | – | – | – | Yes | – | Yes | – | – | Yes |
| Age (mean±sd) | 64±9 | 63 | 65 ± 10 | 52 ± 11 | 59 ± 12 | 58 (range 20–84) | 62 ± 11 | 67 | 60 | 63 ± 11 | 64 |
| Male | 84% | 92% | 85% | 80% | 70% | 71% | 76% | 68% | 77% | 77% | 73% |
| ACEi/ARB | 54% | 62% | 70% | 96% | 86% | 97% | 95% | 89% | 96% | 82% | 97% |
| BB | 21% | 23% | 70% | 4% | 52% | 85% | 87% | 68% | 69% | 98% | 92% |
| CRT | 0% | 0% | NR | NR | NR | 2% | NR | 100% | NR | NR | 58% |
| LVEF | 27% (Mean) | 26% (Mean) | 23% (Mean) | 24% (Mean) | 23% (Mean) | 21% (Mean) | 28% (Mean) | 21% (Median) | 25% (Median) | 35% (Mean) | 25% (Median) |
| QRS width (ms) | NR (73% >100 ms) | NR | NR (51% > 120 ms) | 108 | NR | 115 | 106 | 160 | NR | NR | CRT 160No CRT 108 |
| QRS normal | NR | NR | 49% | 64% | NR | NR | NR | NR | NR | NR | NR |
| QRS abnormal | LBBB 11% | LBBB 8% | LBBB 19% | LBBB 30% | LBBB 48% | LBBB 20% | NR | LBBB 71% | NR | LBBB 8% | CRT LBBB 94%, RBBB 3% No CRT LBBB 17%, RBBB 5% |
| RBBB 8% | RBBB 1% | RBBB 12% | RBBB 3% | RBBB 11% | |||||||
| NYHA I | 37% | 0% | 16% | 22% | 13% | 0% | Excluded | Recruited | 0% | ||
| NYHA II | 73% (II and III) | 65% (II and III) | 35% | 65% | 64% | 57% | 60% | 0% | Recruited | Recruited | 53% |
| NYHA III | 24% | 35% | 20% | 21% | 27% | 86% | Recruited | Recruited | 45% | ||
| NYHA IV | 4% | 0% | 0% | 0% | 0% | 14% | Excluded | Excluded | 1% | ||
| Hypertension | NR | 42% | 53% | NR | 63% | NR | 46% | NR | 56% | 66% | 31% |
| Diabetes | 38% | 6% (IDDM) | 36% | NR | 34% | 23% | 30% | NR | 31% | 34% | 19% |
| Atrial fibrillation | NR | NR | NR | NR | NR | 25% | NR | NR | 15% | 14% | 22% |
ICD, implantable cardioverter defibrillator; CRT-D/P, cardiac resynchronization therapy-defibrillator/pacemaker; SMT, standard medical therapy; ACM, all-cause mortality; MI, myocardial infarction; NSVT, non-sustained ventricular tachycardia; NYHA, New York Heart Association Functional Classification; DCM, dilated cardiomyopathy; HF, heart failure; OMT, optimal medical therapy; VE, ventricular ectopics; VT, ventricular tachycardia; VF, ventricular fibrillation; CAD, coronary artery disease; MPS, myocardial perfusion scintigraphy; CABG, coronary artery bypass graft; NA, not applicable.
Risk of bias assessment
Trial quality was assessed using Cochrane risk of bias tool (Table 2). There was no effective blinding of therapy in any of the trials. We assessed our primary end-point of all-cause mortality as having a low risk of bias. End-points requiring clinical judgement, such as sudden cardiac death and cardiovascular death, are at risk of bias if assessors are not blinded. Only five6,8,20,21,26 of the eleven trials reported on procedures to blind end-point assessment. Secondary outcomes were poorly reported, and often used different statistical measures to the primary outcome.
Table 2.
