Abstract
Background:
Polycystic ovary syndrome (PCOS) is the most common female endocrine disorder with unknown etiology and with different complications. The aim of this study was to evaluate the effect of omega-3 supplementation on PCOS symptoms and metabolic syndrome.
Materials and Methods:
This double-blind clinical trial was performed in 2015 in Alzahra and Shahid Beheshti Hospitals, Isfahan, Iran, on 88 patients with PCOS. Intervention group took omega-3 supplements with dose of 2 g/day for 6 months (two capsules), but control group received two olive oil capsules. Finally, ultrasound and laboratory findings and the recovery rate of menstrual disorders in both groups were compared.
Results:
After 6 months’ intervention, waist circumference (WC) was significantly lower in omega-3 as compared to control (81.18 ± 2.87 vs. 84.22 ± 2.61 cm, respectively, P < 0.0001). High-density lipoprotein was increased (47.2 ± 1.37 vs. 41.56 ± 1.34 mg/dl, respectively, P < 0.0001) while low-density lipoprotein (107.79 ± 1.68 vs. 117.4 ± 1.57 mg/dl, respectively), triglyceride (116.02 ± 3.13 vs. 125.06 ± 2.91 mg/dl, respectively), and cholesterol (180.34 ± 6.34 vs. 189.56 ± 5.93 mg/dl, respectively) in omega-3 were significantly lower than control (P < 0.0001). The interval between periods in omega-3 was significantly shorter than control (29.83 ± 4.68 vs. 47.11 ± 8.72 days, respectively, P < 0.001).
Conclusion:
Omega-3 decrease lipid profiles, WC, and interval between periods while weight, hip circumference, fasting blood sugar, number of ovarian follicle, size of ovary, bleeding volume, menstrual bleeding, and hirsutism score did not change by administration of omega-3.
Keywords: Metabolic syndrome, omega-3, polycystic ovary syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common female endocrine disorder.[1] The prevalence of PCOS in the general population varies according to the diagnostic criteria between 5% and 18%[2,3] and in reproductive age women is between 6% and 10%.[1,4] The estimated prevalence of PCOS in Iran was 7% based on the National Institutes of Health (NIH) criteria, 15.2% under the Rotterdam criteria, and 7.92% according to the AES criteria.[5]
PCOS can be associated with varying effects on a person's life, and these women have many of the established risk factors (include lack of progesterone, irregular menses, unopposed estrogen exposure, obesity, insulin resistance, and diabetes) for endometrial cancer and endometrial hyperplasia.[6] The main complications of the disease in adolescence are the incidence of amenorrhea, oligomenorrhea, hirsutism, obesity, and acne. In fertility ages, the patient's hief complaint is infertility and irregular ovulation.[7] The risk for endometrial cancer in women almost threefold increased in the presence of PCOS.[8]
The pathogenesis of PCOS is not known, both genetic and lifestyle factors contribute to the development of the PCOS phenotype. Reproductive disorders such as irregular menstrual cycle, chronic anovulation, and hyperandrogenism manifestation as hirsutism and infertility and metabolic disorders have evaluated as potential contributors.[9] Metabolic syndrome is more frequent in these patients than general population, and 33%–50% of US women with PCOS have metabolic syndrome compared with only 12% in a similarly aged in general population.[10] Furthermore, in these women, dyslipidemia is more prevalent compared with controls.[11]
The successful treatment in women with PCOS is lifestyle and nutritional interventions along with weight loss.[12,13] Metabolic disorders of PCOS may have improved in the presence of dietary factors such as anti-inflammatory foods. Among dietary factors, omega-3 is known off fatty acids that has an important role in immune regulation, insulin sensitivity, cellular differentiation, and ovulation. It reduces the synthesis of prostaglandins through competitive inhibition of cyclooxygenase 2 (COX-2) as well as competing with arachidonic acid as the substrate for COX-2.[14] Fish oil feeding reduces cholesterol absorption and low-density lipoprotein-cholesterol (LDL-C) synthesis, improves LDL receptor activity in liver, and increases fractional rate of catabolism of LDL-C.[15,16] Moreover, omega-3 fatty acid supplementation had a beneficial effect on some cardiometabolic risk factors in women with PCOS.[17]
Previous studies have evaluated the effect of omega-3 fatty acids in women with PCOS and reported different findings. Results of a meta-analysis of randomized controlled trials reported that supplementation with omega-3 fatty acids may not have a beneficial effect on improving insulin resistance in women with PCOS.[18] Other randomized controlled clinical trial showed that omega-3 fatty acids may contribute to the improvement of metabolic complications and had some beneficial effects on serum adiponectin levels, insulin resistance, and lipid profile in patients with PCOS.[19] Some other studies have different findings, and there are controversial results in studies on patients with PCOS. On the other hand, data about the effect of omega-3 on menstrual status are limited, so the study was designed to evaluate effectiveness of omega-3 supplementation on metabolic syndrome and symptoms in women with PCOS.
