Abstract
The small ubiquitin-like modifier (SUMO) is as a regulator of many cellular functions by reversible conjugation to a broad number of substrates. Under endogenous or exogenous perturbations, the SUMO network becomes a fine sensor of stress conditions by alterations in the expression level of SUMO enzymes and consequently changing the status of SUMOylated proteins. The diaphragm is the major inspiratory muscle, which is continuously active under physiological conditions, but its structure and function is severely affected when passively displaced for long extents during mechanical ventilation (MV). An iatrogenic condition called Ventilator-Induced Diaphragm Dysfunction (VIDD) is a major cause of failure to wean patients from ventilator support but the molecular mechanisms underlying this dysfunction are not fully understood. Using a unique experimental Intensive Care Unit (ICU) rat model allowing long-term MV, diaphragm muscles were collected in rats control and exposed to controlled MV (CMV) for durations varying between 1 and 10 days. Endogenous SUMOylated diaphragm proteins were identified by mass spectrometry and validated with in vitro SUMOylation systems. Contractile, calcium regulator and mitochondrial proteins were of specific interest due to their putative involvement in VIDD. Differences were observed in the abundance of SUMOylated proteins between glycolytic and oxidative muscle fibers in control animals and high levels of SUMOylated proteins were present in all fibers during CMV. Finally, previously reported VIDD biomarkers and therapeutic targets were also identified in our datasets which may play an important role in response to muscle weakness seen in ICU patients. Data are available via ProteomeXchange with identifier PXD006085. Username: reviewer26663@ebi.ac.uk, Password: rwcP5W0o.
There is increasing evidence that post-translational modification mediated by the small ubiquitin modifiers SUMO1, SUMO2, and SUMO3 control a wide range of cellular functions (1–4), such as gene expression regulation and organ development (5, 6). SUMOylation is an ATP-dependent reaction where proteins are covalently attached to specific lysine residues in target proteins by a multistep process involving a heterodimer-activating enzyme E1 (SAE1/SAE2), a conjugating enzyme E2 (UBC9), and a group of E3 ligases, including the PIAS family (7, 8), TOPORS (9, 10), RanBP2 (11), Polycomb2 (12), TRAF7 (13), the mitochondrial protein MUL1 (14), and the TRIMs family (15). The SUMO E3 ligases drive the conjugation efficiently by promoting poly-SUMO2/3 chain formation or by generating additional mono-SUMO1 conjugates to targets or to poly-SUMO2/3 chains as a moiety terminator (16). Maturation of SUMO precursors and removal of SUMO moieties from the targeted substrates are catalyzed by either one of six SUMO-specific peptidases, SENPs (17, 18), or by the newly identified USPL1 (19). Another important class of proteins associated with SUMOylation that contain SUMO-interacting motifs (SIMs) are the SUMO-targeted ubiquitin ligases (STUbL) (20) that bind ubiquitin to poly-SUMOylated substrates for the proteasome degradation (21).
Several model systems (yeast (22), Trypanosoma (23), Drosophila (24), Caenorhabditis elegans (25, 26), Arabidopsis thaliana (27), and mammalian organs like cardiac muscle (28) and brain (29)) have been used to study the roles of the SUMO machinery components. In these studies, SUMOylated targets were identified under normal physiological and stressful (cellular stress (30), heat shock (31), bacteria-virus infections (32–34)) and pathological conditions (35) (cancers (36–40), vascular-cerebral and ischemia/stroke (41), central nervous system (42, 43), and cardiac diseases (6, 44)).
The diaphragm is the dominant inspiratory muscle, and the continuous rhythmic activation during breathing makes it one of the most active muscles in the body (45). The respiratory muscle function can be affected by severe diaphragmatic weakness, most frequently observed in intensive care units (ICUs) 1 where patients are subjected to CMV. CMV is a lifesaving intervention in critically ill ICU patients involving ventilator support to maintain sufficient pulmonary gas exchange. Positive-pressure ventilators increase the patient's airway pressure into the lungs through an endotracheal or tracheostomy tube subjecting the respiratory muscle to a passive load (46). However, prolonged CMV results in the rapid development of VIDD (47). VIDD has significant clinical consequences for the patient's quality of life, mortality/morbidity, and staggering costs for modern health care (48).
In our previous experimental studies, time-resolved analyses of the effects of CMV on the diaphragm from 6 h to 2 weeks have shown that CMV has a severe negative impact on the diaphragm structure and function, such as muscle fiber atrophy, and decreases muscle fiber force-generating capacity (maximum force normalized to muscle fiber area or specific force) (49). When the anti-oxidant and anti-inflammatory BGP-15 drug was administered to long-term CMV rats (10 days), we discovered that diaphragm fibers rescued the force-generating capacity by ∼100% in parallel with a mitochondrial respiration improvement and a reduction in reactive oxygen species production. Those factors were crucial to prove the drug effects to alleviate VIDD (50).
In this study, for the first time, we identified and characterized endogenous SUMO-related proteins and the SUMO-conjugating/deconjugating enzymes in the diaphragm from rats exposed to neuromuscular blockade, sedation, and CMV for duration variables between 6 h and 14 days. The SUMO network was severely altered in rat diaphragm muscle during mechanical ventilation compared with controls. A significant increase of poly-SUMOylated muscle proteins was observed immediately after 1 day of CMV, in parallel with changes in transcript and protein levels and muscle localization of the major SUMO regulatory enzymes.
EXPERIMENTAL PROCEDURES
Experimental Design and Statistical Rationale
To cover the intrinsic variability associated with the single experimental rat, we collected different numbers of diaphragms isolated from various animals within the same period of mechanical ventilation treatment to have approximately a pool of 200 mg of muscles per group (supplemental Table 1). For each group, 5 muscles were collected for the control, 10 muscles for the 1st day, 9 muscles for the 5 days, 10 muscles for the 10 days, and 5 muscle for the 10 days on BGP-15. The aim was to obtain biological replicates that allowed us to achieve adequate reliability and consistency for the proteomics analyses.
Muscle Lysates for SUMOylated Protein Enrichment and Mass Spectrometry
For each control and mechanically ventilated group, a total of ∼200 mg of diaphragm muscles tissue were collected from the samples listed in supplemental Table 2. Solutions, muscle lysates, preparation of protein G-agarose beads coupled with monoclonal anti-SUMO1 and anti-SUMO2 produced by hybridoma cells, SUMO immunoprecipitation, peptide elution, and recycling of the affinity matrices were performed according to the Becker et al. (51) and Barysch et al. (52) protocols. In our procedure, muscles were lysed in an optimized lysis buffer (Tris-Cl 150 mm, NaCl 150 mm, SDS 0.5%, Nonidet P-40 1%, deoxycholate 0.5%, EDTA 5 mm, DTT 1 mm, fresh N-ethylmaleimide 20 mm, and protease inhibitors, pH 7.6) before the incubation with protein G-agarose beads coupled to anti-SUMO antibodies; muscle lysates were also precleared with protein G-agarose beads coupled to anti-normal mouse IgG for 3 h and gently rotated at 4 °C. Precleared muscle lysates were divided into two aliquots: one aliquot was incubated with protein G-agarose beads coupled to anti-SUMO1 antibodies (50% 21C7 and 50% 76–86) for SUMO1 complex enrichment or with protein G-agarose beads coupled with anti-SUMO2/3 antibodies (8A2) for SUMO2/3 complex enrichment. To analyze potential SUMO targets with mass spectrometry (MS), a total of 150 μg of TCA-precipitated peptide-eluted samples were fractionated and separated with SDS-PAGE using a 4–12% gradient gel and subsequent Coomassie Blue staining.
Mass Spectrometry Sample Preparation
The proteins were in-gel digested essentially according to the method described by Shevchenko et al. (53). Briefly, 80 gel sections were cut into small (1 mm3) pieces, destained using acetonitrile (ACN), washed, and exposed to dithiothreitol (DTT) reduction and iodoacetamide alkylation. Thereafter, the proteins were digested by sequencing grade modified trypsin at a concentration of 12.5 ng/μl in 25 mm ammonium bicarbonate, pH 8, overnight at 37 °C. The peptides were extracted by sonication in 60% ACN and 5% formic acid (FA). Finally, the extracted peptides were dried to completion and dissolved in 0.1% FA.
Liquid Chromatography-Tandem Mass Spectrometric (LC-MS/MS) Analysis
The peptides from each gel section were analyzed using a Q Exactive HF Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with an EASY-Spray ion source (Thermo Fisher Scientific). The peptides were separated by reversed phase LC using a Dionex UltiMate 3000 RSLC nanosystem (Thermo Fisher Scientific). The separation was performed on an Acclaim PepMap C18 (2-μm particles, 75 μm inner diameter × 50 cm) column. Peptides were eluted with a 70-min long gradient: 2% B for 5 min, 2–20% B in 50 min, 20–32% B in 10 min, 32–95% B in 1 min, and 95% B for 4 min. Solvent A was 0.1% FA in MilliQ H2O, and solvent B was 0.1% FA in 20% in MilliQ H2O 80% ACN. The peptides were ionized by positive electrospray and then introduced to the MS. The MS was operated in positive ion mode (m/z 375–1500) using an automated gain control target of 3 × 106 at a resolution of 60,000 and an injection time of 60 ms. Data-dependent acquisition was applied. Consecutive higher-energy collisional dissociation fragmentation spectra of the 20 most abundant ions were collected at a resolution of 15,000. These MS/MS spectra were generated with an automated gain control target of 1 × 105. The injection time was 200 ms; the normalized collision energy was set to 27, and the dynamic exclusion time was 20 s. Matching between runs were not enabled. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (www.proteomexchange.org) via the PRIDE (54) partner repository with the data set identifier PXD006085 (username: reviewer26663@ebi.ac.uk; password: rwcP5W0o). The acquired raw data files were analyzed with the Proteome Discoverer 1.4.0.288 (Thermo Fisher Scientific) software using the SEQUEST HT® (University of Washington) search engine against proteins from Rattus norvegicus in the UniProtKB/SwissProt database downloaded August, 2015, containing 8046 sequences and 4,074,405 residues. The search parameters included the following: maximum 10 ppm and 0.6-Da error tolerance for the survey scan and MS/MS analysis, respectively; enzyme specificity was trypsin; maximum of two missed cleavage sites allowed; cysteine carbamidomethylation was set as static modification; oxidation (M) and deamidation (N,Q) were set as variable modifications. The Percolator node and a decoy database were used to estimate false discovery rate (FDR) and an FDR of 5% for peptide identification was accepted. The protein identifications were based on at least two matching peptides per protein. Two approaches were used for the database analyses. First, each sample (gel section) was searched individually, and second, the raw data for all eight samples from one gel lane were combined into one search, respectively, using Multidimensional Protein Identification Technology processing. Functional annotations of the identified proteins in the Multidimensional Protein Identification Technology searches were performed in Proteome Discoverer 2.0 (Thermo Fisher Scientific) using the Gene Ontology (GO) database. Multiconsensus reports and Venn diagrams for comparisons of the control and treated samples for each category, SUMO1 and SUMO2/3, respectively, were created in Proteome Discoverer 1.4.0.288 (Thermo Fisher Scientific). The pathway analysis was through the use of Qiagen's Ingenuity Pathway Analysis (IPA®,Qiagen Redwood City, CA). Unique identified proteins from SUMO1 and SUMO2 were uploaded to the analysis server (IPA 2015 winter release) and mapped to the entire ingenuity pathway knowledgebase. The significance of pathway overlapping was calculated by Fisher's exact t test. A p value lower than 0.05 was considered as statistically significant.
