Abstract
Background:
Prophylactic salpingectomy for the prevention of ovarian cancer has been recommended strongly. The aim of this study was to compare ovarian function in patients who undergo hysterectomy for benign reasons with or without bilateral salpingectomy.
Materials and Methods:
This was a clinical-trial study on patients undergone hysterectomy with/without bilateral salpingectomy in Al-Zahra Hospital, in 2015–2016. Demographic information (age, height, and weight) were recorded. Follicle stimulating hormone (FSH) and luteinizing hormone (LH) were measured in 2–5 days of menstrual cycle before operation. Patients were asked to refer in 6 months for follow-up, including FSH and LH re-measurement and also menopausal status examination. Patients were divided into age groups of 39–45, 46–50, and ≥51 and also groups of body mass index including 18.5–24.9, 25–29.9, and 30–34.9.
Results:
A total of 37 patients divided into two groups, including 22 patients undergone hysterectomy without salpingectomy (H) and 15 undergone hysterectomy with bilateral salpingectomy (H-bS). The mean age (standard deviation) of Group H was 47.77 (3.03) and Group H-bS was 48.47 (2.03) (P > 0.05). Furthermore, the mean level of FSH and LH before surgery was not significantly different (P > 0.05). The mean level of FSH and LH changes was not significant between H and H-bS groups (P = 0.17), (P = 0.16).
Conclusion:
Bilateral salpingectomy during hysterectomy did not increase the risk of ovarian dysfunction after 6 months follow-up.
Keywords: Follicular stimulating hormone, hysterectomy, luteinizing hormone, salpingectomy
INTRODUCTION
Ovarian cancer is the most common gynecological cancer of females and fifth common cancer in terms of mortality.[1,2] The most lethal ovarian malignancy is serous carcinoma that initially forms in the distal mucosal part of the fallopian tube and will extend to ovaries and other pelvic organs.[2,3,4,5] Because the hypothesis of the possible effect of salpingectomy in ovarian cancer prophylaxis is designed.[6,7] Since not having any special screening test for ovarian cancer and high rate of diagnosis in late stages, this hypothesis can play an important role.[8] Other factors for malignancy risk reduction are: OCP consumption, use of flavonoids which exists in leafy vegetables and apple and also quercetin therapy as another flavonoid, hysterectomy, bilateral salpingo-oophorectomy, tube ligation, multiparity, and breastfeeding.[2,9,10]
Due to mentioned hypothesis, numerous Gynecologists have recommended prophylactic tube resection.[11] Moreover, in a meta-analysis study, prophylactic salpingectomy for ovarian cancer prevention is strongly suggested in general population.[12]
Hysterectomy without salpingectomy can also cause postsurgical complications for patients such as hydrosalpinx. Patients undergone hysterectomy without salpingectomy are at higher risk of hydrosalpinx, due to the closure of both sides of ovarian tubes, in comparison to those undergone hysterectomy with bilateral salpingectomy (H-bS).[13]
Due to the common blood supply of ovaries and fallopian tubes, some gynecologists prefer preserving ovaries in a hysterectomy, especially for benign reasons, to conserve ovarian function. Based on this fact, numerous studies have been conducted for evaluating the effect of salpingectomy on ovarian function.[8]
In the case of confirmation that salpingectomy may not affect ovarian function negatively, gynecologists can operate salpingectomy during hysterectomy surgery.[1]
Another important point that should be considered is ovary preservation benefits in women with low risk of ovarian cancer like those who do not carry BRCA. The advantages and disadvantages of tubal preservation during hysterectomy for benign reasons has not been widely assessed, and results are controversial, also the number of studies conducted in Iran are rare.
As bilateral salpingectomy during hysterectomy for benign reasons is controversial, we have decided to conduct the current study. In this study, we also assessed the effect of body mass index (BMI) and age of follicle stimulating hormone (FSH) and luteinizing hormone (LH) change in patients underwent salpingectomy and those whose salpinx were saved. Since the race and ethnicity is important, we compared ovarian function in patients who undergo hysterectomy with or without bilateral salpingectomy and also compare FSH and LH change in different BMI and ages in these two methods.
MATERIALS AND METHODS
This was a clinical-trial study on 37 patients undergone hysterectomy with/without bilateral salpingectomy for benign reasons in Al-Zahra Hospital (affiliated to Isfahan University of Medical Sciences), 2015–2016.