MATERIALS AND METHODS
This double-blind clinical trial was conducted from August to December 2015. Patients, who referred to Obstetrics and Gynecology Department of Alzahra and Shahid Beheshti Hospitals, Isfahan, Iran, with diagnosis of PCOS based on the NIH criteria,[20] were enrolled in the study. Women between 25 and 40 years old were eligible if they were not menopause, did not consumed omega-3 in the last 3 months or followed a special diet, did not used tobaccos, and did not have diabetes or hypothyroidism. Furthermore, patients were not eligible if they consuming contraceptive, were pregnant, had kidney disease, liver disease, cardiovascular disorders, hyperprolactinemia, Cushing syndrome, and congenital adrenal hyperplasia. Infertile women if they did not receive any treatment and did not infertility treatment plans in the next 6 months were entering to the study. Patients were excluded from the study if they need treatment with oral conceptive or did not desire to continue. This study approved by the Ethics Committee of Isfahan University of Medical Sciences (394057), and all participants signed written informed consent.
Eligible patients were randomly divided into two 44-member groups of case and control, using random-maker software “random allocation.” Patients in case group received omega-3 supplements (with mercury free from Zahravi Construction Company) with dose of 2 g/day for 6 months (each capsule 1000 g) which contains 180 g eicosapentaenoic acid and 120 mg docosahexaenoic acid. Patients in control group received two olive oil capsules (Barij Esans Construction Company).
A checklist was completed for each participant. This checklist contained symptoms of hyperandrogenism and hyperinsulinemia, and age, sex, weight without shoes with wearing light clothing, height without shoes, body mass indices (BMIs), number of children, waist circumference (WC) and hip circumference (HipC) (taken with a soft tape in standing position, waist being defined as the narrowest circumference between the costal margin and the iliac crest and hip as the widest circumference between the waist and tight), size of ovary, and the number of ovarian follicle were measured by the same ultrasound device with the same operator. Bleeding volume, the number of interval and bleeding duration, fasting blood sugar (FBS), and lipid profile, triglyceride (TG), cholesterol, LDL, and high-density lipoprotein (HDL) were measured using the standard methods in all participants. All variables were measured before and after treatment periods. All patients in both studied groups were asked to maintain their usual dietary intakes and physical activity during study period and reported any change in their physical activity (cut or increasing the regular physical activity) and nutritional status (cut or increasing a special diet).
The sample size was calculated using the comparison of means formula with α =0.05 and 80% power for the level of cholesterol; the values used for sample calculation based on a study by Mohammadi et al.[19] were as follows: difference in the mean of cholesterol level between groups was 17.5 mg/dL and standard deviation for groups was 25.9 and 32 mg/dL. Statistical analysis of data was performed using SPSS software for windows, version 22 (SPSS, Inc., Chicago, IL, USA). To compare qualitative variables between groups, Chi-square test was performed. The normal distribution of all studied parameters was checked with Kolmogorov–Smirnov test. Student's t-test was used for variables which were distributed in a normal way; besides, Mann–Whitney test was performed for variables that have not normal distribution. P < 0.05 was considered significant.
