Abstract
The widespread distribution of mercury (Hg) threatens wildlife health, particularly piscivorous birds. Western North America is a diverse region that provides critical habitat to many piscivorous bird species, and also has a well-documented history of mercury contamination from legacy mining and atmospheric deposition. The diversity of landscapes in the west limit the distribution of avian piscivore species, complicating broad comparisons across the region. Mercury risk to avian piscivores was evaluated across the Western United States and Canada using a suite of avian piscivore species representing a variety of foraging strategies that together occur broadly across the region. Prey fish Hg concentrations were size-adjusted to the preferred size class of the diet for each avian piscivore (Bald Eagle=36 cm, Osprey=30 cm, Common and Yellow-billed Loon=15 cm, Western and Clark’s Grebe=6 cm, and Belted Kingfisher=5 cm) across each species breeding range. Using a combination of field and lab-based studies on Hg effect in a variety of species, wet weight blood estimates were grouped into five relative risk categories including: background (<0.5 μg/g), low (0.5 – 1 μg/g), moderate (1 – 2 μg/g), high (2 – 3 μg/g), and extra high (>3 μg/g). These risk categories were used to estimate potential mercury risk to avian piscivores across the west at a 1 degree-by-1 degree grid cell resolution. Avian piscivores foraging on larger-sized fish generally were at higher relative risk to Hg. Habitats with relatively high risk included wetland complexes (e.g., prairie pothole in Saskatchewan), river deltas (e.g., San Francisco Bay, Puget Sound, Columbia River), and arid lands (Great Basin and central Arizona). These results indicate that more intensive avian piscivore sampling is needed across western North America to generate a more robust assessment of exposure risk.
Keywords: bioindicator, fish, methylmercury, risk assessment
Graphical abstract
1.0 Introduction
Avian piscivores (fish-eating birds) are valuable bioindicators of environmental health and exposure to aquatic contaminants, such as mercury (Hg) (Becker 2003; Scheuhammer et al., 2007). Their aquatic foraging habits and high trophic level place them among the most at-risk wildlife groups with regard to Hg exposure (Evers et al., 2005). Published literature has demonstrated field-based Hg exposure and related effects in many species of avian piscivores (Ackerman et al., this issue.; Burgess and Meyer 2008; Evers et al., 2008a; Depew et al., 2012; Schoch et al., 2014), and avian piscivores are key bioindicators for many long-term monitoring programs (Braune 2007; Evers et al., 1998; Monteiro and Furness, 1995) and site-specific assessments (Guigueno et al., 2012; Weech et al., 2006). As part of a cross-national assessment of Hg cycling, distribution, and bioaccumulation (Western North America Mercury Synthesis), Hg risk to avian piscivores was assessed for Western North America – a region spanning north to Alaska, south to Arizona, west to the Pacific, and east to the Dakotas (see overview in Eagles-Smith et al., this issue [a]). Understanding Hg risk to avian piscivores across this large region is important for conservation of these sensitive species.
Efforts elsewhere to evaluate avian risk to Hg across similarly broad geographic areas have relied on assessments of key prey fish species and often a wide distribution of only one target avian piscivore species (Depew et al., 2013; Scheuhammer et al., 2016). For example, the Common Loon (Gavia immer) and Yellow Perch (Perca flavescens) have been successfully used as effective bioindicators of risk in previous Hg syntheses in the Northeastern United States (Evers et al. 2008a), Great Lakes region (Evers et al. 2011) and across central and eastern Canada (Depew et al. 2013). In these studies, investigators mathematically transformed Hg concentrations of numerous fish species into single prey species equivalents to facilitate assessing risk to Common Loons across broad spatial extents. For those geographic regions, using a single widely distributed and well-studied sentinel species, (i.e., Common Loons) allowed for the identification of areas with greatest biological risk to Hg exposure (Evers et al., 2007; Scheuhammer et al., 2016).
Understanding risk to avian piscivores across Western North America is complicated by two important factors. First, Western North America covers a large diversity of freshwater and marine ecosystems, incorporating at least 19 bird conservation regions that ultimately support a diverse assemblage of avian piscivore species (North American Bird Conservation Initiative, 2015). Second, Hg distribution across Western North America is highly variable because of diverse landscape factors that facilitate methylation, an extensive mining history, and climatological variation that influences Hg transport and fate (Pinkney et al., 2015; Eagles-Smith et al. this issue [a]). The diverse landscape and climatological gradients of the West limit the geographical utility of single species assessments, because prey fish and avian piscivore assemblages vary greatly across the region, which presents unrealistic assessments of risk to avian taxa that do not exist in certain regions. Limiting the analysis to only one species would not be representative of piscivores in this region, thereby necessitating the current approach.
The goal of this paper is to illustrate relative risk of Hg to freshwater avian piscivores across their Western North America breeding ranges. Avian piscivores were selected from freshwater systems that encompassed a range of foraging strategies, body sizes, and distributions; they include the Bald Eagle (Haliaeetus leucocephalus), Osprey (Pandion haliaetus), Belted Kingfisher (Megaceryle alcyon), and two closely-related species groups: loons (Common, Gavia immer and Yellow-billed, Gavia adamsii) and grebes (Western, Aechmophorus occidentalis and Clark’s, Aechmophorus clarkii). First, bird Hg concentrations in 20 common marine and freshwater avian piscivores were compared to better understand the Hg concentration ranges of the target freshwater piscivores across the study region. Because the geographic scope of the bird data was limited, the large fish monitoring and synthesis effort across the West (summarized in Eagles-Smith et al., this issue [b]) was leveraged to use fish Hg concentrations for modelling risk to the target avian piscivores in Western North America. This approach allows us to identify areas of concern for future research, and sets the context for understanding future changes to Hg bioaccumulation across this region. This approach can be used in the future across the entirety of North America to understand the location and patterns of biological Hg hotspots on a continental scale.
2.0 Materials and Methods
2.1 Fish and avian piscivore database compilation
Fish total Hg (THg) concentrations were compiled from numerous sources, summarized in more detail by Eagles-Smith et al., (this issue [b]). Briefly, fish samples were compiled from federal, state, and provincial databases, constraining data to Alaska, Yukon Territories, Northwest Territories, British Columbia, Washington, Oregon, Idaho, California, Nevada, Arizona, New Mexico, Colorado, Utah, Wyoming, Montana, Alberta, Saskatchewan, North Dakota, and South Dakota. While the larger fish dataset included fish from Hawaii, for this analysis, Hawaiian and any marine fish were excluded. All fish THg concentrations were standardized to skinless, boneless fillet in wet weight. More information on these specific conversions can be found in Eagles-Smith et al., (this issue [b]).
Contaminant databases that contained avian piscivore THg concentrations were also compiled, including data from the U.S. Fish and Wildlife Service Environmental Contaminants Data Management System (ECDMS), the Biodiversity Research Institute’s Center for Mercury Studies, U.S. Geological Survey, Environment Canada, and the multi-partner Seabird Tissue Archival and Monitoring Project (STAMP). More extensive summaries of the bird datasets can be found in Ackerman et al. (this issue). Mercury concentrations were determined in established laboratories using a variety of instruments (e.g., ICP-MS, Direct Hg Analyzers) based on established standard operating procedures.
2.2 Avian Hg sample risk assessment
Risk assessment was focused on modeling five target piscivore species or closely related species groups: Bald Eagle, Osprey, Loons (Common and Yellow-billed) and Aechmophorus Grebes (Clark’s and Western), and Belted Kingfisher (scientific names in Table 2). These species were chosen based upon their use in a variety of Hg monitoring projects and to represent a variety of foraging strategies and geographic areas (Table 1). To understand how these target piscivores compared to other bird species across western U.S. and Canada, avian piscivore tissue (whole blood, egg, and liver) THg concentrations for 20 commonly sampled species were compared across the west (Table 2). All tissue concentrations were converted to a whole blood equivalent THg concentration in order to standardize THg concentrations, and facilitate comparisons and risk categorization. For blood and liver samples, only adult birds were selected. To convert eggs to whole blood, the equation developed in Ackerman et al. (2016) was applied to the egg data compiled for this paper (Eq. 1). Liver samples compiled for this paper were converted to whole blood using an equation developed by Eagles-Smith et al. (2008), which was derived from data for 4 species of waterbirds (Eq. 2).
Table 2.
