Abstract
Background:
Fungal sinusitis is increasing worldwide in the past two decades. It is divided into two types including invasive and noninvasive. Noninvasive types contain allergic fungal sinusitis (AFS) and fungus ball. AFS is a hypersensitivity reaction to fungal allergens in the mucosa of the sinonasal tract in atopic individuals. The fungus ball is a different type of noninvasive fungal rhinosinusitis which is delineated as an accumulation of debris and fungal elements inside a paranasal sinus. Fungal sinusitis caused by various fungi such as Aspergillus species, Penicillium, Mucor, Rhizopus, and phaeohyphomycetes. The aim of the present study is to identify fungal species isolated from noninvasive fungal sinusitis by molecular methods.
Materials and Methods:
During 2015–2016, a total of 100 suspected patients were examined for fungal sinusitis. Functional endoscopic sinus surgery was performed using the Messerklinger technique. Clinical samples were identified by phenotypic and molecular methods. Polymerase chain reaction (PCR) sequencing of ITS1-5.8S-ITS2 region and PCR-restriction fragment length polymorphism with MspI restriction enzyme was performed for molecular identification of molds and yeasts, respectively.
Results:
Twenty-seven out of 100 suspected cases (27%) had fungal sinusitis. Nasal congestion (59%) and headache (19%) were the most common clinical signs among patients. Fifteen patients (55.5%) were male and 12 patients (44.5%) were female. Aspergillus flavus was the most prevalent fungal species (26%), followed by Penicillium chrysogenum (18.5%) and Candida glabrata species complex (15%).
Conclusion:
Since clinical manifestations, computed tomography scan, endoscopy, and histopathological findings are very nonspecific in AFS and fungus ball; therefore, molecular investigations are compulsory for precise identification of etiologic agents and appropriate management of these fungal infections.
Keywords: Fungal agents, molecular identification, non-invasive, sinusitis
INTRODUCTION
Allergic fungal sinusitis (AFS) is a hypersensitivity reaction to fungal allergens including surface antigens and fungal metabolites in the mucosa of the sinonasal tract in atopic individuals. It was first reported by Katzenstein et al. in 1983.[1] Clinical manifestations present with different signs containing nasal congestion, facial deformity, diplopia, osteolytic impairment, and entanglement of the skull base.[2] The incidence of disease is unknown and seems account for up to 10% of surgical cases;[3] however, mixed bacterial and fungal infections represent 13%–28.5% of all maxillary sinusitis cases.[4] The diagnosis of AFS is supported by the presence of thickened allergic mucin that is coarsely evident at surgery and a positive histopathological staining for fungal hyphae.[5] Fungus ball is a type of noninvasive chronic fungal sinusitis without thickened allergic mucin. It is composed of fungal hypha, fibrin, inflammatory cell, shapeless debris, and mucous in pulmonary cavities. It usually appears in the maxillary and sphenoid sinuses.[4] Fungus ball is commonly found in older individuals and females.[6,7] A variety of fungal agents such as yeasts, dematiaceous fungi, and hyaline fungi have been involved in fungal sinusitis.[8,9] The aim of the present study is to identify the etiologic agents of fungal sinusitis isolated from AFS and fungus ball using molecular methods.
MATERIALS AND METHODS
Clinical samples
During December 2015 to December 2016, a total of 100 cases referred to the two university hospitals (Alzahra and Kashani Hospitals) of Isfahan University of Medical Sciences, Isfahan, Iran, due to the acute or chronic sinusitis. All patients were nominated for computed tomography (CT) scan of the paranasal sinuses (PNS). Boney erosion, inspissated mucus in sinuses that cause heterogeneous soft tissue density and calcification were clinical symptoms among patients. Functional endoscopic sinus surgery was performed using the Messerklinger technique by means of Karl Storz Endoskope (Germany). The Messerklinger technique is a mainly diagnostic endoscopic concept presenting that the maxillary and frontal sinuses are subordinate cavities. It can be used for various scopes of indications, excluding nasal polyposis. Sufficient experience and training are needed for the surgical approach.[10] All samples were sent to the medical mycology laboratory in serum saline for direct microscopy and culture. Potassium hydroxide 20% and Sabouraud Dextrose Agar with chloramphenicol (BD, USA) were used for direct microscopic examination and culture, respectively. Periodic acid-Schiff (PAS) (Sigma-Aldrich, USA) was applied for histopathological staining.
