Abstract
Background:
Brain metastases often lead to serious neurological impairment and life threatening states. Their acute management remains complex, particularly in the case of rare malignancies with aggressive evolution. In large single lesions, open surgery followed by radiation to the surgical cavity is widely regarded as the best approach; yet in many cases, microsurgery is not feasible due to the lesion's critical location and/or the number of brain metastases present. We report the effects of adaptive hypofractionated gamma knife radiosurgery in the acute management of critically located thymic carcinoma metastases.
Case Description:
A 50-year-old male with metastatic thymic carcinoma was treated with radiosurgery for two large supratentorial brain metastases (M3 and M4) adjacent to eloquent areas and one smaller cerebellar metastasis (M2). M3 and M4 were treated with adaptive hypofractionated gamma knife radiosurgery, showing a dramatic volume reduction 4 weeks after treatment completion without radiation-induced side effects. Thirteen months later, two new small, threatening supratentorial lesions (M5-M6) were treated with the same technique. Interestingly, M2 (treated with standard single fraction) and M5-M6 developed local adverse radiation events. The patient's general and neurological status remained next to normal by the time of paper submission.
Conclusion:
The application of adaptive hypofractionated radiosurgery in this acute setting proved effective in terms of rapid tumor ablation, with salvage of neurological functionality and limited toxicity. We have called the overall procedure rapid rescue radiosurgery (RRR). A systematic study of past and ongoing RRR-treatments is warranted and in progress.
Keywords: Adaptive hypofractionation, adverse radiation event, critical areas, gamma knife radiosurgery, recursive partitioning analysis, whole brain radiation induced cognitive impairment
INTRODUCTION
Brain metastases often lead to serious neurological impairment and life threatening states. Their acute management remains complex, particularly in the case of rare malignancies such as thymic carcinoma. In large single lesions, open surgery followed by radiation to the surgical cavity is widely regarded as the best approach; yet in many cases, microsurgery is not feasible due to the lesion's critical location and/or the number of brain metastases present. Our case report describes the effects of adaptive hypofractionated gamma knife radiosurgery in the acute management of critically located brain metastases.
CASE PRESENTATION
We present the case of a 50-year-old male patient reporting intermittent upper abdominal pain throughout 2012 and 2013. In February 2014, the patient experienced breathing difficulties and exacerbation of pain. Fludeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) revealed high FDG uptake in a large, heterogeneous intrathoracic mass in the middle mediastinum abutting the right atrium and the right hemidiaphragm, without evidence of distant metastases. The patient underwent subtotal primary tumor resection in April 2014. The histopathology demonstrated a poorly differentiated thymic carcinoma though without primary malignant manifestation in the gland itself. The patient received three adjuvant chemotherapy sessions (Carboplatine/Vepesid) followed by fractionated radiotherapy (2 Gy/day, 30 sessions = 2 Gy × 30 = 60 Gy) to the right thoracic region (July to September 2014). The patient developed paroxysmal atrial fibrillation after thoracic surgery as well as a series of respiratory infections during and after his radiochemotherapy, though without serious sequelae. His general condition remained otherwise stable. By February 2015, the patient developed progressive expressive dysphasia. A brain magnetic resonance imaging (MRI) in May 2015 showed a 3.5 cm ring-enhancing lesion in the left temporal lobe with extensive edema (metastasis # 1 = M1). Speech impairment resolved shortly after gross total resection (GTR). The histopathology proved the lesion to be a thymic carcinoma metastasis with MIB 1 proliferation index of up to 50%. Postsurgical hypofractionated LINAC-radiotherapy (6 Gy/day, 5 sessions = 30 Gy) was delivered to the surgical cavity in June 2015. CT of the neck, thorax, and abdomen in September 2015 showed no signs of distant metastases. However, a follow-up brain MRI in October 2015 demonstrated three new intracranial lesions – the first a 1.9 cm lesion in the right cerebellar hemisphere (metastasis # 2 = M2), the second, a larger extra-axial left temporal mass measuring 3.9 cm with adjacent edema and mass effect threatening the hippocampus, uncus, and language areas (metastasis # 3 = M3), and the third, a 3.0 cm dural-based left parietal lesion exerting mass effect on the sensory cortex (metastasis # 4 = M4). There was no evidence of recurrence in the surgical cavity (M1). Although M2 was relatively small, M3 and M4 were quite large (≥3 cm) and both threatened eloquent areas of the brain. By this time, the patient once again developed expressive dysphasia and mental fatigue [Karnofsky Performance Score (KPS) 90–100], though without evidence of extracranial tumour proliferation (RPA 1) on prior screening CT scan September 2015; the patient remained free from systemic treatment at this stage and was referred to our unit for further management (October 2015).
