Abstract
Resveratrol is a polyphenol found in grape skins and peanuts that has demonstrated many health benefits including protection against aging, cardiovascular and metabolic disease, neurological decline, and cancer. The anticancer properties of resveratrol have been attributed to a variety of mechanisms, including its general inhibition of phase I metabolism and induction of phase II metabolism. The effects of resveratrol on these enzymes, however, are still unclear, as in vitro evidence often contrasts with animal studies and clinical trials. Reasons for these variances could include the low bioavailability of resveratrol and the effects of resveratrol metabolites. Due to resveratrol's interactions with drug‐metabolizing enzymes and drug transporters, individuals concurrently taking pharmacological doses of resveratrol with other supplements or medications could potentially experience nutrient‐drug interactions. This review summarizes the known effects of resveratrol and its main metabolites on drug metabolism in order to help characterize which populations might benefit from resveratrol for the prevention of cancer, as well as those that may need to avoid supplementation due to potential drug interactions.
Keywords: Chemoprevention, cytochrome P450, resveratrol
Abbreviations
- ABCATP
‐binding cassette
- AHR
aryl hydrocarbon receptor
- AUC
area under the curve
- B[a]
Pbenzo‐a‐pyrene
- BCRP
breast cancer resistance protein
- BROD
7‐benzoxy resorufin O‐deethylation
- CDNB
1‐chloro‐2,4‐dinitrobenzene
- COX‐2
cyclooxygenase‐2
- CYP
cytochrome P450
- DBF
O‐benzylfluorescein benzyl ester
- DMBA
dimethylbenz[a]anthracene
- EROD
ethoxyresorufin‐O‐deethylase
- GST
glutathione‐S‐transferase
- HED
human equivalent dose
- MRP3
multidrug resistance protein 3
- NQO1
NAD(P)H dehydrogenase, quinone 1
- NQO2
NAD(P)H dehydrogenase, quinone 2
- OATP
organic anion transporting polypeptide
- P‐gp
P‐glycoprotein
- PNPH
p‐nitrophenol hydroxylase
- R34S
resveratrol‐3‐O‐4′‐O‐disulfate
- R3G
resveratrol‐3‐O‐glucuronide
- R3S
resveratrol‐3‐O‐sulfate
- R4S
resveratrol‐ 4′‐O‐sulfate
- ROS
reactive oxygen species
- TCDD
2,37,8‐tetrachloro‐dibenzo‐p‐dioxin
- TMS
2,3,3′5′‐tetramethoxystilbene
- UGT
Uridine diphosphate‐glucuronosyl transferase
- XRE
xenobiotic response element
Introduction
Resveratrol (3, 4′,5‐trihydroxy‐trans‐stilbene; see Fig. 1) is a polyphenol found in grapes and peanuts that has demonstrated anticancer, antiaging, and anti‐inflammatory properties in preclinical models (Zordoky et al. 2015). Evidence supporting the clinical benefit of resveratrol supplementation is mixed, but recent reviews examine the evidence for resveratrol as an agent to prevent and/or treat obesity (de Ligt et al. 2015), cardiovascular disease (Zordoky et al. 2015), diabetes (Szkudelski and Szkudelska 2015), neurological disease (Bastianetto et al. 2015), aging (Ramis et al. 2015), and cancer (Patel et al. 2013; Carter et al. 2014). Many questions remain regarding the dose and duration of resveratrol supplementation for either prevention or as a therapeutic and for which conditions. To determine who might benefit from resveratrol for cancer prevention, it is imperative to understand the ideal dose, the activity of resveratrol metabolites, and pharmacological targets.
Figure 1.
Structure of transresveratrol, a trihydroxystilbene naturally found in red wine and peanuts.
Resveratrol Dosing
Resveratrol is available over the counter with single capsules containing between 20 to 500 mg pure resveratrol (Chachay et al. 2011). With pharmaceutical doses (i.e. doses attainable only through supplementation and not by a balanced diet alone) being not only achievable but also easily accessible, questions of toxicity and safety have emerged. Detailed reviews of the bioavailability, metabolism, and toxicity of resveratrol are available (Wenzel and Somoza 2005; Cottart et al. 2014). Based on clinical trials (Brown et al. 2010; Chow et al. 2010; Kalash et al. 2014) and on human equivalent dose (HED) calculations from animal models (Crowell et al. 2004), the majority of the available evidence supports an upper limit of 1 g daily for most individuals. However, in one study, doses as high as 2 g daily were safe and well‐tolerated in older adults with Alzheimer disease when starting with a dose of 500 mg daily and increasing by an additional 500 mg every 13 weeks (Turner et al. 2015). It is possible that the higher tolerable upper limit in this trial could be due to the gradual dose escalation schedule, or could be population dependent.