Molecular identification
DNA extraction
DNA of fungi was extracted using glass beads (Sigma-Aldrich, USA) and boiling method.[11] Briefly, a bit of fresh colonies were suspended in 50 μl of double distilled water and simmered for 10 min, afterward centrifuged for 5 min at 5000 rpm, and supernatant was separated for polymerase chain reaction (PCR). ITS1-5.8S-ITS2 rDNA region of fungi was amplified using ITS1 (5’-TCC GTA GGT GAA CCT GCG G-3’) and ITS4 (5’-TCC TCC GCT TAT TGA TAT GC-3’) primers.[12]
Polymerase chain reaction
The PCR cycling was performed as follows: initial denaturation phase at 95°C for 5 min, followed by 32 cycles of denaturation at 94°C for 35 s, annealing at 55°C for 45 s, and extension at 72°C for 1 min, with a final extension phase at 72°C for 7 min. PCR products were purified by QIAquick PCR Purification Kit (Qiagen, USA). Five microliters of each amplicon was run onto 1.5% agarose gel, electrophoresed in TBE buffer (90 mM boric acid, 2 mM 127 ethylenediaminetetraacetic acid, and 90 mM Tris) at 10 V/cm for 90 min, and stained with 0.5 μg/ml ethidium bromide (Sigma-Aldrich, USA).
Sequencing
Purified PCR products were employed for cycle sequencing reactions in forward direction (Bioneer, Korea). Sequence products were analyzed with Chromas Software 2.4 (http://www.chromas.software.informer.com/2.4/) and evaluated using of NCBI BLAST searches against fungal sequences existing in DNA databases (http://www.blast.ncbi.nlm.nih.gov/Blast.cgi).
Restriction fragment length polymorphism
All clinical yeasts were identified by restriction fragment length polymorphism patterns of PCR products.[13,14] MspI (HpaII) restriction enzyme (Fermentas, Vilnius, Lithuania) was used in this step of identification.
RESULTS
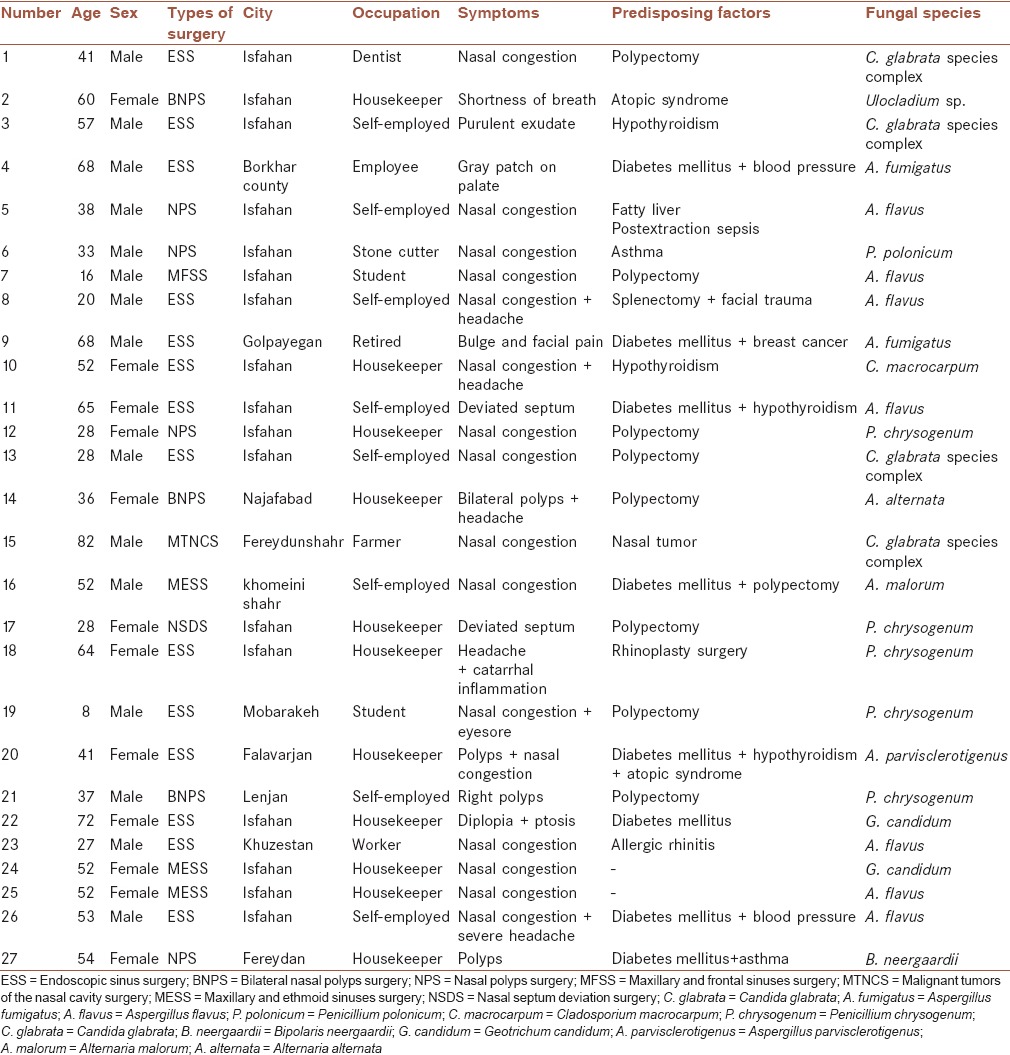
Fungal sinusitis was diagnosed for 27 out of 100 suspected cases (27%). The most patients were house keeper (41%) [Table 1]. Nasal congestion (n = 16; 59%) and headache (n = 5; 19%) were the most common clinical signs among patients [Table 1]. Fifteen patients (55.5%) were male and 12 patients (44.5%) were female. Polyps (33%), diabetes mellitus (30%), and hypothyroidism (15%) were the most risk factors among patients. Age range of patients was between 8 and 88 years, with a mean age of 46 years. Aspergillus flavus was the most prevalent fungal species (26%), followed by Penicillium chrysogenum (18.5%) and Candida glabrata species complex (15%). Table 1 summarizes characteristics of patients of the present study.
Table 1.
Details of patients with allergic fungal sinusitis and fungus ball in the present study
DISCUSSION
Fungal sinusitis is increasing worldwide in the past two decades. It is divided into two types including invasive and non-invasive. Invasive fungal sinusitis (IFS) represents acute, chronic, and granulomatous forms of infection. Many fungi are implicated containing: Zygomycetes (usually in diabetic patients), Aspergillus species (in neutropenic patients), Mucor spp., Rhizopus spp., Rhizomucor spp., and Lichtheimia spp. Noninvasive types contain AFS and fungus ball.