Abstract
Recent evidence exists that enoxaparin can reduce brain injury because of its anticoagulant activity. To investigate the potential therapeutic effect of enoxaparin on cold-induced traumatic brain injury, at 20 minutes after modeling, male BALB/c mouse models of cold-induced traumatic brain injury were intraperitoneally administered 3 and 10 mg/kg enoxaparin or isotonic saline solution. Twenty-four hours later, enoxaparin at 10 mg/kg greatly reduced infarct volume, decreased cell apoptosis in the cortex and obviously increased serum level of total antioxidant status. By contrast, administration of enoxaparin at 3 mg/kg did not lead to these changes. These findings suggest that enoxaparin exhibits neuroprotective effect on cold-induced traumatic brain injury in a dose-dependent manner.
Keywords: nerve regeneration, neuroprotection, traumatic brain injury, cold-induced brain injury, enoxaparin, anti-oxidative, apoptosis, neural regeneration
Introduction
Traumatic brain injury (TBI) is the leading cause of disability, and in humans it carries a posttraumatic risk of embolism (Aloizos et al., 2015). Although many researchers continue to investigate neuroprotective agents against damaged nervous tissue, no distinct clinical improvements have been rendered during TBI treatment. Initial trauma causes structural and functional deficits, but the damage continues to evolve through a secondary injury mechanism (Andriessen et al., 2010). Every effort should be made to inhibit the activation of secondary pathways (Župan et al., 2011).
A body of evidence points out the potency of enoxaparin, a low-molecular-weight heparin that is utilized for anticoagulation, as a neuroprotective agent (Stutzmann et al., 2002). When peripherally administered, enoxaparin has the potential to penetrate the blood-brain barrier (BBB) (Sen et al., 2011). The inhibition of coagulation is thought to cut down post-TBI intravascular microthrombosis and neuronal tissue loss (Jonas et al., 1997; Pratt et al., 1998; Mary et al., 2001; Stutzmann et al., 2002; Quartermain et al., 2003; Li et al., 2015, 2016). Adjunct to its anticoagulant effect, enoxaparin possesses powerful anti-inflammatory properties that limit the progression of tissue injury in different organs and may contribute to neuroprotection (Gikakis et al., 1996; Jonas et al., 1997; Mary et al., 2001).
Neuroprotective effects of enoxaparin have not been previously demonstrated in a cold-induced brain injury model. This model type represents several pathophysiological characteristics of human focal cortical contusion, and hence it allows for the assessment of the desired effect of compounds that have a neuroprotective potential (Jonas et al., 1997). The cold-induced brain injury model used in this study is a well-characterized and reproducible model of experimental TBI (Michinaga et al., 2014, 2015).
We designed the present study to further investigate the role of enoxaparin use following cold-induced TBI. To induce TBI, animals were submitted to cold-induced TBI (i.e., a cryogenic injury model), an animal model commonly used to produce brain lesions that, in some respects, resemble TBI in human patients (Murakami et al., 1999; Grasso et al., 2007; Kelestemur et al., 2016).
Materials and Methods
Ethics statement
Our study was approved by the animal ethics committee of Medipol University (approval number: 23-03-2016/29) and all experiments were carried out according to the internationally approved principles for laboratory animal use and care as found in European Community Guidelines. The article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines). All efforts were made to minimize animal suffering and minimize the number of animals used.
Animals
Male BALB/c mice, 8–10 weeks old, weighing 20–25 g, were used in this study. Animals were randomly divided into three groups (n = 7 per group) as follows. In the control group, 0.2 mL isotonic saline solution was intraperitoneally administered at 20 minutes after TBI. In the low-dose enoxaparin group, enoxaparin (Clexane, Sanofi-Aventis, Istanbul, Turkey) was applied as a single intraperitoneal dose of 3 mg/kg at 20 minutes after TBI. In the high-dose enoxaparin group, enoxaparin was applied as a single intraperitoneal dose of 10 mg/kg at 20 minutes after TBI.
Cold-induced TBI
After general anesthesia by intraperitoneal administration of ketamine hydrochloride (90 mg/kg) combined with xylazine hydrochloride (10 mg/kg), the animals were placed in a stereotactic device (WPI Instruments, Saradota, FL, USA). Throughout the experiments, rectal temperature was kept between 36.5 and 37.0°C through the use of a homoeothermic blanket. Cold induced TBI was executed using the model described by Kelestemur et al. (2016). The skull was exposed via midline scalp incision. A 3-mm diameter craniotomy was performed at the parietal bone 2.5 mm posterior to and 2.5 mm lateral to bregma (The Allen Mouse Brain Atlas). The tip (2.5 mm) of liquid nitrogen-cooled (−78°C) copper cylinder rod (Habas Ltd, Istanbul, Turkey) was applied for 60 seconds to produce a cryogenic lesion. Scalp was closed in layers and the animals (except those in the control group) were treated with enoxaparin 20 minutes after TBI induction. Twenty-four hours after trauma, all animals were sacrificed by decapitation.