Radiosurgical account
The radiosurgical plan was designed based on a series of factors – past/present radiological evolution, tumor localization, identification of organs at risk (OAR), tumor volume, histopathological traits of prior resected metastasis (M1), general and neurological health status (KPS/RPA), absence of extracranial tumor activity at the time of radiosurgery, positive outcome after microsurgery, and good response to prior anti-tumoral treatments (including chemotherapy and extra/intracranial radiation). Our strategy aimed to rapidly relieve/salvage the language areas, the hippocampus, and the post-central gyrus from the larger left-sided lesions (M3 and M4) within a time frame of 7 days (treatment time between RS1 and RS3) to 4 weeks (MRI at 1 month). Because of the patient's previous positive response to extra and intracranial radiotherapy, we considered the tumor to be radiosensitive and proceeded to plot a peripheral prescription dose biologically isoeffective to the cranial hypofractionated treatment delivered to the surgical cavity earlier (6 Gy × 5). To achieve maximal probability of local tumor control while minimizing the risk of focal radiation-induced adverse reaction, treatment settings included an adaptive and MRI-guided hypofractionated radiosurgical approach; the procedure would allow tumor bed dose distribution readjustments in relation to local tumor volume variations during the course of treatment. The treatment was structured on three radiosurgeries (RS) delivered every third day i.e., day 1 (RS 1), day 4 (RS 2), and day 7 (RS 3). Cranial fixation was achieved by applying a Leksell Coordinate Frame G (Elekta AB, Stockholm) prior to each RS. A stereotactic MRI was performed before each RS session (s-MRI 1, 2, 3) for proper gross tumor volume (GTV) delineation; we set no margins to the GTV (GTV = CTV = PTV). As described in Tables 1 and 2, the s-MRI at the onset of treatment (day 1) showed further growth of the tumors with substantial perifocal edema, particularly adjacent to M3 (see Table 1 for tumor volumes at Day 1). M2 was volumetrically optimal for conventional single dose treatment (volume = 2.7 cc). Its marginal dose (GTV = CTV) was set at 19 Gy at the 50% isodose line [Table 3]. Because of their volumetric traits, aggressive growth pattern, and critical localization, we chose RRR as the best approach to treat M3 and M4. Using standard biological effective dose calculations, we set the initial GTV-prescription dose (fraction 1) for M3 and M4 to 8 Gy at the 35% isodose line [Table 4]. Dural tail and peripheral zones of hemorrhage were to be included in the field of treatment. Due to underlying extensive perifocal edema, the patient was to be kept on cortisone during the course of the treatment and approximately 4 weeks thereafter.
Table 1.
LGP-based tumor volume estimates covering time of treatment (Day 1 to Day 7) and follow up period (1 to 7 months). *M3 showed signs of hemorrhage at RS 2 (Day 4) and RS 3 (Day 7); bleeding zones were included in the field of treatment (GTV); see table 2. M4 experienced a tumor volume reduction of approximately 8% between RS 1 (day1) and RS 3 (day 7). M3 and M4 experienced significant tumor volume reduction at 1 month (89% and 90% respectively) and long lasting local control

Table 2.
LGP-based volume estimates for M3s solid component (hemorrhagic zone excluded) : 17% volume reduction between RS 1 (day 1) and RS 3 (day 7)

Table 3.
M2: Dose distribution on the GTV based on a marginal dose of 19 Gy

Table 4.
