Abstract
Background:
Omentin-1, vaspin, and apelin are novel adipokines which closely associate with obesity, nonalcoholic fatty liver disease (NAFLD), and inflammation. The aim of this study was to investigate the circulating levels of omentin-1, vaspin, and apelin in NAFLD patients and to clarify their relationship with biochemical parameters, abdominal obesity, and high sensitive C-reactive protein.
Materials and Methods:
In a case–control study, serum levels of omentin-1, vaspin, and apelin were measured in 41 NAFLD patients and 41 healthy volunteers. The study was performed in the outpatients’ clinic of Imam-Ali Hospital in Zahedan, Iran, during February to July 2015. Fatty liver was confirmed by ultrasonography. The association of the adipokines with lipid profile and anthropometric parameters was assessed using multivariable linear regression models. In this model, those variables that showed P < 0.05 were included in the study.
Results:
NAFLD patients presented a significantly higher apelin levels compared to the controls (P < 0.01), whereas serum omentin-1 and vaspin levels did not differ between two groups (both P > 0.05). Multiple regression analysis showed that the serum levels of apelin and vaspin correlated positively with waist circumference (WC) (P < 0.01 and P < 0.05, respectively) and low-density lipoprotein (P < 0.05 and P < 0.01, respectively) while serum omentin-1 was inversely correlated with WC (P < 0.01) and positively corrected with high-density lipoprotein (P < 0.05).
Conclusion:
The findings showed that among the analyzed adipokines only apelin was different in patients with NAFLD when compared to controls. Considering the multivariate regression analysis, apelin seems be more suitable diagnostic marker in predicting of NAFLD and omentin might be considered as a protective factor in occurrence of NAFLD, particularly in those with central obesity.
Keywords: Adipokines, apelin, central obesity, nonalcoholic fatty liver disease, omentin, vaspin
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common of liver disorder with multifactorial etiology, which is closely associated with obesity, insulin resistance, and dyslipidemia.[1,2,3] It is expected that the prevalence of NAFLD increases with the global epidemic of obesity.[1,3,4,5] The mechanisms which develop NAFLD in obese persons and the effects on liver function are not fully known.[3,6] The regional distribution of fat tissue is an important factor for the development of the metabolic complications of obesity.[7,8] Nonfatty liver disease expresses hepatic component of the metabolic syndrome, especially when it is associated with increased intra-abdominal fat,[9] visceral adipose tissue “as an endocrine organ” contributes to the pathogenicity of obesity and secretes large amounts cytokines[10] and adipokines.[7] There is evidence that the increased the production of adipokines “adipose tissue-derived hormones” might have a strong link to insulin resistance, metabolic syndrome, fatty liver and contribute to the acceleration of atherosclerosis.[1,5,7,11,12,13]
Vaspin,[2,14,15] apelin,[1,16] and omentin-1[17] (secreted from visceral adipose tissue) are newly adipokines that are probably associated with hyperinsulinemia, inflammation, and the components of metabolic syndrome.[1,5,15,17,18,19] Vaspin is first detected in obese type 2 diabetic animal models,[15,18] which is highly correlated to the presence of coronary artery plaque and severity of coronary atherosclerosis.[15] Apelin is produced and secreted by the brain, hypothalamus, stomach and fat tissue in both human and mouse.[16,20] Apelin is involved in the mechanisms underlying the neuroendocrine function, energy homeostasis, and food intake regulation.[20] Omentin-1 (intelectin-1) “a new adipokine described in 2003,”[8] acts centrally to modulate the insulin resistance, body weight, and inflammation.[8,17,19,21] However, its role in the liver pathogenesis and in the metabolic consequences of the liver disorders is not fully elucidated. In this study, we measured the circulating levels of omentin-1, vaspin, and apelin in patients with NAFLD compared with healthy controls, and evaluated their association with C-reactive protein (CRP) and lipid profile in obese patients, particularly those with central obesity.