Assessment of brain infarct volume
The brains from traumatized rats were removed and brain sections were obtained at 1 mm intervals spanning the length of the brain. A total of 12 consecutive coronal sections (5 μm thick) throughout the brain were stained with Cresyl Violet (Sigma, St. Louis, MO, USA). Image J software program (NIH, Bethesda, MD, USA) was used to trace the boundary between the injured and non-injured areas. The area of injury was assessed by subtracting the area of the nonlesioned ipsilateral hemisphere from that on the contralateral side. The volume of injury was calculated by integrating these lesioned areas. All 12 cross sections were individually measured and corresponding volumes were calculated.
TUNEL staining
Brain sections were fixed for 20 minutes at 4°C with 4% paraformaldehyde/0.1 M PBS for DNA fragmentation analysis. TUNEL staining was then performed after labeling with terminal deoxynucleotidyl transferase mix, which contained 12.5 mg/mL terminal deoxynucleotidyl transferase and 25 mg/mL biotinylated dUTP (both Boehringer-Mannheim, Mannheim, Germany); sections were stained with streptavidin-FITC (Sigma-Aldrich). DNA-fragmented cells (apoptotic cells) were microscopically evaluated under 180× magnification using an AxioZoom V16 microscope (Carl Zeiss AG; Oberkochen, Germany) by counting TUNEL-positive cell profiles in predefined arrays consisting of six regions of interest (ROI) in the cortex, 250 μm apart (each ROI measuring 62,500 μm2). Mean values were calculated for all areas.
Measurement of serum levels of total antioxidant status (TAS) and total oxidant status (TOS)
Before sacrifice of animals, blood samples obtained via jugular vein were centrifuged for 5 minutes at 4.500 r/min at 4°C to separate the serum and plasma. Serum TAS and TOS levels were determined using an automated analyzer (Chromate Manager 4300, Palm City, FL, USA). The values are expressed in terms of micromolar hydrogen peroxide equivalent per liter (μmol H2O2 equiv/L).
Statistical analysis
All data were analyzed with SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Differences among groups were analyzed by Kruskal-Wallis tests followed by Mann-Whitney U tests. Values for P ≤ 0.05 were considered statistically significant. All values are given as the mean ± SEM.
Results
Brain infarct volume
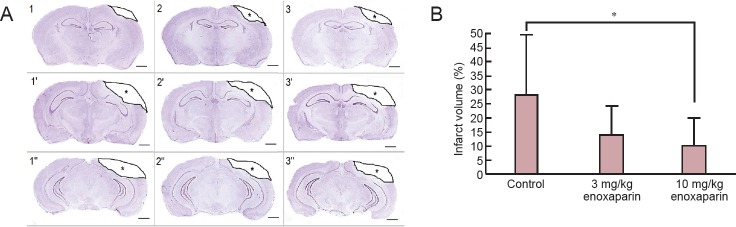
At 24 hours after surgery, the reduction in infarct volume was found to be statically significant in the 10 mg/kg enoxaparin-treated group than in the control group (P < 0.05; Figure 1).
Figure 1.
Effect of enoxaparin on infarct volume in the cortex of mice with cold-induced traumatic brain injury (TBI).
(A) Damaged tissue is defined by a decrease in staining intensity and an example of border demarcation is illustrated in these consecutive images (represented as 1, 1’, 1’’ and so on) (Cresyl violet staining) (scale bars: 1mm). 1–1’’ indicates control group (TBI + isotonic saline solution), 2–2’’ indicates “3 mg/kg enoxaparin group (TBI + 3 mg/kg enoxaparin)” and 3–3’’ indicates “10 mg/kg enoxaparin group (TBI + 10 mg/kg enoxaparin)”. * indicates the infarct area. (B) Infarct volume at 24 hours after surgery. All values are given as the mean ± SEM (mm3). Seven rats were used in each group. *P < 0.05, vs. control group (Kruskal-Wallis tests followed by Mann-Whitney U tests).