Bacteria SUMOylation Assay
The plasmids with the complete SUMO-conjugating system (pSUMO1, -2, -3, containing streptomycin resistance) were purchased from Addgene plasmid IDs: 52258, 52259, and 52260 (55). Escherichia coli BL21 cells were used for chemical transformation with the pGEX-5X-1 plasmids containing the SUMO candidate ORFs together with the SUMO-conjugating system vectors. Exponential cultures were grown in the presence of 100 mg/liter ampicillin or 50 mg/liter streptomycin. Protein expression was induced by the addition of the isopropyl β-d-thiogalactoside (I1000-10 from Saveen Werner, Sweden) to the final concentrations of 250 μm for 3 h at 30 °C and then transferred at 37 °C for 30 min. Finally, cells were harvested by centrifugation (3000 rpm for 10 min at 4 °C), and soluble protein fractions were extracted by sonication in lysis buffer (50 mm Tris-Cl, pH 7.5, 300 mm NaCl, 0.1% Nonidet P-40, 0.05% SDS, 1 mm DTT, 20 mm N-ethylmaleimide, protease inhibitors). Crude lysates were cleared by centrifugation (13,000 rpm for 10 min at 4 °C) and separated with SDS-polyacrylamide gel after denaturation in the presence of loading buffer.
Eukaryotic SUMO Assay
Two μg of eukaryotic plasmids containing the SUMO candidate ORFs were transfected in HeLa cells in presence of Mock vector and with 0.5 and 2 μg of recombinant Myc-Ubc9 using a commercial TurboFect transfection reagent kit (R0531, Thermo Fisher Scientific). After 24 h, cells were harvested, washed with cold PBS in the presence of 0.2 m iodoacetamide, and lysed with lysis buffer. Protein concentration was measured, and 20 μg were loaded and fractionated in an SDS-polyacrylamide gel. PVDF membranes were incubated with the respective antibodies to detect the overexpression and SUMOylation of the candidates.
Rat Mechanical Ventilation Treatment and Muscle Isolation
The experimental female Sprague-Dawley rats (Taconic Biosciences, Inc., Denmark) were deeply sedated by isoflorane inhalation and paralyzed with α-cobratoxin (Biotoxin, Inc., St. Cloud, FL) from the beginning to the end of the mechanical ventilation experimental period. All surgeries were performed under sterile techniques and conditions (56, 57). Animals were maintained in protein and fluid balance with an intra-arterial solution (0.6 ml/h) prepared with 50 ml of H2O, 50 ml of 0.5 n lactated Ringer's solution, 1.25 g of sodium oxacillin, 2.8 mg of α-cobratoxin, 0.3 mg of vitamin K (Synkavite), and 20 meq of K+ (KCl), as well as with an intravenous solution (0.6 ml/h) consisting of 50 ml of H2O, 50 ml of 0.5 n lactated Ringer's solution, 20% glucose, and 1.25 g of sodium oxacillin. The sham-operated control rats underwent the same intervention as the experimental animals but were not pharmacologically paralyzed with α-cobratoxin. Isoflurane was delivered into the inspiratory gas stream by a precision mass-flow controller. During surgery or at any possible irritating manipulation, isoflurane level was >1.5%, and after the initial surgery, isoflurane was gradually lowered over 1–2 days and maintained at <0.5% during all the treatment. Rats were ventilated through a per os coaxial tracheal cannula at 72 breaths/min with an inspiratory and expiratory ratio of 1:2 and a minute volume of 180–200 ml. Neuromuscular blockade was induced on the 1st day and maintained by continuous infusion. Mechanical ventilation was started immediately after the neuromuscular blockade induction. All necessary steps were taken to minimize animal suffering, and animals were monitored continuously to detect any pain reaction (EEG activity, heart rate, and intra-arterial blood pressure). At the end the experiment, animals were sacrificed by thoracotomy, and hearts were removed, followed by tissue harvesting. The ethics committee at Karolinska Institute approved all aspects of this study (N263/14).
Statistical Analysis
One-way analyses of variance and Tukey's test were used to compare multiple groups, and p < 0.05 was considered statistically significant. Data are presented as average ± S.D. See supplemental Methods for full details.
RESULTS
SUMOylated Proteins Were Increased in the Diaphragm during Controlled Mechanical Ventilation
The abundance of SUMOylated proteins was monitored in muscle biopsies listed in supplemental Table 1 using immunoblotting labeled with anti-SUMO1 and anti-SUMO2/3 antibodies are shown in representative Western blottings (Fig. 1, A and C). In control samples, the SUMO1 and SUMO2/3 antibodies detected weak SUMOylation protein profiles. In contrast, the intensity of the SUMO1- and SUMO2/3-conjugated proteins were detectable in muscle samples from animals ventilated for 6 h (1 day group) and increased progressively with increasing CMV duration, i.e. a 5- and 15-fold increase was observed for SUMO1 and SUMO2/3, respectively, after 10 days CMV compared with controls (Fig. 1, B and D). After 6 h CMV, additional distinct SUMO1 bands appeared above 75 kDa, corresponding to mono-SUMOylated proteins. In SUMO2/3 blots, poly-SUMO2/3-conjugated proteins appeared as a smear of high molecular weight species in diaphragm samples from rats ventilated for more than 1 day, and the intensity was increased from 7- to 15-fold during 5–10 days of CMV (Fig. 1D). In line with the notion that different types of stress induce the rapid accumulation of poly-SUMO2/3 conjugases (58), this result suggests a considerable involvement of several muscle proteins being SUMOylated during CMV by the SUMO2/3 moieties. A relevant accumulation of free SUMO1 was detected in muscle samples from day 1 and increased progressively with increasing CMV duration. On the contrary, free SUMO2/3 protein levels remained constant in all samples (Fig. 1, B and D). The effect of SUMO conjugation induced by CMV was reduced in diaphragm muscles when rats were administered the chaperone co-inducer BGP-15 drug (50, 59), but the level of free SUMO1 remained high compared with the 10-day group without drug treatment. Re-probing of the blots with antibodies to ubiquitin revealed an increase of ubiquitin conjugates between 5 and 10 days CMV, while no changes were detectable at a shorter duration of CMV (supplemental Fig. 1). This observation proposes that in the early stage of the CMV, most of the muscle proteins are not targeted from the poly-ubiquitination conjugation.
Fig. 1.
Poly-SUMOylated proteins accumulate during mechanical ventilation treatment in rat diaphragms. A and C, muscle samples listed in supplemental Table 1 were fractionated by SDS-polyacrylamide gel and probed with antibodies to SUMO1 and SUMO2/3. Representative Western blottings of diaphragm muscle lysates from controls (ctrl) and from ventilated rats from 6 h to 1 day (1d), from 1 to 5 days (5d), from 5 to 10 days without (10d), and in the presence of the drug BGP-15 (10d BGP-15) were fractionated by SDS-polyacrylamide gel, and PVDF membranes were probed with anti-SUMO1 (A) and anti-SUMO2 antibodies (C). B and D, quantification of free and conjugated SUMO1 and SUMO2/3. The intensities of free SUMO and SUMO conjugates identified by the dashed areas in A and B were quantified by densitometry in two independent experiments. The intensity band quantifications for each area were expressed in AU after normalization by the correspondent GAPDH loading control and referred to the control samples described in supplemental Table 1.