Inclusion criteria were (1) regular menstruation cycle, (2) no history of malignancy, (3) not being menopause, (4) underlying reason of myxomatosis uterus or menorrhagia, and (5) Exclusion criteria were (1) operation canceling, (2) not the accessibility of hormone measurement before or after operation due to any reason and (3) postsurgical pathology of malignancy.
Numbers of 43 patients were candidate for hysterectomy. Two of them were excluded because of menopausal status, three patients did not return for 6-month follow-up and pathology result of endometrial curettage of one patient with abnormal uterine bleeding was endometrial cancer [Figure 1].
Figure 1.
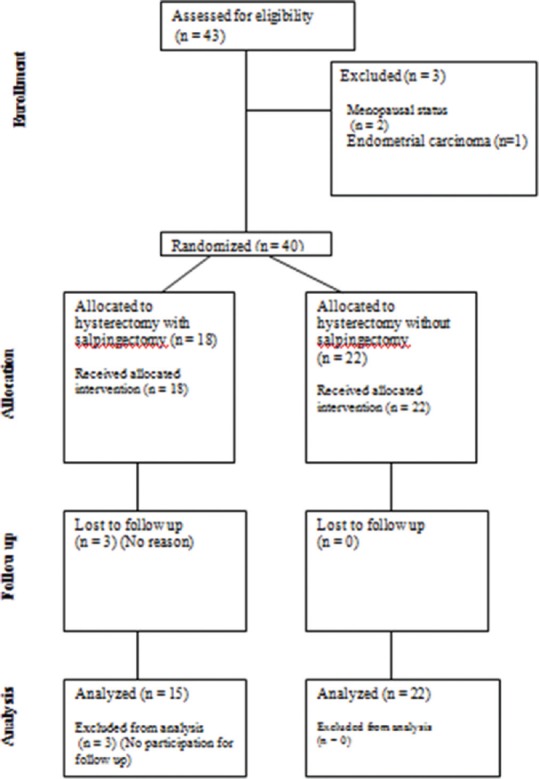
Consort diagram of studied patients
Consent forms for participating and all needed information about the study were given to patients. This study was approved based on IRCT2015112025139N1code from the Research Council and Ethics Committee of School of Medicine of Isfahan University of Medical Sciences.
Patients were divided into two groups of (A) hysterectomy and (B) H-bS. They were enrolled in a list and the method of surgery was chosen based on simple sampling (sortition). All patients underwent operation performed by an expert Gynecologist.
Demographic information of patients including age, height, and weight were recorded.
All included patients had regular menses of 21–45[14] days and FSH and LH were measured in 2–5 days of the menstrual cycle[15] before operation. Patients were asked to refer in 6 months for follow-up. FSH and LH were measured again to evaluate and compare ovarian function. Symptoms of menopause, including hot-flash and vaginal atrophy was evaluated too.
Patients were also divided into age groups of (A) 39–45, (B) 46–50 and (C) ≥51.
Furthermore, patients were divided into three groups based on BMI including; 18.5–24.9, 25–29.9, and 30–34.9.
FSH and LH were measured with chemiluminescence method by Elecsyscobas e411 Roche device.
Then, data were analyzed withIBM® SPSS® version 20, software - USA. Descriptive data were reported in mean ± standard deviation. For analytic data, paired t-test, Chi-square, regression analysis, and ANOVA and The correlation coefficient and Pearson correlation coefficient were used. P < 0.05 considered statistically significant.
RESULTS
This is a study on 37 patients divided into two groups including 22 patients’ undergone hysterectomy without salpingectomy (H) and 15 patients’ undergone H-bS.
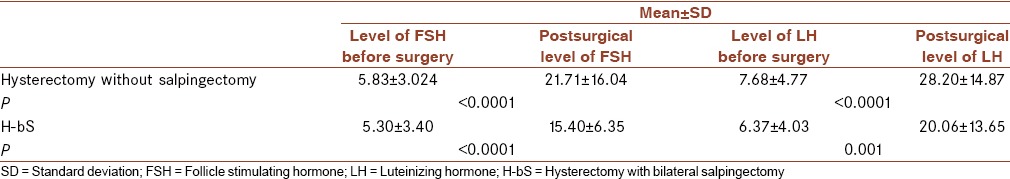
The mean age of Group H was 47.77 ± 3.03 and Group H-bS was 48.47 ± 2.03 that was not significantly different (P > 0.05). Furthermore, the mean level of FSH and LH before surgery was not significantly different in two groups (P > 0.05). FSH and LH in two groups before and postsurgery is compared in Table 1.