Cell apoptosis
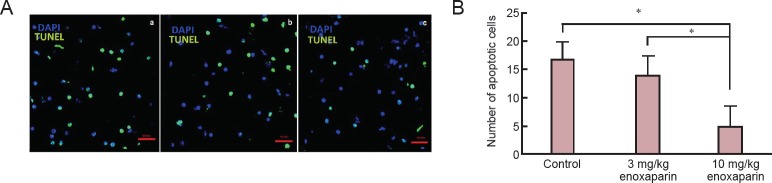
The number of apoptotic cells in the 10 mg/kg enoxaparin group was significantly decreased than that in the control group (P < 0.05; Figure 2).
Figure 2.
Effect of enoxaparin on cell apoptosis in the cortex of mice with cold-induced traumatic brain injury.
(A) Representative fluorescent microscopic images of brain sections of (a) control rats receiving isotonic saline solution, (b) 3 mg/kg enoxaparin-treated rats, (c) 10 mg/kg enoxaparin-treated rats (TUNEL staining). Green staining represents the apoptotic cells. (B) Quantification of TUNEL-positive cells (/μm2). All values are given as the mean ± SEM. *P < 0.05, vs. control group (Kruskal-Wallis tests followed by Mann-Whitney U tests). Seven rats were used in each group.
Serum TAS and TOS levels
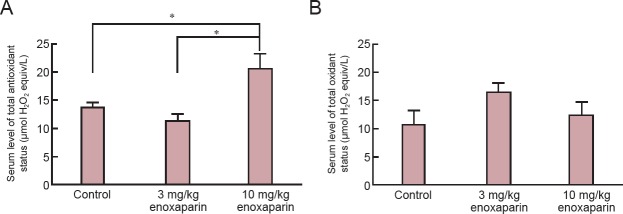
Serum TAS level was significantly increased in the 10 mg/kg enoxaparin than in the control group (P < 0.05). However, there was no significant difference in serum TOS level between enoxaparin-treated groups and control group (P > 0.05; Figure 3).
Figure 3.
Effect of enoxaparin on serum levels of total antioxidant and total oxidant status in mice with cold-induced traumatic brain injury.
(A) Serum total antioxidant status. (B) Serum total oxidant status. All values are given as the mean ± SEM. *P < 0.05 (Kruskal-Wallis tests followed by Mann-Whitney U tests). Seven rats were used in each group.
Discussion
Results from this study confirm the neuroprotective effect of enoxaparin use in a cold-injury TBI model. Enoxaparin reduced cell apoptosis and alleviated brain injury greatly. In addition, the use of enoxaparin significantly increased total antioxidant activity.
It is well known that multiple mechanisms underlie secondary brain damage following TBI. Mechanical trauma can kill neural cells by rupturing their membranes. But ischemia that accompanies brain swelling and elevated intracranial pressure to trauma can indirectly harm neurons (Stoicaand and Faden, 2010). The formation of microthrombi has been reported to occur in TBI and may lead to secondary ischemic injury (Babaee et al., 2015). Fibrin has been reported to be deposited in cerebral microvessels and, along with platelets and leukocytes, contributes to the occlusion of microvessels (Sen et al., 2011).
Stutzmann et al. (2002) reported the neuroprotective character of enoxaparin in an experimental model of TBI. They also pointed out that enoxaparin is free from the risk of harm at the doses used. Later, other animal studies showed the neuroprotective effects of enoxaparin, thus proposing its inherent capacity in the treatment of TBI (Jonas et al., 1997; Pratt et al., 1998; Wahl et al., 2000; Mary et al., 2001; Grasso et al., 2007; Li et al., 2015, 2016). In these studies, enoxaparin decreased the extent of brain edema, reduced the lesion size, and ameliorated cognitive impairment and neurological deficits following experimental TBI. In subsequent years, the number of studies regarding how enoxaparin demonstrates neuroprotective action in TBI has increased, and these studies demonstrated the molecular mechanism between the two entities. Sen et al. (2011) demonstrated that enoxaparin reduced cell death, inflammation, and apoptosis in the brain tissue after experimentally induced severe focal TBI. Župan et al. (2011) showed that following TBI, enoxaparin use significantly reduced hippocampal thiobarbituric acid-reactive substances and oxidized protein levels, COX-2 overexpression, and reactive gliosis. These evidences suggest that enoxaparin may reduce oxidative damage and inflammation following TBI.