MATERIALS AND METHODS
In a case-control study, a total of 41 NAFLD patients (13 men, 28 women; mean age 39.8 ± 8.9-year-old, body mass index [BMI]: 28.3 ± 4.2 kg/m2), and 41 healthy controls without NAFLD (17 men, 24 women; mean age 36.7 ± 8.5-year-old, BMI: 25.1 ± 3.6 kg/m2 matched by age, sex and BMI) were recruited from the outpatients’ clinic of Imam-Ali Education and Research Hospital in Zahedan, located in South-East of Iran. The study was carried out during February to July 2015. The sample size was estimated based on a previous study.[2] Hepatic ultrasonography (US) (Grade1–3) was performed to diagnose and evaluate the severity of NAFLD. The inclusion criterion included females and males the aged 20–70 years and ranged 18–35 kg/m2. Exclusion criteria for all participants included history of alcohol consumption and food supplements (Vitamins A, C, E), smoking, endocrine and metabolic or kidney diseases, hepatitis Type B and C, infectious diseases, and the consumption of drugs that could affect lipid or carbohydrate metabolism.
Laboratory measurements
Fasting blood samples were collected after at least 12 h fasting. Routine laboratory tests such as fasting blood sugar, total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), were performed by the commercial kits (Pars Azmun, Tehran, Iran) using an auto-analyzer (Hitachi, Japan). Serum levels of highly sensitive CRP (hs-CRP were assessed by latex-enhanced nephelometry (Behring BN II Nephelometer, Germany). For the measurement of adipokines, the rest of the sera were immediately distributed in aliquots and frozen at −70°C.
Adipokines measurement
Serum levels of adipokines (omentin-1, vaspin and apelin) were measured by enzyme-linked immune-sorbent assay (ELISA) using commercial kits: Human Intelectin-1(ITLN1/Omentin) ELISA kit (Cat No. E0155Hu: Shanghai Crystal Day Biotech Co., Ltd.); human viceral adipose-specific serine protease inhibitor (vaspin) ELISA kit (Cat No. E092Hu: Shanghai Crystal Day Biotech Co., Ltd.), and Human Apelin ELISA kit (Cat No. E2014Hu: Shanghai Crystal Day Biotech Co., Ltd.). The intra- and inter-assay coefficient of variation (CV) of vaspin was ranged from 6.7% to 9.5%, respectively. The intra- and inter-assay CV of omentin-1 and apelin was ranged from 3.3%–4.3% to 5.1%–8.5%, respectively.
Anthropometric evaluation
Waist circumference (WC) was measured with an inelastic tape at the minimum circumference between the iliac crest and the rib cage. According the proposed Iranian National Committee of Obesity, which has revised ATP III criteria with regional cutoff, values of WC >95 cm for men and women were defined as abdominal obesity.[22]
Weight and height were measured by Seca scale to the nearest 0.1 kg and 0.5 cm. BMI was calculated using weight (kg) divided by the square of height (m2).
The Ethical Committee of the Zahedan University of Medical Sciences approved the protocol of study (Approval: 93-6994; Date: November 30, 2014). Oral informed consent was obtained from all participants for participation in the study. The study was performed in accordance with the Declaration of Helsinki recommendations.
Statistical analysis
Statistical analysis was performed using SPSS for Windows, version 21. The results of biochemical tests and anthropometric parameters were presented as mean ± standard deviation. The serum levels of hs-CRP and adipokines were expressed as median (range) because the data were not normally distributed. Data were tested for normal distribution using the Kolmogorov–Smirnov test. Student's t-test and one-way ANOVA with the Turkey's post hoc were performed for variables with normal distribution. Mann–Whitney U and Kruskal–Wallis tests were used for nonnormally distribution variables. Odds ratio (OR) and 95% confidence intervals (CIs) were calculated. Spearman's orrelation coefficient and multivariable linear regression models were used for the relationship between adipokines and other variables. P < 0.05 was considered statistically significant.
RESULTS
Table 1 shows demographic and biochemical characteristics of studied population. A significant difference was found between NAFLD patients and control group in terms of BMI, WC, lipid profile, and AST. US findings demonstrated that 27 participants (65.9%) had fatty liver with Grade I (mild) and 14 of them (34.1%) had moderate fatty liver (Grade II).
Table 1.
Demographic and biochemical characteristics in the studied population
Mean levels of apelin were significantly higher (P < 0.01), while, omentin-1 and vaspin serum levels were not significantly different in patients with NAFLD when compared with controls [P > 0.05, Figure 1].
Figure 1.