The ideal dose for resveratrol supplementation, however, may be less. Metabolic disturbance is a risk factor for multiple cancer types (Uzunlulu et al. 2016) and reversal of metabolic disturbances by low‐dose resveratrol may reduce cancer risk as well as improve cancer outcomes (Fay et al. 2009; Seyfried and Shelton 2010). In recent clinical trials, 5–250 mg doses of resveratrol showed a positive effect on metabolic parameters [e.g. glycemic control, insulin sensitivity, the Homeostasis Model Assessment (HOMA) index, and oxidative stress] in diabetics (Brasnyó et al. 2011; Bhatt et al. 2012) as well as healthy obese adults (Timmers et al. 2011; Witte et al. 2014). However, in a recent, randomized, controlled cross‐over trial with diet‐controlled diabetics, 1 g daily resveratrol had no effect on glycemic control or other metabolic parameters (Thazhath et al. 2016). Similarly, doses ranging from 1 to 2 g daily resveratrol had little or no benefit in older adults with glucose intolerance (Crandall et al. 2012) or healthy obese males (Dash et al. 2013; Poulsen et al. 2013). While the specific metabolic parameters measured are study dependent, taken together, these clinical trials suggest higher efficacy of resveratrol on metabolic control at a lower dose. Conversely, one small, randomized clinical trial in individuals with metabolic syndrome showed that 1.5 g resveratrol daily improved AUC of insulin and insulinogenic index compared to placebo (Méndez‐del Villar et al. 2014). Only one animal study has investigated differential dosing of resveratrol; in mice fed a high fat diet, 5 mg resveratrol (HED of 28 mg for a 70 kg human) reduced intestinal adenoma number and volume more potently than a 1 g dose, this efficacy was correlated with activation of adenosine monophosphate‐activated protein kinase (AMPK), a central regulator of metabolic control (Cai et al. 2015). One explanation for this nonlinear dose response is that lower doses of antioxidants allow for low levels of reactive oxygen species (ROS) to activate natural cellular defense mechanisms (Cai et al. 2015). It is also possible that higher doses of resveratrol compete with other polyphenols for transporters, reducing their uptake and potential synergistic effects.
Resveratrol Metabolites
The benefits of oral resveratrol could also be derived from its metabolites, which have demonstrated more potent activity than resveratrol against multiple cancer types in some preclinical models (Aires et al. 2013; Ruotolo et al. 2013). Resveratrol has high oral absorption (up to 70%) (Walle et al. 2004) but is rapidly metabolized by sulfotransferases (Miksits et al. 2005) and UDP‐glucuronosyltransferases (Brill et al. 2006). Emerging evidence also indicates a role for the gut microbiome in resveratrol metabolism (Qiao et al. 2014; Cai et al. 2015).
The primary metabolite in humans is the sulfated metabolite resveratrol‐3‐O‐sulfate (R3S). Other sulfated metabolites include resveratrol‐4′‐O‐sulfate (R4S) and resveratrol‐3‐O‐4′‐O‐disulfate (R34S) (Miksits et al. 2005). Glucuronidated metabolites include resveratrol‐3‐O‐glucuronide (R3G) and resveratrol‐4′‐O‐glucuronide (R4G). A glucuronide‐sulfate metabolite has also been detected in humans after resveratrol administration (Chow et al. 2010). Piceatannol (3,4,3′,5′‐tetrahydroxy‐trans‐stilbene) is a minor metabolite which results from metabolism of resveratrol by cytochrome P450 enzymes (CYPs) (Chang et al. 2007). Figure 2 illustrates the structures of these main metabolites of resveratrol, including piceatannol. In addition, 3,4′‐dihydroxy‐trans‐stilbene, 3,4′‐dihydroxybibenzyl (lunularin), and dihydroresveratrol have been identified as human gut microbial metabolites (Bode et al. 2013).
Figure 2.