Risk of bias
| Trial | CABG-Patch | MADIT I | MADIT II | CAT | AMIOVIRT | DEFINITE | DINAMIT | COMPANION | SCD-HeFT | IRIS | DANISH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 1996 | 1996 | 2002 | 2002 | 2003 | 2004 | 2004 | 2004 | 2005 | 2009 | 2016 |
| Author | Bigger | Moss | Moss | Bansch | Strickberger | Kadish | Hohnloser | Bristow | Brady | Steinbeck | Køber |
| Random sequence generation (selection bias) | Low risk | Unclear–not reported | Unclear–not reported | Low risk–central randomization | Unclear–not reported | Unclear-not reported | Low risk–central randomization with stratification | Unclear–not reported | Low risk | Low risk | Low risk–Web-based randomization with stratification |
| Allocation concealment (selection bias) | Unclear | Unclear–not reported | Unclear–not reported | Low risk– “closed envelopes with the assigned study group were sent to each centre … envelopes were opened when a patient was enrolled” | Unclear–not reported | Unclear-not reported | Unclear–not reported | Unclear–not reported | Low risk | Unclear–not reported | Low risk |
| Blinding of participants and personnel (performance bias) | High–“nature of the intervention precluded the blinding of investigators or patients” | High risk | High risk | High risk | High risk | High risk | High risk | High risk–“patients, physicians… were not blinded to the treatment assignments” | High risk | High risk | High risk |
| Blinding of outcome assessment (performance bias) | Unclear–“accumulating data were reviewed by an independent Data and Safety Monitoring Board”, but no report of whether outcomes were blindly assessed | Unclear–“two member end-point subcommittee reviewed information on the causes and circumstances of deaths”, but no report on whether blinded | Unclear–not reported | Unclear–not reported | Low–“events committee determined the cause of death” … “independently evalutated all information available” and “to assure a blinded review, all references to amiodarone or ICD therapy was removed from the reviewed documents” | Low - “cause of death was determined by an events committee… unaware of patients’ treatment assignment” | High – “ascertainment of the cause of death was the responsibility of the local investigators”, but a “blinded central validation committee independently reviewed information on all deaths” | Low–“steering committee and endpoint committee were unaware of the treatment assignments” | Unclear–not reported | Low–“adverse-event committee that was unaware of the treatment assignments classified” the causes of death | Low–“endpoint classification committee, the members of which were unaware of the treatment assignments, used prespecified criteria to adjudicate all prespecified cinical outcomes” |
| Incomplete outcome data (attrition bias) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Selective reporting (reporting bias) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Other bias | Trial funded by CPI/Guidant who supplied devices, but had no role in design, analysis, interpretation or writing. | Trial funded by CPI/Guidant who supplied devices, but had no role in design, analysis, interpretation or writing. | Trial funded by CPI/Guidant who supplied devices, but had no role in design, analysis, interpretation or writing. | Trial funded by CPI/Guidant who supplied devices, but had no role in design, analysis, interpretation or writing. | Supported in part by an unrestricted research grant from the Guidant Corporation | Trial funded by St Jude who supplied devices, but had no role in design, analysis, interpretation or writing. | Trial funded by St Jude who supplied devices, but had no role in design, analysis, interpretation or writing. | Trial funded by Guidant who supplied devices, but had no role in design, analysis, interpretation or writing. | Trial funded by Medtronic who supplied devices, but had no role in design, analysis, interpretation or writing. | Trial funded by Medtronic who supplied devices and had access to the final pre-submission manuscript | Trial funded by Medtronic, St Jude, TrygFonden, but had no role in design, analysis, interpretation or writing. |
Populations studied
Across the 11 trials, the mean age was 63.1 years. Most trials enrolled patients with an EF ≤ 35%; two trials enrolled those with an EF ≤ 30%,19,23 and one enrolled those with an LVEF ≤ 40%.26
All trials included patients with NYHA Class III symptoms. In 5 trials only patients who were NYHA Class II and III were included. Three trials included patients with NYHA Class IV symptoms, but these accounted for only a small proportion of patients (14%, 4%, 1%). One trial6 did not recruit NYHA Class II patients. 5 trials8,21,23,25,26 included NYHA Class I patients.
The electrophysiology inclusion criteria varied between the trials with 6 trials enrolling based on previous NSVT or ectopics, and 5 having no specific electrophysiological inclusion criteria.