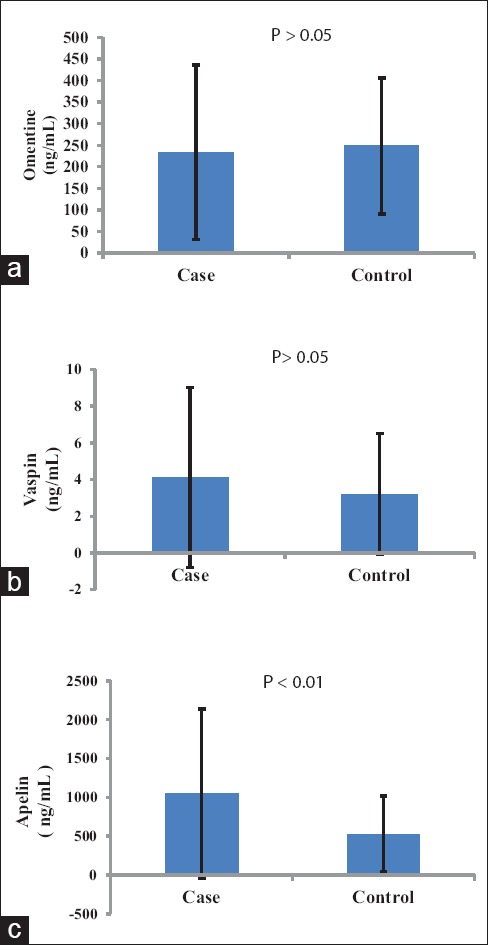
Mean and error bar of serum dipokines (omentin-1 (a), vaspin (b), apelin (c)) in studied population
As shown in Table 2, significantly higher serum concentrations of apelin (both P < 0.001) and LDL (P = 0.025 and P < 0.001, respectively) and significantly lower concentrations of omentin-1 (P = 0.03 and P < 0.01, respectively) were found in obese patients with NAFLD compared to the nonobese patients and controls. Serum levels of vaspin and cholesterol were markedly higher in obese patients than controls (both P < 0.01). Furthermore, the levels of serum TG (P < 0.0001 and P < 0.05, respectively) and hs-CRP (both P < 0.001) in obese and nonobese patients with NAFLD were significantly higher, and HDL levels were significantly lower than that of controls. No difference was found between obese and nonobese control subjects for all of the variables.
Table 2.
The risk factors related to central obesity in the studied population
The serum levels of hs-CRP and adipokines according to the fatty liver grade using US findings showed that the serum levels of hs-CRP (P > 0.05), vaspin (P < 0.05) and apelin (P < 0.01) were higher, and the levels of serum omentin-1 (P > 0.05) were lower in patients who had mild fatty liver (Grade II) as compared with those who had moderate fatty liver (Grade I) [Table 3].
Table 3.
The levels of the high sensitive C-reactive protein and adipokines according to the fatty liver grade using ultrasonography findings in the studied population
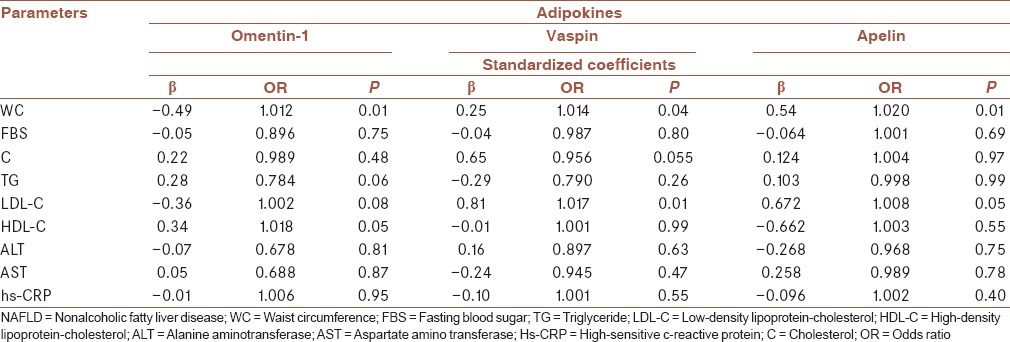
Spearman correlation test showed that apelin levels were positively correlated with BMI (r = 0.33, P < 0.05), WC (r = 0.34, P < 0.05), cholesterol (r = 0.77, P < 0.05), LDL (r = 0.575, P < 0.05) and CRP (r = 0.25, P < 0.05), and negatively with omentin-1 (r = −0.35, P < 0.05). A significant positive correlation was found between the serum levels of vaspin with apelin (r = 0.57, P < 0.001), WC (r = 0.35, P < 0.05), and LDL (r = 0.375, P < 0.05). Whereas, serum omentin-1 levels were adversely correlated with WC (r = −0.50, P < 0.001) and positively correlated with HDL (r = 0.36, P < 0.05). In addition, in multivariate regression analysis, serum omentin-1 levels showed a significant negative correlation with WC (OR: 1.012, CI: 1.010–1.014; P < 0.01), and a positive correlation with HDL (OR: 1.018, CI: 1.006–1.030; P < 0.05). As well, significant positive correlations were found between serum vaspin and apelin with WC (OR: 1.014, CI: 1.004–1.024; P < 0.05) and (OR: 1.020, CI: 1.012–1.028; P < 0.01), respectively, and LDL (OR: 1.017, CI: 1.002–1.032; P < 0.01) and (OR: 1.008, CI: 1.006–1.010; P < 0.05), respectively [Table 4].