Geometric mean blood Hg (equivalent) concentrations for 20 avian piscivore species in taxonomic order. Liver and egg tissues were standardized to whole blood equivalent (μg/g, ww).
| Percent of samples in each Hg category | ||||||||
|---|---|---|---|---|---|---|---|---|
| Avian Piscivore Species | N | Mean Hg (μg/g) | Standard Error | Background 0 – 0.5 (μg/g) | Low 0.5 – 1 (μg/g) | Moderate 1 – 2 (μg/g) | High 2 – 3 (μg/g) | Extra High > 3 (μg/g) |
| Marbled Murrelet Brachyramphus marmoratus |
69 | 0.19 | 0.01 | 97% | 3% | 0% | 0% | 0% |
| Thick-billed Murre Uria lomvia |
141 | 0.12 | 0.01 | 94% | 6% | 0% | 0% | 0% |
| Common Murre Uria aalge | 198 | 0.18 | 0.01 | 92% | 8% | 0% | 0% | 0% |
| Least Tern Sternula antillarum | 131 | 0.76 | 0.04 | 24% | 43% | 27% | 7% | 0% |
| Bald Eagle Haliaeetus leucocephalus |
334 | 0.69 | 0.02 | 30% | 44% | 22% | 4% | 0% |
| Osprey Pandion haliaetus |
229 | 0.12 | 0.01 | 98% | 2% | 0% | 0% | 0% |
| Black-crowned Night Heron Nycticorax nycticorax | 210 | 0.29 | 0.02 | 75% | 16% | 7% | 1% | 0% |
| Tricolored Heron Egretta tricolor |
220 | 0.15 | 0.01 | 99% | 0% | 0% | 0% | 0% |
| Great Blue Heron Ardea herodias | 238 | 0.19 | 0.01 | 90% | 7% | 2% | 1% | 0% |
| Brown Pelican Pelecanus occidentalis |
161 | 0.35 | 0.02 | 68% | 23% | 6% | 2% | 1% |
| American White Pelican Pelecanus erythrorhynchos |
185 | 1.02 | 0.09 | 28% | 21% | 26% | 14% | 11% |
| Brown Booby Sula leucogaster |
19 | 0.27 | 0.03 | 84% | 16% | 0% | 0% | 0% |
| Black-footed Albatross Phoebastria nigripes |
16 | 2.72 | 0.31 | 0% | 0% | 25% | 56% | 19% |
| Laysan Albatross Phoebastria immutabilis |
204 | 0.65 | 0.02 | 26% | 56% | 17% | 0% | 0% |
| Clark’s Grebe Aechmophorus clarkii |
103 | 0.90 | 0.09 | 28% | 30% | 27% | 5% | 10% |
| Western Grebe Aechmophorus occidentalis |
259 | 0.53 | 0.03 | 51% | 28% | 14% | 5% | 3% |
| Eared Grebe Podiceps nigricollis |
358 | 0.30 | 0.02 | 59% | 20% | 17% | 4% | 0% |
| Yellow-billed Loon Gavia adamsii |
176 | 0.64 | 0.04 | 40% | 34% | 18% | 7% | 1% |
| Common Loon Gavia immer |
626 | 0.91 | 0.03 | 21% | 35% | 27% | 12% | 5% |
| Red-throated Loon Gavia stellata |
150 | 0.33 | 0.03 | 59% | 28% | 8% | 3% | 2% |
| Overall | 4027 | 0.41 | 0.01 | 55% | 24% | 14% | 5% | 2% |
Table 1.
Characteristics of target avian piscivore species or species groups. For consistency, piscivores from each ecosystem are shown in order from large to small prey.
| Species or group | Foraging Habits during Breeding Season | Fish Size Range | Fish Size Preference |
|---|---|---|---|
| Bald Eaglea | Opportunistic forager (active fishing and scavenger) | 20–75 cm | 36 cm |
| Ospreyb | Obligate piscivore, limited by weight of fish to carry | 10–45 cm | 30 cm |
| Loonsc | Obligate piscivore, limited by size able to swallow | 10–25 cm | 15 cm |
| Grebesd | Small fish specialist (also some invertebrates) | 2–15 cm | 6 cm |
| Belted Kingfishere | Small fish specialist (also some invertebrates) | 1–10 cm | 5 cm |
Common Loon Evers et al., 2010; Yellow-billed Loon North, 1994
Clark’s Grebe, Western Grebe, Ackerman et al., 2015
Equation 1. (Ackerman et al., 2016)
Equation 2. (Eagles-Smith et al., 2008)
After converting THg in all tissues to the common unit of THg concentrations in whole blood, concentrations were compared across all 20 species (Fig. 1). Although the sample size for each target species was relatively large (Table 2), they had relatively constrained geographic distributions, making it difficult to estimate risk across their ranges (Fig. 2). As a result, risk maps were developed based on models derived from fish THg concentrations.
Figure 1.
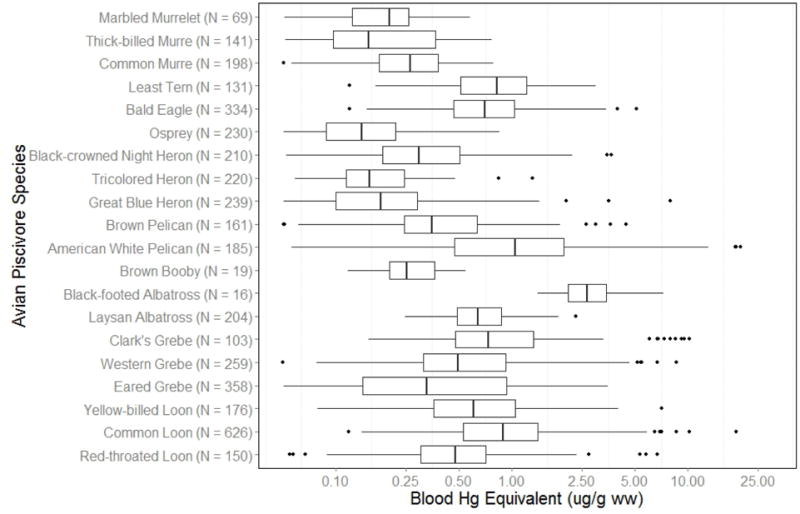
Whole blood Hg (μg/g wet weight) comparison across 20 avian piscivores in western United States and Canada. Birds (including sample size) are shown in taxonomic order. Egg and liver samples were converted into whole blood equivalent to aid in comparison using Equation 1 and 2 (see text).
Figure 2.
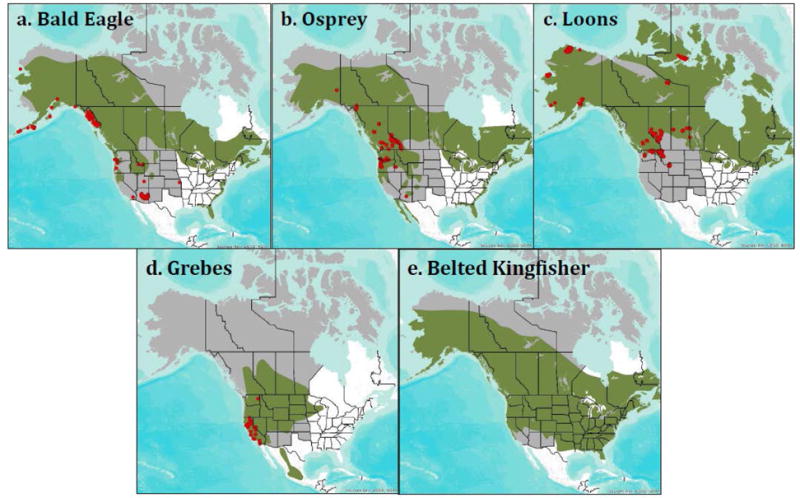
Distribution of avian samples (blood, egg, and liver combined) for target piscivores across their breeding ranges in western United States and Canada. The study area in western United States and Canada is shown in gray. Each species breeding range is show in green. Loons include Common Loons and Yellow-billed Loons (c). Grebes include Western Grebes and Clark’s Grebes (d). There were no bird data for Belted Kingfishers (e).
2.3 Freshwater fish Hg sample risk assessment
The preferred prey fish size for each target piscivore species was determined using published literature (Table 1). For each avian piscivore species, the fish length that best fit their foraging preferences was used in the calculation of risk based upon prey fish THg concentration. Size adjustment of fish THg concentrations was conducted in R (version 3.1.0; www.r-proiect.org) and JMP (Version 12, SAS Institute Inc., Cary, NC) was used for all subsequent statistical analyses. Because THg concentrations often vary with fish length, the raw fish THg concentrations were size-adjusted to the preferred prey size of each piscivore (Table 1). For each target piscivore, the available fish data was constrained to only those species with individuals that fell within the birds prey size range, and where a likelihood ratio test suggested that the inclusion of length improved the fit of the data. Fish species with similar ecologies and physiologies were grouped into aggregate species for size-adjustment, because some species were poorly represented in the dataset (Eagles-Smith et al. this issue [b]). Species groups included in the dataset are shown in Table 2.
Sites were defined separately for lentic and lotic systems; fish locations in lentic systems were aggregated to the centroid of the water body, whereas fish locations in lotic systems were aggregated such that samples within 10 km of each other were categorized as originating from the same location. For each of these fish species groups, a linear mixed-effects model that contained fish total length as a fixed continuous factor; and site, fish species, and a species × total length interaction as random effects was used to predict the whole body total THg concentration of each fish at the preferred prey size of each piscivore (Bald Eagle = 36 cm, Osprey = 30 cm, Loons = 15 cm, Grebes = 6 cm, Belted Kingfisher = 5 cm; Table 1). For each predicted THg concentration, each individual fish’s residual was added from the size-adjusted model to reincorporate variation not related to length into the size-adjusted THg concentrations for each individual fish. In some species, a likelihood ratio test indicated that the model including length did not fit the fish THg concentrations better than a null model; for these species, non size-adjusted, whole body THg concentrations were used.