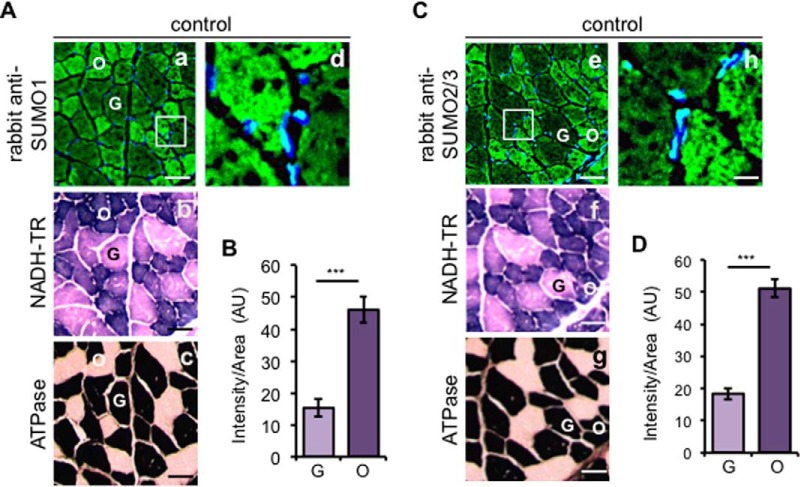
SUMOylated Proteins Are Distinctly Distributed between Different Types of Muscle Fibers
In the rat diaphragms, the majority of the muscle fibers are expressing type IIx myosin heavy chain isoform (MyHC), either alone or in combination with the IIb MyHC isoform, which have a low resistance to fatigue with a limited mitochondrial volume density (60, 61). The remaining fibers express the β/slow type I MyHC isoforms, which are fatigue-resistant and have a greater mitochondrial volume density (62). To explore the SUMO1 and SUMO2/3 distribution in the muscle fibers, cryosections from control diaphragm muscles were probed with specific SUMO1 and SUMO2/3 antibodies generated in rabbit. Confocal images revealed a high fluorescence intensity signal for both SUMO1 (Fig. 2A, panel a) and SUMO2/3 (Fig. 2C, panel e) staining on fibers with small cross-sectional areas (O) compared with large fibers (G). To understand which muscle fiber types contained distinct levels of SUMOylated proteins, consecutive muscle sections were probed with two different protocols to distinguish muscle fiber type according to their metabolism (oxidative and glycolytic) and myosin ATPase activity (type I and type II). According to the mitochondrial content, we classified in oxidative type (O) the small section of fibers colored in dark violet, and the large section area fibers, colored in pink, in glycolytic type (G) (Fig. 2, A, panel b, and C, panel f). The histochemical alkaline myosin ATPase activity, pH 10.3, classified fibers in slow-twitch type I (white) and fast-twitch type II (black) (Fig. 2, A, panel c, and C, panel g). The SUMO fluorescence distribution merged perfectly with the sections that distinguished the fibers into oxidative and glycolytic types by following the metabolic fiber classification instead of the myosin ATPase activity. The correspondent fluorescence quantification indicated a significant (***, p < 0.001) presence of SUMO signal in the oxidative fibers almost 3-fold higher than the glycolytic fibers: 15–54 for SUMO1 and 20–60 for SUMO2/3 fluorescence density (Fig. 2, B and D). To validate the experiment performed with anti-SUMO antibodies developed in rabbit and to exclude nonspecific staining, we repeated the staining of control muscle slides with anti-SUMO1 and anti-SUMO2/3 antibodies prepared from hybridoma mouse cells with and without peptide neutralization. The correspondent confocal images confirmed the previous results: oxidative fibers were highly abundant in SUMOylated proteins compared with the glycolytic type by giving the same fluorescence intensity and staining profile. We also validated the specific immunofluorescence generated by the hybridoma SUMO antibodies because the signal was abolished when immunoglobulin was preincubated with the specific SUMO peptides (supplemental Fig. 2). In line with the observation that SUMOs localized in cell nuclei, we demonstrate the presence of SUMO in myonuclei, with all SUMO antibodies raised in rabbits and in mice (Fig. 2, A, panel d, and C, panel h, and supplemental Fig. 2).
Fig. 2.
Immunofluorescence analysis of SUMO conjugates in control diaphragm. A and C, muscle fibers are differently stained with antibodies to SUMO1 and SUMO2/3. Cellular localization of free and conjugated SUMO1 (A, panel a) and SUMO2/3 proteins (C, panel e) on cryo-cross-sections were detected by immunofluorescence with anti-SUMO1 and anti-SUMO2/3 antibodies raised in rabbit and with secondary anti-rabbit AlexaFluor 488-conjugated antibodies. The white box areas are enlarged (A, panel d, and C, panel h), showing the nuclear localization of both SUMO1 and SUMO2/3. A, panel b, and C, panel f, consecutive serial muscle sections were subjected to NADH-TR staining to recognize the oxidative (O, dark purple) and glycolytic (G, light purple) fibers. A, panel c, and C, panel g, representation of ATPase, pH 10.3, staining to distinguish type I (white) and type II (black) fibers. B and D, mean fluorescence intensity per area and ±S.D. for SUMO1 (B) and SUMO2/3 (D) immunofluorescences were measured considering a number of 35 oxidative and 35 glycolytic fibers, respectively, from four different diaphragm control sections (***, p < 0.001) and expressed in AU. Scale bars, 50 μm (panels a–c and panels e–g) and 20 μm (panels d and h). Nuclei are stained with DAPI (blue). TR, Tetrazolium Reductase.
Distribution of SUMOylated Proteins Is Modulated between the Different Fibers in Mechanically Ventilated Rat Diaphragm
Combining the results presented in Figs. 1 and 2, we asked whether this general progressive and reproducible increase of conjugated SUMO1 and SUMO2/3 proteins affects independently all muscle fibers or is referred to a specific fiber type. For this purpose, at least three muscle samples for each time point of ventilation treatment were stained with anti-SUMO1 (Fig. 3A) and anti-SUMO2/3 (Fig. 3B) antibodies, and the consecutive serial muscle slides were treated with NADH-TR staining to identify the respective oxidative and glycolytic fibers. First, with SUMO1 staining, we observed no difference of fluorescence signal between oxidative and glycolytic fibers from the control to the ventilated samples until 5 days (Fig. 3A, ctrl, 1d, and 5d). In accordance with the Western blotting results (Fig. 1, A and B), the increase of conjugated SUMO1 proteins was paralleled by an increased fluorescence signal of SUMO1 in animals ventilated for 10 days (Fig. 3A, 10d). In this group, the fluorescence density of the glycolytic fibers had an equal and high value of 45 A.U., i.e. comparable with the signal detected in the oxidative fibers within the same section. In contrast, the SUMO2/3 immunofluorescence showed an equal and high intensity value for both fiber types even in diaphragm samples after 1 day of ventilation, and it remained stably independent on the duration of CMV. This observation validates the Western blotting results during the early period of CMV where the SUMO2/3 conjugate signals increased 5-fold compared with controls and increased progressively during CMV duration. Consistently for SUMO1 staining, the glycolytic fibers were most affected by the accumulation of SUMOylated proteins or were also selectively involved in enhancing the SUMO conjugation reaction. A significant variation in the fluorescence signal in oxidative fibers was detected after 1 day of CMV compared with controls, i.e. 35–40 A.U. for SUMO1 (*, p < 0.05) and 32–42 A.U. for SUMO2/3 (*, p < 0.05), and they remained stable with increasing CMV duration independent of BGP-15 administration. This result suggests that the SUMO network of oxidative fibers is primarily modulated during the early phase of CMV. Finally, we observed a considerable reduction of fluorescence signal, especially in the glycolytic fibers, in response to 10 days of CMV during administration of BGP-15, in accordance with the down-regulation of poly-SUMOylated proteins (Fig. 1). Myonuclear SUMO localization was consistently observed in all the analyzed immunofluorescence stainings in the diaphragms (Fig. 3).
Fig. 3.
Localization of SUMO1 and SUMO2/3 proteins on diaphragm muscle cross-sections from mechanically ventilated rats. The distribution of SUMOylated proteins changes at different durations of CMV. A and B, left part, cryo-sections stained for NADH-TR, and A and B, right part, immunocytochemical stainings with SUMO1 and SUMO2/3 antibodies in diaphragms from control (ctrl) and mechanically ventilated rats 1 day, 5 days, 10 days, and with BGP-15 for 10 days (10d BGP-15) (means and ±S.D. of SUMO fluorescence intensity per area of 35 oxidative (O, light purple) and 35 glycolytic (G, dark purple) fibers were quantified and plotted (three animals per time point). Scale bars, 50 μm. ***, p < 0.001; **, p < 0.005; *, p < 0.05.
Analysis and Identification of Enriched SUMO1 and SUMO2/3 Muscle Proteins in Rat Diaphragms by Mass Spectrometry and Immunoblotting
To identify the SUMO-conjugated proteins in muscle diaphragms, SUMOylated proteins were isolated from the five groups of muscle samples indicated as control, 1 day, 5 days, 10 days, and 10 days of BGP-15, respectively, and are described in supplemental Table 1. Preparative SDS-PAGE was used to separate the proteins immunoprecipitated with anti-SUMO1 and SUMO2/3 antibodies, and after staining (Fig. 4, A and B) the in-gel digestion peptides were analyzed with high resolution tandem MS with a subsequent database search for protein identification. The complete lists of identified candidates are listed in supplemental Data Files 1 and 2. To verify the successful isolation of SUMOylated proteins, an aliquot of 20 μg from the peptide-eluted samples was separated on SDS-PAGE, transferred to membranes, and probed with anti-SUMO1 and anti-SUMO2/3 antibodies (Fig. 4, C and D). A different protein profile, compared with the previous Western blotting with total muscle lysates (Fig. 1), was observed, suggesting an efficacious extraction and enrichment of SUMOylated proteins within the total muscle lysates. Different profiles in terms of SUMOylated proteins among the control group and the other ventilated sample groups were detected (Fig. 4C). This result is in line with the observation that during CMV treatment the SUMOylation protein profile is altered. We observed a set of three new bands that appeared between 37 and 75 kDa and disappeared in the BGP-15-treated samples. An intense high molecular mass band localized between 150 and 250 kDa was detectable together with an ∼90-kDa band showing the well known SUMO1 target RanGAP1. The SUMO2/3 immunocomplex Western blottings highlighted a remarkable profile difference between control, ventilated, and BGP-15 muscle samples groups. A continuous smear from 20 to 150 kDa that corresponded to enriched SUMO2/3 protein was observed in the control group; meanwhile, a predominant intense smear of high molecular mass species above 100 kDa appeared in all the mechanically ventilated groups. On the contrary, in the BGP-15 group, this high molecular weight signal corresponding to the accumulation of poly-SUMO2/3 proteins was clearly reduced, and the profile was comparable with the control diaphragm muscle samples (Fig. 4D), suggesting a strong role of this drug in the remodeling of the altered SUMO2/3 pathway caused by CMV. The Western blotting results (Fig. 4, C and D) clearly indicated a considerable difference in the protein profile between the SUMO1- and SUMO2/3-enriched proteins from the control, mechanically ventilated, and BGP-15-treated animals for both SUMO immunoprecipitation assays. To further investigate these differences, we performed an MS-based approach to identify the SUMO immunoprecipitated proteins in normal diaphragm muscle and at different durations of CMV with and without BGP-15 treatment to investigate new muscle SUMO-related targets.
Fig. 4.