Table 1.
Mean level of follicle stimulating hormone and luteinizing hormone prior to and postsurgery
FSH changes were assessed with linear regression model. In this model, the main predictor variable was the type of surgery and confounding variables were age and BMI. The controlled results of this model for age and BMI showed no difference between these two methods (β = −6.8, 95% confidence interval [CI]: −14.9–1.2, P = 0.92). The LH results with same method showed same results (β = −7.8, 95% CI: −17.6-2, P = 0.114).
In 6-month follow-up, three patients had whether signs, symptoms, or laboratory findings of menopause. One of the patients in the group who had undergone hysterectomy without salpingectomy had hot-flush, and in another group, menopause occurred in two of the patients, one of them had FSH = 71 (cut-off > 45)[16] and vaginal atrophy was found in vaginal examination of other one.
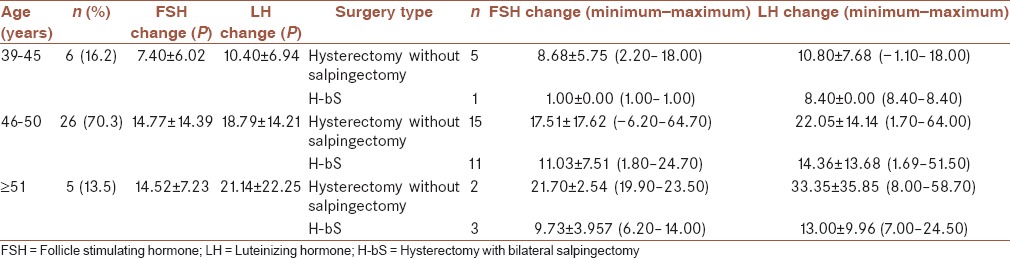
Patients’ information based on different age is shown in Table 2.
Table 2.
Patients’ information based on different ages
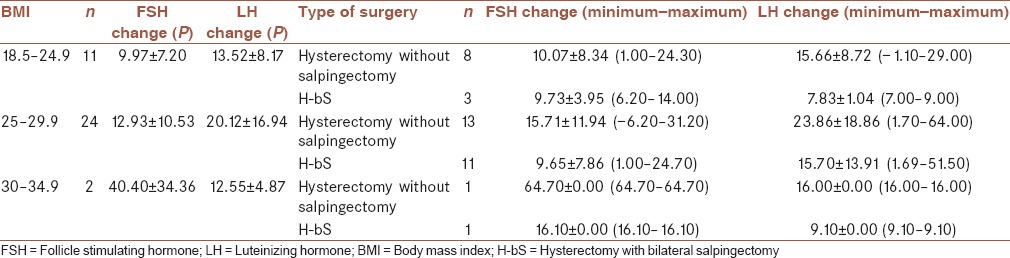
The last assessment is FSH and LH changes based on BMI presented in Table 3.
Table 3.
Patients’ information based on different body mass index
The correlation coefficient between changes in FSH and age (r = 0.28, P = 0.096) and FSH and BMI (r = 0.32, P = 0.051) show direct relationship between age and BMI increase and FSH change. Spearman's orrelation coefficient shows the same results about FSH change and age (r = 0.35, P = 0.035). This significance is probably due to low number of cases. With a unit increase in age and BMI, FSH will be increased 1.3 and 1.5 units, respectively.
The results for LH were similar but not statistically significant (r = 0.22, P = 0.183 for LH change and age) and (r = 0.12, P = 0.48 for LH change and BMI). With a unit increase in age and BMI, FSH will be increased 1.3 and 0.5 units, respectively.
DISCUSSION
Prophylactic salpingectomy with the aim of ovarian cancer prevention has been increased by Medeiros et al. in 2006 for the first time[17] and it was strongly recommended in further studies,[18] but concerns about postsurgical ovarian function may negatively affect making decision for resection of fallopian tubes in hysterectomy for benign reasons.[19] In this study, ovarian function was assessed by hormonal markers measurement.