The geospatial selection of fish samples for statistical analysis (using ArcGIS v 10.2.2) was conducted by constraining the dataset to only those locations that fell within each target piscivores breeding range using species-specific range maps (Buehler 2000; Evers et al. 2010; Kelly et al. 2009; LaPorte et al., 2013; North 1994; Poole et al. 2002; Storer and Nuechterlein 1992). The study region— including the states and provinces listed previously—does not cover the entire North American range of any of these target avian piscivore species. Western and Clark’s Grebes are mostly western species, and the study area covered 89% of their entire range. Other species had breeding distributions that expanded far beyond Western North America. For the Bald Eagle, the study area encompasses 78% of its breeding range. For the Osprey (found on all continents except Antarctica), the study area encompassed 72% of its North American breeding range. For the Belted Kingfisher, the study area covered only 69% of its breeding range. Common and Yellow-billed Loons had 78% of their North American breeding range within the western study area; Yellow-billed Loons also breed in Russia.
Grid cells (1-degree-by-1-degree) were created to cover the extent of the geographic range of each species to aid in creating species-specific risk maps. Grid cells have been used in previous synthesis efforts to illustrate risk across large geographic area with a wide range of representation of fish and bird THg concentrations (Evers et al. 2007, 2011). Grid cells covered multiple water bodies within a region and were drawn not based on state or provincial boundaries. Any grid cells with low number of fish samples (n < 3) were excluded and results were classified based on the confidence in the estimate. Because each grid contains a different data density, confidence in the least squares mean was shown based on whether model-calculated standard error divided by the least squares mean was less than 0.25 (indicating low confidence in the least squares mean estimate). Because datasets for each avian piscivore were both corrected for fish that fell within their preferred size and within their breeding range, sample sizes of individual fish THg concentrations were different for each avian piscivore (Bald Eagle n = 56,096; Osprey n = 58,890; Loon n = 46,840; Grebe n = 46,203; Belted Kingfisher n = 54,673). By using this modeling approach, the fish dataset varies in species composition and sample size (Table 2).
All THg concentrations in fish samples were natural log (ln) transformed to improve normality of residuals. From this subset of data, modeled the least squares mean prey fish THg concentration was modeled for each 1-degree-by-1-degree grid cell, including site and species group as random effects. Back-transformed standard errors were estimated using the delta method (Seber 1982). In order to standardize the relative risk estimates, the least squares mean prey fish THg concentration in each grid was converted to the equivalent avian blood THg concentrations using the global equation from Ackerman et al. (2015), which synthesized seven different study’s equations (Eq. 3).
Equation 3. (Ackerman et al., 2015)
2.2.1 Important assumptions
The use of prey fish data THg concentrations to estimate avian THg exposure comes with four important assumptions. First, preferred prey size was used to model fish THg concentrations. Although using the preferred prey size likely provides an accurate estimate of typical risk to each piscivore, each species actually consumes variable sized fish depending on availability. Further, the size range of potential prey varies tremendously among avian piscivores (Table 1) suggesting that the variability in the predictions of risk may be underestimated more in some species in comparison to others. Bald Eagles, in particular, will eat much larger fish if scavenging dead prey, whereas Osprey are limited by the size of fish that they can carry (Buehler, 2000; Poole et al., 2002). Second, the model also assumed that the birds were eating primarily fish. Loons and Osprey are known to focus on fish prey, but Bald Eagles often consume carrion or terrestrial prey not related to fish. Grebes and Kingfishers will eat a variety of invertebrates in addition to small fish (Kelly et al., 2009; Storer and Nuechterlien, 1992; LaPorte et al., 2013), but prey fish THg concentrations are very highly correlated to grebe blood THg concentrations (Ackerman et al. 2015). Third, it was assumed that birds were eating the same fish species that were included within the fish Hg database. Although the size correction process likely accounted for much of this issue, it is still possible that avian piscivores select different fish species or sizes than those that are monitored by Hg monitoring programs.Foraging habitat also has a large impact on Hg exposure in fish and avian piscivores; this is partially taken into account by including site in the model for fish. Fourth, it is assumed that fish THg is correlated with bird blood THg and that this correlation is similar across species. Ackerman et al. (2015) synthesized 7 study’s equations into one global equation which was used to correlate prey fish to bird Hg concentrations. Because published correlations between fish prey and bird Hg do not exist for all of the individual target piscivores, it is assumed that this global correlation is applicable across species. While there may be relatively few studies that correlate prey fish to bird blood, the correlations that do exist are remarkably consistent across taxa and geographic areas, supporting the use of the global equation (Ackerman et al. 2015, Evers et al. 2011; Scheuhammer et al. 1998; Champoux et al. 2006; Burgess and Meyer 2008; Yu et al. 2011; Scheuhammer et al., 2016).
2.3 Hg Risk Categories for Avian Piscivores
Selection of risk benchmarks for avian piscivores is greatly complicated by substantial variation in sensitivity among taxa (Heinz et al. 2009), and limited data available on effects. A comprehensive assessment of risk benchmarks for birds based upon their THg concentrations is compiled elsewhere (Ackerman et al., this issue) and their categorical approach was applied to standardize interpretation among papers. Blood THg concentrations (μg/g, ww) were classified into five categories that included: background (< 0.5 μg/g), generally below effects thresholds, low (0.5 – 1 μg/g) or elevated above background but below most effects thresholds, moderate (1 – 2 μg/g) or where adult physiological and behavioral abnormalities likely occur, high (2 – 3 μg/g) or likely reproductive impairment, to extra high (> 3 μg/g) or a high likelihood of significant reproductive failure.
Given the scale of this synthesis, it was most appropriate to use these broad categories, because more precise estimates may have limited basis for use across taxa. Although there are some studies of relative risk across different bird species (Heinz et al. 2009), there are few comprehensive benchmarks for the five target piscivores highlighted here. Although these risk categories approximately align with Hg risk thresholds developed for Common Loons (Evers et al., 2008a; Depew et al., 2012), interpreting their meaning for the different species outlined in this study is complicated by uncertainty in interspecies differences. Thus, qualitative risk categories were presented to be used as a meaningful guide to evaluate concern at a population level.
3. Results
3.1 Avian piscivore Hg samples
THg concentrations in 4407 samples were compiled from avian piscivore tissues across western North America, and converted into blood Hg equivalent (μg/g, wet weight throughout) for comparison (Fig. 1, Table 2). More than half of the bird samples (55%) fell into the background Hg risk category (0 μg/g – 0.5 μg/g), whereas 24% were in the low category (0.5 μg/g – 1 μg/g), 14% were in the moderate category (1 μg/g – 2 μg/g), 5% were in the high category (2 μg/g – 3 μg/g), and 2% were in the extra high category (> 3 μg/g). While the majority of samples were less than 1 μg/g, individual species exhibited variation in the proportion of samples in each Hg category (Table 2). The species with the highest proportion of samples above 2 μg/g (high or extra high category) were Black-footed Albatross (75% above 2 μg/g), American White Pelican (25% above 2 μg/g), Common Loon (17% above 2 μg/g), Clark’s Grebe (15% above 2 μg/g), Western Grebe (8% above 2 μg/g), Yellow-billed Loon (8% above 2 μg/g), and Least Tern (7% above 2 μg/g).
The geometric mean for all samples was 0.41 μg/g ww (SE = 0.01). On an individual species basis, geometric mean Hg concentrations in 11 of the 20 species were within the background Hg category (0 μg/g – 0.5 μg/g; Table 2). Black-footed Albatross had the highest geometric mean (± SE) concentration (2.72 μg/g ± 0.31) but also the lowest sample size (N = 16). American White Pelican samples had a geometric mean concentration of 1.02 μg/g ± 0.09 (moderate risk category), while seven other species exhibited geometric mean THg concentrations in the low Hg category (0.5 – 1 μg/g), including: Common Loon (0.91 μg/g ± 0.03), Clark’s Grebe (0.90 μg/g ± 0.09), Least Tern (0.76 μg/g ± 0.4), Bald Eagle (0.69 μg/g ± 0.02), Laysan Albatross (0.65 μg/g ± 0.02), Yellow-billed Loon (0.64 μg/g ± 0.04), and Western Grebe (0.53 μg/g ± 0.03). Although the sample size for many of these species was relatively large (Table 2), the geographic distribution was quite constrained (Fig. 2). Therefore, the focused analysis is based upon predicted THg concentrations from the fish database, which provides a more robust spatial representation.