In one trial,22 ICDs were placed with epicardial leads during coronary artery bypass grafting (CABG) surgery. In one trial,24 47% were placed with epicardial leads and 53% placed with transvenous leads. In all other studies transvenous leads were used. The studies enrolling patients with chronic IHD recruited patients at least 3 weeks after previous MI; those enrolling patients with acute MI within 31 days26 or 40 days of an MI.25 Baseline characteristics, inclusion, and exclusion criteria are detailed in Table 1.
Effect on all-cause mortality
Left ventricular dysfunction without ischaemic heart disease
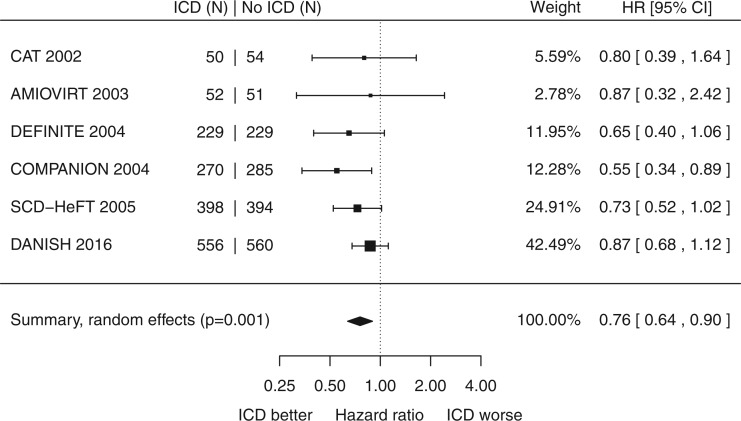
Across the 3128 patients without ischaemic heart disease, there was a significant reduction in all-cause mortality with minor heterogeneity (HR 0.76, 95% CI 0.64 to 0.90, P = 0.001, I2 = 3%, Figure 2). The annualized mortality rate in control patients was 5.4%.
Figure 2.
Title: Left ventricular dysfunction without ischaemic heart disease: impact of primary prevention ICD on all-cause mortality.
A sensitivity analyses, carried out by omitting each of the trials in turn, in each case shows a statistically significant consensus reduction in mortality (see Supplementary material online, Appendix S4). A funnel plot did not show any significant asymmetry (Egger’s test P = 0.5, Supplementary material online, Appendix S5).
Left ventricular dysfunction with ischaemic heart disease
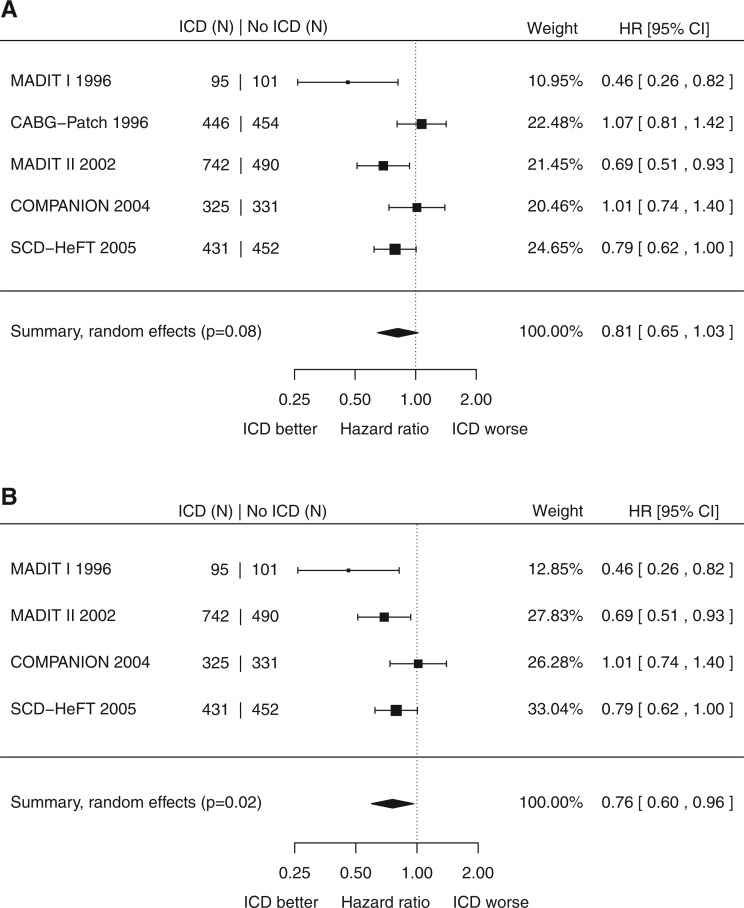
Across the 3867 patients in all trials of primary prevention ICD therapy with ischaemic heart disease and no recent MI, there was a non-significant reduction in all-cause mortality (pooled HR 0.81, 95% CI 0.65 to 1.03, P = 0.08, Figure 3A). However, there was substantial heterogeneity (I2 = 62%). One trial22 was unique in inserting the ICD at the time of CABG surgery. There was a 16% higher infection rate in the ICD