Table 4.
Results of multivariate linear regression analysis between omentin-1, vaspin, and apelin and various parameters in patients with nonalcoholic fatty liver disease
DISCUSSION
In this study, we reported the serum levels of adipokinesomentin-1, vaspin, and apelin in patients with NAFLD. Omentin-1 is a novel adipokine, which is produced in visceral but not in subcutaneous fat tissue. Although several studies have declared that circulating omentin-1 levels are significantly increased in NAFLD,[17] in liver cirrhosis[23] and in hepatitis C patients,[19] in our study; the levels of serum omentin-1 (intelectin-1) did not differ in patients with NAFLD when compared with the controls. This is in agreement with the study by Klusek-Oksiuta et al.[13] A study also showed that the levels of serum omentin-1 in type 2 diabetic patients were significantly lower than controls, suggesting that glucose and insulin concentrations may have a repressive effect on omentin-1 concentrations.[8]
In the current study, serum omentin-1 levels of obese patients were significantly lower than that of controls. Whereas in several studies, there was no significant difference in omentin-1 serum levels between the nonobese group with type 2 diabetes and the nonobese healthy control group.[19,24]
Unlike the previous studies[8,24] which have shown BMI is one of the major determinants of omentin-1, in our study, serum omentin-1 levels did not correlate with BMI, whereas a negative correlation was found between serum omentin-1 and WC, suggesting that BMI seems to be a weaker surrogate for body fat distribution than WC. On the other hand, the previous studies suggested a complex relationship between omentin-1 and liver function. In a study, omentin-1 level showed a significant positive correlation with liver enzymes AST and ALT.[19] While, in the study of Eisinger et al.,[23] omentin-1was not associated with liver function in patients with liver cirrhosis. In another study, serum omentin-1 level was positively correlated with CRP and hepatocyte ballooning,[17] which declares omentin-1, is linked to the hepatocyte degeneration.[17,19] Klusek-Oksiuta et al.[13] found a positive correlation between omentin-1 with TC and LDL-C in children with NAFLD.
In this study, we did not find any correlation between omentin-1 with ALT, AST, CRP, and lipid profile, but similar to Gursoy et al.[8] study, serum levels of omentin-1 were positively associated with HDL-C levels in NAFLD patients, suggesting that omentin-1 might be a protective factor in the occurrence of NAFLD.
Apelin “fat-derived hormone” is produced in the brain, hypothalamus, and the stomach and is also secreted by both human and mouse adipocytes.[16,20] The role of apelin in the pathogenesis of NAFLD may be considered as a cause of the ectopic fat accumulation in the liver. Because it is closely associated with the insulin resistance, which has been distinguished as one of the most important factors affecting NAFLD.[13]
In this study, serum apelin levels were markedly higher in patients than controls and increased with obesity. Apelin levels were positively correlated with WC and BMI. This finding was in agreement with others.[1,16,18] It has been reported that abdominal visceral fat is linked to risk of metabolic disturbances more often than subcutaneous fat[7,8] because increased abdominal fat is more associated with insulin resistance and fatty liver.[8] The previous studies have reported that increased levels of plasma apelin in the hyperinsulinemia-associated obese states in obese humans may be linked to complex relationship between the apelin regulations by insulin which could affect serum levels of apelin.[21] However, insulin concentration was no evaluated, in our study.
