Abstract
There is currently very little research regarding the dynamics of the subcellular degenerative events that occur in the central nervous system in response to injury. To date, multi-photon excitation has been primarily used for imaging applications; however, it has been recently used to selectively disrupt neural structures in living animals. However, understanding the complicated processes and the essential underlying molecular pathways involved in these dynamic events is necessary for studying the underlying process that promotes neuronal regeneration. In this study, we introduced a novel method allowing in vivo use of low energy (less than 30 mW) two-photon nanosurgery to selectively disrupt individual dendrites, axons, and dendritic spines in the murine brain and spinal cord to accurately monitor the time-lapse changes in the injured neuronal structures. Individual axons, dendrites, and dendritic spines in the brain and spinal cord were successfully ablated and in vivo imaging revealed the time-lapse alterations in these structures in response to the two-photon nanosurgery induced lesion. The energy (less than 30 mW) used in this study was very low and caused no observable additional damage in the neuronal sub-structures that occur frequently, especially in dendritic spines, with current commonly used methods using high energy levels. In addition, our approach includes the option of monitoring the time-varying dynamics to control the degree of lesion. The method presented here may be used to provide new insight into the growth of axons and dendrites in response to acute injury.
Keywords: nerve regeneration, dendrite, dendritic spine, axon, spinal cord, two-photon nanosurgery, single-synapse resolution, neural regeneration
Introduction
Two-photon microscopy has been widely used in the fields of neurobiology, embryology, physiology, and tissue engineering due to its high resolution (Masters et al., 1997), efficient light detection, increased tissue penetration (Denk et al., 1990). When combined with fluorescent protein expression methods, two-photon microscopy has been used to monitor the plasticity and activity of specific neurons (Chen et al., 2011), particularly cortical neurons (Helmchen and Denk, 2005) in living mice. In addition to the wide range of applications in imaging, multi-photon absorption was also considered a tool for selectively disrupting intracellular structures (Konig et al., 1999; Sacconi et al., 2005). In combination with laser-induced lesions, two-photon microscopy has also been applied in vivo (Galbraith and Terasaki, 2003; Nishimura et al., 2006), ablating or dissecting individual neurons. To date, multiphoton nanosurgery has been used to determine the role of specific neurons in animal behavior (Chung et al., 2006) and to study axon regeneration in worms (Yanik et al., 2004). When combined with in vivo imaging, this technique helped to record detailed remodeling processes and highlighted the cellular adaptive structural remodeling in response to injury.
It is well known that function of the nervous system relies on both axon and dendrite integrity through the pool of neurons. The ability of adult neurons in the central nervous system to regenerate damaged axons in response to injury is limited due to both intrinsic and extrinsic factors (Rossi et al., 1991; Horner and Gage, 2000; Snider et al., 2002; Kerschensteiner et al., 2005; Hawthorne et al., 2011; Tuszynski and Steward, 2012). Although a great deal of efforts have been made to counteract these factors, direct observation of axon degeneration and regeneration has remained elusive due to the lack of information regarding axonal dynamics and plasticity within the mammalian central nervous system, hampering the effectiveness of current therapeutic strategies.
Dendrites exert essential roles in the integration of synaptic inputs and in determining the scope of the action potentials generated by the neuron (Urbanska et al., 2008). The morphological alterations in the dendritic spines were considered to correlate with the underlying cognitive functions (Yuste and Bonhoeffer, 2001) in both healthy and damaged systems (Tavosanis, 2012). To date, it is not known whether mature neurons are capable of detecting the dendrite injury and responding by initiating the regenerative process (Stone et al., 2014). While some studies using cultured neurons have shown that dendrites can be regrown in vitro (Barnes and Polleux, 2009), they lacked the environmental context of in vivo reality.