[9] AFS is pathologically and clinically a specific form of rhinosinusitis in atopic immunocompetent people. It is most frequent among young individuals; the mean age of occurrence is about 21 years, whereas the mean age of patients in the present study was 46 years. Many studies reported that the male-to-female sex ratio is same;[15,16] however, in the present investigation, the sex ratio of infected individuals was nearly the same (15/12). Hypersensitivity reaction in the mucosa of the sinonasal tract, tissue edema, and reproduction of organisms intensifies inflammation that cause polyposis and chronic rhinosinusitis;[16] here, we found nine patients (33%) with polyposis in this study, too. This condition occurs with a wide variety of fungi such as Aspergillus species, Penicillium, Mucor, Rhizopus, and many phaeohyphomycetes.[17] Patients with AFS usually show signs of chronic sinusitis and allergic rhinitis containing purulent rhinorrhea, nasal obstruction, bony erosion, postnasal drainage, and headache. Pain is a rare sign; however, seven patients (26%) had made a complaint of headache, eyesore, and facial pain, in the present survey. Mucin in the AFS is plentiful, thick, firm, and adhesive. It has better appearance leading to the nonspecific radiographic findings of AFS on CT scans, i.e. heterogeneous areas within the PNS that are seen due to the collection of heavy metals such as manganese, iron, accompanied by calcium crystals in the inspissated mucin.[18] All PNS and nasal cavity are usually involved, with the maxillary and ethmoid sinuses being the most common.[15] In the present investigation, 15% of cases were diagnosed with the involvement of maxillary and ethmoid sinuses. In terms of histology, AFS has a typical appearance of “tree rings” or “tide lines” of mucin interchanging with inflammatory fragments, which reveals as alternating blue and pink lines. The debris is composed of aggregated neutrophils, eosinophils, and deteriorated eosinophils forming Charcot–Leyden crystals that are bipyramidal, refractile, and needle shaped.[3] Identification of fungal elements is complicated on hematoxylin and eosin staining and may be seen with PAS and Grocott's methenamine silver. For this reason, all clinical samples in this study were stained with PAS, and fungal hyphae were diagnosed clearly. If fungal elements are not seen using of culture and staining, the entity of AFS may be diagnosed as eosinophilic mucin rhinosinusitis.[19] Treatment of AFS is efficient with debridement of involved tissue, complete evacuation of inspissated mucus, and polypectomy. Table 1 show the different types of surgery were performed for each patient in the present study. Postoperative steroids’ therapy is usually recommended to decrease allergy, inflammation, and sensitization for long-term control, but recurrences are usual.[15,16,20] All patients are usually treated with intranasal steroid sprays, similar to the patients of this investigation. AFS is treated much differently than IFS and must be marked with discerning histopathologic features of IFS that is inflammatory cells within the tissue instead of free-floating in mucin, presence of fungal elements within the vascular spaces and tissues.