3.2 Fish Hg models
Although coverage was better than the bird dataset, the fish data did not cover the entire western breeding range of any of the avian piscivores. The amount of each species’ western breeding range that was covered by the fish model was 44% for Bald Eagle, 51% for Osprey, 25% for loons, 79% for grebes, and 41% for Belted Kingfisher (Fig. 3). All THg concentrations are reported as μg/g, wet weight (ww) ± standard error. Fish size distributions are reported in cm ± standard error.
Figure 3.
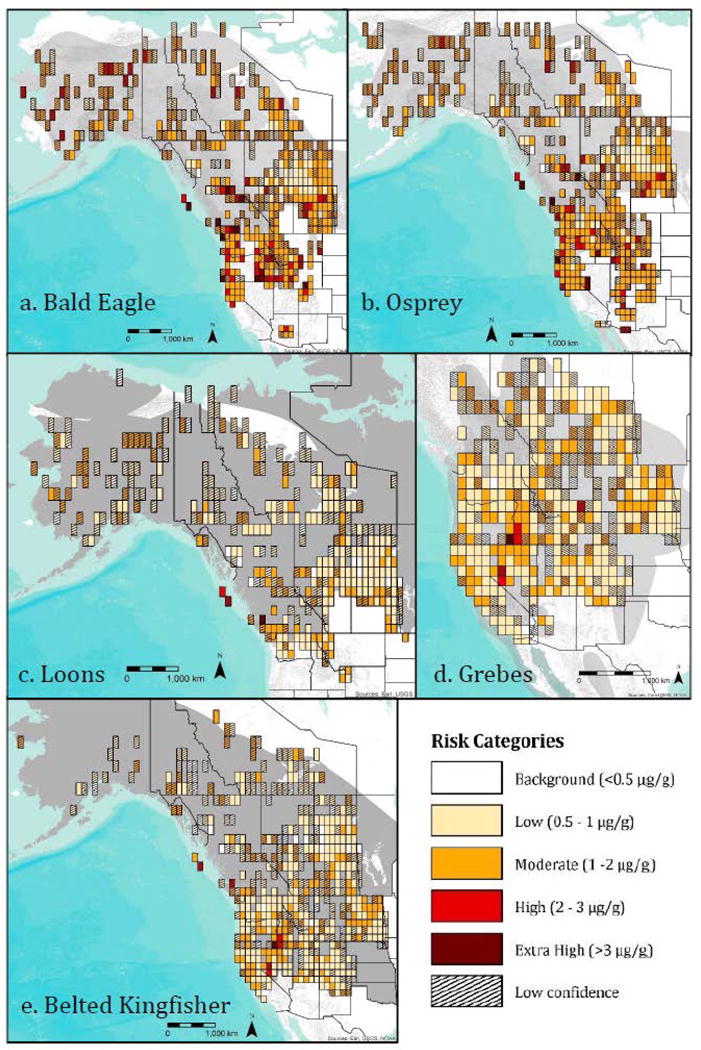
Relative Hg risk per 1 degree grid cell across the North American breeding range of five target freshwater piscivore species: a) Bald Eagle, b) Osprey, c) Loons (Common and Yellow-billed), d) Grebes (Clark’s and Western), and e) Belted Kingfisher. The prey fish were size-corrected according to the preference of each species: Bald Eagle = 36 cm, Osprey = 30 cm, Loons = 15 cm, Grebes = 6 cm, Belted Kingfisher = 5 cm. All Hg concentrations have been converted to wet weight whole bird blood for comparison. Low confidence is estimated as any grid cell where the standard error is more than one quarter of the mean.
Bald Eagle
Fish data used to estimate Bald Eagle exposure (N = 56,096) was size corrected to 36 cm before the THg concentration of each fish was converted to its avian blood equivalent using Equation 1. Original fish total lengths ranged from 1.5 cm to 138 cm, with a mean of 45 cm ± 0.08. Blood THg concentrations derived from the Bald Eagle fish dataset ranged from 0.02 μg/g to 20.4 μg/g (geometric mean = 1.47 μg/g ± 0.004). The mixed effects model used to compute the least squares mean THg concentration of each grid cell had an adjusted R2 of 0.72, with a significant effect of grid cell (F443,1382 = 3.7,p < 0.0001). Least squares mean THg concentrations were estimated for 444 grid cells, ranging from 0.37 μg/g –8.03 μg/g, with a mean THg concentration of 1.5 μg/g ± 0.03. The majority (71%) of grid cells with estimates that had high confidence (SE/mean < 0.25) fell into the moderate risk (1 – 2 μg/g) category, whereas 17% and 11% of grid cells fell into the low (0.5 – 1 μg/g) and high (2 – 3 μg/g) categories, respectively (Table 4). Relatively few grid cells were in the extra high (> 3 μg/g) range (1%). Based upon the grid maps, several geographic areas had relatively higher risk in comparison with the rest of the Bald Eagle range fish data occurred. High confidence grid cells greater than 2 μg/g occurred in the Great Basin (southeastern Oregon and southern Idaho) and the Prairie Pothole region (Saskatchewan). Other high confidence, greater than 2 μg/g grid cells occurred near Puget Sound, the Columbia River, Pinchi Lake region (British Columbia), central Arizona, and San Francisco Bay (Fig. 3a).
Table 4.
Percentage of grid cells assigned to each risk category for each freshwater avian piscivore. High confidence grids had standard error that was within one quarter of the mean. All grids includes both those grids with high and low confidence (total dataset).
| Species | Subset of Data | Background (< 0.5 μg/g) | Low (0.5 – 1 μg/g) | Moderate (1 – 2 μg/g) | High (2 – 3 μg/g) | Extra High (> 3 μg/g) | Total |
|---|---|---|---|---|---|---|---|
| Bald Eagle | High Confidence | 1 (0%) | 35 (17%) | 148 (71%) | 22 (11%) | 3 (1%) | 209 |
| All grid cells | 3 (1%) | 85 (19%) | 294 (66%) | 50 (11%) | 12 (3%) | 444 | |
| Osprey | High Confidence | 3 (1%) | 54 (21%) | 182 (71%) | 14 (5%) | 2 (1%) | 255 |
| All grid cells | 7 (1%) | 121 (25%) | 323 (66%) | 32 (7%) | 7 (1%) | 490 | |
| Loons | High Confidence | 9 (5%) | 125 (69%) | 46 (25%) | 1 (1%) | 0 (0%) | 181 |
| All grid cells | 23 (7%) | 218 (64%) | 97 (28%) | 2 (1%) | 1 (0%) | 341 | |
| Grebes | High Confidence | 8 (3%) | 176 (65%) | 82 (30%) | 3 (1%) | 0 (0%) | 269 |
| All grid cells | 22 (6%) | 252 (63%) | 117 (29%) | 5 (1%) | 1 (0%) | 397 | |
| Belted Kingfisher | High Confidence | 11 (4%) | 211 (69%) | 81 (26%) | 3 (1%) | 0 (0%) | 306 |
| All grid cells | 36 (7%) | 339 (65%) | 138 (27%) | 6 (1%) | 1 (0%) | 520 |
Osprey
Fish data used to estimate Osprey exposure (N = 58,890) was size corrected to 30 cm before each the THg concentration of each fish was converted to its avian blood equivalent using Equation 1. Original fish total lengths ranged from 1.5 cm to 138 cm, with a mean of 43.8 cm ± 0.08. Blood THg concentrations derived from the Osprey fish dataset ranged from 0.02 μg/g to 21.7 μg/g (geometric mean = 1.29 μg/g ± 0.003). The mixed effects model to compute the least squares mean of each grid cell for osprey prey fish had an adjusted R2 of 0.70, with a significant effect of grid cell (F489,1840 = 3.32,p < 0.0001). Least squares mean THg concentrations were estimated for 490 grid cells, ranging from 0.33 μg/g to 4.57 μg/g, with a mean THg concentration of 1.31 μg/g ± 0.02 across all grid cells. A high proportion of grid cells fell into the 0.5 – 1 μg/g category (low risk, 21%) and 1 – 2 μg/g category (moderate risk, 71%) (Table 4). Parts of the Great Basin in Oregon, Idaho, and Nevada, along with the Prairie Pothole region of Saskatchewan contained areas of relatively higher risk in comparison to other geographic areas within their range (Fig. 3b).