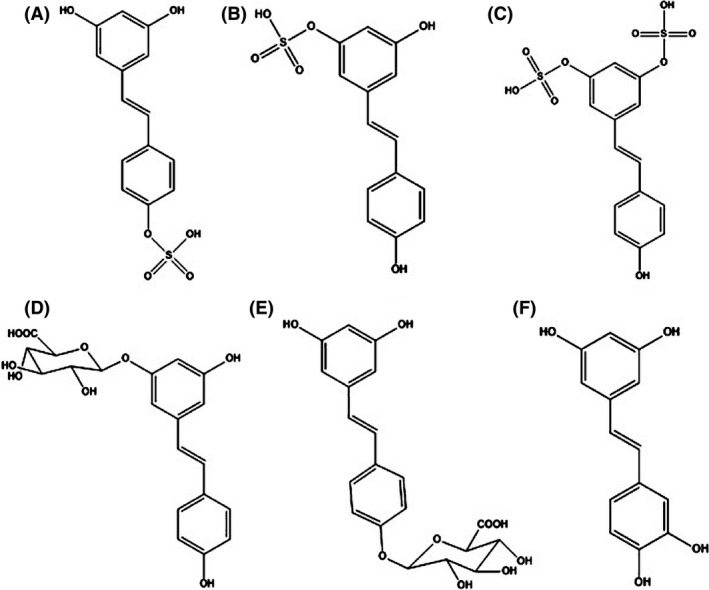
Structures of resveratrol's main metabolites. (A) transresveratrol‐4′‐O‐sulfate (main metabolite found in mice) (B) transresveratrol‐3‐O‐sulfate (main metabolite found in humans) (C) transresveratrol‐3‐O‐4′‐O‐disulfate (D) transresveratrol‐3‐O‐glucuronide (major glucuronide product) (E) transresveratrol‐4′‐O‐glucuronide, (F) piceatannol (a minor resveratrol metabolite that is quickly metabolized by phase II enzymes).
Resveratrol and Chemoprevention
Resveratrol has been shown to act as a calorie‐restriction mimetic, antioxidant, and anti‐inflammatory agent, supporting an anticancer environment (Kulkarni and Cantó 2014; Bitterman and Chung 2015). Less attention has been given to another mechanism by which resveratrol may prevent carcinogenesis, namely, through the inhibition of procarcinogen‐activating enzymes (phase I metabolism, or CYPs) and induction of conjugation enzymes (phase II metabolism). While some of resveratrol's interactions with CYP enzymes have been summarized in an existing review (Detampel et al. 2012), we will present the updated evidence and include a discussion of resveratrol's effects on phase II metabolism, focusing on implications for cancer prevention. Figure 3 illustrates the systemic actions of resveratrol based on the available evidence. We will also discuss the anticancer activities of resveratrol's main glucuronide and sulfate metabolites when evidence is available.
Figure 3.
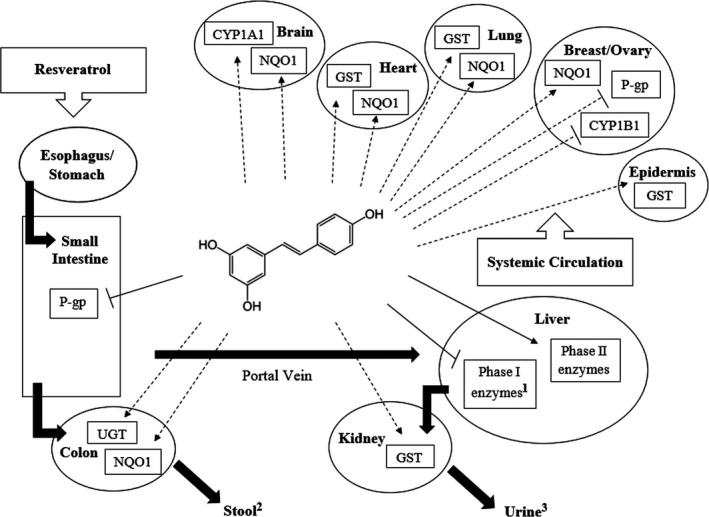
Systemic effects of resveratrol on drug‐ and carcinogen‐metabolizing enzymes. Solid lines within the figure refer to effects that have been demonstrated in both preclinical and clinical models. Dotted lines indicate effects that have only been demonstrated in cell culture and/or animal studies. 1 Denotes the exception of CYP1A2, which was induced by resveratrol in a clinical study (Chow et al. 2010).2 Concentration of resveratrol in stool ranges from 0 to 23 μg resveratrol/g dry weight feces, with the concentration of metabolites <1% (Boocock et al., 2007). 3 77% of all urinary species excreted within 4 h post consumption, likely due to enterohepatic recirculation (Boocock et al., 2007).CYP, cytochrome P450; GST, glutathione S‐transferase; NQO1, NAD(P)H dehydrogenase, quinone 1; P‐gp, P‐glycoprotein; UGT, Uridine diphosphate.