group, with 4.3% requiring removal. Current practice is to minimize infection risk by implanting the cardiac device separately from any open surgery. Running the analysis for the trials that tested this approach showed a significant reduction in mortality (HR 0.76, 95% CI 0.60 to 0.96, P = 0.02, I2 52%, Figure 3B). The annualized mortality rate in the control patients was 11.3%. A funnel plot did not show any significant asymmetry (Egger’s test P = 0.2, Supplementary material online, Appendix S5).
Figure 3.
(A) Title: Left ventricular dysfunction with ischaemic heart disease: impact of primary prevention ICD on all-cause mortality. (B). Title: Left ventricular dysfunction with ischaemic heart disease: impact of primary prevention ICD implanted during a dedicated procedure on all-cause mortality.
Left ventricular dysfunction with acute myocardial infarction
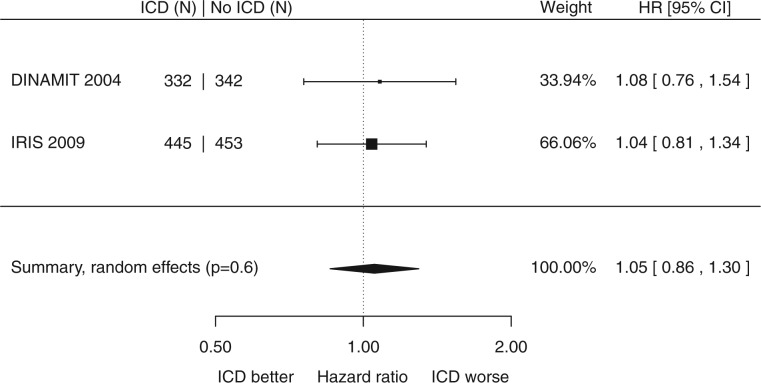
In the 2 trials that enrolled 1572 patients after an acute MI, ICD therapy did not cause a significant reduction in mortality (HR 1.05, 95% CI 0.86 to 1.30, P = 0.6, I2 = 0%, Figure 4). The annualized event rate in the control patients was 7.6%. Supplementary material online, Appendix S5 contains the funnel plot.
Figure 4.
Left ventricular dysfunction with acute myocardial infarction: impact of primary prevention ICD on all-cause mortality.
Effect on secondary outcomes
Secondary outcomes were inconsistently reported with not all trials presenting data. Some data were presented as raw counts from which risk ratios could be derived, and some as hazard ratios. ICD therapy was consistently associated with a statistically significant reduction in hazard ratio and risk ratios for all three groups (without IHD, with IHD, and acute MI) for sudden cardiac death (without IHD HR 0.4 RR 0.29; with IHD HR 0.38 RR 0.41; acute MI HR 0.49, RR 0.57, Supplementary material online, Appendix S3).