Loon
Fish data used to estimate Loon exposure (N = 42,840) was size corrected to 15 cm before the THg concentration of each fish was converted to its avian blood equivalent using Equation 1. Original fish total lengths ranged from 1.5 cm to 138 cm, with a mean of 48.8 cm ± 0.08. Converted blood THg concentrations from the loon dataset ranged from 0.01 μg/g to 13.84 μg/g (geometric mean = 0.95 μg/g ± 0.003). The mixed effects model to compute the least squares mean per grid cell for loons had an adjusted R2 of 0.56, with a significant effect of grid cell (F340,941 = 2.75, p < 0.0001). Least squares mean THg concentrations were estimated for 341 grid cells, ranging from 0.22 μg/g to 3.05 μg/g, with a mean THg concentration of 0.90 μg/g ± 0.02. Most of the grid cells fell into the low (0.5 – 1 μg/g) and moderate risk (1 – 2 μg/g) categories (69% and 25%, respectively) with no grid exceeding the extra high risk threshold (> 3 μg/g; Table 4). Compared to Bald Eagle and Osprey, the loon map showed relatively fewer areas of high or extra high concern (> 2 μg/g). Only three grid cells along the Pacific Ocean in British Columbia were modeled to high risk categories (Fig. 3c)
Grebe
Fish data used to estimate Grebe exposure (N = 46,203) was size corrected to 6 cm before the THg concentration of each fish was converted to its avian blood equivalent using Equation 1. Original fish total lengths ranged from 1.7 cm to 104.6 cm, with a mean of 29.4 cm ± 0.08. Converted blood THg concentrations from the grebe dataset ranged from 0.01 μg/g to 11.72 μg/g (geometric mean = 0.92 μg/g ± 0.002). The mixed effects model to compute the least squares mean of each grid cell for grebes had an adjusted R2 of 0.64, with a significant effect of grid cell (F396,1957 = 3.06,p < 0.0001). Least squares mean THg concentrations were estimated for 397 grid cells, ranging from 0.21 μg/g to 4.1 μg/g with a mean THg concentration of 0.91 μg/g ± 0.02. Over 90% of the grids fell within the low and moderate risk category (65% within 0.5 – 1 μg/g, 30% within 1 – 2 μg/g, Table 4). This fish prey model indicated areas of concern in the Great Basin of Oregon and Nevada (Fig. 3d).
Belted Kingfisher
Fish data used to estimate Belted Kingfisher exposure (N = 54,673) was size corrected to 6 cm before the THg concentration of each fish was converted to its avian blood equivalent using Equation 1. Original fish total lengths ranged from 1.1 cm to 121.7 cm, with a mean of 32.7 cm ± 0.08. Converted blood THg concentrations from the Belted Kingfisher dataset ranged from 0.01 μg/g to 11.5 μg/g (geometric mean = 0.88 μg/g ± 0.002). The mixed effects model to compute the least squares mean of each grid cell for Belted Kingfisher had an adjusted R2 of 0.64, with a significant effect of grid cell (F519,2178 = 2.92, p < 0.0001). Least squares mean THg concentrations were estimated for 520 grid cells, ranging from 0.21 μg/g to 4.19 μg/g, with a mean THg concentration of 0.89 μg/g ± 0.02. Similar to grebes, over 90% of the grids fell within the low (0.5 – 1 μg/g) and moderate (1 – 2 μg/g) risk category (69% and 26%, respectively, Table 4). The risk map for kingfisher prey fish was similar to that for grebes, with increased risk in the Great Basin (Fig. 3e) and additional areas with low confidence in British Columbia.
4.0 Discussion
4.1 Comparison to previous Hg synthesis work
In this study, Hg risk to multiple avian piscivores was assessed across a broad geographic area – the western United States and Canada. Unlike previous synthesis efforts that focused on a single avian piscivore and prey species for assessing risk from environmental Hg loads (Ackerman et al. 2015; Burgess and Meyer 2008; Depew et al., 2013; Evers et al., 2007; 2008b; 2011), there was not a single bird species that could be effectively used across the region. Instead, the approach targeted five breeding avian piscivores that collectively span the majority of the freshwater habitats occurring within western North America. The use of fish Hg concentrations to predict risk to avian piscivores had several advantages. First, fish are the most widely measured taxonomic group for Hg exposure. Second, there are numerous national-, state-, and provincial-scale fish Hg monitoring programs used as a basis for fish consumption guidelines for human health. Third, fish Hg concentrations can be converted to avian piscivore risk. Other papers within this special issue used either fish (Eagles-Smith et al. this issue [b], Lepak et al. this issue) or bird (Ackerman et al., this issue) Hg concentrations to characterize risk across the study region. By leveraging the established strong linkages between prey fish and avian piscivore Hg concentrations (Ackerman et al. 2015), risk for 5 target avian piscivores was characterized at a finer scale than piscivorous bird data alone would allow. Many areas in Western North America remain uncharacterized and using fish Hg concentrations as a proxy for bird Hg exposure allows for an examination of risk at a landscape level to better understand the spatial patterns of biological Hg hotspots.
In freshwater ecosystems, the positive modeled relationship between fish length and Hg concentration demonstrated that avian piscivores that consume larger fish were classified into higher Hg categories across their breeding range (Table 4). Proportion of grid cells above 1.0 μg/g declined from 83% for Bald Eagle and 77% for Osprey to 26% for loons, 31% for grebes, and 27% for kingfishers. While these Hg categories are helpful for comparing risk on a standardized scale, this does not necessarily mean that smaller avian piscivores are not at risk. For example, in San Francisco Bay, Forster’s Terns and invertivorous Black-necked Stilts had similar or higher THg concentrations than Caspian Terns despite the fact that Caspian Terns feed on larger fish at a higher trophic position (Eagles-Smith et al. 2009). Space use data showed that Forster’s Terns and stilts selected foraging habitats with much higher baseline Hg concentrations than did Caspian Terns (Ackerman et al. 2007; 2008).
In addition to variation in habitat drivers of differential Hg bioaccumulation that can put birds that eat smaller fish at risk, there is also tremendous variation in the sensitivity of different avian species that can complicate toxicological risk (Heinz et al. 2009). Aside from the thresholds developed for Common Loons, there are few field-based effects thresholds that are well-developed for other bird species. Artificially incubated eggs dosed with Hg have shown that species show wide variation in their sensitivities to Hg, based on hatching success (Heinz et al. 2009; Braune et al. 2012). In a comparative egg dosing study of 21 bird species by Heinz et al. (2009), Osprey were among the most sensitive species to Hg in egg injections, which might translate to lower effects thresholds than presented here. Comparatively, loons are moderately sensitive (Kenow et al., 2011; based on similar protocols as Heinz et al. 2009). However, it is widely acknowledged that the MeHg injected into eggs is likely more toxic than maternally derived Hg, complicating the application of these effects benchmarks to successful reproduction in the wild, where parental incubation behavior and ability to catch prey (and subsequent ability to raise young) may be impaired by Hg contamination.
4.2 Ecosystems and regions of Hg concern
Although the focus of the analysis did not identify conclusive “biological Hg hotspots” as were developed in previous synthesis efforts in the Northeast (Evers et al. 2007) and Great Lakes (Evers et al. 2011), the results do show certain areas of concern for multiple avian piscivores. To better visualize the results, grid cells across all target species that were in the moderate category or above (> 1 μg/g) were compiled (Fig. 4). Some regions highlighted in the risk map have known sources of elevated Hg. For example, the Pinchi Lake region has been well characterized as an area with elevated environmental Hg loads due to cinnabar deposits and mining (Weech et al., 2004). Similarly, fish and birds in the Carson River complex in Nevada have well-documented elevated Hg concentrations as a result of Hg from to legacy mining transported to wetlands areas with high methylmercury production capacity (Henny et al. 2002; Hill et al. 2008; Seiler et al. 2004).
Figure 4.
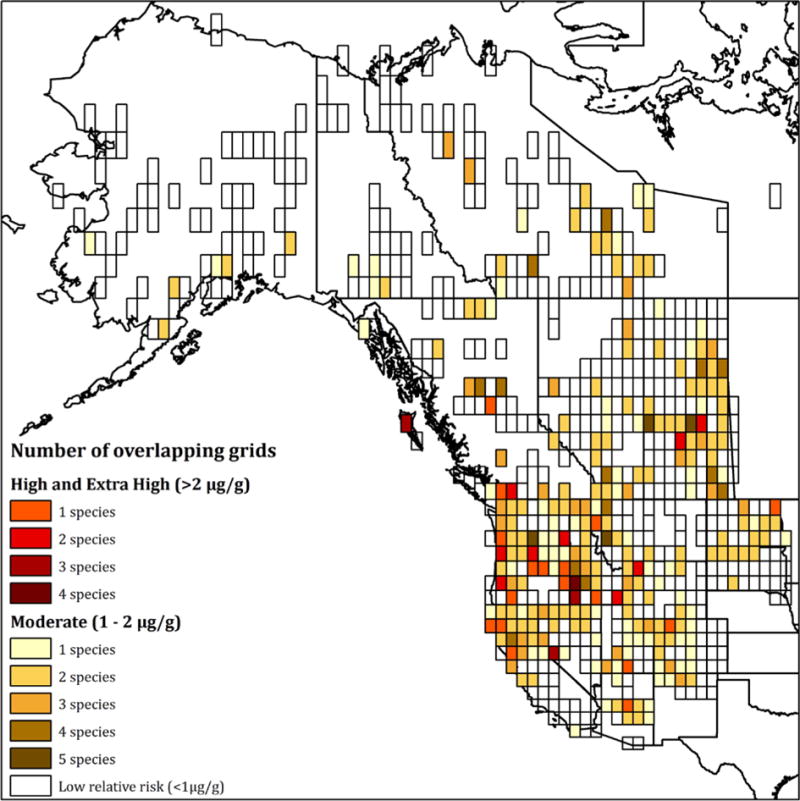
Areas of western United States and Canada where fish modeling indicates high confidence in moderate or higher relative Hg risk. Light colors indicate fewer species with overlapping risk. Empty grid cells indicate areas where fish data was modeled but risk was estimated to be low. Areas not covered by grid cells either have least square mean estimates with low confidence or no fish data to use in the model.