Resveratrol and Phase I Metabolism
Phase I metabolism of pharmaceutical drugs, phytochemicals, environmental pollutants, and other endogenous and exogenous compounds is accomplished mainly by the CYP enzymes. CYPs catalyze oxidation, reduction, hydrolysis, deamination, and other reactions that add or expose polar substituents to a compound (Omiecinski et al. 2011). This increased polarity may facilitate the elimination of these compounds, or perhaps create a bioactive metabolite. Altering the activity of these enzymes could result in reduced efficacy or increased toxicity of treatment regimens. The effects of resveratrol on isolated isozymes and in microsomes are summarized in Table 1. Table 2 summarizes the effects of resveratrol on drug‐ and carcinogen‐metabolizing enzymes in clinical studies and animal models.
Table 1.
Inhibitory effect of resveratrol in liver microsomes and heterologously expressed isozymes (only studies that measured enzyme activity are included)
| Enzyme | Substrate | Model | IC50 (μmol/L) | Ki (μmol/L) | Reference |
|---|---|---|---|---|---|
| CYP3A4 | Testosterone | Human liver microsome | 4.0 | Piver et al. 2001 | |
| Rat liver microsome | 20 | Piver et al. 2001 | |||
| Recombinant isozyme | 10 | Piver et al. 2001 | |||
| Recombinant isozyme | 15 | Piver et al. 2003 | |||
| Human liver microsome | 25 | Piver et al. 2003 | |||
| Recombinant isozyme | 1.1 | Yu et al. 2003; | |||
| CYP3A11 (homolog) | Aripiprazole Nifedipine | Recombinant isozyme Mouse liver microsome |
6.8 15.8 |
Zhan et al. 2015Wang et al. 2015 | |
| CYP1A1 | EROD | Human HepG2 microsome | 1.0 | 0.42 | Ciolino and Yeh 1999 |
| Human HepG2 cell | 1.0 | Ciolino and Yeh 1999 | |||
| Human MCF‐7 cell | 0.5 | Ciolino and Yeh 1999 | |||
| Recombinant isozyme | 1.2 | Chang et al. 2001 | |||
| Recombinant isozyme | 40 | Piver et al. 2001 | |||
| Recombinant isozyme | 30 | Piver et al. 2003; | |||
| CYP1A2 | EROD | Recombinant isozyme | 15.5 | Chang et al. 2001 | |
| Recombinant isozyme | 30 | Piver et al. 2001 | |||
| Human liver microsome | 25 | Piver et al. 2003 | |||
| Phenacetin | Mouse liver microsome | 127.3 | Wang et al. 2015 | ||
| CYP1B1 | EROD | Recombinant isozyme | 0.8 | Chang et al. 2001 | |
| Recombinant isozyme | 40 | Piver et al. 2003 | |||
| CYP2B1 | EFC | Rat liver microsomea | Huynh and Teel 2002 | ||
| CYP2B6 | BROD | Recombinant isozyme | >50 | Piver et al. 2003 | |
| Recombinant isozyme | 100 | Piver et al. 2003 | |||
| Human liver microsome | 100 | Piver et al. 2003 | |||
| CYP2D6 | Bufuralol | Human liver microsome | > 50 | Yu et al. 2003 | |
| Aripiprazole | Recombinant isozyme | 87.9 | Zhan et al. 2015 | ||
| CYP2E1 | Chlorzoxazone | Human liver microsome | 150 | Piver et al. 2001 | |
| Recombinant isozyme | 50 | Piver et al. 2003; | |||
| Human liver microsome Mouse liver microsome | 74.3 | 50 | Piver et al. 2003Wang et al. 2015 | ||
| PNPH | Rat liver microsome | 18.5 | Mikstacka et al. 2002 | ||
| CYP2C9 | Diclofenac | Human liver microsome | >50 | Yu et al. 2003 | |
| CYP 2C19 | DBF | Human liver microsome | 22.5 | Orsini et al. 2016 |
BROD, 7‐benzoxy resorufin; EFC, 7‐ethoxy‐4‐trifluormethyl courmarin; EROD, 7‐ethoxyresorufin; DBF, O‐benzylfluorescein benzyl ester; PNPH, p‐nitrophenol hydroxylase.