All in all, investigating the response to varying degrees of injury in individual neurons may be essential for understanding the overall general trends of neuronal regeneration, and may contribute to establishing therapeutic strategies for neurological disorders involving dendritic injury. With this in mind, in the present study, we aimed to build an in vivo model to accurately and efficiently monitor axon and dendrite regeneration following precise lesions using low energy (less than 30 mW) two-photon nanosurgery.
Materials and Methods
Animals
All experimental and surgical procedures were approved by the Laboratory Animal Ethics Committee at Jinan University, China (approval number: 20111008001). One-month-old male mice (003782-B6.Cg-Tg (Thy1-YFP) HJrs) expressing yellow fluorescent protein (YFP) in pyramidal cells predominantly in cortical layer V were purchased from Jackson Laboratory (Bar Harbor, ME USA) (YFP-H line) (Feng et al., 2000). The animals were kept on a 12-hour light/dark cycle with ad libitum access to food and water. The temperature of the animal holding area was kept at 21 ± 2°C, and the humidity at 50–60%. After imaging, the surgical wounds were sutured and the animals were allowed to recover at 37°C after imaging. All efforts were made to minimize the suffering of animals and to reduce the number of animals used in the experiments.
Preparations for procedures on the brain
To selectively disrupt individual dendrites, spines, and axons in the brain, a thinned-skull preparation was used as previously described (Grutzendler et al., 2002; Zuo et al., 2005).
One-month-old mice (YFP-H line) were anesthetized with 1.25% avertin (intraperitoneal injection, 20 μL/g). The head of the mouse was fixed using a head holder (Narishige STS-A SG-4N) and the tail was clamped (RWD-68091) to avoid the impact of movement due to breathing when imaging (Figure 1A).
Figure 1.
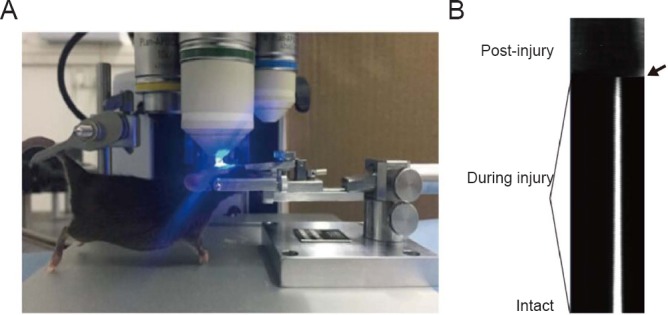
The basic setup for nanosurgery.
(A) The imaging setup consists of the schematic optical instrument and an anesthetized mouse with a thinned-skull window. (B) Line-scan time series images indicated the processes of dissecting before, during and after lesion. The arrow indicates the scan stop point.
An approximately 1 cm in length incision of the skin/scalp was cut using a surgical scalpel to expose the skull. Thinned-skull windows were generated using a high-speed micro drill, with a diameter of 200 μm. Further grinding of the skull, using a fine microsurgical blade, brought the bone in its thinnest area to a thickness of 20 μm.
The vascular topography was mapped using a charge-coupled device (Leica DFC 7000T, Solms, Germany) (Yang et al., 2009) and used as a guide for selecting the same region in the subsequent imaging sessions. The image area located in the motor cortex is 1.3 mm anterior to the bregma and 1.2 mm lateral from the midline (Yang et al., 2009).
Preparations for spinal cord procedures
To selectively disrupt individual axons in the spinal cord, the spinal cord window set up was based on a previously described protocol (Figley et al., 2013), and was performed with the following modifications: 1) The segments of the spinal cord were identified starting with the T2 as a reference, as its protruding spine can be easily identified (Ding et al., 2014). 2) An incision measuring ~1.5 cm in length was made into the skin and muscle, and the tissue layers were retracted to expose the lamina. 3) A laminectomy was performed on spinal vertebrae from segments C5 to T1 and the corresponding spinal cord segments were exposed. For each imaging session, the C4 and T2 vertebrae and the tail were affixed to an adaptor (RWD-68091) and the animal was lifted to minimize the effect of breathing on image capture (Kerschensteiner et al., 2005).