[16,21] The fungus ball (mycetoma) is a different type of noninvasive fungal rhinosinusitis which is delineated as an accumulation of debris and fungal elements inside a PNS. Fungus ball of the PNS is characterized by a mass of fungal elements and fruiting heads of fungi and minimal host immune responses. It is always located in the maxillary sinus and sphenoid sinus, respectively.[22] Various fungal species have been isolated from fungus ball such as Aspergillus, Alternaria, Mucor, Penicillium, and Bipolaris.[22,23] Some risk factors have been suggested for fungus ball such as diabetes mellitus,[24] previous surgery on the sinonasal area,[25] estrogens,[26] and living in a rural area.[25] In the present study, we recognized a patient with fungus ball (patient no. 24) [Table 1]; however, she did not live in rural regions, and also she did not have any predisposing factor. The etiologic agent of fungus ball was Geotrichum candidum, which is yeast-like fungus with worldwide distribution. The pathogenicity of G. candidum in humans has not been obviously determined.[27] To the best of our knowledge, this is the first case of fungus ball owing to G. candidum reported in Iran. A. flavus was the most common species in the present study, whereas Marglani and Shaikh[9] reported Aspergillus terreus and Aspergillus fumigatus as the etiologic agent of AFS by phenotypic methods. Cavanna et al.[28] isolated a dematiaceous fungus as the Curvularia lunata from an immunocompetent male with allergic fungal rhinosinusitis. Similar to the present study, the fungus was identified by sequencing of internal transcribed spacer regions of fungal ribosomal DNA. Gupta et al.[29] reported a case of chronic rhinosinusitis with the involvement of orbit by Exserohilum rostratum in an immunocompetent child. In opposition to the present study, the fungus was only identified on the basis of morphological features such as direct microscopy, culture, and histopathology. Azar et al.[30] reported the frequency of various fungi using histological assays and culture from biopsy samples of patients with rhinosinusitis, in Khorramabad. Candida albicans, A. flavus, and A. fumigatus were the most common fungal species in their study. Nazeri et al.[31] showed A. flavus as the most prevalent causative agent of allergic fungal rhinosinusitis, chronic invasive type, and fungus ball. They also isolated a few infrequent fungal species such as Fusarium proliferatum and Schizophyllum commune from clinical samples based on PCR sequencing. Phenotypic methods were used in Jahromi and Khaksar in a retrospective investigation.[32] They reported A. flavus and C. albicans as predominant fungal species isolated from PNS.
CONCLUSION
Gene sequencing can be helpful for identification of etiologic agents of AFS and fungus ball and may be functional for discriminating of phaeohyphomycetes from other filamentous fungi, especially if nonsporulating molds that are fast growing at 37°C; fails to identify on general media with actidione. Since clinical manifestations, CT scan, endoscopy, and histopathological reactions are very nonspecific in AFS and fungus ball; therefore, molecular investigations are compulsory for precise identification of etiologic agents and appropriate management of these fungal infections.