Analysis of the endogenous SUMO1- and SUMO2/3-modified proteins in diaphragms of controls and rats exposed to different times of mechanical ventilation and GO comparison between SUMO1 and SUMO2/3 control samples. Preparative gels for the mass spectrometry analysis. A, SUMOylated myofibrillar diaphragm proteins from non-ventilated (control) and from different durations of CMV (1d, 5d, and 10d) without and with BGP-15 (10d BGP-15) were pulled down with monoclonal SUMO1 (21C7 and 76–86); and B, SUMO2 (8A2) antibodies purified from hybridoma cell line, separated by SDS-polyacrylamide gel, stained with Coomassie Blue, cut based on protein molecular weights (dotted lines), and analyzed by high-resolution LC-MS/MS. 1/100 of the total SUMO1 and SUMO2/3 pulldowns samples were probed with anti-SUMO1 (C) and anti-SUMO2/3 (D) antibodies to evaluate the SUMO protein profile and the quality of eluted SUMO immune complexes. Hashtag (#) indicates the SUMOylated RanGAP1 protein. E, Venn diagram comparison between SUMO1 (red circle) and SUMO2 (green circle) identified proteins from control diaphragm. F, Gene Ontology Molecular Function terms were used to find statistical enrichment within the common SUMO1 + SUMO2/3 (blue bars), unique SUMO1 (red bars), and unique SUMO2/3 (green bars) identified on the enriched SUMOylated proteins from not ventilated control muscle diaphragms. All listed terms are significant with −log (p value) >1.30, dashed line. G, classification and comparison using Gene Ontology Cellular Compartments between unique SUMO1 (red bars) and unique SUMO2 (green bars) identified protein targets from control muscle diaphragm compared with the total proteins. The numbers close to the bars indicated the ratios between the unique identified proteins in the cellular compartments and the total SUMO proteins. H, list of six top overlapping pathways; column length indicates the −log (p value) of overlapping p value from Fisher's exact test. Differential yellow line and markers refer to the coverage ratio of identified SUMO1 and SUMO2 proteins in the canonical pathway within the IPA knowledge base. The filling color indicates the predicted activation status of pathways.
Bioinformatics Analysis of Endogenous SUMO1 and SUMO2/3 Mass Spectrometry-identified Proteins in Control Diaphragm Muscles
A total number of 426 SUMO1 and 310 SUMO2/3 endogenous muscle SUMOylated proteins with a coverage score threshold above 5 were identified. Among those numbers, we predicted a total of 1610 SUMO sites for SUMO1 and 1054 SUMO sites for SUMO2 target proteins, respectively, with the consensus ΨKXE sequence (63). In our study, the coverage of endogenous SUMO proteins containing the SUMO sites is almost 90% to the total SUMO-identified targets (supplemental Fig. 6). In the Venn diagram, a total of 280 common proteins, 146 unique SUMO1, and 30 unique SUMO2/3 candidates were observed (Fig. 4E). We compared our identified SUMOylated proteins to one of the most comprehensive site-specific SUMO target protein data sets (64). The dataset summarized 11 SUMO proteomic studies and included 6799 proteins. In uniquely SUMO1 proteins, 82 of 146 proteins were reported previously as SUMO-targeted protein. In uniquely SUMO2 proteins, 11 of 30 proteins were previously reported. For common proteins, 160 of 120 were reported (supplemental Fig. 6). The most statistically significant molecular functions, with a −log (p value) of >1.33, which were common for SUMO1- and SUMO2/3-identified proteins (Fig. 4F, blue bars) were related to generate metabolic precursors, respiratory electron transport chain, cellular component morphogenesis, metabolic processes, and muscle contraction functions. The unique SUMO1 candidates were connected mainly with fatty acid, lipid, and protein metabolic processes (Fig. 4F, red bars), and the unique SUMO2/3 candidates were associated mainly with cellular components, anatomical structure morphogenesis, and respiratory electron transport chain functions (Fig. 4F, green bars).
Many of the SUMOylated proteins are known to reside in the nucleus (65), and the Gene Ontology analysis revealed the following distribution for SUMO1- and SUMO2/3-enriched substrates: 25–30% of SUMOylated proteins had their localization in the mitochondria, 20–25% in the nuclei, and 10–15% in the sarcolemma, cytoplasm, or endoplasmic reticulum (Fig. 4G). The co-localization of SUMOylated proteins in mitochondria was supported by immunofluorescence performed on control diaphragm sections using both anti-SUMO1 and anti-SUMO2/3 antibodies together with the mitochondrial marker, anti-ATP5 antibodies (supplemental Fig. 4). To cover a deep analysis of the SUMOylated protein identified in the control group, specific cellular pathways were investigated. Two major statistically significant cellular pathways were identified, the “Gβγ signaling pathway” specific for the SUMO1-associated proteins with −log (p value) = 4.2, ratio = 0.08 (supplemental Fig. 3) and the “oxidative phosphorylation pathway” shared for both SUMO1- (red color) and SUMO2 (green color)-recognized proteins with −log (p value) = 5.6, ratio = 0.08 (Fig. 4H). The results indicate that SUMOylated proteins may have an important role in the mitochondrial ATP production under physiological conditions in the diaphragm.
Validation of Some Potential SUMO Targets Identified by Mass Spectrometry Analysis in the Control Group Samples
We selected 10 highly abundant SUMO-protein candidates with different roles and localizations for the muscle activity/physiology and validated in both bacteria and in HeLa cells the potential SUMOylation reaction. First, we performed a bioinformatics approach analysis using two available software (www.jassa.fr/index.php?m=jassa or www.abgent.com/sumoplot/) to identify the SUMO-binding lysines (with a threshold of >60% of the consensus score) in the amino acid sequence of our targets and the SIMs along the protein sequence (supplemental Table 4). Second, we cloned from muscle cDNA the correspondent open reading frames (ORFs) of the following selected candidate proteins: TRIM63 (MuRF1-Q91Z63); TRIM54 (MuRF3-Q5XIH6); aspartate aminotransferase (AATM-P00507); ornithine aminotransferase (OAT-P04182); ATP synthase subunit-α (ATP5A-P15999); ATP synthase subunit-ε (ATP5E-P29418); calsequestrin 1 (CASQ1-P19633); calsequestrin 2 (CASQ2-P51868); Triadin (TRDN-Q9QX75); and myosin ATPase catalytic domain (common domain of all myosin) in prokaryotic and eukaryotic expression vectors (supplemental Table 3). After sequencing, the corrected prokaryotic plasmids were introduced into BL21-competent bacteria together with plasmids codifying the complete SUMO conjugation system (pSUMO1, -2, and -3) (55), and after induction and lysis the proteins were separated with SDS-PAGE and probed with anti-GST antibodies or with some antibodies specific for the expressed protein (Fig. 5). We discovered and validated two important E3 muscle ubiquitin ligases, TRIM63 and TRIM54, as unique SUMO1 targets; in particular, the recombinant GST-TRIM63 showed two active potential SUMO conjugation sites suggested by the two slow migrating bands above the not modified protein. TRIM54 had only one active conjugation site with SUMO1 (Fig. 5A). Different myosin isoforms (myosin-4, -7, -6, -3, -8, and -9) were identified in both SUMO1 and SUMO2/3 immunoprecipitation assays. Because myosin is a dominant protein in the muscle fibers, it could potentially be a contamination in the pulldowns. To prove true SUMO conjugation on this protein, it was studied in detail. Because of the high molecular weight of myosin and the different isoforms, we restricted the analysis only to the conserved myosin ATPase domain (N terminus 1–780 amino acids), containing six potential SUMO-conjugated lysines (supplemental Table 4), and we validated in bacteria that SUMO1 and SUMO2/3 are bound to this myosin region (Fig. 5B).
Fig. 5.
Validation of potential identified SUMOylated targets with recombinant SUMOylation system in E. coli and in eukaryotic cells. The mass spectrometry SUMO-identified proteins: A, TRIM63, TRIM54. B, myosin-ATPase domain (Myo-ATPase). C, aspartate aminotransferase (AATM), ornithine aminotransferase (OAT), ATP synthase subunit-ε (ATP5E),and ATP synthase subunit-α (ATP5A). D, calsequestrin 1 (CASQ1), calsequestrin 2 (CASQ2), and Triadin (cTRDN) luminal C-terminal portion, tagged with GST, were expressed in BL21 bacteria together with pSUMO1 (lane 1), pSUMO2 (lane 2), pSUMO3 (lane 3) vectors containing the SUMOylation system described under “Experimental Procedures,” and empty vector (mock, M). Bacteria lysates were probed with anti-GST antibodies to detect the expression of not modified protein indicated with an arrow (lane M), and the corresponding SUMOylated bands appeared as low speed bands above the native GST-tagged protein. Specific antibodies raised against native proteins (TRIM63 and ATP5A) were used to validate the results obtained with anti-GST antibodies. E, candidates were co-expressed in eukaryotic cells with different amounts of the recombinant SUMO E2 ligase, Ubc9. SUMOylated bands appeared as new low migrating bands in presence of Ubc9. Square brackets indicate poly-SUMOylation of the recombinant proteins expressed both in bacteria and in eukaryotic cells. All blots are representative of three independent experiments performed in triplicate.
Mitochondrial proteins were also identified as SUMO targets. We selected some of the candidates with high scores from the MS analysis and validated the SUMO conjugation in the bacterial SUMO assay. Particularly we observed that aspartate aminotransferase protein was preferentially conjugated with SUMO1 in two regions giving a double band in the SUMO1 reaction, above the native protein. Ornithine aminotransferase was mono-SUMOylated by SUMO1, but it potentially forms poly-SUMO chains in presence of SUMO2 and SUMO3 moieties. The ATP synthase subunit-ε denoted a single post-translational modification for all the three SUMO moieties. Finally, we found that the ATP synthase subunit-α was detected in the SUMO pulldowns, although denoted to be a substrate not modified by SUMO and confirmed in the bacterially GST-ATP synthase subunit-α recombinant expression with the specific anti-ATPase subunit-α antibodies (Fig. 5C). In the SUMO pulldowns we identified proteins associated with the ryanodine receptor structure as calsequestrin1, calsequestrin2, triadin, and ryanodine receptor1 and -2. We validated calsequestrin1 and calsequestrin2 as SUMO1 and SUMO2/3 targets (Fig. 5D). Due to the transmembrane domain localized at the N terminus of the full-length triadin, a non-soluble protein was produced that turned out to be not suitable for the SUMO bacteria assay. To overcome this problem, we cloned and tested the luminal portion of triadin that still covers >90% of the total protein sequence. Interestingly, this portion has 19 potential SUMO consensus lysines predicted in the bioinformatics analysis (supplemental Table 4) that can explain the multiple bands above the recombinant GST protein in the presence of the SUMO conjugation machinery in bacteria (Fig. 5D).