In vivo two-photon nanosurgery
A two-photon microscope (Zeiss LM780, Oberkochen, Germany) was equipped with a Ti:Sapphire laser source (120 fs width pulses, 90 MHz repetition rate). The two-photon excitation wavelength was set to 920 nm. Imaging was achieved using 1.2 numerical aperture × 20 water-immersion objectives.
The immobilized mice were placed under the microscope (Figure 1A). The frame imaging module (Zen, 2012) was used to select a larger operating range (200 × 200 μm2). Using the 3D crop option, the target dendrites, dendritic spines or axons were chosen to be disrupted. Typically, brighter ones were selected. The “linescan” module was selected to program the specific position to disrupt. The selected line position was repeatedly dissected by the laser using lower energy (less than 30 mW). A time series module was used to dissect the line position for thousands of times. Typically, the frequency of scan was 500 Hz, and for dissecting the dendrite and axon, 10,000 times was chosen; for dissecting the dendritic spine, 1,000 times was needed.
As shown in Figure 1B, the resulting line scan is an image of spacetime, typically displayed with the individual scan line accumulating to the top. The fluorescence of each line represented the scanned target at a time. The degree of injury was monitored when dissecting based on the time-lapse images, the diameter of targeted position became smaller with increasing times for scanning and the fluorescence would disappear once the dissecting succeed (indicated by the arrow in Figure 1B). Once the dissecting succeeded, the scan should be stopped to avoid the excessive damage.
The in vivo images were captured at the same position at different time points after injury. The same set-up was used to image the same position at different time points after injury.
Image processing
Z-stack images (view scope was 100 × 100 μm2, at 512 × 512 pixels, 0.75 μm z-axis step) were acquired to obtain a 3D reconstructions of the targeted structures. 3D-stacks were analyzed using ImageJ software (version 1.48; National Institutes of Health, Bethesda, MD, USA).
Results
Dendrite ablation in the brain
Time-lapse morphological alterations were monitored for the dendrite disrupted by two-photon nano-surgery. In vivo imaging revealed that a single injured dendrite was completely ablated, and although the proximal end remained stable, the distal end began to rapidly fragment, contributing to the formation of a bulb, and the interval between the proximal and distal ends increased over-time after injury (Figure 2).
Figure 2.
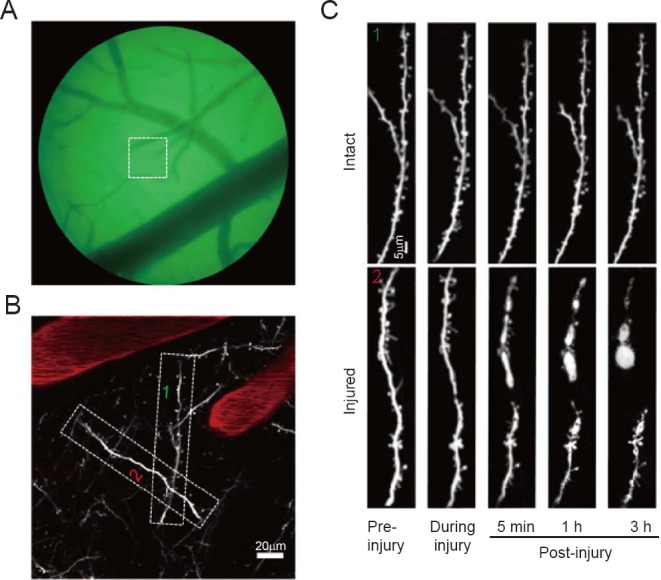
Time-lapse images, showing changes in a single dendrite in the mouse brain after a laser-induced disruption.
(A) Representative immunofluorescence images under the thin-skull window taken under the microscope with a charge-coupled device. (B) Two-photon image of view from the boxed region in A. Selected dissected area images, with rhodamine-dextran stained vessels shown in red. 1 represents the intact dendrite and 2 represents the injured one. (C) Detailed view of proximal and distal dendrite ends of another dendrite after lesion, illustrating bulb formation (proximal and distal) before, during, 5 minutes (min), 1 hour (h) and 3 h after lesion.