Financial support and sponsorship
This investigation has been sponsored by Isfahan University of Medical Sciences, Isfahan, Iran (Thesis No. 395196).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Alzahra and Kashani Hospitals personnel for their cooperation.
REFERENCES
- 1.Katzenstein AL, Sale SR, Greenberger PA. Allergic Aspergillus sinusitis: A newly recognized form of sinusitis. J Allergy Clin Immunol. 1983;72:89–93. doi: 10.1016/0091-6749(83)90057-x. [DOI] [PubMed] [Google Scholar]
- 2.Manning SC, Merkel M, Kriesel K, Vuitch F, Marple B. Computed tomography and magnetic resonance diagnosis of allergic fungal sinusitis. Laryngoscope. 1997;107:170–6. doi: 10.1097/00005537-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Marple BF. Allergic fungal rhinosinusitis: Current theories and management strategies. Laryngoscope. 2001;111:1006–19. doi: 10.1097/00005537-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Grosjean P, Weber R. Fungus balls of the paranasal sinuses: A review. Eur Arch Otorhinolaryngol. 2007;264:461–70. doi: 10.1007/s00405-007-0281-5. [DOI] [PubMed] [Google Scholar]
- 5.Schubert MS. Allergic fungal sinusitis: Pathophysiology, diagnosis and management. Med Mycol. 2009;47(Suppl 1):S324–30. doi: 10.1080/13693780802314809. [DOI] [PubMed] [Google Scholar]
- 6.Lai JC, Lee HS, Chen MK, Tsai YL. Patient satisfaction and treatment outcome of fungus ball rhinosinusitis treated by functional endoscopic sinus surgery. Eur Arch Otorhinolaryngol. 2011;268:227–30. doi: 10.1007/s00405-010-1299-7. [DOI] [PubMed] [Google Scholar]
- 7.Nicolai P, Lombardi D, Tomenzoli D, Villaret AB, Piccioni M, Mensi M, et al. Fungus ball of the paranasal sinuses: Experience in 160 patients treated with endoscopic surgery. Laryngoscope. 2009;119:2275–9. doi: 10.1002/lary.20578. [DOI] [PubMed] [Google Scholar]
- 8.Al-Dousary SH. Allergic fungal sinusitis: Radiological and microbiological features of 59 cases. Ann Saudi Med. 2008;28:17–21. doi: 10.5144/0256-4947.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marglani O, Shaikh AM. Allergic fungal sinusitis eroding the pterygoid plates: A rare case series. Braz J Otorhinolaryngol. 2015;81:109–12. doi: 10.1016/j.bjorl.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stammberger H, Posawetz W. Functional endoscopic sinus surgery. Concept, indications and results of the Messerklinger technique. Eur Arch Otorhinolaryngol. 1990;247:63–76. doi: 10.1007/BF00183169. [DOI] [PubMed] [Google Scholar]
- 11.Silva GA, Bernardi TL, Schaker PD, Menegotto M, Valente P. Rapid yeast DNA extraction by boiling and freeze-thawing without using chemical reagents and DNA purification. Braz Arch Biol Technol. 2012;55:319–27. [Google Scholar]
- 12.Mohammadi R, Abastabar M, Mirhendi H, Badali H, Shadzi S, Chadeganipour M, et al. Use of restriction fragment length polymorphism to rapidly identify dermatophyte species related to dermatophytosis. Jundishapur J Microbiol. 2015;8:e17296. doi: 10.5812/jjm.8(5)2015.17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chadeganipour M, Mohammadi R. Causative agents of onychomycosis: A 7-year study. J Clin Lab Anal. 2016;30:1013–20. doi: 10.1002/jcla.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazdani MR, Foroughifar E, Mohammadi R. Identification of Candida species isolated from renal transplant recipients with candiduria. Int J Organ Transplant Med. 2016;7:206–11. [PMC free article] [PubMed] [Google Scholar]