Several major river-fed systems across western North America were isolated as areas of moderate or high relative risk for multiple piscivores (e.g., Columbia River, Puget Sound, San Francisco Bay). San Francisco Bay in particular appears to be an area with highly elevated biotic Hg concentrations that are related to legacy mining activities and methylating habitats (Ackerman et al. 2008; Eagles-Smith 2008, 2009). Relatively elevated Hg concentrations were observed in areas of the Puget Sound and Columbia River; however, no direct estimates exist regarding specific source in these systems. Models have suggested that delta regions of rivers associated with marine ecosystems can be affected by deposition of watershed transport of Hg, with climate change projections suggesting increased methylation rates and Hg releases (Amos et al. 2014, Fisher et al. 2012).
The Prairie Pothole region in Canada (particularly in southern Saskatchewan) was shown to have moderate or high relative risk for multiple piscivores. The Prairie Pothole region is a unique system with relatively high methylation potential due to high density of wetlands (Hall et al. 2009; Bates and Hall 2012). Although relatively few contaminant studies with avian bioindicators have occurred there (Allen et al., 1998; Winder et al., 2011; Fox et al., 2005), the Prairie Pothole is consistently cited as one of the largest waterfowl producing areas in North America making further study of Hg in this area critically important (Greenwood et al., 1995). This study also highlighted two arid land ecosystems: the Great Basin in Idaho, Oregon and Nevada and central Arizona. Parts of the Great Basin also are identified using only bird Hg concentrations (Ackerman et al. this issue). Different areas of this large region have been studied previously because of concerns from mining legacy Hg use (Henny et al., 2002) or risk to waterfowl (i.e., the Great Salt Lake; Vest et al., 2009), but the area also supports a vast network of seasonal and permanent wetlands (Sada and Vinyard 2002) that may enhance methylmercury potential. A more integrated monitoring is needed across the region to better develop causality. New predictions of increased Hg deposition in the western United States that likely originate from increased global Hg emissions (Weiss-Penzias et al., 2016) also indicates that ecosystems sensitive to Hg input need to be monitored to track potential increases of biotic Hg body burdens.
4.3 Conclusions
Results highlight the need for future monitoring of Hg exposure in both bird and fish across North America. Compared to the risk maps created using bird Hg concentrations (Ackerman et al., this issue), our results using prey fish identified additional areas of potential concern where bird sampling has not occurred. Although using fish Hg concentrations allow more extensive spatial coverage than only bird tissue sampling, the ability to draw conclusions about the overall Hg risk to each bird species was limited because of uncertainties related to the sensitivities of birds to Hg (Heinz et al., 2009). Fish Hg concentrations covered from 25% to 79% of the western breeding ranges of the target piscivores, better geographic coverage and a defined sampling design is needed to fully evaluate the proportion of the breeding population impacted. Using the approach within this paper, it is important to next examine Hg concentrations in prey fish and associated target avian piscivores across their entire North American breeding ranges. Standardized continental comparisons by Evers et al. (1998) for Common Loons found a significant west (lower) to east (higher) gradient of loon Hg concentrations, but this has not been established for other avian piscivores. Due to the climatological gradients of western United States and Canada, many aquatic-specialist bird species concentrate in relatively small areas to breed and forage, making those areas potentially of higher conservation concern. The next step for assessing risk of environmental Hg loads to avian piscivores is to understand their breeding density areas across the broad and varied ecosystems that they occupy.
An approach that can scale the risk of Hg to breeding populations of avian piscivores across a mosaic of ecosystems at continental levels is important for assessing potential impacts at global levels. In October 2013, 128 countries signed a new global treaty called the Minamata Convention (UNEP 2013) with the overall objective to “protect the human health and the environment from anthropogenic emissions and releases of mercury and mercury compounds.” This Convention will be instrumental for reducing the use and release of Hg into the environment and metrics are being developed that could be used for evaluating its effectiveness (Evers et al. submitted). As such, one metric will likely be based on biotic Hg concentrations from key bioindicators. In the Convention (Article 19), “geographically representative monitoring of Hg and Hg compounds in vulnerable populations and in environmental media, including biotic media such as fish, marine mammals, sea turtles, and birds” is needed by the Parties to develop a plan for assessing improvements in ecological and human health. Monitoring strategies for Hg in fish, birds, marine mammals and other biota have been developed (Mason et al. 2005; Evers et al. 2008b) and inclusion of avian piscivores as sensitive and high profile bioindicators is important for identifying biological Hg hotspots and tracking trends of environmental Hg loads over time. The results from the assessment of potential impacts from Hg on avian piscivores in Western North America will help meet the demands for evaluating the effectiveness of the Minamata Convention.
Supplementary Material
Table 3.
Sample size for fish species groups used in each avian piscivore Hg risk model. Sample sizes vary for each piscivore because our modeling process only included fish species groups if they fell within the range preferred by avian piscivores. All fish Hg concentrations used in models were size adjusted to the preferred size of each piscivore (size shown in parenthesis in heading).
| Fish Species Group | Bald Eagle (36cm) | Osprey (30cm) | Loons (15cm) | Grebes (6cm) | Belted Kingfisher (5cm) | Fish Species Total |
|---|---|---|---|---|---|---|
| Anadromous salmonid | 288 | 335 | 623 | |||
| Arctic grayling | 263 | 274 | 284 | 821 | ||
| Black basses | 2744 | 3817 | 770 | 6015 | 4953 | 18299 |
| Burbot | 821 | 821 | 636 | 2278 | ||
| Carp | 355 | 533 | 85 | 1054 | 909 | 2936 |
| catfishes | 540 | 828 | 185 | 1845 | 1780 | 5178 |
| Char | 5001 | 5811 | 4479 | 2057 | 5473 | 22821 |
| Chub | 224 | 247 | 39 | 327 | 342 | 1179 |
| Cichlids | 8 | 7 | 15 | |||
| Cisco | 245 | 232 | 265 | 742 | ||
| Clupeiformes | 3 | 4 | 8 | 4 | 19 | |
| Crappie | 251 | 325 | 24 | 625 | 605 | 1830 |
| Freshwater drum | 24 | 24 | 48 | |||
| Goldeye | 1154 | 1236 | 1053 | 3443 | ||
| Killifish | 3948 | 4169 | 8117 | |||
| Minnows | 1952 | 1932 | 3884 | |||
| Morone bass | 29 | 193 | 222 | |||
| Pike | 13995 | 13303 | 12853 | 40151 | ||
| Pikeminnow | 1844 | 1899 | 226 | 1905 | 1913 | 7787 |
| Sculpin | 184 | 243 | 427 | |||
| Stickleback | 412 | 415 | 827 | |||
| Suckers | 3482 | 3585 | 1913 | 3554 | 4221 | 16755 |
| Sunfishes | 318 | 545 | 74 | 847 | 797 | 2581 |
| Trout | 3798 | 5681 | 1777 | 6189 | 6506 | 23951 |
| Walleye/Sauger | 13894 | 12437 | 12016 | 11337 | 17829 | 67513 |
| Whitefish | 5065 | 5334 | 4889 | 1561 | 16849 | |
| Yellow perch | 1758 | 1442 | 1248 | 2376 | 2582 | 9406 |
| Total | 56096 | 58890 | 42840 | 46203 | 54673 | 258702 |
Highlights.
Avian piscivores are important sentinel species for Hg across Western North America
No single avian piscivore occurs across this large geographic area
Hg risk evaluated for five avian piscivores across their breeding ranges
Geographic spread of bird samples currently lacking; used fish to estimate risk
Novel way of assessing risk provides information about areas of Hg concern
Acknowledgments
The authors would like to thank everyone who contributed data to both the fish and bird datasets. We would like to thank Jim Wiener for his guidance throughout the Powell Center meetings and Madeline Turnquist and Kiira Siitari for their early help organizing the fish database. Michelle Lutz, and Michael Tate provided GIS support and advice. Jeffrey Tash was integral for help with maps. This work was conducted as a part of the Western North American Mercury Synthesis Working Group supported by the John Wesley Powell Center for Analysis and Synthesis, with additional funding from the U.S. Geological Survey Contaminant Biology Program. We thank the Government of Canada - Environment Canada - Clean Air Regulatory Agenda - Mercury Science Program for supporting the program and enabling the development of the western North America project, in particular Linda Campbell, Neil Burgess, and David Depew. We are very thankful for funding providing by the Region-10 US EPA Regional Applied Research Effort (RARE) grant; and our ORD Principal Investigator Heather Golden (Ecological Exposure Research Division, National Exposure Research Laboratory), and Regional Science Liaison Bruce Duncan. For the marine pelagic seabird eggs, please see Supplemental Table S1 for a list of contributors to the Seabird Tissue Archival and Monitoring Project (STAMP). We also thank Paul Becker, the Marine Environmental Specimen Bank staff, Austin Ahmasuk, Bureau of Indian Affairs, North Pacific Research Board, Beth Flint, Ty Benally, and Jason Omick for their continuing help and support of STAMP. Eggs were collected under MBTA permits MB025914 and MB28525A, and permits from states of AK, HI, tribal nations, Kaena Point Natural Area Reserve and Papahānaumokuākea Marine National Monument. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Any mention of commercial products is for information only; it does not imply recommendation or endorsement by the U.S. government.