RESULTS
Figure 1 shows the study flowchart. Ninety-four patients were screened for eligibility, two patients were not eligible (one was pregnant and one consumed omega-3 1 month before the study), and four patients did not desire to participate and refused informed consent. Eighty-eight eligible patients were randomly assigned in two omega-3 or control groups and followed for 6 months. During follow-up, one patient changed her usual dietary and followed a special diet so was excluded from the study. Finally, 43 patients in omega-3 group and 44 patients in control groups completed the study and were analyzed.
Figure 1.
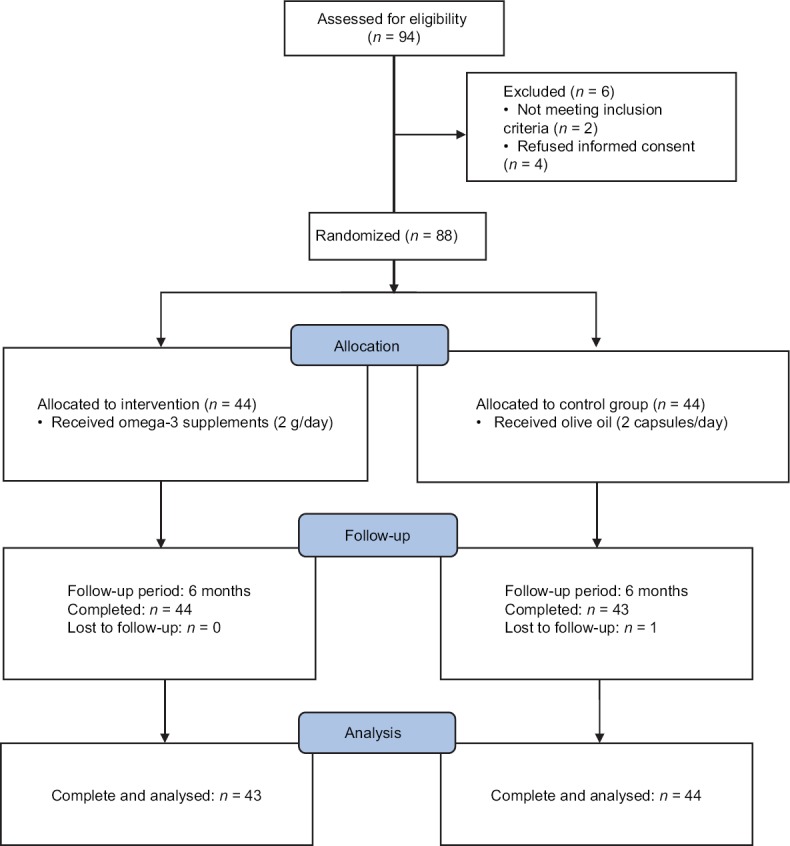
Study flowchart
Table 1 shows the characteristics of the studied patients. The mean age of patients in omega-3 group was 31 years and in control group was 29.23 years (P = 0.16). The number of children between groups was not significantly different (P = 0.86). Fourteen patients in omega-3 group were employed, and 17 patients in control group were employed (P = 0.73). Twelve of all studied patients were infertile, seven in omega-3 group and five in control group (P = 0.51). Furthermore, having regular physical activity was not significantly different between groups.
Table 1.