Discussion
Based on high-quality data from RCTs, this meta-analysis finds that primary prevention ICDs reduce all-cause mortality in patients with left ventricular dysfunction both with and without IHD. No benefit from ICDs is seen in the setting of acute myocardial infarction. These findings are consistent with the current ESC guideline recommended management.31,32
Patients without ischaemic heart disease
There has been controversy over the utility of ICDs in patients without IHD. Many of the published guidelines make a distinction between the aetiologies with respect to the level of evidence on which their recommendations are made. The 2015 European Society of Cardiology (ESC) ventricular arrhythmia guidelines,31 and the 2016 ESC heart failure guideline32 give ICDs for primary prevention a 1A recommendation for an ischaemic aetiology, and 1B for a non-ischaemic aetiology. Indeed, this uncertainty was the stimulus for conducting the recent DANISH study. Subsequent commentary3 has added to the uncertainty.
Part of this uncertainty may have arisen as mortality rate in patients without IHD is lower than those with IHD (5.4%/year vs. 11.3%/year, respectively), and consequently the confidence intervals are wider for individual trials.
However, all the point estimates lie in the range 0.55 to 0.87, and the trials showed minimal heterogeneity (I2 = 3%). The group without IHD in COMPANION was, even on its own, statistically significant for a reduction of all-cause mortality with ICD (see Supplementary material online, Appendix S2), although this was not the chosen central message of the COMPANION primary publication.
Our meta-analysis confirms a statistically significant reduction in all-cause mortality by primary prevention ICD in patients without IHD. Whilst only one trial was individually significant, the point estimates from all 6 trials were in the same direction, suggestive of benefit. Furthermore, even omitting both COMPANION and the recent DANISH trial from the meta-analysis still produces a statistically significant consensus reduction in mortality (see Supplementary material online, Appendix S4).
Patients with ischaemic heart disease
This meta-analysis supports the current consensus that ICDs reduce all-cause mortality in left ventricular dysfunction with IHD, in the trials that use the current clinical convention of a dedicated device implant procedure. Interestingly, the reduction in hazard ratio is numerically the same (24%) in patients with and without IHD. Consequently, when considering ICD therapy, distinctions between the two groups may be unnecessary.
In acute myocardial infarction, however, there is no indication of a reduction in all-cause mortality.
Difference between this meta-analysis and previous meta-analyses
Our meta-analysis is the first to include the results of the patients without IHD from the COMPANION and DANISH trials. Other meta-analyses33 have omitted COMPANION, presumably because the paper did not display the hazard ratio explicitly. However, the hazard ratio and its confidence interval can be calculated from the steps shown in Supplementary material online, Appendix S2. The current meta-analysis therefore provides important new information regarding the role of ICD therapy in patients with left ventricular dysfunction without IHD.
Study limitations
Any meta-analysis can only examine studies that have actually been carried out. Different studies took different approaches to recruitment. However, it is notable that all six non-ischaemic trial results were concordant not only in the direction of effect, but also the approximate magnitude, with the I2 statistical test showing minor heterogeneity.
In the case of the COMPANION trial, the hazard ratio was calculated using the information published in the primary publication by steps shown in Supplementary material online, Appendix S2. The original publication did not comment on this hazard ratio. It is wise to be cautious of results of sub-group analyses, because many such analyses are possible and some will be positive by chance alone. However, the single most important dichotomy in current guidelines31,32 for primary prevention ICDs in left ventricular systolic dysfunction is the presence vs. absence of ischaemic heart disease. Therefore, this sub-group analysis need not be assumed to be a random result selected from many possible sub-groups analyses. Moreover, all six groups of patients without ischaemic heart disease showed the same direction of effect. Furthermore, the finding is stable to the removal of any one trial (see Supplementary material online, Appendix S4).
Background medical therapy has improved over the time-course of these trials, with only 4% treated with beta-blockers in the CAT (2002), but 92% in DANISH (2016). Whilst the relative mortality-reduction effect size has remained remarkably consistent over time this will reduce the absolute effect size (when analysed over a fixed time window) of ICDs for primary prevention.