Moreover, positive correlation between apelin with cholesterol, LDL and CRP and negative correlation with omentin, suggesting that apelin can be considered as a biomarker of inflammation process in the liver that may contribute to atherosclerosis complications. It has been reported that vaspin is also an adipokine with insulin-sensitizing effects, which may be linked to visceral obesity, type 2 diabetes, atherosclerosis, and risk factors of liver complications.[13,15,25,26] In opposition to earlier studies,[18,27] in the current study, serum vaspin levels were not different between patients with NAFLD than in controls. A study by Choi et al.[15] reporting that plasma vaspin concentrations correlated with BMI and WC in men with the metabolic syndrome compared with those without the metabolic syndrome. In a study by Jian et al.,[26] the serum vaspin concentration was also significantly correlated with BMI and waist-hip ratio in type 2 diabetic patients than in controls. They indicated that low serum concentration of vaspin is a risk factor for the progression of type 2 diabetes mellitus. In another study by Körner et al.,[25] obese girls had lower vaspin serum levels than those of lean controls, but there was no significant correlation between vaspin with BMI obese children. Suleymanoglu et al.[28] demonstrated that the serum vaspin levels were significantly higher in obese pubertal girls than control subjects, and vaspin levels were positively correlated with BMI, TGs, and fasting insulin. In a study by Klusek-Oksiuta et al.,[13] a significant positive correlation was found between vaspin and ALT in obese children with NAFLD compared to the control. It is notable that some of these studies were performed on diabetic patients, children, and normal population, not patients with NAFLD.
In our study, the concentrations of vaspin were significantly higher in the central obese patients compared with healthy control subjects. Furthermore, after stepwise linear regression analysis, serum concentrations of vaspin were positively correlated with apelin, WC, and LDL. This finding supports further investigations of this adipokine in atherosclerosis and metabolic liver diseases.
In general, the relationship between the three novel adipokines (omentin-1, apelin, and vaspin) with WC suggests that these adipokines reflect body fat mass, and WC might be considered as an independent factor that affects the serum levels of adipokines.
Aside from obesity and dyslipidemia, another lifestyle-related factors such as diet[29] or supplements[30,31] have also contradictory effects on liver fat content; however, we did not assess the nutrient intakes in this study.
At present study, we evaluated the severity of liver involvement using US findings. Almost 65.9% of patients had Grade I (mild) and 34.1% had Grade II (moderate fatty liver). None of the patients showed severe fatty liver (Grade III). Given that the grading of NAFLD by ultrasound is based on the visual scale; it has limitations in differentiating moderate from severe groups. So that in some patients with the borderline findings of ultrasound might be incorrectly classified the moderate from severe grade.[32]
The levels of the adipokines according to the fatty liver grade demonstrated that in Grade II (moderate fatty liver), serum level of omentine-1 was slightly decreased while serum levels vaspin and apelin were markedly increased. The serum levels of hs-CRP in NAFLD patients with Grade II were slightly higher than in those with Grade I. However, no correlation was found between hs-CRP and three adipokines and severity of fatty liver, which may be related to small sample size.
Overall, the findings suggest that these adipokines are involved in the development of obesity and pathogenesis of fatty liver.[29]
Our study has several potential limitations, including the relatively small sample size, financial constraints, failure to do a liver biopsy, and just use of ultrasound findings for the diagnosis of fatty liver, which restrict the generalizability of our results. Thus, further longitudinal studies with more sample size are needed to clarify the link of these adipokines with obesity and other metabolic disorders in patients with NAFLD.
CONCLUSIONS
The findings showed that among the analyzed adipokines only apelin was different in patients with NAFLD when compared to controls. Apelin seems be more suitable diagnostic marker in predicting of NAFLD. Considering the multivariate regression analysis, the findings suggest that vaspin may reflect body fat mass, but its association with fatty liver remains controversial. On the other hand, omentin-1 might be considered as a protective factor in the evaluation of occurrence of NAFLD, especially in central obese patients. Thus, the measurement of these novel adipokines may contribute to the evaluation of NAFLD occurrence.
Financial support and sponsorship
This study was granted by the Zahedan University of Medical Sciences, Zahedan, Iran (No.: 2014-860).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank the participants who willingly participated in the study.