Baseline characteristics in the study patients by groups
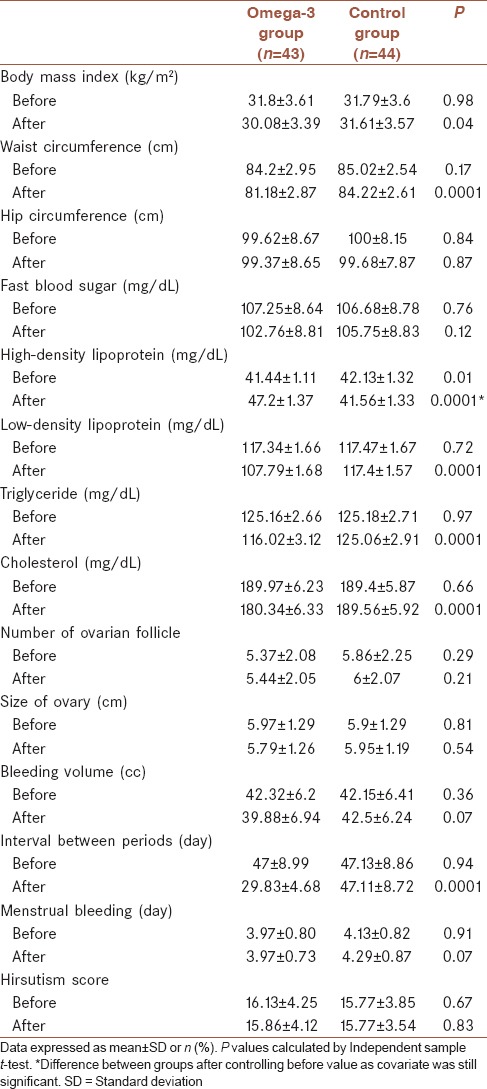
Table 2 shows the comparison of studied variables between case and control groups before and after treatment. Before treatment, all variables between groups were similar, and no significant differences were noted between two groups (P > 0.05) and only HDL between groups was significantly different (P = 0.01). After 6 months’ intervention, WC was significantly lower in omega-3 group (81.18 ± 2.87) as compared to control group (84.22 ± 2.61) (P < 0.0001). Moreover, lipid profiles significantly changed in omega-3 group. HDL was significantly lower in omega-3 group than control group (47.2 ± 1.37 mg/dl versus 41.56 ± 1.34 mg/dl, respectively, P < 0.0001), difference between groups after controlling baseline values as covariate was still significant. LDL was significantly lower in omega-3 group than control group (107.79 ± 1.68 mg/dl versus 117.4 ± 1.57 mg/dl, respectively, P < 0.0001). Also, TG (116.02 mg/dl in omega-3 group vs. 125.06 mg/dl in control group, P < 0.0001) and cholesterol (180.34 mg/dl in omega-3 group vs. 189.56 mg/dl in control group, P < 0.0001) in omega-3 group were significantly lower than control group. Furthermore, we found that regularity of periods becomes much shorter in omega-3 group compare to control group (29.83 ± 4.68 vs. 47.11 ± 8.72 days, respectively, P < 0.0001). BMI was significantly lower in omega-3 group than control group (P = 0.044) while HipC (P = 0.87), FBS (P = 0.12), number of ovarian follicle (P = 0.21), size of ovary (P = 0.54), bleeding volume (P = 0.07), menstrual bleeding duration (P = 0.07), and hirsutism score (P = 0.83) were similar in both groups and were statistically not significant.
Table 2.
Comparison of studied variables between groups before and after treatment
DISCUSSION
Results of the present study show that 6 months’ treatment with omega-3 in women with PCOS improved the values of WC, HDL, LDL, TG, and regularity of periods in compared to control group. However, no significant changes were observed in weight, HipC, FBS, number of ovarian follicle, size of ovary, bleeding volume, menstrual bleeding, and hirsutism score between intervention and control groups after intervention.
Previous studies in patients with PCOS had similar findings for anthropometric measurements. Cussons et al. studied the effect of omega-3 supplementation in patients with PCOS with nonalcoholic fatty liver disease and found that BMI and waist to hip ratio after 8 weeks’ treatment by omega-3 (4 g/day) were not significantly different from control group.[17] In other study, Vargas et al. found that 6 weeks’ supplementation with 3.5 g/day fish oil in patients with PCOS did not significantly change weight, BMI, and WC in compare to control group.[21] Results of Hajianfar et al’s. study showed that consumption of fish oil reduces BMI, WC, and waist to hip ratio in women with type 2 diabetes compared with the placebo group.[22] In the present study like these studies, most of anthropometric measurements did not significantly changed after study, but BMI in our study in omega-3 group was significantly decreased after treatment in compare to control group, which was different from the results of the previous studies. This controversy can be explained by the differences between studies in regard to studied population and treatment period, whereas Cussons et al.[17] studied population were patients with PCOS with nonalcoholic fatty liver disease with 8 weeks’ treatment, Hajianfar et al’s.[22] study were patients with PCOS with type 2 diabetes, and Vargas et al’s.[21] study treatment period was 6 weeks; in contrast in our study, patients with PCOS without type 2 diabetes or nonalcoholic fatty liver disease were treated for 6 months.