Peripheral dose prescription summary for M3 and M4. Doses were adapted according to local volumetric evolution

RESULTS
The radiological evolution during the course of treatment proved interesting for all concerned lesions. M2 developed increased central necrosis between s-MRI 1 (day 1) and s-MRI 2 (day 4). M3 developed local hemorrhage at s-MRI 2 (day 4) while its solid component suffered a slight volume reduction at s-MRI 3 (day 7) [Tables 1 and 2]. The area of hemorrhage was adaptively incorporated in the field of treatment. M4 showed volume reduction between s-MRI 2 (day 4) and s-MRI 3 (day 7) [Table 1]. Repeated frame application was well tolerated. The first follow-up MRI was performed 4 weeks after RS 3. M2 demonstrated a size reduction of 26% [Table 5] while M3 and M4 had almost completely resolved despite their initial overwhelming size [Table 1, Figures 1 and 2]. Edema adjacent to M2 had dramatically decreased and edema adjacent to M1 and M3 had resolved. The patient showed no signs of speech impairment or other neurological deficit at this time. Follow-up MRI at 2, 4, 6, and 7 months demonstrated further volume reduction and no signs of adverse radiation event/effect (ARE) for M3 and M4 [Table 1, Figures 1 and 2]. Interestingly, follow-up MRI at 6 and 7 months showed focal edema and increase in tumor volume of M2 [Table 5, Figure 3]. At this particular point, the patient was suffering from intermittent occipital headaches and minor balance issues, which promptly resolved with oral cortisone. A 11C-methionine PET (MET PET) fused with MRI at 7 months demonstrated focal, patchy intermediate uptake limited to parts of the ring enhancing M2 in the right cerebellar hemisphere with T/N ratio of 1.5 (relative mean uptake of tumor to normal contralateral mirrored cerebellar hemisphere), decreasing in uptake on follow-up MET PET at 10 and 13 months, both with calculated T/N ratio of 1.3 [Figure 4a and b], together suggesting a focal ARE. Perfusion MRI at 8.5 and 11.5 months showed no local increase in relative cerebral blood volume (rCBV) or flow (rCVF) and diminished edema surrounding M2, confirming the above diagnosis of focal ARE [Figure 5a–c]. Follow-up MRI at 10.5 months showed unchanged status of M3 and M4 compared to the previous MRIs at 7 and 8.5 months; however, M2's ARE had further evolved with increased lesion size and increased surrounding edema [Figure 6]. On MRI follow-up at 10.5 months, two new small rim enhancing metastases presented in two areas in the left temporal lobe (M5 and M6), one located 5 mm medial to the subsequently nodularly enhancing surgical cavity and an adjacent one which extended inferiorly to within 1 cm medial to the prior location of treated M3. There was no increase in rCBV in the two new areas of contrast enhancement on MR perfusion. Subsequent progress of the lesions in this region of the left temporal lobe, including two small linear areas of restricted diffusion, prompted an additional MET PET at 13 months, which did not show an increase in MET uptake in the new lesions, most likely because the lesions were too small to detect on PET. A multidisciplinary decision to treat M5 and M6 with RRR was made, due to the strong clinical concern that the lesions may represent viable malignant tumor, being either new metastases or recurrence of either the resected lesion M1 or, less likely of M3 [Figure 7]. One month post RRR-treatment #2, the new lesions (M5 and M6) had increased in diameter with increased perilesional edema without significant mass effect, interpreted as an early form of ARE [Figure 2a], probably due to prior LINAC-based radiotherapy to the surgical cavity (M1) and previous RRR-treatment of M3. M2, M3, and M4 remained under control at this stage (Follow-up at 14 months).
Table 5.
LGP-based volume estimates showing M2s evolution after single dose treatment (s-MRI + RS)

Figure 1.
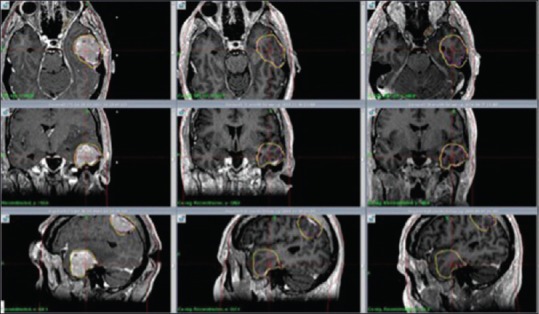
M3: Axial, coronal and sagittal contrast enhanced T1 weighted MR images show dramatic tumor reduction at 1 month (center column) and 7 months (right column) respectively after RRR. No signs of ARE at 1 and 7 months after treatment were identified. Left column shows tumor size at day 1 (RS1)
Figure 2.
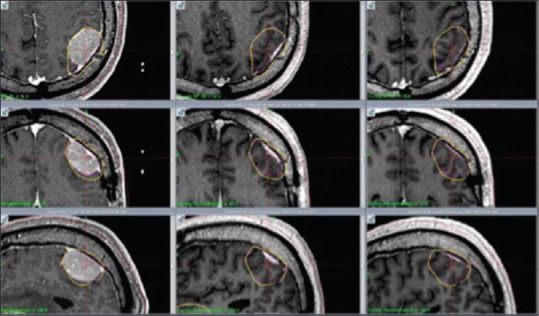
M4: Axial, coronal and sagittal contrast enhanced T1 weighted MR images show significant tumor reduction at 1 month (center column) and 7 months (right column) respectively after RRR. Left column shows tumor size at day 1 (RS 1). Significant tumor reduction at 1 month (center column) and 7 months (right column)
Figure 3.