Dendritic spine nanosurgery in the brain
Time-lapse morphological alterations of dendritic spine were monitored after two-photon nanosurgery. The dendritic spine was ablated and the proximal end was still fully separated from the distal end after 5 minutes, leaving a singular bulb in the distal end. One hour after surgery, the proximal end attempted to reconnect with the distal bulb by forming a new intermediary structure, however, the singular bulb had disappeared by the 3rd hour, and no new structure was observed 6 hours after nano-surgery (Figure 3).
Figure 3.
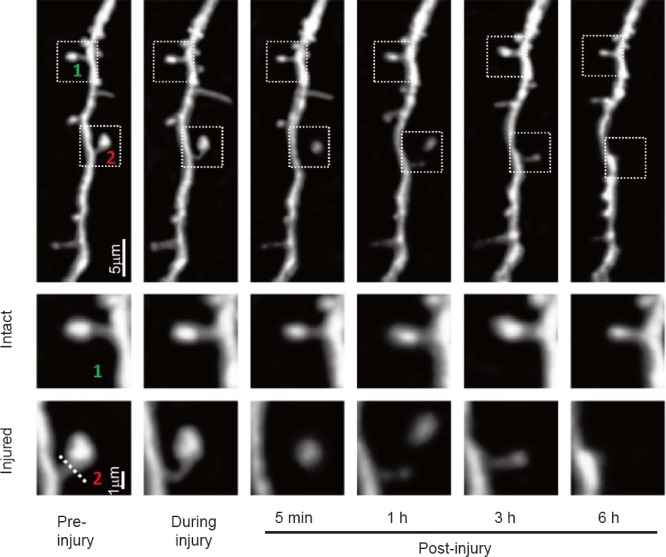
Determination of time-lapse alterations in response to a laser-induced disruption in a single dendritic spine from the mouse brain.
Time-varying representative images of dendrite where the dendritic spines were disrupted in the first row. Two-photon images of the (1) intact and (2) injured dendritic spines from the boxed region in the first row were taken before, during, 5 minutes (min), 1 hour (h), 3 h and 6 h after lesion, with bulb formation.
Nanosurgical axon dissection in the brain
Time-lapse morphological changes in the injury site of the targeted axon in the murine brain were captured. As described in Figure 4, a rapid bulb formation was observed in both the proximal and distal ends following transection, and while these structures were present at the 3 hour time point, they had disappeared 6 hours after injury as the distance between the two segments increased.
Figure 4.
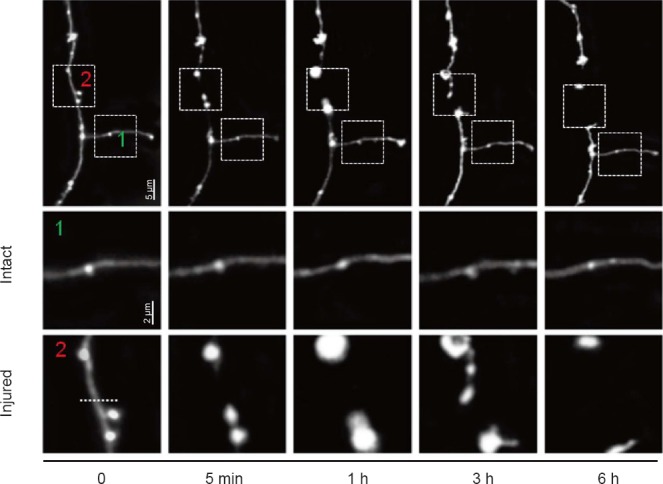
Time-lapse images showing changes in a single axon in the mouse brain after a laser-induced disruption.