REFERENCES
- 1.Ercin CN, Dogru T, Tapan S, Kara M, Haymana C, Karadurmus N, et al. Plasma apelin levels in subjects with nonalcoholic fatty liver disease. Metabolism. 2010;59:977–81. doi: 10.1016/j.metabol.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Kukla M, Zwirska-Korczala K, Hartleb M, Waluga M, Chwist A, Kajor M, et al. Serum chemerin and vaspin in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2010;45:235–42. doi: 10.3109/00365520903443852. [DOI] [PubMed] [Google Scholar]
- 3.Montazerifar F, Karajibani M, Ansari-Moghaddam AR. Evaluation of some risk factors of non alcoholic fatty liver disease in adult population of Zahedan, Southeast of Iran. Zahedan J Res Med Sci. 2014;16:12–5. [Google Scholar]
- 4.Hatziagelaki E, Herder C, Tsiavou A, Teichert T, Chounta A, Nowotny P, et al. Serum chemerin concentrations associate with beta-cell function, but not with insulin resistance in individuals with non-alcoholic fatty liver disease (NAFLD) PLoS One. 2015;10:e0124935. doi: 10.1371/journal.pone.0124935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abenavoli L, Luigiano C, Guzzi PH, Milic N, Morace C, Stelitano L, et al. Serum adipokine levels in overweight patients and their relationship with non-alcoholic fatty liver disease. Panminerva Med. 2014;56:189–93. [PubMed] [Google Scholar]
- 6.Tock L, Prado WL, Caranti DA, Cristofalo DM, Lederman H, Fisberg M, et al. Nonalcoholic fatty liver disease decrease in obese adolescents after multidisciplinary therapy. Eur J Gastroenterol Hepatol. 2006;18:1241–5. doi: 10.1097/01.meg.0000243872.86949.95. [DOI] [PubMed] [Google Scholar]
- 7.Mirza MS. Obesity, visceral fat, and NAFLD: Querying the role of adipokines in the progression of nonalcoholic fatty liver disease. ISRN Gastroenterol. 2011;2011:592404. doi: 10.5402/2011/592404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gursoy G, Kirnap NG, Esbah O, Acar Y, Demirbas B, Akcayoz S, et al. The relationship between plasma omentin-1 levels and insulin resistance in newly diagnosed type 2 diabetic women. Clin Rev Opin. 2010;2:49–54. [Google Scholar]
- 9.Angelico F, Del Ben M, Conti R, Francioso S, Feole K, Maccioni D, et al. Non-alcoholic fatty liver syndrome: A hepatic consequence of common metabolic diseases. J Gastroenterol Hepatol. 2003;18:588–94. doi: 10.1046/j.1440-1746.2003.02958.x. [DOI] [PubMed] [Google Scholar]
- 10.Giorgino F, Laviola L, Eriksson JW. Regional differences of insulin action in adipose tissue: Insights from in vivo and in vitro studies. Acta Physiol Scand. 2005;183:13–30. doi: 10.1111/j.1365-201X.2004.01385.x. [DOI] [PubMed] [Google Scholar]
- 11.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 12.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 13.Klusek-Oksiuta M, Bialokoz-Kalinowska I, Tarasów E, Wojtkowska M, Werpachowska I, Lebensztejn DM. Chemerin as a novel non-invasive serum marker of intrahepatic lipid content in obese children. Ital J Pediatr. 2014;40:84. doi: 10.1186/s13052-014-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klöting N, Kovacs P, Kern M, Heiker JT, Fasshauer M, Schön MR, et al. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia. 2011;54:1819–23. doi: 10.1007/s00125-011-2137-1. [DOI] [PubMed] [Google Scholar]
- 15.Choi SH, Kwak SH, Lee Y, Moon MK, Lim S, Park YJ, et al. Plasma vaspin concentrations are elevated in metabolic syndrome in men and are correlated with coronary atherosclerosis in women. Clin Endocrinol (Oxf) 2011;75:628–35. doi: 10.1111/j.1365-2265.2011.04095.x. [DOI] [PubMed] [Google Scholar]
- 16.Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–71. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz Y, Yonal O, Kurt R, Alahdab YO, Eren F, Ozdogan O, et al. Serum levels of omentin, chemerin and adipsin in patients with biopsy-proven nonalcoholic fatty liver disease. Scand J Gastroenterol. 2011;46:91–7. doi: 10.3109/00365521.2010.516452. [DOI] [PubMed] [Google Scholar]