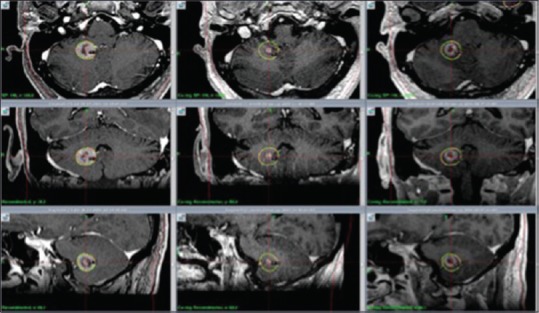
M2: Axial, coronal and sagittal contrast enhanced T1 weighted MR images. RS 1 (day 1, left column); significant tumor volume reduction at 1 month (center column); volume increase, although still smaller than original treatment volume at 7 months (right column)
Figure 4.
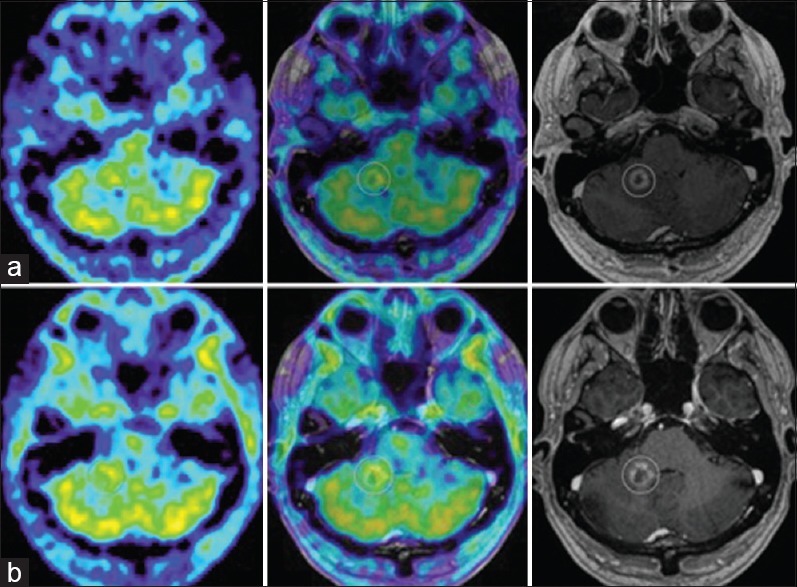
(a) M2's evolution on amino acid PET. 11C-Methionine PET (MET PET, left), MET PET fused with MRI CE T1 (middle) and MRI CE T1 weighted (right) axial images at 7 months demonstrating intermediate focal MET uptake in the medial aspect of the ring enhancing right cerebellar lesion (T/N ratio = 1.5 compared to contralateral mirrored tissue) and local edema. (b) MET PET (left) at 10 months now demonstrating T/N ratio = 1.3, diminished compared to MET PET 3 months prior, MET PET fused with MRI CE T1 (middle) and MRI CE T1 weighted (right): suspected ARE
Figure 5.
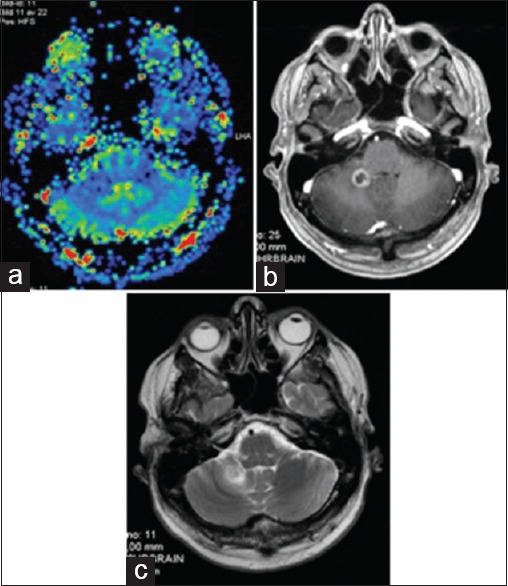
(a) M2: MR Perfusion imaging at 8.5 months (M2): No increase in CBV. (b) M2: Slight increase in tumor volume / central necrosis on MRI CE T1. (c) M2: Limited perilesional edema on MRI T2 with intermittent cortisone treatment: probable focal ARE
Figure 6.