P < 0.05 for percent inhibition.
Table 2.
Effect of resveratrol on drug‐ and carcinogen‐metabolizing enzymes in clinical trials and animal studies (only studies which measured enzymatic activity are included)
| Enzyme | Substrate | Species | Dose per day (HED) | Administration | Effect | Reference |
|---|---|---|---|---|---|---|
| CYP3A4 | Diltiazem | Rat |
2.5 mg/kg (28 mg)b |
Oral | InhibitionW | Hong et al. 2008 |
| Nicardipine |
0.5 mg/kg (6 mg)b 2.5 mg/kg (28 mg)b |
Oral | InhibitionW | Choi et al. 2009 | ||
| Buspirone | Human | 1.0 ga | Oral | InhibitionM ** | Choi et al. 2009 | |
| Carbamazepine | Human | 0.5 gb | Oral | InhibitionW * | Chow et al. 2010 | |
| CYP3A11 (homolog) | Nifedipine | Mice |
2.5 mg/kg (14 mg)b |
Oral |
InhibitionW
Inhibition** |
Bedada and Nearati 2015
Wang et al. 2015 |
| CYP3A1/2 | Testosterone | Mice |
50 mg/kg (284 mg)b |
Intraperitoneal | Inhibition*** | Canistro et al. 2009 |
| CYP1A2 | Caffeine | Human | 1.0 ga | Oral | Induction* | Chow et al. 2010 |
| Mice | 10 mg/kg (56 mg)b | Oral | Inhibition** | Wang et al. 2015 | ||
| CYP2B1/2 | Testosterone | Mice |
50 mg/kg (284 mg)b |
Intraperitoneal | Inhibition** | Canistro et al. 2009 |
| CYP2D6 | Dextromethorphan | Human | 1.0 ga | Oral | Inhibition* | Chow et al. 2010 |
| CYP2E1 | Chlorzoxazone | Mice |
5 mg/kg (28 mg)b |
Oral | Inhibition*** | Wang et al. 2015 |
| CYP2C9 | Losartan | Human | 1.0 ga | Oral | Inhibition* | Chow et al. 2010 |
| GST | CDNB | Mice |
25 mg/kg (142 mg)b |
Intraperitoneal | Inhibition** | Canistro et al. 2009 |
| Human | 1.0 ga | Oral | InductionNS | Chow et al. 2010 | ||
| Mice | Low dosec | Oral | Induction* | Liu et al. 2015 | ||
| Mice |
16.7 mg/kg (95 mg)b |
Oral | Induction* | Tung et al. 2015 | ||
| Rat |
10 mg/kg (114 mg)b |
Oral | Induction* | Ali et al. 2015 | ||
| Rat |
20 mg/kg (227 mg)b |
Oral | Induction* | Sadi et al. 2015 | ||
| Measured directly | Rat |
~2.5 mg (28 mg)b |
Oral | Induction* | Javkhedkar et al. 2015 | |
| NQO1 | 2.6‐dicholorophenol‐indophenol | Mice |
16.7 mg/kg (95 mg)b |
Oral | Induction* | Tung et al. 2013 |
| 2.6‐dicholorophenol‐indophenol | Mice |
16.7 mg/kg (95 mg)b |
Oral | Induction * | Tung et al. 2015 | |
| UGT | 1‐naphthol | Mice |
25 mg/kg (142 mg)b |
Intraperitoneal | Induction** | Canistro et al. 2009 |
| Bilirubin as indirect measure | Human | 1.0 gb | Oral | InductionNS | Chow et al. 2010 | |
| P‐gp (transporter) | Nicardipine | Rat |
0.5 mg/kg (6 mg)b |
Oral | InhibitionW | Choi et al. 2009 |
|
2.5 mg/kg (28 mg)b |
Oral | InhibitionM ** | Choi et al. 2009 | |||
| Fexofenadine | Human | 500 mg | Oral | InhibitionW * | Bedada et al. 2014 |
CDNB, 1‐chloro‐2,4‐dinitrobenzene; HED, human equivalent dose (based on 70 kg human); PROD, pentoxyresorufin dealkylase.
Blood concentrations averaged 72.7 ng/mL (0.32 μmol/L).