We performed a parallel SUMO validation assay by overexpressing the recombinant candidates in the presence of different amounts of the E2 SUMO ligase Ubc9 in HeLa cells (Fig. 5E). We confirmed that Ubc9 conjugated SUMO to almost all candidates. The SUMOylated proteins appeared with a high molecular weight bands above the recombinant native proteins. Interestingly, TRIM54 was shown not to be a Ubc9 SUMO target because no modified bands were detected in this assay. We assumed that TRIM54 could require another E3 ligase to become SUMOylated.
In the total MS analysis, we also identified already known muscle proteins as SUMO targets: the sarcoplasmic reticulum calcium ATPase (66, 67) and the α-skeletal muscle actin (68), but other classes of highly expressed muscle proteins like the rod-shaped cytoplasmic protein dystrophin (427 kDa) or proteins derived from the transmembrane dystroglycan or sarcoglycan complexes were missing. This further confirmed the validity of the immunoprecipitation approach.
Gene Ontology Analysis of Identified SUMO1 and SUMO2/3 Substrates during Mechanical Ventilation
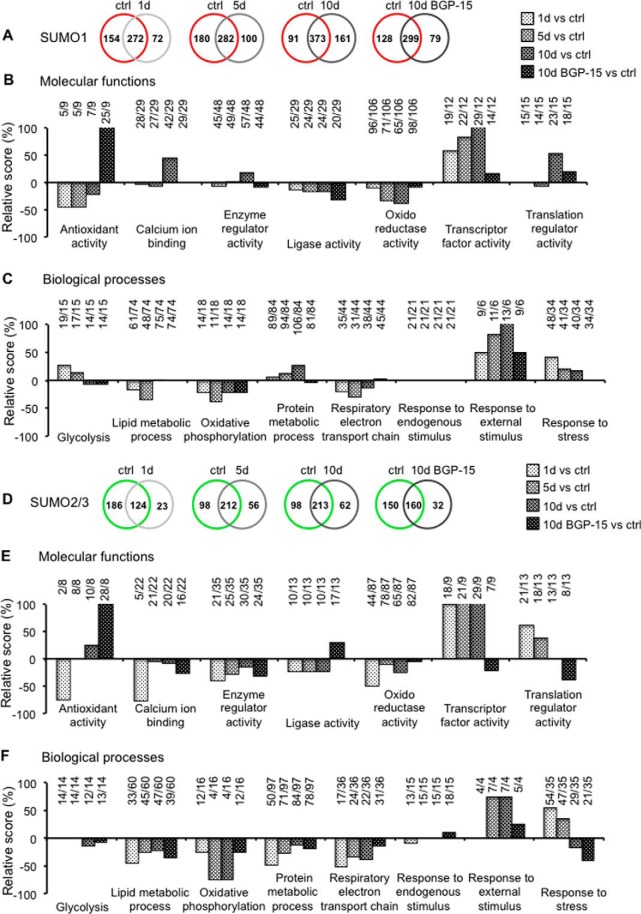
The dynamic nature of SUMOylated protein accumulation in diaphragm muscles in mechanically ventilated rats and the consequent reduction in response to BGP-15 treatment is shown in Fig. 1. Furthermore, we generated Venn comparisons for SUMO1 (Fig. 6A and supplemental Data File 3) and separately for the SUMO2/3 (Fig. 6D and supplemental Data File 4) pulldowns among the diaphragm control group with 1, 5, 10, and 10 days with BGP-15 groups to investigate the potential overlap of the MS-identified SUMOylated proteins (Fig. 6, A and D). From the combined overlaps between the control with the 1-, 5-, and 10-day groups, we observed an increased number of unique SUMO1 (from 72, 100 to 161) and unique SUMO2/3 (from 23, 56 to 62)-related proteins. Nevertheless, we found a reduction in the number of unique SUMO1 (from 161 to 79) and unique SUMO2/3 (from 62 to 32) targets comparing the controls to the 10 days of CMV with the BGP-15 treatment. This result suggested that the new SUMO substrates generated during CMV are important for VIDD disease. The predicted SUMO sites for the endogenous SUMO proteins identified during mechanical ventilation and the coverage of endogenous SUMO proteins containing the SUMO sites are described in supplemental Fig. 6.
Fig. 6.
Gene Ontology analysis from identified SUMO1- and SUMO2/3-modified proteins. Venn diagram comparisons between controls SUMO1 (A, red circles) and SUMO2/3 (D, green circles) proteins with the MS/MS-identified SUMO substrates along the ventilation treatment after 1 day (1d, very light gray), 5 days (5d, light gray), 10 days without (10d, gray), and with (10d BGP-15, dark gray) BGP-15 drug. B and E, Statistical Gene Ontology analysis for selected molecular functions, and C and F, biological process terms were used to investigate the effects of mechanical ventilation treatment along the identified SUMOylated substrates per each time group compared with the control. Bars indicate the percentage of variation between the number of SUMO-identified proteins related to the specific GO subjects for each time of treatment compared with the correspondent SUMO identified proteins control (1d versus ctrl, 5d versus ctrl, 10d versus ctrl, and 10d BGP-15 versus ctrl). Ratios (X/Y) on top of each bar indicate X is the number of identified proteins in the treatment and Y is the number of protein in the control. The internal figure legend indicates the different fillings of the histogram bar represented the respective comparisons.
To understand which molecular functions and biological processes were modulated in the diaphragm muscles at different durations of CMV, we performed a restricted GO analysis particularly related to the previously validated proteins within the different groups of SUMO1 and SUMO2/3 MS-identified proteins (Fig. 6, B, C, E, and F). The molecular functions in which CMV promoted a reduction were the antioxidant activity, ligase activity, and oxidoreductase activity, and the biological processes were lipid metabolic process, oxidative phosphorylation, respiratory electron transport chain, and response to stress. On the contrary, CMV had a positive effect on SUMO-related proteins involved in the transcription factor, translation regulator activity, response to external stimulus, and response to stress. The treatment with BGP-15 had a predominantly positive effect to increase the antioxidant activity and to ameliorate the oxidoreductase activity, the transcriptor factor activity (Fig. 6, B and E), the oxidative phosphorylation, the respiratory electron transport chain, and the response to external stimulus and to stress (Fig. 6, C and F).
Modulation of Diaphragm Proteins by SUMO1 and SUMO2/3 during Mechanical Ventilation
In addition to analyses of MS-identified SUMOylated proteins performed with bacteria assay, the enriched SUMO1 and SUMO2/3 peptide-eluted immunocomplexes were used to validate in vivo putative SUMO targets. In accordance with previous results (50), demonstrating post-translational modifications of myosin by SUMO2/3 and the formation of poly-SUMO2 species in diaphragm muscle in response to 10 days of CMV, here we show that the SUMO2/3 conjugation started after 1 day of CMV and then decreased but was still detectable during long-term CMV and reduced by BGP-15 treatment. Moreover, a fraction of myosin was also targeted by SUMO1 controls and shown as two bands above 200 kDa. This modification persisted during ventilation and was reduced with by BGP-15 treatment (Fig. 7A).
Fig. 7.
Modulation of identified SUMO1 and SUMO2/3 muscle proteins during mechanical ventilation. Mechanical ventilation promotes peculiar SUMO post-translational modifications in muscle proteins. Enriched SUMO1 and SUMO2/3 immunocomplexes of control (ctrl) and at different durations of CMV (1d, 5d, 10d, 10d, BGP-15), together with 5 μg of crude control diaphragm lysate (ML) were separated by SDS-polyacrylamide gel and probed with anti-myosin (A), anti-calsequestrin1 (B), anti-triadin (C) and anti-ATPase synthase α-subunit (D). The bands corresponding to the proteins conjugated by SUMO1 are indicated with black arrows, and the protein conjugation by poly-SUMO2/3 is indicated with a square bracket. The white hashtags (#) indicated the low immunoglobulin chains as residues of the immunoprecipitation experiment. Anti-mouse IgG conjugated to Sepharose beads (b) were loaded in parallel to the myosin immunoprecipitants (IPs) as negative control.
To detect other SUMO post-translational protein modifications induced by CMV, MS-identified SUMOylated proteins from gel sections above 100 kDa (Fig. 4, A and B; sections 1, 2, and 3) and proteins with native molecular masses below 100 kDa were considered. High molecular mass bands, above the native form of calsequestrin1, reacting with specific calsequestrin1 antibodies were observed after 1 day of CMV, increased at longer durations, and were decreased with BGP-15 treatment (Fig. 7B). In response to long-term CMV (5 and 10 days), a potential SUMOylated form of triadin was observed with a molecular mass above the native form detected in the muscle lysates (Fig. 7C). Finally, SUMOylated mitochondrial proteins were detected as SUMO targets. To understand why the ATPase synthase α-subunit was detected in the SUMO pulldowns but was validated in bacteria as a no-SUMO substrate (Fig. 5C), anti-ATPase synthase α-subunit antibodies were used on the enriched SUMO1 and SUMO2/3 fractions. Unexpectedly, the native protein was highly abundant at the early stage of CMV (1 day) and possibly SUMOylated forms mediated by SUMO1 between 5 and 10 days of CMV (Fig. 7D), suggesting late post-translational modification events exclusively mediated by SUMO1. The abundant enrichment of the native protein observed at an early stage of CMV was possibly generated by a new rearrangement in the mitochondrial protein interactions mediated by mitochondrial substrates containing SIM domains only in ventilated samples. These findings were consistent with the bacterial SUMO validation assay (Fig. 5), i.e. MS-identified SUMOylated muscle proteins showing a broad spectrum of protein functions were differently SUMOylated during CMV.