Time-varying representative images of disrupted axons in the first row. Two-photon images of the (1) intact and (2) injured axons from the boxed region in the first row were taken before, 5 minutes (min), 1 hour (h), 3 h and 6 h after lesion, indicating bulb formation (proximal and distal).
Nanosurgical axon dissection in the spinal cord
As shown in Figure 5, the axon in mouse spinal cord was also accessible to sectioning by nanosurgery, with clearly observed spacing between the proximal and distal ends.
Figure 5.
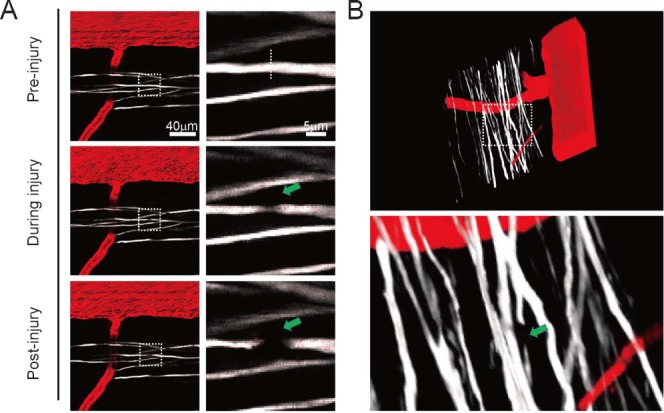
Time-lapse morphological alterations in a single axon in the mouse spinal cord after a laser-induced lesion.
(A) The two-photon images of spinal cord before, during, and after lesion. (B) Representative 3D images of a lesioned axon 3 hours after injury. Arrows indicate lesioned axons.
Discussion
Using this protocol, we successfully applied two-photon nano-surgery with low energy to dissect the axon, dendrite and especially dendrite spine in the brain or spinal cord in mice. Using in vivo imaging technique, we showed the time-lapse alterations in these subsets in response to the lesion. To the best of our knowledge, this is the first report of dendritic spine dissection without any visualized damage to the near structures in the living mice using two-photon nanosurgery with low energy. Our approach allows the investigation of the pre- and post-injury dynamics in the neuronal substructures in vivo.
It has been proposed that the mechanical or chemical methods of generating commonly used injury models cause significant disruption in tissues, with high spatial specificity and confinement (Allegra Mascaro et al., 2014). Techniques using laser emission have been successfully used in the manipulation of single cells, as any structure resolved by light microscopy was amenable to laser ablation. Laser surgery has also been widely used in several medical procedures, in cell lysis for single-cell characterization (Quinto-Su et al., 2008), in developmental studies of insect embryos (Supatto et al., 2005), and in ablating individual synapses to modify neural networks in C. elegans (Allen et al., 2008). Previous studies have shown that ablation of progenitor cells or some very small neurons influences the development and behavior (Bargmann and Avery, 1995; Gray et al., 2005). Moreover, individual axon branches can be ablated without generating a persistent glial scar (Allegra Mascaro et al., 2013) and single spines were able to be disrupted without influence on the structural integrity of the parent dendrite (Sacconi et al., 2007). When applying laser nanosurgery, three possible mechanisms of generating supplementary damage in the target structure must be considered: a) thermal and chemical processes induced by multi-photon absorption; b) production of huge thermoelastic stress; and c) mechanical, thermal, and chemical processes released from optical breakdown (formation of plasma) generated by multi-photon combined with the cascade ionization processes (Vogel et al., 2005). As a consequence, the nonlinear optical methods applied in these fields require further development and refinement (Zipfel et al., 2003). A previous study has reported that the non-linear nature of multi-photon processes supplies an absorption volume confined spatially to the focused zone (Zipfel et al., 2003). Multi-photon nanosurgery with spatial precision is applied in live brain to disrupt a single neuron, even in the previous study using high energy to dissect (Allegra Mascaro et al., 2010), no obvious collateral injury to the surrounding structure was observed. Here, our low level energy approach allows in vivo targeted dissection of axons, dendrites, and even dendritic spines.