In the current study, FSH and LH levels as indirect markers were measured to assess ovarian function after hysterectomy with/without salpingectomy. A few numbers of studies have evaluated LH and FSH for ovarian function assessment while anti-Mullerian hormone (AMH), estradiol and inhibin B, as direct markers of ovarian function are used more frequently. Specificity of AMH in ovarian function prediction is 84%, whereas FSH is 82%, on the other hand, FSH checking cost is remarkably lower and it is more accessible, thus we decided to assess FSH levels for ovarian function prediction.[20]
In this study, we found that level of both LH and FSH rose significantly after hysterectomy with/without salpingectomy by 6 months but only a patient in group of hysterectomy with salpingectomy had FSH >45. This finding may reflect ovarian reserve has decreased regardless type of surgery. Our results are consistent with the study of Atalay et al. that presented elevated levels of FSH and LH within 6 months after surgery[21] but is inconsistent with the study of Song et al.[14]
FSH and LH changes neither in Group H, nor in the other group were not significant (P = 0.17, 0.166 for FSH and LH, respectively). These results manifest that type of surgery is not in association with postsurgical ovarian function. This finding is consistent with the study of Findley et al. in which they measured AMH for ovarian function assessment and found no significant difference in postsurgical level of AMH whether in hysterectomy or H-bS.[6]
Posthysterectomy menopause has been mentioned as one of the complications of this procedure.[22] In the current study, three of patients showed whether signs, symptoms, or laboratory findings (FSH >45) of menopause that might be induced by surgery.
Postsurgical FSH and LH levels were not in association with patients’ age, neither in Group H nor in Group H-bS. Other study has reported similar result for patients’ undergone hysterectomy that found no correlation between patients’ age underwent hysterectomy and level of markers showing ovarian function. However, this effect in hysterectomy versus hysterectomy with salpingectomy was not compared.[23] Atalay et al. reported the same results among patients >40 years versus <40 years undergone total abdominal hysterectomy-bilateral salpingectomy or laparoscopic hysterectomy-bilateral salpingectomy.[21]
The most LH and FSH change occurred among patients ≥51 years that may be due to low number of patients in this group or being near to their menopause.
Both FSH and LH increase were positively affected by age and also BMI. LH relation with age and BMI was not statistically significant, but FSH was, although this significance is probably affected with low number of sample. These findings are presenting a need for more considerations in limited ages or BMIs in further studies.
In summary, H-bS has some advantages in comparison with tube preservation and based on our study, resection of tubes during hysterectomy had not significantly posed menopausal status.
Furthermore, there was no difference in various ages and BMIs. Thus, hysterectomy with salpingectomy is recommended in order of reducing complications and also the risk of serous ovarian cancer.
CONCLUSION
Based on the current study, it seems that bilateral salpingectomy during hysterectomy with the aim of ovarian cancer prevention would not increase risk of ovarian dysfunction.
Limitations
Higher number of participants can improve validity of further studies. Longer duration of patients follow-up may improve our overview of procedure. A cohort study to evaluate the incidence of ovarian cancer in these patients is suggested as well.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CK, Wallace S, Guiahi M, Sheeder J, Behbakht K, Spillman MA. Risk-reducing salpingectomy as preventative strategy for pelvic serous cancer. Int J Gynecol Cancer. 2013;23:417–21. doi: 10.1097/IGC.0b013e3182849dba. [DOI] [PubMed] [Google Scholar]
- 3.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10:803–8. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Shih IeM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer – Shifting the paradigm. Hum Pathol. 2011;42:918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tone AA, Salvador S, Finlayson SJ, Tinker AV, Kwon JS, Lee CH, et al. The role of the fallopian tube in ovarian cancer. Clin Adv Hematol Oncol. 2012;10:296–306. [PubMed] [Google Scholar]