Diaphragm Muscle SUMO Conjugation and Deconjugation Machinery Is Affected during Mechanical Ventilation and Partially Restored by BGP-15 Treatment
A pool of muscle proteins was SUMOylated in the control diaphragm muscle in response to CMV, and the SUMO conjugation reaction was partially impaired by BGP-15 treatment during CMV. A transcriptome analysis was performed on total RNAs isolated from the samples listed in supplemental Table S1. Transcripts of the SUMO isoforms (Fig. 8A), the most common and described SUMO conjugases (Fig. 8, B and C) and deconjugases (Fig. 8D), the E3 ubiquitin ligase STUbL RNF4 (Fig. 8E), Atrogin, TRIM63, and the Per2 (69) circadian gene were evaluated (Fig. 8F). The variation of transcript abundance was normalized with Gapdh and referred to the control. An increase in gene transcripts was observed of the SUMO moieties, the component of E1 dimers Sae1 and Uba1, the E2 Ubc9, some E3 ligases Pias1, Pias3, Pias4, RanBP2, TOPORS, and some deconjugases SENP3, -5, -7, and -8, and particularly the BGP-15 treatment did not reduce the transcript overexpression of the described genes. However, the transcripts of some E3 ligases Pias1, Pias3, Pias4, and Traf7 and deconjugases SENP1, -2, and USPL1 were down-regulated with BGP-15 treatment. Opposite results were found with the RNF4 gene. The down-regulation in CMV samples was restored in BGP-15 treatment to control values. We confirmed that the observed changes during the period of mechanical ventilation treatment were not influenced by circadian cycles because the Per2 transcript gene expression remained stable and comparable with the control samples indicating that the observed variations in gene expression were generated by CMV treatment. Specific antibodies to some SUMO conjugases and deconjugases were used to validate the transcriptome data (Fig. 8G). The gene expression variations observed in the transcriptome analysis were confirmed at the protein level by using specific antibodies, and the quantifications are reported in Fig. 8H. The Western blot panel in Fig. 8G highlights how protein levels of SUMO enzymes were differently modulated along the CMV treatment. Notably the SUMO conjugases and deconjugases, PIAS1, PIAS3, SENP1, and TRAF7, were up-regulated only at specific CMV durations and then restored as control levels. To improve our understanding of how CMV modulated two important key regulator enzymes, the E2 SUMO ligase UBC9 and the E3 ubiquitin ligase RNF4, the localization and abundance on diaphragm muscles at different durations of CMV and BGP-15 treatment were detected. Immunofluorescences were performed with specific anti-UBC9 and anti-RNF4 antibodies previously clarified to remove all cross-reactivities with muscle proteins (supplemental Fig. 5). In line with the transcript and protein quantification results, a significant correlation between increasing fluorescence with anti-UBC9 antibodies (Fig. 8I, upper panel) and the reduction in the signal with anti-RNF4 antibodies was observed (Fig. 8I, lower panel) in the diaphragm during CMV. The fluorescence signal was only restored for RNF4 during BGP-15 treatment and not for UBC9.
Fig. 8.
Transcriptome analysis, protein quantification, and diaphragm muscle localization of SUMO-related enzymes in control and in mechanically ventilated rats without and with drug treatment. The diaphragm muscle SUMO network was altered during CMV treatment and partially restored by BGP-15. Quantitative PCRs performed on SUMOs (A), E1- and E2 SUMO-conjugating enzymes (B and C), SUMO deconjugases transcripts genes (D), STUbL RNF4 (E), and ATR1, TRIM63, and the circadian Per2 (F) transcripts genes were analyzed on the same samples listed in the supplemental Table 1. Fold changes are normalized by GAPDH housekeeping gene and compared with the control diaphragms (ctrl). Mean ± S.D. of indicated fold changes were performed for each sample in triplicate. G, representative Western blotting panel of total myofibrillar proteins from diaphragm sample lysates were probed with specific antibodies to detect some SUMO conjugases (UBC9, PIAS1, PIAS3, and TRAF7), deconjugases (SENP1, SENP2, SENP5, and SENP6) and E3 ubiquitin ligase RNF4. PIAS3 is detected as a double band indicated with a black bracket immediately below the SENP5 bands. H, protein quantifications of the above-described blot panel. The intensities of the analyzed proteins were quantified by densitometry, normalized with the correspondent loading control (GAPDH), and referred to the control signal. Mean ± S.D. of the indicated fold changes were performed for each sample listed in the supplemental Table 1 in triplicate. The internal figure legend indicates the different fillings of the histogram. I, confocal images of diaphragm cryo-cross-sections from control (ctrl) and different duration of CMV (1d, 5d, and 10d) without and with BGP-15 (10d BGP-15) were incubated with depleted muscle protein anti-UBC9 (upper panel), and anti-RNF4 (lower panel) antibodies show the localization and the variation of the UBC9 and RNF4 protein content along muscle fibers. Pictures are representative of four different sections derived from four independent muscle samples within the same group. Scale bars, 50 μm. Nuclei are stained with DAPI.
DISCUSSION
The molecular mechanism of SUMO conjugation/deconjugation to specific targets has been particularly well defined. On the contrary, its role in in vivo, like mammalian tissue, has just begun to be investigated. It is clear that SUMOylation provides a rapid and sensitive system that can dynamically regulate the activity, function, and responses of a broad type of proteins in multiple cell types and tissues, followed by alteration of normal condition due to external or internal stimuli. In skeletal muscles, the role of the SUMO pathway as a regulator of gene activity and cell development and/or disorders is just arising, and it has been mainly studied on cardiac tissue (5, 28). Our study uncovers a new facet about the role of SUMO in the diaphragm in response to long-term CMV, leading to the severely impaired function VIDD. During oxidative stress circumstances, many different changes in the biological process occur, and SUMOylation is one of them. In line with the observation that variations of oxidative stress are able to alter the global protein SUMO conjugation profile (70), here we documented a novel respiratory muscle response to the external ventilation treatments in terms of a significant remodeling of the global levels of SUMOylated proteins and the alteration of the SUMO machinery.
A mass spectrometry-based proteomic approach was used to identify diverse targets of SUMO in the diaphragm from controls and animals exposed to CMV. Among the hundreds identified targets, new classes of proteins related to SUMO important for muscle contraction, i.e. myosin isoforms (Myo-4, -7, -6, -3, and -8), calsequestrin1 and triadin, muscle remodeling, i.e. TRIM63 and TRIM54, and several mitochondrial proteins were validated. We then chose to investigate which proteins were severely modified by SUMO1 or SUMO2/3 in control muscle and during the CMV. In controls, the total pool of fast myosin possessed a low protein fraction conjugated to SUMO1, but an early conjugation of poly-SUMO2/3 chains on myosin was observed in response to CMV. At this moment, we can only speculate that the SUMOylation intervention to myosin may have a protective effect to avoid immediate protein degradation when the diaphragm is not actively contracted. This is supported by previous results demonstrating a maintained muscle fiber size during the first 5 days of CMV in the experimental ICU model used in this study (49), as well as studies demonstrating the protective role of SUMO conjugation in neurons exposed to ischemia-induced damage (43, 71).
Furthermore, components of the dihydropyridine receptor t-tubule membrane complex in the diaphragm, such as calsequestrin1 and triadin, were involved in SUMO modifications in response to CMV. The presence of the ryanodine complex proteins as SUMO targets suggests an important role of SUMO in the regulation of calcium homeostasis during diaphragm displacement that may also affect the muscle contraction/adaptation in CMV and contributes as one co-factor of the VIDD. The E3 muscle ubiquitin-protein ligases TRIM63, -55, and -54 were exclusively identified in enriched SUMO1 proteins in the control diaphragm group, a new post-translational modification mediated by SUMO1 affecting these E3 ubiquitin ligases.
According to MS analyses, a consistent group of SUMO-related proteins was located in the inner mitochondrial membrane and was validated by immunofluorescence demonstrating signals generated by anti-SUMO1 and anti-SUMO2/3 antibodies co-localized with the mitochondrial tracker, anti-ATPase synthase α-subunit antibodies (supplemental Fig. 4). In particular, we focused our attention on the mitochondrial membrane ATP synthase (F1F0-ATP synthase or complex V) α- and ε-subunits as components of the oxidative phosphorylation pathway. In bacteria and cell assays, both were validated as potential SUMO targets. Thus, diaphragm muscle mitochondrial proteins were SUMOylated in response to CMV and modulated by treatment with the chaperone co-inducer BGP-15. This result addresses a new field of investigation related to the role of SUMO modification in muscle mitochondrial proteins related to ATP production.
The connection between SUMO and mitochondria were highlighted with the Gene Ontology Molecular Function and Biological Process analysis performed with purified SUMOylated proteins in diaphragm muscles from control and mechanically ventilated animals. The major functions and activities that were affected by the treatment were glycolysis, oxidative phosphorylation, and antioxidant activity, which are directly linked to mitochondrial functions. This result supports and extends previous observations in the rat experimental ICU model demonstrating severe changes in mitochondrial structure and function in the diaphragm in response to 10 days of CMV and a partial restoration in function with BGP-15 treatment (50).
A weakness of our mass spectrometry approach was to identify the peptides where lysines directly involved in the conjugation to SUMO, contained the -Gly-Gly residues. This is explained because our material was extracted not from transgenic animals where a mutant form of endogenous SUMO leading a trypsin restriction site close to -Gly-Gly motifs would help in the identification of the SUMO sites in the purified proteins. To overcome this inconvenience, by using bioinformatics approach we discovered that almost 90% of identified SUMOylated protein detected by mass spectrometry contained the SUMO sites for each group of muscles. The lack of complete coverage is due to the presence of false-positive proteins, such as proteins without the SUMO sites but containing the SIM motifs or simply no specific proteins that can be identified with the unbiased method we adopted in this study. We provided the comparison between our identified proteins with a complete published data set to determine the overlaps. Few common targets were identified between the groups possibly because the starting material and the stress were different as we used muscle tissue of control and mechanically ventilated rat diaphragms.
In addition, all SUMO-conjugating and -deconjugating enzymes from the analyzed diaphragm muscles were investigated in a transcriptome study. In response to CMV, the diaphragm SUMO machinery was significantly altered. To adapt to this treatment, the diaphragm established a new set of SUMO enzyme transcripts and proteins, determining the observed change in the muscle SUMO protein profile. The protein abundance of specific SUMO enzymes showed a specific temporal pattern during CMV; PIAS1 was up-regulated early, whereas PIAS3, SENP1, SENP2, and TRAF7 showed an up-regulation after a long period of CMV. CMV also affected UBC9 and RNF4, the two major key components regulating the SUMO conjugation and degradation of poly-SUMOylated substrates both at the transcript and protein levels. The observed opposite abundance in muscle section immunofluorescence may also explain the stabilization of poly-SUMOylated proteins along CMV.