Secondary ischemia is due in part to the edema-induced compression of blood vessels. Even when administered at 18 hours post-insult, the use of enoxaparin was found to significantly reduce cerebral edema in a dose-dependent manner in rat models of cerebral trauma (Wahl et al., 2000). Enoxaparin was shown to attenuate brain edema and improve neurological recovery after TBI, through the blunting of cerebral leukocyte recruitment diminished live leukocytes rolling on the pial endothelium, and endothelial cell activation while accelerating neurological recovery (Li et al., 2015). This was associated with concurrent reductions in microvascular leakage. Enoxaparin was found to reduce the intensity of tissue inflammation by inhibiting leukocyte activation and adhesion to endothelial cells. In particular, heparinization before hemorrhage and trauma resulted in blunted endothelial activation and restored the ability of endothelial cells to release nitric oxide. In different in vivo TBI models, enoxaparin use has been shown to reduce brain edema and lesion size, without increasing intracranial bleeding (Li et al., 2016). Although it has been frequently emphasized, the risk with such an anticoagulant drug is the possibility of a hemorrhagic transformation. However, enoxaparin shows a much lower tendency for bleeding than heparin for the same anti-Xa activity (Pratt et al., 1998).
The current study has many limitations; therefore, the molecular mechanism underlying enoxaparin in cold-induced TBI requires further investigation. Additionally, the dosage regimens for the neuroprotective effect of enoxaparin should be clarified. The currently recommended dose for thromboembolic complications is reportedly to be 3.5 mg/kg/d (Quartermain et al., 2003). Meanwhile, according to Kobbi et al. (2016), a dose of 20 mg/kg/d is considered the upper toxic dose in rats. Doses of 3.5 mg/kg/d and 5 mg/kg/d showed no undesirable effects, and are therefore recommended for further pharmacodynamics studies. However, some adverse effects were clearly observed at the dose of 20 mg/kg/d, whereas induced lethality at doses of 100 and 40 mg/kg/d have been reported. According to the results of our study, the greatest neuroprotective effect was observed when enoxaparin was used at a dose of 10 mg/kg/d—a dose still below the upper toxic dose, but above the standard therapeutic dose.
In conclusion, although the effectiveness of enoxaparin as a neuroprotectant has been investigated previously in brain injury models, none has used a cryogenic injury model or pattern. This study is the first to provide the in vivo evidence that following cold-induced TBI, enoxaparin is a beneficial drug in treating injuries, as it exhibits neuroprotective effects on the brain tissue.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Li CH, Song LP, Zhao M
References
- Aloizos S, Evodia E, Gourgiotis S, Isaia EC, Seretis C, Baltopoulos GJ. Neuroprotective effects of erythropoietin in patients with severe closed brain injury. Turk Neurosurg. 2015;25:552–558. doi: 10.5137/1019-5149.JTN.9685-14.4. [DOI] [PubMed] [Google Scholar]
- Andriessen TM, Jacobs B, Vos PE. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J Cell Mol. 2010;Med14:2381–2392. doi: 10.1111/j.1582-4934.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaee A, Eftekhar-Vaghefi SH, Asadi-Shekaari M, Shahrokhi N, Soltani SD, Malekpour-Afshar R, Basiri M. Melatonin treatment reduces astrogliosis and apoptosis in rats with traumatic brain injury. Iran J Basic Med Sci. 2015;18:867–872. [PMC free article] [PubMed] [Google Scholar]
- Gikakis N, Khan MD, Hiramatsu Y, Gorman JH, III, Hack CE, Sun L, Rao AK, Niewiarowski S, Colman RW, Edmunds LH., Jr Effect of factor Xa inhibitors on thrombin formation and complement and neutrophil activation during in vitro extracorporeal circulation. Circulation. 1996;94:341–346. [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Meli F, Fodale V, Buemi M, Iacopino DG. Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res. 2007;1182:99–105. doi: 10.1016/j.brainres.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Jonas S, Sugimori M, Llinas R. Is low molecular weight heparin a neuroprotectant? Ann N Y Acad Sci. 1997;825:389–393. doi: 10.1111/j.1749-6632.1997.tb48449.x. [DOI] [PubMed] [Google Scholar]
- Kelestemur T, Yulug B, Caglayan AB, Beker MC, Kilic U, Caglayan B, Yalcin E, Gundogdu RZ, Kilic E. Targeting different pathophysiological events after traumatic brain injury in mice: role of melatonin and memantine. Neurosci Lett. 2016;612:92–97. doi: 10.1016/j.neulet.2015.11.043. [DOI] [PubMed] [Google Scholar]