- 18.Aktas B, Yilmaz Y, Eren F, Yonal O, Kurt R, Alahdab YO, et al. Serum levels of vaspin, obestatin, and apelin-36 in patients with nonalcoholic fatty liver disease. Metabolism. 2011;60:544–9. doi: 10.1016/j.metabol.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Nassif WM, Amin AI, Hassan ZA, Abdelaziz DH. Changes of serum omentin-1 levels and relationship between omentin-1 and insulin resistance in chronic hepatitis C patients. EXCLI J. 2013;12:924–32. [PMC free article] [PubMed] [Google Scholar]
- 20.Sörhede Winzell M, Magnusson C, Ahrén B. The apj receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept. 2005;131:12–7. doi: 10.1016/j.regpep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Zhu L, Zheng M, Fan C, Li Y, Zhang D, et al. Changes of serum omentin-1 levels in normal subjects, type 2 diabetes and type 2 diabetes with overweight and obesity in Chinese adults. Ann Endocrinol (Paris) 2014;75:171–5. doi: 10.1016/j.ando.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Hajian-Tilaki K. Metabolic syndrome and its associated risk factors in Iranian adults: A systematic review. Caspian J Intern Med. 2015;6:51–61. [PMC free article] [PubMed] [Google Scholar]
- 23.Eisinger K, Krautbauer S, Wiest R, Karrasch T, Hader Y, Scherer MN, et al. Portal vein omentin is increased in patients with liver cirrhosis but is not associated with complications of portal hypertension. Eur J Clin Invest. 2013;43:926–32. doi: 10.1111/eci.12122. [DOI] [PubMed] [Google Scholar]
- 24.El-Mesallamy HO, El-Derany MO, Hamdy NM. Serum omentin-1 and chemerin levels are interrelated in patients with type 2 diabetes mellitus with or without ischaemic heart disease. Diabet Med. 2011;28:1194–200. doi: 10.1111/j.1464-5491.2011.03353.x. [DOI] [PubMed] [Google Scholar]
- 25.Körner A, Neef M, Friebe D, Erbs S, Kratzsch J, Dittrich K, et al. Vaspin is related to gender, puberty and deteriorating insulin sensitivity in children. Int J Obes (Lond) 2011;35:578–86. doi: 10.1038/ijo.2010.196. [DOI] [PubMed] [Google Scholar]
- 26.Jian W, Peng W, Xiao S, Li H, Jin J, Qin L, et al. Role of serum vaspin in progression of type 2 diabetes: A 2-year cohort study. PLoS One. 2014;9:e94763. doi: 10.1371/journal.pone.0094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genc H, Dogru T, Tapan S, Kara M, Ercin CN, Aslan F, et al. Circulating vaspin and its relationship with insulin sensitivity, adiponectin, and liver histology in subjects with non-alcoholic steatohepatitis. Scand J Gastroenterol. 2011;46:1355–61. doi: 10.3109/00365521.2011.603163. [DOI] [PubMed] [Google Scholar]
- 28.Suleymanoglu S, Tascilar E, Pirgon O, Tapan S, Meral C, Abaci A. Vaspin and its correlation with insulin sensitivity indices in obese children. Diabetes Res Clin Pract. 2009;84:325–8. doi: 10.1016/j.diabres.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Haghighatdoost F, Salehi-Abargouei A, Surkan PJ, Azadbakht L. The effects of low carbohydrate diets on liver function tests in nonalcoholic fatty liver disease: A systematic review and meta-analysis of clinical trials. J Res Med Sci. 2016;21:53. doi: 10.4103/1735-1995.187269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekhlasi G, Kolahdouz Mohammadi R, Agah S, Zarrati M, Hosseini AF, Arabshahi SS, et al. Do symbiotic and Vitamin E supplementation have favorite effects in nonalcoholic fatty liver disease? A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2016;21:106. doi: 10.4103/1735-1995.193178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pezeshki A, Safi S, Feizi A, Askari GH, Karami F. The effect of green tea extract supplementation on liver enzymes in patients with non-alcoholic fatty liver disease. Int J Prev Med. 2016;7:28. doi: 10.4103/2008-7802.173051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamali R, Arj A, Razavizade M, Aarabi MH. Prediction of nonalcoholic fatty liver disease via a novel panel of serum adipokines. Medicine (Baltimore) 2016;95:e2630. doi: 10.1097/MD.0000000000002630. [DOI] [PMC free article] [PubMed] [Google Scholar]