In summary, this study has added significant knowledge to our understanding of the mechanisms underlying as well as initiated continuing investigation of specific SUMO enzymes as biomarkers in the monitoring of VIDD in critically ill ICU patients as well as potential targets for therapeutic interventions.
Supplementary Material
Acknowledgments
We are grateful to Drs. Frauke Melchior (Ruprecht-Karls University, Heidelberg, Germany) and Ron T. Hay (College of Life Sciences, Dundee University, Dundee, Scotland, United Kingdom) for providing plasmids, antibodies, and technical advice; Dr. Marcela Ferella (SciLifeLab, Eukaryotic Single Cell Genomic Facility, Stockholm, Sweden) for useful comments and critical reading of the manuscript; and Dr. Oriano Marin (CRIBI Peptide Facility, University of Padua, Italy) for providing peptides used for SUMO immunocomplex elutions. We thank Yvette Hedström for excellent technical assistance. The contributions from undergraduate students Hasina Nasser, Dardan Konjusha, and Leonardo Traini are gratefully acknowledged. We thank Åsa Eriksson and Alexander Falk (SciLifeLab, Mass Spectrometry-based Proteomic Facility, Uppsala University, Sweden) for mass spectrometry analysis. We also thank the DNA Sequencing KI-Gene Facility at the Department of Molecular Medicine and Surgery, Neurogenetics Unit, Karolinska Institutet, Stockholm, Sweden.
Footnotes
Author contributions: S.G. designed research; A.V.N., G.H., J.M., N.C., K.H., A.K., S.B., L.L., and S.G. performed research; J.M., K.H., S.B., and S.G. analyzed data; A.V.N., J.M., K.H., S.B., L.L., and S.G. wrote the paper.
* This work was supported by Swedish Research Council Grant Vetenskapsrådet Grants 2013-3074, Erik och Edith Fernströms Foundation Grant 2012 (to S.G.); Åke Wiberg Foundation Grant, M14-0127; Carl Trygger Foundation Grant, CST 15:57; Magnus Bergvall Foundation Grant 2015-01200; Swedish Research Council Grant 8651 (to S.B.L.); the Swedish Foundation for International Cooperation in Research and Higher Education (STINT); the Erling-Persson Family Foundation; the Karolinska Institutet; the Stockholm County Council (ALF Project to L.L.), and National Natural Science Foundation of China Grant 31671139 (to J.M.). The authors declare that they have no conflicts of interest with the contents of this article.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- ICU
- intensive care unit
- VIDD
- ventilator-induced diaphragmatic dysfunction
- MV
- mechanical ventilation
- MS
- mass spectrometry
- CMV
- controlled MV
- SIM
- SUMO-interacting motif
- STUbL
- SUMO-targeted ubiquitin ligase
- MyHC
- myosin heavy chain isoform
- GO
- Gene Ontology
- AU
- arbitrary unit
- ACN
- acetonitrile
- FA
- formic acid
- FDR
- false discovery rate
- IPA
- Ingenuity Pathway Analysis.
REFERENCES
- 1. Flotho A., and Melchior F. (2013) Sumoylation: a regulatory protein modification in health and disease. Annu. Rev. Biochem. 82, 357–385 [DOI] [PubMed] [Google Scholar]
- 2. Geiss-Friedlander R., and Melchior F. (2007) Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 8, 947–956 [DOI] [PubMed] [Google Scholar]
- 3. Dohmen R. J. (2004) SUMO protein modification. Biochim. Biophys. Acta 1695, 113–131 [DOI] [PubMed] [Google Scholar]
- 4. Hannoun Z., Greenhough S., Jaffray E., Hay R. T., and Hay D. C. (2010) Post-translational modification by SUMO. Toxicology 278, 288–293 [DOI] [PubMed] [Google Scholar]
- 5. Wang J., and Schwartz R. J. (2010) Sumoylation and regulation of cardiac gene expression. Circ. Res. 107, 19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang J., Chen L., Wen S., Zhu H., Yu W., Moskowitz I. P., Shaw G. M., Finnell R. H., and Schwartz R. J. (2011) Defective sumoylation pathway directs congenital heart disease. Birth Defects Res. A Clin. Mol. Teratol. 91, 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rytinki M. M., Kaikkonen S., Pehkonen P., Jääskeläinen T., and Palvimo J. J. (2009) PIAS proteins: pleiotropic interactors associated with SUMO. Cell. Mol. Life Sci. 66, 3029–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson P. K. (2001) A new RING for SUMO: wrestling transcriptional responses into nuclear bodies with PIAS family E3 SUMO ligases. Genes Dev. 15, 3053–3058 [DOI] [PubMed] [Google Scholar]
- 9. Pungaliya P., Kulkarni D., Park H. J., Marshall H., Zheng H., Lackland H., Saleem A., and Rubin E. H. (2007) TOPORS functions as a SUMO-1 E3 ligase for chromatin-modifying proteins. J. Proteome Res. 6, 3918–3923 [DOI] [PubMed] [Google Scholar]
- 10. Weger S., Hammer E., and Heilbronn R. (2005) Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett. 579, 5007–5012 [DOI] [PubMed] [Google Scholar]
- 11. Pichler A., Gast A., Seeler J. S., Dejean A., and Melchior F. (2002) The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 108, 109–120 [DOI] [PubMed] [Google Scholar]
- 12. Kagey M. H., Melhuish T. A., and Wotton D. (2003) The polycomb protein Pc2 is a SUMO E3. Cell 113, 127–137 [DOI] [PubMed] [Google Scholar]
- 13. Morita Y., Kanei-Ishii C., Nomura T., and Ishii S. (2005) TRAF7 sequesters c-Myb to the cytoplasm by stimulating its sumoylation. Mol. Biol. Cell 16, 5433–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prudent J., Zunino R., Sugiura A., Mattie S., Shore G. C., and McBride H. M. (2015) MAPL SUMOylation of Drp1 stabilizes an ER/mitochondrial platform required for cell death. Mol. Cell 59, 941–955 [DOI] [PubMed] [Google Scholar]
- 15. Chu Y., and Yang X. (2011) SUMO E3 ligase activity of TRIM proteins. Oncogene 30, 1108–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tatham M. H., Jaffray E., Vaughan O. A., Desterro J. M., Botting C. H., Naismith J. H., and Hay R. T. (2001) Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J. Biol. Chem. 276, 35368–35374 [DOI] [PubMed] [Google Scholar]
- 17. Kolli N., Mikolajczyk J., Drag M., Mukhopadhyay D., Moffatt N., Dasso M., Salvesen G., and Wilkinson K. D. (2010) Distribution and paralogue specificity of mammalian deSUMOylating enzymes. Biochem. J. 430, 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drag M., and Salvesen G. S. (2008) DeSUMOylating enzymes–SENPs. IUBMB Life 60, 734–742 [DOI] [PubMed] [Google Scholar]
- 19. Schulz S., Chachami G., Kozaczkiewicz L., Winter U., Stankovic-Valentin N., Haas P., Hofmann K., Urlaub H., Ovaa H., Wittbrodt J., Meulmeester E., and Melchior F. (2012) Ubiquitin-specific protease-like 1 (USPL1) is a SUMO isopeptidase with essential, non-catalytic functions. EMBO Rep. 13, 930–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nie M., and Boddy M. N. (2016) Cooperativity of the SUMO and ubiquitin pathways in genome stability. Biomolecules 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Praefcke G. J., Hofmann K., and Dohmen R. J. (2012) SUMO playing tag with ubiquitin. Trends Biochem. Sci. 37, 23–31 [DOI] [PubMed] [Google Scholar]
- 22. Wohlschlegel J. A., Johnson E. S., Reed S. I., and Yates J. R. 3rd (2004) Global analysis of protein sumoylation in Saccharomyces cerevisiae. J. Biol. Chem. 279, 45662–45668 [DOI] [PubMed] [Google Scholar]
- 23. Iribarren P. A., Berazategui M. A., Cazzulo J. J., and Alvarez V. E. (2015) Biosynthesis of SUMOylated proteins in bacteria using the Trypanosoma brucei enzymatic system. PLoS ONE 10, e0134950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Talamillo A., Sánchez J., and Barrio R. (2008) Functional analysis of the SUMOylation pathway in Drosophila. Biochem. Soc. Trans. 36, 868–873 [DOI] [PubMed] [Google Scholar]
- 25. Zhang H., Smolen G. A., Palmer R., Christoforou A., van den Heuvel S., and Haber D. A. (2004) SUMO modification is required for in vivo Hox gene regulation by the Caenorhabditis elegans polycomb group protein SOP-2. Nat. Genet. 36, 507–511 [DOI] [PubMed] [Google Scholar]
- 26. Kaminsky R., Denison C., Bening-Abu-Shach U., Chisholm A. D., Gygi S. P., and Broday L. (2009) SUMO regulates the assembly and function of a cytoplasmic intermediate filament protein in C. elegans. Dev. Cell 17, 724–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elrouby N., and Coupland G. (2010) Proteome-wide screens for small ubiquitin-like modifier (SUMO) substrates identify Arabidopsis proteins implicated in diverse biological processes. Proc. Natl. Acad. Sci. U.S.A. 107, 17415–17420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang J. (2011) Cardiac function and disease: emerging role of small ubiquitin-related modifier. Wiley Interdiscip. Rev. Syst. Biol. Med. 3, 446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilkinson K. A., Nakamura Y., and Henley J. M. (2010) Targets and consequences of protein SUMOylation in neurons. Brain Res. Rev. 64, 195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Enserink J. M. (2015) Sumo and the cellular stress response. Cell Div. 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Golebiowski F., Matic I., Tatham M. H., Cole C., Yin Y., Nakamura A., Cox J., Barton G. J., Mann M., and Hay R. T. (2009) System-wide changes to SUMO modifications in response to heat shock. Sci. Signal. 2, ra24. [DOI] [PubMed] [Google Scholar]