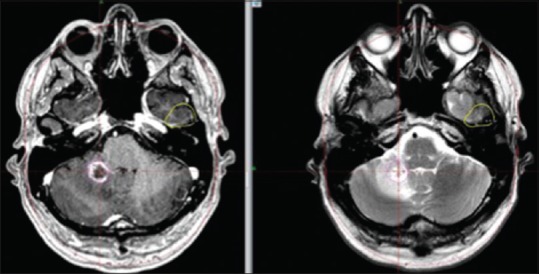
M2: Further increase in volume (CE T1 weighted MR images) and perifocal edema (T2 weighted MR images) at 10.5 months
Figure 7.
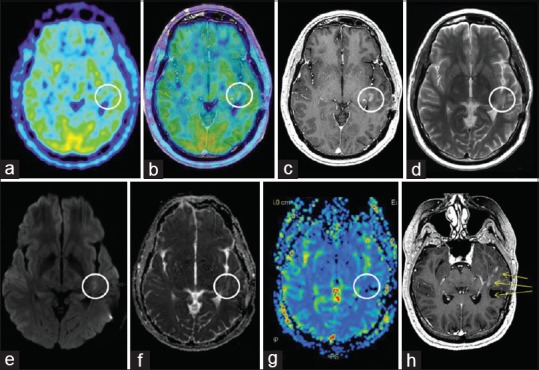
MET PET and MR imaging at 13 months: (a) Axial MET PET (b) axial MET PET + MRI CET1 (c) axial MRI CET1 (d) axial T2 (e) axial DWI (f) axial ADC (g) MR perfusion rCBV (h) reoriented CET1 demonstrating M5 and M6's proximity to the surgical area as well as new nodular rim enhancement of the surgical cavity
Extracranial tumor screening in November 2016 showed no recurrence. By the time of paper submission, the patient's clinical condition was assessed as good (KPS 90–100/RPA 1) without ongoing antitumoral therapy. The patient remains on low dose cortisone due to M5-M6's ARE. Further clinical and radiological follow up (including MRI and PET scans) are planned to carefully monitor M5-M6's evolution and identify further brain metastatic development.
DISCUSSION
The management of brain metastases remains complex and requires tailored treatment, often including microsurgery, different radiotherapeutic modalities, chemotherapy, as well as targeted therapy.[25] In the case of rare metastatic entities, the challenge of tailoring a multimodal treatment becomes even greater as available data in the literature is generally limited. Thymic carcinoma is a rare malignancy with typically aggressive evolution and, in many cases, poor prognosis; however, brain metastatic activity for this particular tumor group remains uncommon.[1,4,9,22,30,32,33,36] In general, open surgery with adjunctive radiotherapy delivered to the surgical cavity has proven effective on single large metastasis with more “common”/frequent histopathological traits.[2,8,1,24] Nevertheless, in many cases, this approach may not be possible due to the tumor's critical location and/or number of metastases present at that particular time. In terms of tumor control and local toxicity, single fraction gamma knife radiosurgery (SF-GKRS) has proven effective when treating small (single or multiple) metastatic lesions with different histopathological background.[19,23,29] However, in the case of large (>8–10 cc) unresectable metastasis, the effectiveness of SF-GKRS, particularly in terms of local radiation-induced toxicity, remains a subject of debate.[14,17,19] For these tumors, other radiation treatment modalities such as whole brain radiation therapy (WBRT) and local hypofractionated regimens are often considered. Because of the risk for long-term cognitive impairment,[11,13,23,24,29] WBRT is increasingly avoided as an upfront treatment. LINAC and Gamma Knife based hypofractionated regimens have proven effective in the treatment of metastatic lesions,[8,12,15,17,21,27,28,29,37] particularly for larger ones.[21] RRR aims to deal with life-threatening, neurologically destructive neoplasms by achieving next to comparable debulking/decompressing surgery results within the course of treatment (days) or shortly after (weeks). As described earlier, the treatment's key factor is to adapt peripheral prescription doses and tumor bed dose distributions to ongoing tumor volume dynamics within the week of the treatment. This enables the surgeon to increase dose heterogeneity/dose escalation within tumor boundaries while sparing adjacent healthy tissue. LQ-model based calculations are crucial to assess tolerable biologically effective dose distributions in healthy tissues in relation to required ablative peripheral dose prescriptions.