- 15.Correll DP, Luzi SA, Nelson BL. Allergic fungal sinusitis. Head Neck Pathol. 2015;9:488–91. doi: 10.1007/s12105-014-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huchton DM, editor. Allergic fungal sinusitis: An otorhinolaryngologic perspective. Allergy and Asthma Proceedings. USA: OceanSide Publications, Inc; 2003. [PubMed] [Google Scholar]
- 17.Arsenijevic VA, Barac A, Pekmezovic M, Stosovic R, Pender I. Allergic fungal sinusitis – New aspects of clinical features, laboratory diagnosis and therapy. Srp Arh Celok Lek. 2013;141:698–704. doi: 10.2298/sarh1310698a. [DOI] [PubMed] [Google Scholar]
- 18.deShazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Swain R, Lyons M, et al. Criteria for the diagnosis of sinus mycetoma. J Allergy Clin Immunol. 1997;99:475–85. doi: 10.1016/s0091-6749(97)70073-3. [DOI] [PubMed] [Google Scholar]
- 19.Thompson LD, Wenig BM. Diagnostic Pathology: Head and Neck. Amirsys: Elsevier Health Sciences; 2016. [Google Scholar]
- 20.Zeid NG, Mohammed AS, El-Fouly ME, El-Essawy MS. The relationship between systemic strioid therapy and the level of serum IgE in allergic fungal rhinosinusitis. Zagazig Univ Med J. 2016;22:250–7. [Google Scholar]
- 21.Driemel O, Wagner C, Hurrass S, Müller-Richter U, Kühnel T, Reichert TE, et al. Allergic fungal sinusitis, fungus ball and invasive sinonasal mycosis – Three fungal-related diseases. Mund Kiefer Gesichtschir. 2007;11:153–9. doi: 10.1007/s10006-007-0058-4. [DOI] [PubMed] [Google Scholar]
- 22.Lop-Gros J, Gras-Cabrerizo JR, Bothe-González C, Montserrat-Gili JR, Sumarroca-Trouboul A, Massegur-Solench H. Fungus ball of the paranasal sinuses: Analysis of our serie of patients. Acta Otorrinolaringol Esp. 2016;67:220–5. doi: 10.1016/j.otorri.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25:195–202. doi: 10.1016/j.clindermatol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Chusakul S. Is high blood sugar a risk factor for paranasal sinus fungus ball? Otolaryngol Head Neck Surg. 2013;149(2 Suppl):P261. [Google Scholar]
- 25.Nomura K, Asaka D, Nakayama T, Okushi T, Matsuwaki Y, Yoshimura T, et al. Sinus fungus ball in the Japanese population: Clinical and imaging characteristics of 104 cases. Int J Otolaryngol 2013. 2013:731640. doi: 10.1155/2013/731640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chobillon MA, Jankowski R. What are the advantages of the endoscopic canine fossa approach in treating maxillary sinus aspergillomas? Rhinology. 2004;42:230–5. [PubMed] [Google Scholar]
- 27.Yegneswaran Prakash P, Seetaramaiah VK, Thomas J, Khanna V, Rao SP. Renal fungal bezoar owing to Geotrichum candidum. Med Mycol Case Rep. 2012;1:63–5. doi: 10.1016/j.mmcr.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavanna C, Seminari E, Pusateri A, Mangione F, Lallitto F, Esposto MC, et al. Allergic fungal rhinosinusitis due to Curvularia lunata. New Microbiol. 2014;37:241–5. [PubMed] [Google Scholar]
- 29.Gupta A, Xess I, Sharma SC, Mallick S. Invasive rhinosinusitis by Exserohilum rostratum in an immunocompetent child. BMJ Case Rep 2014. 2014 doi: 10.1136/bcr-2013-202380. pii: Bcr2013202380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azar S, Mansour B, Parivash K, Babak B. Fungal rhinosinusitis in hospitalized patients in Khorramabad, Iran. Middle East J Sci Res. 2011;7:387–91. [Google Scholar]
- 31.Nazeri M, Hashemi SJ, Ardehali M, Rezaei S, Seyedmousavi S, Zareei M, et al. Fungal rhino sinusitisin in Tehran, Iran. Iran J Public Health. 2015;44:374–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Jahromi SB, Khaksar AA. Paranasal sinus mycosis in suspected fungal sinusitis. Arch Clin Infect Dis. 2006;1:25–9. [Google Scholar]