- 32. Ribet D., and Cossart P. (2010) SUMOylation and bacterial pathogens. Virulence 1, 532–534 [DOI] [PubMed] [Google Scholar]
- 33. Ribet D., Hamon M., Gouin E., Nahori M. A., Impens F., Neyret-Kahn H., Gevaert K., Vandekerckhove J., Dejean A., and Cossart P. (2010) Listeria monocytogenes impairs SUMOylation for efficient infection. Nature 464, 1192–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Citro S., and Chiocca S. (2010) Listeria monocytogenes: a bacterial pathogen to hit on the SUMO pathway. Cell Res. 20, 738–740 [DOI] [PubMed] [Google Scholar]
- 35. Sarge K. D., and Park-Sarge O. K. (2011) SUMO and its role in human diseases. Int. Rev. Cell Mol. Biol. 288, 167–183 [DOI] [PubMed] [Google Scholar]
- 36. Song J. G., Xie H. H., Li N., Wu K., Qiu J. G., Shen D. M., and Huang C. J. (2015) SUMO-specific protease 6 promotes gastric cancer cell growth via deSUMOylation of FoxM1. Tumour Biol. 36, 9865–9871 [DOI] [PubMed] [Google Scholar]
- 37. Mattoscio D., and Chiocca S. (2015) SUMO pathway components as possible cancer biomarkers. Future Oncol. 11, 1599–1610 [DOI] [PubMed] [Google Scholar]
- 38. Eifler K., and Vertegaal A. C. (2015) SUMOylation-mediated regulation of cell cycle progression and cancer. Trends Biochem. Sci. 40, 779–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tuccilli C., Baldini E., Sorrenti S., Di Gioia C., Bosco D., Ascoli V., Mian C., Barollo S., Rendina R., Coccaro C., Pepe M., Catania A., Bononi M., Tartaglia F., De Antoni E., et al. (2015) Papillary thyroid cancer is characterized by altered expression of genes involved in the sumoylation process. J. Biol. Regul. Homeost. Agents 29, 655–662 [PubMed] [Google Scholar]
- 40. Alshareeda A. T., Negm O. H., Green A. R., Nolan C., Tighe P., Albarakati N., Sultana R., Madhusudan S., Ellis I. O., and Rakha E. A. (2014) SUMOylation proteins in breast cancer. Breast Cancer Res. Treat. 144, 519–530 [DOI] [PubMed] [Google Scholar]
- 41. Yang W., Sheng H., Thompson J. W., Zhao S., Wang L., Miao P., Liu X., Moseley M. A., and Paschen W. (2014) Small ubiquitin-like modifier 3-modified proteome regulated by brain ischemia in novel small ubiquitin-like modifier transgenic mice: putative protective proteins/pathways. Stroke 45, 1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nisticò R., Ferraina C., Marconi V., Blandini F., Negri L., Egebjerg J., and Feligioni M. (2014) Age-related changes of protein SUMOylation balance in the AβPP Tg2576 mouse model of Alzheimer's disease. Front. Pharmacol. 5, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee Y. J., Castri P., Bembry J., Maric D., Auh S., and Hallenbeck J. M. (2009) SUMOylation participates in induction of ischemic tolerance. J. Neurochem. 109, 257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He X., Riceberg J., Pulukuri S. M., Grossman S., Shinde V., Shah P., Brownell J. E., Dick L., Newcomb J., and Bence N. (2015) Characterization of the loss of SUMO pathway function on cancer cells and tumor proliferation. PLoS ONE 10, e0123882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poole D. C., Sexton W. L., Farkas G. A., Powers S. K., and Reid M. B. (1997) Diaphragm structure and function in health and disease. Med. Sci. Sports Exerc. 29, 738–754 [DOI] [PubMed] [Google Scholar]
- 46. Vassilakopoulos T. (2008) Ventilator-induced diaphragm dysfunction: the clinical relevance of animal models. Intensive Care Med. 34, 7–16 [DOI] [PubMed] [Google Scholar]
- 47. Cacciani N., Ogilvie H., and Larsson L. (2014) Age related differences in diaphragm muscle fiber response to mid/long term controlled mechanical ventilation. Exp. Gerontol. 59, 28–33 [DOI] [PubMed] [Google Scholar]
- 48. Petrof B. J., and Hussain S. N. (2016) Ventilator-induced diaphragmatic dysfunction: what have we learned? Curr. Opin. Crit. Care 22, 67–72 [DOI] [PubMed] [Google Scholar]
- 49. Corpeno R., Dworkin B., Cacciani N., Salah H., Bergman H. M., Ravara B., Vitadello M., Gorza L., Gustafson A. M., Hedström Y., Petersson J., Feng H. Z., Jin J. P., Iwamoto H., Yagi N., et al. (2014) Time course analysis of mechanical ventilation-induced diaphragm contractile muscle dysfunction in the rat. J. Physiol. 592, 3859–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salah H., Li M., Cacciani N., Gastaldello S., Ogilvie H., Akkad H., Namuduri A. V., Morbidoni V., Artemenko K. A., Balogh G., Martinez-Redondo V., Jannig P., Hedström Y., Dworkin B., Bergquist J., et al. (2016) The chaperone co-inducer BGP-15 alleviates ventilation-induced diaphragm dysfunction. Sci. Transl. Med. 8, 350ra103. [DOI] [PubMed] [Google Scholar]
- 51. Becker J., Barysch S. V., Karaca S., Dittner C., Hsiao H. H., Berriel Diaz M., Herzig S., Urlaub H., and Melchior F. (2013) Detecting endogenous SUMO targets in mammalian cells and tissues. Nat. Struct. Mol. Biol. 20, 525–531 [DOI] [PubMed] [Google Scholar]
- 52. Barysch S. V., Dittner C., Flotho A., Becker J., and Melchior F. (2014) Identification and analysis of endogenous SUMO1 and SUMO2/3 targets in mammalian cells and tissues using monoclonal antibodies. Nat. Protoc. 9, 896–909 [DOI] [PubMed] [Google Scholar]
- 53. Shevchenko A., Wilm M., Vorm O., and Mann M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 54. Vizcaíno J. A., Csordas A., del-Toro N., Dianes J. A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T., Xu Q. W., Wang R., and Hermjakob H. (2016) 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weber A. R., Schuermann D., and Schär P. (2014) Versatile recombinant SUMOylation system for the production of SUMO-modified protein. PLoS ONE 9, e102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dworkin B. R., Dworkin S., and Tang X. (2000) Carotid and aortic baroreflexes of the rat: I. Open-loop steady-state properties and blood pressure variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R1910–R1921 [DOI] [PubMed] [Google Scholar]
- 57. Dworkin B. R., and Dworkin S. (1990) Learning of physiological responses: I. Habituation, sensitization, and classical conditioning. Behav. Neurosci. 104, 298–319 [DOI] [PubMed] [Google Scholar]
- 58. Tempé D., Piechaczyk M., and Bossis G. (2008) SUMO under stress. Biochem. Soc. Trans. 36, 874–878 [DOI] [PubMed] [Google Scholar]
- 59. Literáti-Nagy B., Tory K., Peitl B., Bajza Á., Korányi L., Literáti-Nagy Z., Hooper P. L., Vígh L., and Szilvássy Z. (2014) Improvement of insulin sensitivity by a novel drug candidate, BGP-15, in different animal studies. Metab. Syndr. Relat. Disord. 12, 125–131 [DOI] [PubMed] [Google Scholar]
- 60. Schiaffino S., and Reggiani C. (2011) Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531 [DOI] [PubMed] [Google Scholar]
- 61. Geiger P. C., Cody M. J., Macken R. L., and Sieck G. C. (2000) Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J. Appl. Physiol. 89, 695–703 [DOI] [PubMed] [Google Scholar]
- 62. Sieck G. C., Han Y. S., Prakash Y. S., and Jones K. A. (1998) Cross-bridge cycling kinetics, actomyosin ATPase activity and myosin heavy chain isoforms in skeletal and smooth respiratory muscles. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 119, 435–450 [DOI] [PubMed] [Google Scholar]
- 63. Zhao Q., Xie Y., Zheng Y., Jiang S., Liu W., Mu W., Liu Z., Zhao Y., Xue Y., and Ren J. (2014) GPS-SUMO: a tool for the prediction of sumoylation sites and SUMO-interaction motifs. Nucleic Acids Res. 42, W325–W330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hendriks I. A., Lyon D., Young C., Jensen L. J., Vertegaal A. C., and Nielsen M. L. (2017) Site-specific mapping of the human SUMO proteome reveals co-modification with phosphorylation. Nat. Struct. Mol. Biol. 24, 325–336 [DOI] [PubMed] [Google Scholar]
- 65. Hendriks I. A., and Vertegaal A. C. (2016) A comprehensive compilation of SUMO proteomics. Nat. Rev. Mol. Cell Biol. 17, 581–595 [DOI] [PubMed] [Google Scholar]
- 66. Kho C., Lee A., Jeong D., Oh J. G., Chaanine A. H., Kizana E., Park W. J., and Hajjar R. J. (2011) SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477, 601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kranias E. G., and Hajjar R. J. (2012) Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ. Res. 110, 1646–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Uda M., Kawasaki H., Iizumi K., Shigenaga A., Baba T., Naito H., Yoshioka T., and Yamakura F. (2015) Sumoylated α-skeletal muscle actin in the skeletal muscle of adult rats. Mol. Cell. Biochem. 409, 59–66 [DOI] [PubMed] [Google Scholar]
- 69. Yan J., Wang H., Liu Y., and Shao C. (2008) Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput. Biol. 4, e1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Manza L. L., Codreanu S. G., Stamer S. L., Smith D. L., Wells K. S., Roberts R. L., and Liebler D. C. (2004) Global shifts in protein sumoylation in response to electrophile and oxidative stress. Chem. Res. Toxicol. 17, 1706–1715 [DOI] [PubMed] [Google Scholar]
- 71. Datwyler A. L., Lättig-Tünnemann G., Yang W., Paschen W., Lee S. L., Dirnagl U., Endres M., and Harms C. (2011) SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J. Cereb. Blood Flow Metab. 31, 2152–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.