Our study could not consider the degree to which comorbidities might affect results. It has been noted that patients recruited into trials often have fewer comorbidities than those in the general population. The external validity of RCTs is always challenged by this, particularly in conditions such as heart failure where comorbidities may be frequent and severe.34 Furthermore, whilst this meta-analysis finds that stratifying by the presence or absence of ischaemic heart disease does not influence the mortality benefit of ICDs in primary prevention, other factors might. Supplementary material online, Appendix S4 includes data stratified by the presence or absence of CRT, but this analysis is hindered by the limited data in CRT group which is derived from COMPANION6 and a sub-group of DANISH.20 The 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy35 similarly recognize that limited RCT data is available for the comparison between CRT-P and CRT-D. The guidelines suggest clinical conditions such as advanced or end-stage cardiac or renal disease may favour CRT-P over CRT-D.
Clinical implications
The challenge facing clinical trials, as highlighted by McMurray,3 is that skilful modern treatment algorithms have reduced event rates down to low levels in the types of patients who would be eligible for, and willing to enter, randomized controlled trials; the annualized rate is 5.4% in patients without IHD. In light of this perhaps, we should pay maximal attention to information that RCTs give us.
The low event rate in the trials is why viewing multiple trials is necessary to see the survival benefit. However, the 24% risk reduction is as sizable as one might realistically hope for, for any intervention. This meta-analysis provides strong support for the role of primary prevention ICDs in patients with left ventricular dysfunction. A 24% risk reduction in all-cause mortality is comparable with other therapies which we recommend in heart-failure such as candesartan36 or an angiotensin-neprilysin inhibitor (HR 0.77, 0.84, respectively).37
Conclusions
In patients with left ventricular dysfunction, primary prevention ICDs reduce mortality. ICDs reduce mortality by 24% in both patients with (P = 0.03) and without IHD (P = 0.0023).
When deciding on ICD therapy, classification of heart failure by aetiology may therefore not be useful.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the British Heart Foundation [grant numbers FS/14/27/30752 (MJSS), FS/12/12/29294 (GC), FS/13/44/30291 (ZW), FS/10/038 (DPF)].
Conflict of interest: M.J.S.S., S.Z., J.P.H., G.C., and D.P.F. declare no conflict of interest. ZW has received speaker fees from St. Jude, and a research grant unrelated to this work from Medtronic.
Supplementary Material
References
- 1. Betts TR, Sadarmin PP, Tomlinson DR, Rajappan K, Wong KCK, Bono JP. D, Bashir Y.. Absolute risk reduction in total mortality with implantable cardioverter defibrillators: analysis of primary and secondary prevention trial data to aid risk/benefit analysis. Europace 2013;15:813–819. [DOI] [PubMed] [Google Scholar]
- 2. Kusumoto FM, Calkins H, Boehmer J, Buxton AE, Chung MK, Gold MR, Hohnloser SH, Indik J, Lee R, Mehra MR, Menon V, Page RL, Shen W-K, Slotwiner DJ, Stevenson LW, Varosy PD, Welikovitch L.. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation 2014;130:94–125. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJV. The ICD in heart failure–time for a rethink? N Engl J Med 2016;375:1283–1284. [DOI] [PubMed] [Google Scholar]
- 4. Plot Digitizer [Internet]. Available from: http://plotdigitizer.sourceforge.net/. (24 January 2017).
- 5. Guyot P, Ades AE, Ouwens MJNM, Welton NJ.. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, Marco TD, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM.. Comparison of medical therapy, pacing, and defibrillation in heart failure (COMPANION) investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 7. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR.. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strickberger SA, Hummel JD, Bartlett TG, Frumin HI, Schuger CD, Beau SL, Bitar C, Morady F.. AMIOVIRT Investigators. Amiodarone versus implantable cardioverter-defibrillator: randomized trial in patients with nonischaemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia–AMIOVIRT. J Am Coll Cardiol 2003;41:1707–1712. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC.. Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins JPT, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Egger M, Davey Smith G, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2016. Available from: https://www.R-project.org/. (24 January 2017).
- 13. Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. Journal of Statistical Software 2010;36:1–48.