In vivo imaging allows the direct observation and morphological analysis of the injured sub-structures at the single cell level over hours in situ. Based on sensitive time-lapse monitoring, it was possible to directly observe the provisional stages of the remodeling process and to subsequently show the newly-formed connections (Allen et al., 2008). In this paper, expanding on this technique, time-lapse changes in injured dendrite spine, dendrite, axon were monitored in vivo. Based on our current findings, we propose that a common cellular program of self-protection, the formation of the bulb in injured structures, is a localized attempt to protect neurons from lesion. The neuron may attempt to regenerate at the early stage, however, this robust regenerated response seems to fail due to some unknown interrupting factors as the damage is not resolved, and ultimately may lead to the affected neuron undergoing apoptosis.
In view of these observations, our approach which combines nanosurgery and in vivo imaging, may be an exciting tool for neuropharmacological studies on how dendrite spines and dendrites respond to lesion. This may contribute to the development of therapeutic strategies to promote regeneration in pathological conditions related to the neuronal injury or degeneration.
Footnotes
Funding: This work was supported by National Basic Research Program of China (973 Program), No. 20114002002, and 2014CB542205, and by Hong Kong Health and Medical Research Fund, No. 02132826.
Conflicts of interest: The authors declare no competing financial interests.
Ethics: The study protocol was approved by the Ethics Committee of Jinan University (approval number 20111008001). The experimental follows the national guidelines for the Care and Use of Laboratory Animals, and “Consensus author guidelines on animal ethics and welfare” by the International Association for Veterinary Editors (IAVE). The article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines).
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Li CH, Song LP, Zhao M
References
- Allegra Mascaro AL, Sacconi L, Pavone FS. Multi-photon nanosurgery in live brain. Front Neuroenergetics. 2010;2 doi: 10.3389/fnene.2010.00021. pii: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra Mascaro AL, Sacconi L, Pavone FS. Laser nanosurgery of cerebellar axons in vivo. J Vis Exp. 2014:e51371. doi: 10.3791/51371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra Mascaro AL, Cesare P, Sacconi L, Grasselli G, Mandolesi G, Maco B, Knott GW, Huang L, De Paola V, Strata P, Pavone FS. In vivo single branch axotomy induces GAP-43-dependent sprouting and synaptic remodeling in cerebellar cortex. Proc Natl Acad Sci U S A. 2013;110:10824–10829. doi: 10.1073/pnas.1219256110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PB, Sgro AE, Chao DL, Doepker BE, Scott Edgar J, Shen K, Chiu DT. Single-synapse ablation and long-term imaging in live C. elegans. J Neurosci Methods. 2008;173:20–26. doi: 10.1016/j.jneumeth.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Avery L. Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol. 1995;48:225–250. doi: 10.1016/s0091-679x(08)61390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Leischner U, Rochefort NL, Nelken I, Konnerth A. Functional mapping of single spines in cortical neurons in vivo. Nature. 2011;475:501–505. doi: 10.1038/nature10193. [DOI] [PubMed] [Google Scholar]