[10,15] High performance imaging, including MRI and PET examinations are crucial in terms of pretreatment diagnostics, treatment planning, and follow-up settings. The differentiation between adverse radiation event/effect and viable tumor as the cause of increase in size of a contrast-enhancing lesion after stereotactic radiosurgery remains a significant challenge in neuroradiology. There is convincing evidence in the literature with multiple studies, meta-analyses, and review articles confirming that the presence of restricted diffusion,[3,35] increased rCBV on MR perfusion,[5,6] as well as high uptake on amino acid PET such as 11C-methionine[6,7,16,31,34] support a diagnosis of viable malignant tumor, and their absence support the diagnosis of ARE. A meta-analysis of 17 articles investigating glioma recurrence by Deng et al. in 2013 demonstrated that “ 11C-MET PET and dynamic susceptibility contrast-enhanced MR perfusion had comparable sensitivity (0.870 and 0.844, respectively), specificity (0.813 and 0.853, respectively)”[31] In distinguishing recurrent brain metastasis versus radiation injury after stereotactic radiosurgery, Tsuyuguchi et al. examined 21 patients with MET PET resulting in a sensitivity of 77.8% and specificity of 100% for the detection of tumor recurrence.[34] MET PET has also been used successfully in radiosurgery planning. Momose et al. in 2014 in a group of 88 patients demonstrated decreased radiation volume and prolonged survival for patients with previously irradiated brain metastases when dose planning was based on MET PET/MRI fusion versus MRI alone, with a median survival of 18.1 months in the former and 8.6 months in the latter patient group (P = 0.01).[20]
Based on our institutional experience and previous reports,[18,26] we believe the tumor's histopathology/underlying radiosensitivity and RPA-surrogate factors might play a determinant role on RRR-outcome. In this particular patient case, the procedure proved highly successful by promptly ablating M3 and M4, hence avoiding further neurological impairment including aphasia, sensorimotor deficit, and epileptic activity.
We have achieved similar results on a number of cases with large metastatic brain lesions with more common histopathological traits; a retrospective analysis covering the short and long-term outcome of RRR on these cases will be the subject of our next two papers.
CONCLUSION
In this particular case, Rapid Rescue Radiosurgery (RRR) proved highly effective in achieving next to comparable surgical decompression results on two large aggressive metastatic brain lesions. However, particular factors such as the tumor's histopathology/intrinsic radiosensitivity as well as RPA-surrogate factors could have played a substantial role in the outcome of the treatment. We believe this procedure has the potential to become an important surgical tool in the future management of large unresectable metastases. Retrospective analysis of all cases treated with RRR as well as further prospective studies are warranted and indeed ongoing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Georges Sinclair, Email: georges.sinclair@karolinska.se.
Heather Martin, Email: heather.martin@karolinska.se.
Michael Fagerlund, Email: michael.fagerlund@karolinska.se.
Amir Samadi, Email: amir.samadi-ahadi@karolinska.se.
Hamza Benmakhlouf, Email: hamza.benmakhlouf@karolinska.se.
Ernest Doodo, Email: ernest.dodoo@karolinska.se.
REFERENCES
- 1.Al-Barbarawi M, Smith SF, Sekhon LH. Haemorrhagic brain metastasis from a thymic carcinoma. J Clin Neurosci. 2004;11:190–4. doi: 10.1016/j.jocn.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Al-Omair A, Soliman H, Xu W, Karotki A, Mainprize T, Phan N, et al. Hypofractionated stereotactic radiotherapy in five daily fractions for post-operative surgical cavities in brain metastases patients with and without prior whole brain radiation. Technol Cancer Res Treat. 2013;12:493–9. doi: 10.7785/tcrt.2012.500336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asao C, Korogi Y, Kitajima M, Hirai T, Baba Y, Makino K, et al. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol. 2005;26:1455–60. [PMC free article] [PubMed] [Google Scholar]