Serum lipid values in patients with PCOS in our study after 6 months’ supplementation with omega-3 significantly reduced when compared to control group. Some previous studies showed a significant decrease in serum TG levels in patients with PCOS after supplementation with fish oil which was similar to our results.[17,19,22,23,24] These findings show the effect of fish oil on TG levels in patients with PCOS, whereas it was shown that effect of fish oil on TG levels is dose dependent.[23] The effect of omega-3 in patients with PCOS on other serum lipid values reported differently, some studies reported no significant differences in the level of serum TC, LDL, and HDL concentrations after intervention in compare to control group,[17,21] other study by Mohammadi et al.[19] showed that cholesterol and LDL decreased after administration of omega-3, while HDL and TG did not change. There were some similarity and differences between the present study results and other studies findings. Dose of omega-3 fatty acids, duration of intervention period, and differences in studied patients can be noted as possible reasons for controversy between results.
Marshall Keri et al. reported that women with PCOS have clinical problems such as disruption of menstrual cycle, infertility, and hirsutism. They mentioned that if the patients were not treated well, they would be prone to type 2 diabetes, cardiovascular disease, and hyperestrogen-related cancer.[24] All the participants in our study had irregular period. While after the trial, the percentage of regularity in menstrual status in the omega-3 group was significantly higher. We found that just interval between periods decreased. These results were similar to Nadjarzadeh et al’s. study.[24] They show that after 8 weeks’ treatment with omega-3, the percentage of regular menstruation in the omega-3 group was significantly more than the placebo group. The effect omega-3 on regular menstruation can be explained by the decreased in testosterone concentration, whereas in Nadjarzadeh et al’s. study,[24] testosterone concentration was significantly decreased after 8-week use of omega-3 in compare to placebo.
The main limitation of the present study can be that the information about daily energy intake and physical activity as two main interventions in women with PCOS was not collected before and after intervention. However, patients in the present study were asked to maintain their usual dietary intakes and physical activity during study period and reported any change, but in the lack of this information, we could not the possible effects of nutritional status and physical activity between studied groups.
CONCLUSION
Results of the present study showed that, administration of omega-3 decrease lipid profiles, decrease WC, and interval between periods. But weight, HipC, FBS, number of ovarian follicle, size of ovary, bleeding volume, menstrual bleeding, and hirsutism score did not change after administration of omega-3. These data suggest that omega-3 by improving metabolic parameters can make menstrual status regular.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgments
This study was financially supported by Isfahan University of Medical Sciences. We gratefully acknowledge the dedicated efforts of the investigators, the coordinators, the volunteer patients who participated in this study, and the Clinical Research Development Units of Isfahan Alzahra hospital.