- Kobbi Z, Kraiem H, Benlasfar Z, Marouani A, Massoud T, Boubaker S, Bouhaouala-Zahar B, Fenina N. Comparative subcutaneous repeated toxicity study of enoxaparin products in rats. Regul Toxicol Pharmacol. 2016;10:9–17. doi: 10.1016/j.yrtph.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Li S, Marks JA, Eisenstadt R, Kumasaka K, Samadi D, Johnson VE, Holena DN, Allen SR, Browne KD, Smith DH, Pascual JL. Enoxaparin ameliorates posttraumatic brain injury edema and neurologic recovery, reducing cerebral leukocyte endothelial interactions and vessel permeability in vivo. J Trauma Acute Care Surg. 2015;79:78–84. doi: 10.1097/TA.0000000000000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Eisenstadt R, Kumasaka K, Johnson VE, Marks J, Nagata K, Browne KD, Smith DH, Pascual JL. Does enoxaparin interfere with HMGB1 signaling after TBI? A potential mechanism for reduced cerebral edema and neurologic recovery. J Trauma Acute Care Surg. 2016;80:381–387. doi: 10.1097/TA.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary V, Wahl F, Uzan A, Stutzmann JM. Enoxaparin in experimental stroke: neuroprotection and therapeutic window of opportunity. Stroke. 2001;32:993–999. doi: 10.1161/01.str.32.4.993. [DOI] [PubMed] [Google Scholar]
- Michinaga S, Nagase M, Matsuyama E, Yamanaka D, Seno N, Fuka M, Yamamoto Y, Koyama Y. Amelioration of cold injury-induced cortical brain edema formation by selective endothelin ETB receptor antagonists in mice. PLoS One. 2014;9:e102009. doi: 10.1371/journal.pone.0102009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michinaga S, Seno N, Fuka M, Yamamoto Y, Minami S, Kimura A, Hatanaka S, Nagase M, Matsuyama E, Yamanaka D, Koyama Y. Improvement of cold injury-induced mouse brain edema by endothelin ETB antagonists is accompanied by decreases in matrixmetalloproteinase 9 and vascular endothelial growth factor-A. Eur J Neurosci. 2015;42:2356–2370. doi: 10.1111/ejn.13020. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Yang G, Chen SF, Morita-Fujimura Y, Chan PH. Cold injury in mice: a model to study mechanisms of brain edema and neuronal apoptosis. Prog Neurobiol. 1999;57:289–299. doi: 10.1016/s0301-0082(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Pratt J, Boudeau P, Uzan A, Imperato A, Stutzmann J. Enoxaparin reduces cerebral edema after photothrombotic injury in the rat. Haemostasis. 1998;28:78–85. doi: 10.1159/000022416. [DOI] [PubMed] [Google Scholar]
- Quartermain D, Li Y S, Jonas S. The low molecular weight heparin enoxaparin reduces infarct size in a rat model of temporary focal ischemia. Cerebrovasc Dis. 2003;16:346–355. doi: 10.1159/000072556. [DOI] [PubMed] [Google Scholar]
- Schwarzmaier SM, de Chaumont C, Balbi M, Terpolilli NA, Kleinschnitz C, Gruber A, Plesnila N. The formation of microthrombi in parenchymal microvessels after traumatic brain injury is independent of coagulation factor XI. J Neurotrauma. 2016;33:1634–1644. doi: 10.1089/neu.2015.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen O, Sonmez E, Cekinmez M, Ozen O, Caner H. Antithrombin III and enoxaparin treatment inhibit contusion-triggered cell death, inflammation, hemorrhage and apoptosis after severe traumatic brain injury in rats. Turk Neurosurg. 2011;21:203–209. doi: 10.5137/1019-5149.JTN.3646-10.1. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Faden AI. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics. 2010;7:3–12. doi: 10.1016/j.nurt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann JM, Mary V, Wahl F, Grosjean-Piot O, Uzan A, Pratt J. Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: a review. CNS Drug Rev. 2002;8:1–30. doi: 10.1111/j.1527-3458.2002.tb00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl F, Grosjean-Piot O, Bareyre F, Uzan A, Stutzmann JM. Enoxaparin reduces brain edema, cerebral lesions, and improves motor and cognitive impairments induced by a traumatic brain injury in rats. J Neurotrauma. 2000;17:1055–1065. doi: 10.1089/neu.2000.17.1055. [DOI] [PubMed] [Google Scholar]
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Župan Ž, Pilipović K, Dangubić B, Frković V, ŏ ustićA, Župan G. Effects of enoxaparin in the rat hippocampus following traumatic brain injury. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1846–1856. doi: 10.1016/j.pnpbp.2011.08.005. [DOI] [PubMed] [Google Scholar]