- 4.Asao T, Fujiwara Y, Sunami K, Kitahara S, Goto Y, Kanda S, et al. Medical treatment involving investigational drugs and genetic profile of thymic carcinoma. Lung Cancer. 2016;93:77–81. doi: 10.1016/j.lungcan.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2009;30:367–72. doi: 10.3174/ajnr.A1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Souza MM, Sharma R, Jaimini A, Panwar P, Saw S, Kaur P, et al. 11C-MET PET/CT and advanced MRI in the evaluation of tumor recurrence in high-grade gliomas. Clin Nucl Med. 2014;39:791–8. doi: 10.1097/RLU.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 7.Deng SM, Zhang B, Wu YW, Zhang W, Chen YY. Detection of glioma recurrence by (1)(1) C-methionine positron emission tomography and dynamic susceptibility contrast-enhanced magnetic resonance imaging: A meta-analysis. Nucl Med Commun. 2013;34:758–66. doi: 10.1097/MNM.0b013e328361f598. [DOI] [PubMed] [Google Scholar]
- 8.Eaton BR, Gebhardt B, Prabhu R, Shu HK, Curran WJ, Jr, Crocker I. Hypofractionated radiosurgery for intact or resected brain metastases: Defining the optimal dose and fractionation. Radiat Oncol. 2013;8:135. doi: 10.1186/1748-717X-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filosso PL, Guerrera F, Rendina AE, Bora G, Ruffini E, Novero D, et al. Outcome of surgically resected thymic carcinoma: A multicenter experience. Lung Cancer. 2014;83:205–10. doi: 10.1016/j.lungcan.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–68. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio-Morin C, Masson-Cote L, Ezahr Y, Blanchard J, Ebacher A, Mathieu D. Early Gamma Knife stereotactic radiosurgery to the tumor bed of resected brain metastasis for improved local control. J Neurosurg. 2014;121(Suppl):69–74. doi: 10.3171/2014.7.GKS141488. [DOI] [PubMed] [Google Scholar]
- 12.Ishihara T, Yamada K, Harada A, Isogai K, Tonosaki Y, Demizu Y, et al. Hypofractionated stereotactic radiotherapy for brain metastases from lung cancer: Evaluation of indications and predictors of local control. Strahlenther Onkol. 2016;192:386–93. doi: 10.1007/s00066-016-0963-2. [DOI] [PubMed] [Google Scholar]
- 13.Kocher M, Wittig A, Piroth MD, Treuer H, Seegenschmiedt H, Ruge M, et al. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol. 2014;190:521–32. doi: 10.1007/s00066-014-0648-7. [DOI] [PubMed] [Google Scholar]
- 14.Le Rhun E, Dhermain F, Vogin G, Reyns N, Metellus P. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurother. 2016;16:903–14. doi: 10.1080/14737175.2016.1184572. [DOI] [PubMed] [Google Scholar]
- 15.Martens B, Janssen S, Werner M, Fruhauf J, Christiansen H, Bremer M, et al. Hypofractionated stereotactic radiotherapy of limited brain metastases: A single-centre individualized treatment approach. BMC Cancer. 2012;12:497. doi: 10.1186/1471-2407-12-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minamimoto R, Saginoya T, Kondo C, Tomura N, Ito K, Matsuo Y, et al. Differentiation of Brain Tumor Recurrence from Post-Radiotherapy Necrosis with 11C-Methionine PET: Visual Assessment versus Quantitative Assessment. PLoS One. 2015;10:e0132515. doi: 10.1371/journal.pone.0132515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, et al. Single-Fraction Versus Multifraction (3×9 Gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int J Radiat Oncol Biol Phys. 2016;95:1142–8. doi: 10.1016/j.ijrobp.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Mohammadi AM, Recinos PF, Barnett GH, Weil RJ, Vogelbaum MA, Chao ST, et al. Role of Gamma Knife surgery in patients with 5 or more brain metastases. J Neurosurg. 2012;117(Suppl):5–12. doi: 10.3171/2012.8.GKS12983. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi AM, Schroeder JL, Angelov L, Chao ST, Murphy ES, Yu JS, et al. Impact of the radiosurgery prescription dose on the local control of small (2 cm or smaller) brain metastases. J Neurosurg. 2017;126:735–43. doi: 10.3171/2016.3.JNS153014. [DOI] [PubMed] [Google Scholar]