- Chung SH, Clark DA, Gabel CV, Mazur E, Samuel AD. The role of the AFD neuron in C. elegans thermotaxis analyzed using femtosecond laser ablation. BMC Neurosci. 2006;7:30. doi: 10.1186/1471-2202-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Ding Y, Qu Y, Feng J, Wang M, Han Q, So KF, Wu W, Zhou L. Functional motor recovery from motoneuron axotomy is compromised in mice with defective corticospinal projections. PLoS One. 2014;9:e101918. doi: 10.1371/journal.pone.0101918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Figley SA, Chen Y, Maeda A, Conroy L, McMullen JD, Silver JI, Stapleton S, Vitkin A, Lindsay P, Burrell K, Zadeh G, Fehlings MG, DaCosta RS. A spinal cord window chamber model for in vivo longitudinal multimodal optical and acoustic imaging in a murine model. PLoS One. 2013;8:e58081. doi: 10.1371/journal.pone.0058081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith JA, Terasaki M. Controlled damage in thick specimens by multiphoton excitation. Mol Biol Cell. 2003;14:1808–1817. doi: 10.1091/mbc.E02-03-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102:3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Hawthorne AL, Hu H, Kundu B, Steinmetz MP, Wylie CJ, Deneris ES, Silver J. The unusual response of serotonergic neurons after CNS Injury: lack of axonal dieback and enhanced sprouting within the inhibitory environment of the glial scar. J Neurosci. 2011;31:5605–5616. doi: 10.1523/JNEUROSCI.6663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Konig K, Riemann I, Fischer P, Halbhuber KJ. Intracellular nanosurgery with near infrared femtosecond laser pulses. Cell Mol Biol (Noisy-le-grand) 1999;45:195–201. [PubMed] [Google Scholar]
- Masters BR, So PT, Gratton E. Multiphoton excitation fluorescence microscopy and spectroscopy of in vivo human skin. Biophys J. 1997;72:2405–2412. doi: 10.1016/S0006-3495(97)78886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Schaffer CB, Friedman B, Tsai PS, Lyden PD, Kleinfeld D. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat Methods. 2006;3:99–108. doi: 10.1038/nmeth844. [DOI] [PubMed] [Google Scholar]
- Quinto-Su PA, Lai HH, Yoon HH, Sims CE, Allbritton NL, Venugopalan V. Examination of laser microbeam cell lysis in a PDMS microfluidic channel using time-resolved imaging. Lab Chip. 2008;8:408–414. doi: 10.1039/b715708h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Wiklund L, van der Want JJ, Strata P. Reinnervation of cerebellar Purkinje cells by climbing fibres surviving a subtotal lesion of the inferior olive in the adult rat. I. Development of new collateral branches and terminal plexuses. J Comp Neurol. 1991;308:513–535. doi: 10.1002/cne.903080403. [DOI] [PubMed] [Google Scholar]
- Sacconi L, Tolic-Norrelykke IM, Antolini R, Pavone FS. Combined intracellular three-dimensional imaging and selective nanosurgery by a nonlinear microscope. J Biomed Opt. 2005;10:14002. doi: 10.1117/1.1854675. [DOI] [PubMed] [Google Scholar]
- Sacconi L, O’Connor RP, Jasaitis A, Masi A, Buffelli M, Pavone FS. In vivo multiphoton nanosurgery on cortical neurons. J Biomed Opt. 2007;12:050502. doi: 10.1117/1.2798723. [DOI] [PubMed] [Google Scholar]
- Snider WD, Zhou FQ, Zhong J, Markus A. Signaling the pathway to regeneration. Neuron. 2002;35:13–16. doi: 10.1016/s0896-6273(02)00762-6. [DOI] [PubMed] [Google Scholar]
- Stone MC, Albertson RM, Chen L, Rolls MM. Dendrite injury triggers DLK-independent regeneration. Cell Rep. 2014;6:247–253. doi: 10.1016/j.celrep.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supatto W, Debarre D, Moulia B, Brouzes E, Martin JL, Farge E, Beaurepaire E. In vivo modulation of morphogenetic movements in Drosophila embryos with femtosecond laser pulses. Proc Natl Acad Sci U S A. 2005;102:1047–1052. doi: 10.1073/pnas.0405316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavosanis G. Dendritic structural plasticity. Dev Neurobiol. 2012;72:73–86. doi: 10.1002/dneu.20951. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777–791. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanska M, Blazejczyk M, Jaworski J. Molecular basis of dendritic arborization. Acta Neurobiol Exp. 2008;68:264–288. doi: 10.55782/ane-2008-1695. [DOI] [PubMed] [Google Scholar]
- Vogel A, Noack J, Hüttman G, Paltauf G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B. 2005;81:1015–1047. [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–4. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]