- 6.Findley AD, Siedhoff MT, Hobbs KA, Steege JF, Carey ET, McCall CA, et al. Short-term effects of salpingectomy during laparoscopic hysterectomy on ovarian reserve: A pilot randomized controlled trial. Fertil Steril. 2013;100:1704–8. doi: 10.1016/j.fertnstert.2013.07.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandoval C, Fung-Kee-Fung M, Gilks B, Murphy KJ, Rahal R, Bryant H. Examining the use of salpingectomy with hysterectomy in Canada. Curr Oncol. 2013;20:173–5. doi: 10.3747/co.20.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacoby VL, Grady D, Wactawski-Wende J, Manson JE, Allison MA, Kuppermann M, et al. Oophorectomy vs. ovarian conservation with hysterectomy: Cardiovascular disease, hip fracture, and cancer in the Women's Health Initiative Observational Study. Arch Intern Med. 2011;171:760–8. doi: 10.1001/archinternmed.2011.121. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadi V, Dehghani S, Larijani B, Azadbakht L. Ovarian cancer risk and nonisoflavone flavonoids intake: A systematic review of epidemiological studies. J Res Med Sci. 2016;21:123. doi: 10.4103/1735-1995.196605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parvaresh A, Razavi R, Rafie N, Ghiasvand R, Pourmasoumi M, Miraghajani M. Quercetin and ovarian cancer: An evaluation based on a systematic review. J Res Med Sci. 2016;21:34. doi: 10.4103/1735-1995.181994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falconer H, Yin L, Grönberg H, Altman D. Ovarian cancer risk after salpingectomy: A nationwide population-based study. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju410. pii: Dju410. [DOI] [PubMed] [Google Scholar]
- 12.Yoon SH, Kim SN, Shim SH, Kang SB, Lee SJ. Bilateral salpingectomy can reduce the risk of ovarian cancer in the general population: A meta-analysis. Eur J Cancer. 2016;55:38–46. doi: 10.1016/j.ejca.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110:1091–5. doi: 10.1097/01.AOG.0000285997.38553.4b. [DOI] [PubMed] [Google Scholar]
- 14.Song T, Kim MK, Kim ML, Jung YW, Yun BS, Seong SJ, et al. Impact of opportunistic salpingectomy on anti-Müllerian hormone in patients undergoing laparoscopic hysterectomy: A multicentre randomised controlled trial. BJOG. 2017;124:314–320. doi: 10.1111/1471-0528.14182. [DOI] [PubMed] [Google Scholar]
- 15.Sezik M, Ozkaya O, Demir F, Sezik HT, Kaya H. Total salpingectomy during abdominal hysterectomy: Effects on ovarian reserve and ovarian stromal blood flow. J Obstet Gynaecol Res. 2007;33:863–9. doi: 10.1111/j.1447-0756.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 16.Henrich JB, Hughes JP, Kaufman SC, Brody DJ, Curtin LR. Limitations of follicle-stimulating hormone in assessing menopause status: Findings from the National Health and Nutrition Examination Survey (NHANES 1999-2000) Menopause. 2006;13:171–7. doi: 10.1097/01.gme.0000198489.49618.96. [DOI] [PubMed] [Google Scholar]
- 17.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–6. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 18.Foulkes WD. Preventing ovarian cancer by salpingectomy. Curr Oncol. 2013;20:139–42. doi: 10.3747/co.20.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morelli M, Venturella R, Mocciaro R, Di Cello A, Rania E, Lico D, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: Primum non nocere. Gynecol Oncol. 2013;129:448–51. doi: 10.1016/j.ygyno.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Barad DH, Weghofer A, Gleicher N. Comparing anti-Müllerian hormone (AMH) and follicle-stimulating hormone (FSH) as predictors of ovarian function. Fertil Steril. 2009;91 4 Suppl:1553–5. doi: 10.1016/j.fertnstert.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 21.Atalay MA, Cetinkaya Demir B, Ozerkan K. Change in the ovarian environment after hysterectomy with bilateral salpingectomy: Is it the technique or surgery itself? Eur J Obstet Gynecol Reprod Biol. 2016;204:57–61. doi: 10.1016/j.ejogrb.2016.07.483. [DOI] [PubMed] [Google Scholar]
- 22.Ahn EH, Bai SW, Song CH, Kim JY, Jeong KA, Kim SK, et al. Effect of hysterectomy on conserved ovarian function. Yonsei Med J. 2002;43:53–8. doi: 10.3349/ymj.2002.43.1.53. [DOI] [PubMed] [Google Scholar]
- 23.Pitynski K, Szczudrawa A, Basta A, Pawlak M, Oplawski M, Peszek W. Changes in ovaries after hysterectomy. Przegl Lek. 2001;58:805–8. [PubMed] [Google Scholar]