- 20.Momose T, Nariai T, Kawabe T, Inaji M, Tanaka Y, Watanabe S, et al. Clinical benefit of 11C methionine PET imaging as a planning modality for radiosurgery of previously irradiated recurrent brain metastases. Clin Nucl Med. 2014;39:939–43. doi: 10.1097/RLU.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 21.Navarria P, Pessina F, Cozzi L, Ascolese AM, De Rose F, Fogliata A, et al. Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol. 2016;11:76. doi: 10.1186/s13014-016-0653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolato A, Ferraresi P, Bontempini L, Tomazzoli L, Magarotto R, Gerosa M. Multiple brain metastases from “lymphoepithelioma-like” thymic carcinoma: A combined stereotactic-radiosurgical approach. Surg Neurol. 2001;55:232–4. doi: 10.1016/s0090-3019(01)00361-5. [DOI] [PubMed] [Google Scholar]
- 23.Niranjan A, Lunsford LD. Gamma Knife Radiosurgery for 5 to 10 Brain Metastases: A Good Option for Upfront Treatment. Oncology. 2016;30:314–5, 7. [PubMed] [Google Scholar]
- 24.Ojerholm E, Lee JY, Thawani JP, Miller D, O’Rourke DM, Dorsey JF, et al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg. 2014;121(Suppl):75–83. doi: 10.3171/2014.6.GKS14708. [DOI] [PubMed] [Google Scholar]
- 25.Owen S, Souhami L. The management of brain metastases in non-small cell lung cancer. Front Oncol. 2014;4:248. doi: 10.3389/fonc.2014.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell JW, Chung CT, Shah HR, Canute GW, Hodge CJ, Bassano DA, et al. Gamma Knife surgery in the management of radioresistant brain metastases in high-risk patients with melanoma, renal cell carcinoma, and sarcoma. J Neurosurg. 2008;109(Suppl):122–8. doi: 10.3171/JNS/2008/109/12/S19. [DOI] [PubMed] [Google Scholar]
- 27.Shuryak I, Carlson DJ, Brown JM, Brenner DJ. High-dose and fractionation effects in stereotactic radiation therapy: Analysis of tumor control data from 2965 patients. Radiother Oncol. 2015;115:327–34. doi: 10.1016/j.radonc.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Sinclair G, Bartek J, Jr, Martin H, Barsoum P, Dodoo E. Adaptive hypofractionated gamma knife radiosurgery for a large brainstem metastasis. Surg Neurol Int. 2016;7(Suppl 4):S130–8. doi: 10.4103/2152-7806.176138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soliman H, Das S, Larson DA, Sahgal A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016;7:12318–30. doi: 10.18632/oncotarget.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura Y, Kuroiwa T, Doi A, Min KY. Thymic carcinoma presenting as cranial metastasis with intradural and extracranial extension: Case report. Neurosurgery. 2004;54:209–11. doi: 10.1227/01.neu.0000097554.14112.55. [DOI] [PubMed] [Google Scholar]
- 31.Terakawa Y, Tsuyuguchi N, Iwai Y, Yamanaka K, Higashiyama S, Takami T, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49:694–9. doi: 10.2967/jnumed.107.048082. [DOI] [PubMed] [Google Scholar]
- 32.Thomas de Montpreville V, Ghigna MR, Lacroix L, Besse B, Broet P, Dartevelle P, et al. Thymic carcinomas: Clinicopathologic study of 37 cases from a single institution. Virchows Arch. 2013;462:307–13. doi: 10.1007/s00428-013-1371-y. [DOI] [PubMed] [Google Scholar]
- 33.Thompson EM, Sather MD, Reyes CA, Long DJ. Intracranial leptomeningeal metastasis from thymic carcinoma: Case report and review. Surg Neurol. 2007;68:233–8. doi: 10.1016/j.surneu.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 34.Tsuyuguchi N, Sunada I, Iwai Y, Yamanaka K, Tanaka K, Takami T, et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: Is a differential diagnosis possible? J Neurosurg. 2003;98:1056–64. doi: 10.3171/jns.2003.98.5.1056. [DOI] [PubMed] [Google Scholar]
- 35.Xu JL, Li YL, Lian JM, Dou SW, Yan FS, Wu H, et al. Distinction between postoperative recurrent glioma and radiation injury using MR diffusion tensor imaging. Neuroradiology. 2010;52:1193–9. doi: 10.1007/s00234-010-0731-4. [DOI] [PubMed] [Google Scholar]
- 36.Yamamura K, Kubo O, Aoki N, Kagawa M. Falx metastasis of thymic carcinoma: A case report and review of literature. No Shinkei Geka. 1993;21:921–4. [PubMed] [Google Scholar]
- 37.Zada G, Yu C, Pagnini PG, Khalessi AA, Zelman V, Apuzzo ML. Early decreased tumor volume following fractionated Gamma Knife Radiosurgery for metastatic melanoma and the role of “adaptive radiosurgery”: Case report. Neurosurgery. 2010;67:E512–3. doi: 10.1227/01.NEU.0000371984.18490.55. [DOI] [PubMed] [Google Scholar]