6.0 Literature Cited
- Ackerman JT, Eagles-Smith CA, Takekawa JY, Demers SA, Adelsbach TL, Bluso JD, Miles AK, Warnock N, Suchanek TH, Schwarzbach SE. Mercury concentrations and space use of pre-breeding American avocets and black-necked stilts in San Francisco Bay. Sci Total Environ. 2007;384:452–466. doi: 10.1016/j.scitotenv.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Ackerman JT, Eagles-Smith CA, Takekawa JY, Bluso JD, Adelsbach TL. Mercury concentrations in blood and feathers of pre-breeding Forster’s terns in relation to space use of San Francisco Bay habitats. Environ Tox Chem. 2008;27:897–908. doi: 10.1897/07-230.1. [DOI] [PubMed] [Google Scholar]
- Ackerman JT, Hartman CA, Eagles-Smith CA, Herzog MP, Davis JA, Ichikawa G, Bonnema A. Estimating mercury exposure of piscivorous birds and sport fish using prey fish monitoring. Environ Sci Technol. 2015;49:13596–13604. doi: 10.1021/acs.est.5b02691. [DOI] [PubMed] [Google Scholar]
- Ackerman JT, Eagles-Smith CA, Herzog MP, Hartman CA. Maternal transfer of contaminants in birds: mercury and selenium concentrations in parents and their eggs. Environ Poll. 2016;210:145–154. doi: 10.1016/j.envpol.2015.12.016. [DOI] [PubMed] [Google Scholar]
- Ackerman JT, Eagles-Smith CA, Herzog MP, Hartman CA, Peterson SH, Evers DC, Jackson AK, Elliott JE, Vander Pol SS, Bryan CE. Avian mercury exposure and risk across western North America: a synthesis. Sci Total Environ. doi: 10.1016/j.scitotenv.2016.03.071. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GT, Blackford SH, Welsh D. Arsenic, mercury, selenium, and organochlorines and reproduction of interior least terns in the Northern Great Plains, 1992–1994. Colon Waterbirds. 1998;21:356–366. [Google Scholar]
- Amos HM, Jacob DJ, Kocman D, Horowitz HM, Zhang Y, Dutkiewicz S, Horvat M, Corbitt ES, Krabbenhoft DP, Sunderland EM. Global biogeochemical implications of mercury discharges from rivers and sediment burial. Environ Sci Tech. 2014;48:9514–9522. doi: 10.1021/es502134t. [DOI] [PubMed] [Google Scholar]
- Bates LM, Hall BD. Concentrations of methylmercury in invertebrates from wetlands of the prairie pothole region of North America. Environ Poll. 2012;160:153–160. doi: 10.1016/j.envpol.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Becker PH. Biomonitoring with birds. Trace metals and other contaminants in the Environment. 2003;6:677–736 d. doi: 10.1016/S0927-5215(03)80149-2. [DOI] [Google Scholar]
- Braune BM. Temporal trends of organochlorines and mercury in seabird eggs from the Canadian Arctic, 1975-2003. Environmental Pollution. 2007;148:599–613. doi: 10.1016/j.envpol.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Braune BM, Scheuhammer AM, Crump D, Jones S, Porter E, Bond D. Toxicity of methylmercury injected into eggs of thick-billed murres and arctic terns. Ecotoxicol. 2012;21:2143–2152. doi: 10.1007/s10646-012-0967-3. [DOI] [PubMed] [Google Scholar]
- Buehler DA. Bald Eagle (Haliaeetus leucocephalus) In: Poole A, editor. The Birds of North America Online. Vol. 506 Ithaca: Cornell Lab of Ornithology; 2000. [Google Scholar]
- Burgess NM, Meyer MW. Methylmercury exposure associated with reduced productivity in common loons. Ecotoxicology. 2008;17:83–91. doi: 10.1007/s10646-007-0167-8. [DOI] [PubMed] [Google Scholar]
- Champoux L, Masse DC, Evers D, Lane OP, Plante M, Timmermans STA. Assessment of mercury exposure and potential effects on common loons (Gavia immer) in Québec. Hydrobiologia. 2006;567:263–274. [Google Scholar]
- Depew DC, Basu N, Burgess NM, Campbell LM, Evers DC, Grasman KA, Scheuhammer AM. Derivation of screening benchmarks for dietary methylmercury exposure for the common loon (Gavia immer): Rationale for use in ecological risk assessment. Environ Toxicol Chem. 2012;31:2399–2407. doi: 10.1002/etc.1971. [DOI] [PubMed] [Google Scholar]
- Depew DC, Burgess NM, Campbell LM. Modelling mercury concentrations in prey fish: derivation of a national-scale common indicator of dietary mercury exposure for piscivorous fish and wildlife. Environ Pollut. 2013;176:234–43. doi: 10.1016/j.envpol.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Ackerman JT, Adelsbach TL, Takekawa JY, Miles AK, Keister RA. Mercury correlations among six tissues for four waterbird species breeding in San Francisco Bay, California, USA. Environ Toxicol Chem. 2008;27:2136–2153. doi: 10.1897/08-038.1. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Ackerman JT, De La Cruz SEW, Takekawa JY. Mercury bioaccumulation and risk to three waterbird foraging guilds is influenced by foraging ecology and breeding stage. Environ Poll. 2009;157:1993–2002. doi: 10.1016/j.envpol.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Wiener JG, Eckley C, Willacker JJ, Evers DC, Marvin-DiPasquale M, Obrist D, Fleck J, Aiken G, Lepak J, Jackson AK, Webster J, Stewart AR, Davis J, Alpers C, Ackerman JT. Mercury in western North America: a synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Sci Total Environ. doi: 10.1016/j.scitotenv.2016.05.094. this issue (a) [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Ackerman JT, Willacker JJ, Tate MT, Lutz MA, Stewart AR, Wiener JG, Evers DC, Lepak J, Davis JA. Spatial and Temporal Patterns of Mercury Concentrations in Freshwater Fishes of the Western US and Canada. Sci Total Environ. doi: 10.1016/j.scitotenv.2016.03.229. this issue (b) [DOI] [PubMed] [Google Scholar]
- Evers DC, Kaplan JD, Meyer MW, Reaman PS, Major A, Burgess N, Braselton WE. Bioavailability of environmental mercury measured in Common Loon feathers and blood across North American. Environ Tox Chem. 1998;17:173–183. [Google Scholar]
- Evers DC, Burgess NM, Champoux L, Hoskins B, Major A, Goodale WM, Taylor RJ, Poppenga R, Daigle T. Patterns and Interpretation of Mercury Exposure in Freshwater Avian Communities in Northeastern North America. Ecotoxicology. 2005;14:193–221. doi: 10.1007/s10646-004-6269-7. [DOI] [PubMed] [Google Scholar]
- Evers DC, Han YJ, Driscoll CT, Kamman NC, Goodale MW, Lambert KF, Holsen TM, Chen CY, Clair TA, Butler T. Biological Mercury Hotspots in the Northeastern United States and Southeastern Canada. Bioscience. 2007;57:29–43. doi: 10.1641/B570107. [DOI] [Google Scholar]
- Evers DC, Savoy LJ, Desorbo CR, Yates DE, Hanson W, Taylor KM, Siegel LS, Cooley JH, Bank MS, Major A, Munney K, Mower BF, Vogel HS, Schoch N, Pokras M, Goodale MW, Fair J. Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology. 2008a;17:69–81. doi: 10.1007/s10646-007-0168-7. [DOI] [PubMed] [Google Scholar]
- Evers DC, Mason RP, Kamman NC, Chen CY, Bogomolni AL, Taylor DH, Hammerschmidt CR, Jones SH, Burgess NM, Munney K, Parsons KC. An integrated mercury monitoring program for temperate estuarine and marine ecosystems on the North American Atlantic Coast. EcoHealth. 2008b;5:426–441. doi: 10.1007/s10393-008-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers DC, Paruk JD, Mcintyre JW, Barr JF. Common Loon (Gavia immer) In: Poole A, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 2010. p. 313. [Google Scholar]
- Evers DC, Williams KA, Meyer MW, Scheuhammer AM, Schoch N, Gilbert AT, Siegel L, Taylor RJ, Poppenga R, Perkins CR. Spatial gradients of methylmercury for breeding common loons in the Laurentian Great Lakes region. Ecotoxicology. 2011;20:1609–25. doi: 10.1007/s10646-011-0753-7.. [DOI] [PubMed] [Google Scholar]