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG.. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bigger JT, Whang W, Rottman JN, Kleiger RE, Gottlieb CD, Namerow PB, Steinman RC, Estes NA.. Mechanisms of death in the CABG Patch trial: a randomized trial of implantable cardiac defibrillator prophylaxis in patients at high risk of death after coronary artery bypass graft surgery. Circulation 1999;99:1416–1421. [DOI] [PubMed] [Google Scholar]
- 16. Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML.. MADIT-II Investigators. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II). J Am Coll Cardiol 2004;43:1459–1465. [DOI] [PubMed] [Google Scholar]
- 17. Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, Boehmer JP, Carlson MD, Frantz RP, McNulty SE, Rogers JG, Anderson J, Johnson GW, Walsh MN, Poole JE, Mark DB, Lee KL, Bardy GH.. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation 2009;120:2170–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH.. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352: 225–237. [DOI] [PubMed] [Google Scholar]
- 19. Bänsch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, Block M, Gietzen F, Berger J, Kuck KH.. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation 2002;105:1453–1458. [DOI] [PubMed] [Google Scholar]
- 20. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjær H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbæk R, Hassager C, Svendsen JH, Høfsten DE, Torp-Pedersen C, Pehrson S.. DANISH Investigators. Defibrillator implantation in patients with nonischaemic systolic heart failure. N Engl J Med 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 21. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NAM, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH.. Defibrillators in Non-Ischaemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischaemic dilated cardiomyopathy. N Engl J Med 2004;350: 2151–2158. [DOI] [PubMed] [Google Scholar]
- 22. Bigger JT. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med 1997;337:1569–1575. [DOI] [PubMed] [Google Scholar]
- 23. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML.. Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 24. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M.. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 25. Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ.. DINAMIT Investigators. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med 2004;351:2481–2488. [DOI] [PubMed] [Google Scholar]
- 26. Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, Kornacewicz-Jach Z, Sredniawa B, Lupkovics G, Hofgärtner F, Lubinski A, Rosenqvist M, Habets A, Wegscheider K, Senges J.. IRIS Investigators. Defibrillator implantation early after myocardial infarction. N Engl J Med 2009;361:1427–1436. [DOI] [PubMed] [Google Scholar]
- 27. Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, Mitchell LB, Green MS, Klein GJ, O’brien B.. Canadian implantable defibrillator study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation 2000;101:1297–1302. [DOI] [PubMed] [Google Scholar]
- 28. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The antiarrhythmics versus implantable defibrillators (AVID) investigators. N Engl J Med 1997;337: 1576–1583. [DOI] [PubMed] [Google Scholar]
- 29. Kuck KH, Cappato R, Siebels J, Rüppel R.. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation 2000;102:748–754. [DOI] [PubMed] [Google Scholar]
- 30. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G.. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 1999;341:1882–1890. [DOI] [PubMed] [Google Scholar]
- 31. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Veldhuisen DJ., Van. . Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the european society of cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;17:1601–1687. [DOI] [PubMed] [Google Scholar]
- 32. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Meer P., van der. Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 33. Colquitt JL, Mendes D, Clegg AJ, Harris P, Cooper K, Picot J, Bryant J.. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure: systematic review and economic evaluation. Health Technol Assess 2014;18:1–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boriani G, Berti E, Belotti LMB, Biffi M, Palma R, De Malavasi VL, Bottoni N, Rossi L, Maria E, De Mantovan R, Zardini M, Casali E, Marconi M, Bandini A, Tomasi C, Boggian G, Barbato G, Toselli T, Zennaro M, Sassone B.. RERAI (Registry of Emilia Romagna on Arrhythmia Interventions) Investigators. Cardiac device therapy in patients with left ventricular dysfunction and heart failure: “real-world” data on long-term outcomes (mortality, hospitalizations, days alive and out of hospital). Eur J Heart Fail 2016;18:693–702. [DOI] [PubMed] [Google Scholar]
- 35. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt O-A, Cleland J, Deharo J-C, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE.. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34: 2281–2329. [DOI] [PubMed] [Google Scholar]
- 36. Granger CB, McMurray JJV, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K.. Charm Investigators Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. The Lancet 2003;362:772–776. [DOI] [PubMed] [Google Scholar]
- 37. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.