REFERENCES
- 1.Baptiste CG, Battista MC, Trottier A, Baillargeon JP. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2010;122:42–52. doi: 10.1016/j.jsbmb.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelley CE, Brown AJ, Diehl AM, Setji TL. Review of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. stroenterol. 2014;20:14172–84. doi: 10.3748/wjg.v20.i39.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González F, Kirwan JP, Rote NS, Minium J. Glucose ingestion stimulates atherothrombotic inflammation in polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2013;304:E375–83. doi: 10.1152/ajpendo.00491.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khani B, Mehrabian F, Khalesi E, Eshraghi A. Effect of soy phytoestrogen on metabolic and hormonal disturbance of women with polycystic ovary syndrome. J Res Med Sci. 2011;16:297–302. [PMC free article] [PubMed] [Google Scholar]
- 5.Mehrabian F, Khani B, Kelishadi R, Ghanbari E. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol. 2011;62:238–42. [PubMed] [Google Scholar]
- 6.Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: A systematic review. Reprod Biomed Online. 2009;19:398–405. doi: 10.1016/s1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- 7.Haji Shafiha M, Zabiri T, Salari Lak SH. Investigating validity criteria of vaginal ultrasound (ovarian volume, the ovarian stroma and the stromal surface of the ovary) in the diagnosis of polycystic ovary syndrome. Urmia Med J. 2007;3:538–43. [Google Scholar]
- 8.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–92. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril. 2002;77:1095–105. doi: 10.1016/s0015-0282(02)03111-4. [DOI] [PubMed] [Google Scholar]
- 10.Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–15. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 11.Lo Jc, Feigenbaum Sl, Yang J, Pressman Ar, Selby Jv, Go As. Epidemiology And Adverse Cardiovascular Risk Profile Of Diagnosed Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2006;91:1357–63. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 12.Phelan N, O’Connor A, Kyaw Tun T, Correia N, Boran G, Roche HM, et al. Hormonal and metabolic effects of polyunsaturated fatty acids in young women with polycystic ovary syndrome: Results from a cross-sectional analysis and a randomized, placebo-controlled, crossover trial. Am J Clin Nutr. 2011;93:652–62. doi: 10.3945/ajcn.110.005538. [DOI] [PubMed] [Google Scholar]
- 13.Rafraf M, Mohammadi E, Asghari-Jafarabadi M, Farzadi L. Omega-3 fatty acids improve glucose metabolism without effects on obesity values and serum visfatin levels in women with polycystic ovary syndrome. J Am Coll Nutr. 2012;31:361–8. doi: 10.1080/07315724.2012.10720443. [DOI] [PubMed] [Google Scholar]
- 14.Hurst S, Curtis CL, Rees SG, Harwood JL, Caterson B. Effects of n-3 polyunsaturated fatty acids on COX-2 and PGE2 protein levels in articular cartilage chondrocytes. Int J Exp Pathol. 2004;85:A22–3. [Google Scholar]
- 15.Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol. 2006;98:27i–33i. doi: 10.1016/j.amjcard.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Nestel PJ. Fish oil and cardiovascular disease: Lipids and arterial function. Am J Clin Nutr. 2000;71(1 Suppl):228S–31S. doi: 10.1093/ajcn/71.1.228S. [DOI] [PubMed] [Google Scholar]
- 17.Cussons AJ, Watts GF, Mori TA, Stuckey BG. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: A randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab. 2009;94:3842–8. doi: 10.1210/jc.2009-0870. [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi A, Djafarian K, Mohammadi H, Shab-Bidar S. Effect of omega-3 fatty acids supplementation on insulin resistance in women with polycystic ovary syndrome: Meta-analysis of randomized controlled trials. Diabetes Metab Syndr. 2016 doi: 10.1016/j.dsx.2016.06.025. pii: S1871-402130093-5. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi E, Rafraf M, Farzadi L, Asghari-Jafarabadi M, Sabour S. Effects of omega-3 fatty acids supplementation on serum adiponectin levels and some metabolic risk factors in women with polycystic ovary syndrome. Asia Pac J Clin Nutr. 2012;21:511–8. [PubMed] [Google Scholar]
- 20.Azziz R. Diagnostic criteria for polycystic ovary syndrome: A reappraisal. Fertil Steril. 2005;83:1343–6. doi: 10.1016/j.fertnstert.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 21.Vargas ML, Almario RU, Buchan W, Kim K, Karakas SE. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metabolism. 2011;60:1711–8. doi: 10.1016/j.metabol.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajianfar H, Hosseinzadeh MJ, Bahonar A, Mohammad K, Askari GR, Entezari MH, et al. The effect of omega-3 on the serum visfatin concentration in patients with type II diabetes. J Res Med Sci. 2011;16:490–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr. 2002;76:1007–15. doi: 10.1093/ajcn/76.5.1007. [DOI] [PubMed] [Google Scholar]
- 24.Nadjarzadeh A, Dehghani Firouzabadi R, Vaziri N, Daneshbodi H, Lotfi MH, Mozaffari-Khosravi H. The effect of omega-3 supplementation on androgen profile and menstrual status in women with polycystic ovary syndrome: A randomized clinical trial. Iran J Reprod Med. 2013;11:665–72. [PMC free article] [PubMed] [Google Scholar]