- Evers DC, Keane SE, Basu N, Buck D. Evaluating the effectiveness of the Minamata Convention on mercury: Principles for an approach. Science of the Total Environment. doi: 10.1016/j.scitotenv.2016.05.001. Submitted. [DOI] [PubMed] [Google Scholar]
- Fisher JA, Jacob DJ, Soerensen AL, Amos HM, Steffen A, Sunderland EM. Riverine source of Arctic Ocean mercury inferred from atmospheric observations. Nature Geoscience. 2012;5:499–504. [Google Scholar]
- Fox GA, MacCluskie MC, Brook RW. Are current contaminant concentrations in eggs and breeding female lesser scaup of concern? Condor. 2005;107:50–61. [Google Scholar]
- Greenwood RJ, Sargeant AB, Johnson DH, Cowardin LM, Shaffer TL. Factors associated with duck nest success in the prairie pothole region of Canada. Wildlife Monographs. 1995;128:3–57. [Google Scholar]
- Guigueno MF, Elliott KH, Levac J, Wayland M, Elliott JE. Differential exposure of alpine ospreys to mercury: melting glaciers, hydrology or deposition patterns? Environ Int. 2012;40:24–32. doi: 10.1016/j.envint.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Hall BD, Baron LA, Somers CM. Mercury concentrations in surface water and harvested waterfowl from the Prairie Pothole region of Saskatchewan. Environ Sci Technol. 2009;43:8759–8766. doi: 10.1021/es9024589. [DOI] [PubMed] [Google Scholar]
- Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA. Species differences in the sensitivities of avian embryos to methylmercury. Arch Environ Contam Toxicol. 2009;56:129–138. doi: 10.1007/s00244-008-9160-3. [DOI] [PubMed] [Google Scholar]
- Henny CJ, Hill EF, Hoffman DJ, Spalding MG, Grove RA. Nineteenth century mercury: hazard to wading birds and cormorants of the Carson River, Nevada. Ecotoxicol. 2002;11:213–231. doi: 10.1023/a:1016327602656. [DOI] [PubMed] [Google Scholar]
- Hill EF, Henny CJ, Grove RA. Mercury and drought along the lower Carson River, Nevada: II. Snowy egret and black-crowned night-heron reproduction on Lahontan Reservoir, 1997–2006. Ecotoxicol. 2008;17(2):117–131. doi: 10.1007/s10646-007-0180-y. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Bridge ES, Hamas MJ. Belted Kingfisher (Megaceryle alcyon) In: Poole A, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 2009. p. 84. [Google Scholar]
- Kenow KP, Meyer MW, Rossman R, Gendron-Fitzpatrick A, Gray BR. Effects of injected methylmercury on the hatching of Common Loon (Gavia immer) eggs. Ecotoxicol. 2011;20(7):1684–1693. doi: 10.1007/s10646-011-0743-9. [DOI] [PubMed] [Google Scholar]
- LaPorte N, Storer RW, Nuechterlein GL. Western Grebe (Aechmophorus occidentalis) In: Poole A, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 2013. p. 26. [Google Scholar]
- Lepak JM, Hooten MB, Eagles-Smith CA, Lutz MA, Tate MT, Ackerman JT, Willacker JJ, Evers DC, Davis J, Flanagan Pritz C, Wiener JG. Assessing mercury concentrations in fish across western Canada and the United States: potential health risks to fish and humans. Sci Total Environ. doi: 10.1016/j.scitotenv.2016.03.031. this issue. [DOI] [PubMed] [Google Scholar]
- Mason RP, Abbot ML, Bodaly RA, Bullock OR, Driscoll CT, Evers DC, S Lindberg SE, Murray M, Swain EB. Monitoring the environmental response to changes in mercury contamination from the atmosphere: A multi-media challenge. Environ Sci Technol. 2005;39:15A–22A. doi: 10.1021/es053155l. [DOI] [PubMed] [Google Scholar]
- Monteiro LR, Furness RW. Seabirds as monitors of mercury in the marine environment. Water, Air, Soil Pollut. 1995;80:851–870. doi: 10.1007/BF01189736. [DOI] [Google Scholar]
- North American Bird Conservation Initiative. Bird Conservation Regions. www.nabci-us.org/bcrs.htm. Accessed 29 October 2015.
- North MR. Yellow-billed Loon (Gavia adamsii) In: Poole A, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 1994. p. 121. [Google Scholar]
- Pinkney AE, Driscoll CT, Evers DC, Hooper MJ, Horan J, Jones JW, Lazarus RS, Marshall HG, Milliken A, Rattner BA, Schmerfeld J, Sparling DW. Interactive effects of climate change with nutrients, mercury, and freshwater acidification on key taxa in the North Atlantic Landscape Conservation Cooperative region. Integrated Environ Assessment and Manag. 2015;11(3):355–369. doi: 10.1002/ieam.1612. [DOI] [PubMed] [Google Scholar]
- Poole AF, Bierregaard RO, Martell MS. Osprey (Pandion haliaetus) In: Poole A, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 2002. p. 683. [Google Scholar]
- Sada DW, Vinyard GL. Anthropogenic changes in biogeography of Great Basin aquatic biota. Smithsonian Contributions to the Earth Sciences. 2002;33:277–293. [Google Scholar]
- Scheuhammer AM, Atchison CM, Wong AHK, Evers DC. Mercury exposure in breeding common loons (Gavia immer) in central Ontario, Canada. Environ Tox Chem. 1998;17:191–196. [Google Scholar]
- Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio. 2007;36:12–18. doi: 10.1579/0044-7447(2007)36[12:EOEMOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Scheuhammer AM, Lord SI, Wayland M, Burgess NM, Champoux L, Elliott JE. Major correlates of mercury in small fish and common loons (Gavia immer) across four large study areas in Canada. Environ Poll. 2016;210:361–370. doi: 10.1016/j.envpol.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Schoch N, Glennon MJ, Evers DC, Duron M, Jackson AK, Driscoll CT, Ozard JW, Sauer AK. The impact of mercury exposure on the Common Loon (Gavia immer) population in the Adirondack Park, New York, USA. Waterbirds. 2014;37:133–146. [Google Scholar]
- Seber GAF. The estimation of animal abundance and related parameters. Second. Macmillan; New York: 1982. [Google Scholar]
- Seiler RL, Lico MS, Wiemeyer SN, Evers DC. Mercury in the Walker River Basin, Nevada and California—Sources, Distribution, and Potential Effects on the Ecosystem. US Geological Survey, Water Resources Investigative Report. 2004:2004–5157. [Google Scholar]
- Storer RW, Nuechterlein GL. Clark’s Grebe (Aechmophorus clarkii) In: Poole A, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 1992. p. 26b. [Google Scholar]
- UNEP. Minamata Convention on Mercury: text and annexes. 2013 Available online at www.mercuryconvention.org.
- Vest JL, Conover MR, Perschon C, Luft J, Hall JO. Trace element concentrations in wintering waterfowl from the Great Salt Lake, Utah. Arch Environ Contamin Toxicol. 2008;56:302–316. doi: 10.1007/s00244-008-9184-8. [DOI] [PubMed] [Google Scholar]
- Weech SA, Scheuhammer AM, Elliott JE, Cheng KM. Mercury in fish from the Pinchi Lake Region, British Columbia, Canada. Environ Poll. 2004;131:275–286. doi: 10.1016/j.envpol.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Weech SA, Scheuhammer AM, Elliott JE. Mercury exposure and reproduction in fish-eating birds breeding in the Pinchi Lake region, British Columbia, Canada. Environ Toxicol Chem. 2006;25:1433–1440. doi: 10.1897/05-181r.1. [DOI] [PubMed] [Google Scholar]
- Weiss-Penzias PS, Gay DA, Brigham ME, Parsons MT, Gustin MS, ter Schure A. Trends in mercury wet deposition and mercury air concentrations across the U.S. and Canada. Science of the Total Environment. 2016 doi: 10.1016/j.scitotenv.2016.01.061. in press. [DOI] [PubMed] [Google Scholar]
- Winder VL, Emslie SD. Mercury in breeding and wintering Nelson’s Sparrows (Ammodramus nelsoni) Ecotoxicology. 2011;20:218–225. doi: 10.1007/s10646-010-0573-1. [DOI] [PubMed] [Google Scholar]
- Yu X, Driscoll CT, Montesdeoca M, Evers D, Duron M, Williams K, Schoch N, Kamman NC. Spatial patterns of mercury in biota of Adirondack, New York lakes. Ecotoxicol. 2011;20:1543–1554. doi: 10.1007/s10646-011-0717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