Blood concentration not given.
5.7 μg/mL resveratrol mixed with drinking water three times weekly.
*P < 0.05; **P < 0.01; ***P < 0.001; NSNot significant; WWeak (1.25‐ to 2‐fold change in AUC); MModerate (2‐ to 5‐fold change in AUC).
Cyp3a4
CYP3A enzymes, particularly CYP3A4, are among the most important and investigated components of xenobiotic metabolism. CYP3A4 plays a role in the metabolism of 60% known drugs (Zhou et al. 2005) and metabolizes the majority of anticancer medicines, including imatinib, docetaxel, and irinotecan (Tian and Hu 2014). Therefore, when considering resveratrol supplementation it is necessary to predict potential interactions with CYP3A4 to ensure the safety of patients receiving chemotherapeutics.
Resveratrol has been shown to attenuate pregnane X receptor (PXR)‐induced expression of Cyp3a11, a homolog of CYP3A4, in mouse hepatocytes (Deng et al. 2014). It also irreversibly inactivates CYP3A4 by undergoing metabolism by the enzyme, then forming an irreversible complex by means of a reactive intermediate (Chan and Delucchi 2000). Resveratrol has inhibited CYP3A4 at IC50 values of 4.0 μmol/L in human liver microsomes (Piver et al. 2001) and in heterologously expressed isozymes at IC50 values ranging from 1.1 to 15.8 μmol/L (Piver et al. 2001; Yu et al. 2003; Wang et al. 2015; Zhan et al. 2015). Injection of mice with 50 mg/kg resveratrol (HED of 284 mg for a 70 kg human) resulted in an approximately 61% loss of hepatic Cyp3a activity (Canistro et al. 2009).
Consistent with the in vitro data, CYP3A4 is one of the few CYP enzymes with data directly showing resveratrol‐drug interactions in animal models and humans. Rats treated with as little as 2.5 mg/kg resveratrol (HED of 28 mg for a 70 kg human) experienced increased plasma levels of calcium channel blockers, nicardipine (Choi et al. 2009) and diltiazem (Hong et al. 2008), substrates of CYP3A4. In a clinical trial with healthy volunteers, 500 mg/day resveratrol for 10 days significantly decreased the metabolite to parent ratio of carbamazepine (an anticonvulsant) and increased the AUC of carbamazepine by 1.5‐fold, indicating inhibition of CYP3A4 (Bedada and Nearati 2015). In another clinical study, 1 g daily resveratrol significantly inhibited CYP3A4 activity among participants with a high baseline activity, as measured from a 33% geometric mean change in the AUC of buspirone, a CYP3A4 probe drug (Chow et al. 2010). Because of ethical concerns, it is not possible to design a study to determine whether this inhibition of CYP3A4 by resveratrol translates to reduced clearance and subsequent toxicity from other CYP3A4 substrates in humans; however, these studies strongly suggest that individuals receiving CYP3A4 substrates should avoid supplementation with resveratrol due to potential toxicity related to reduced clearance.
CYP1A1 and CYP1A2
CYP1A1 (mainly extrahepatic) and 1A2 (exclusively hepatic) help metabolize a range of medications, including anticancer drugs such as tegafur (solid tumors), flutamide (prostate), and dacarbazine (skin) (Zhou et al. 2010). CYP1A enzymes also activate procarcinogens such as benzo[a]pyrene (B[a]P), a polycyclic aromatic hydrocarbon found in cigarette smoke (Ding et al. 2014) and grilled/smoked food products (Rose et al. 2015) and 2,37,8‐tetrachloro‐dibenzo‐p‐dioxin (TCDD), a contaminant in chlorophenoxy herbicides (Saberi Hosnijeh et al. 2012). Resveratrol has been shown to inhibit both the expression and activity of CYP1A enzymes (Detampel et al. 2012). Theoretically, inhibition of CYP1A through resveratrol supplementation would result in less activation of procarcinogens, making resveratrol a viable cancer prevention strategy for those with exposure to procarcinogens such as B[a]P. This relationship has prompted multiple preclinical studies to study the extent and mechanism of resveratrol's inhibition of CYP1A and the subsequent development of derivatives to enhance this observed inhibition (Orsini et al. 2016).
In preclinical models, resveratrol inhibits CYP1A gene expression by blocking transcription through the aryl hydrocarbon receptor (AHR) pathway. CYP1A gene expression is induced by the binding of a ligand (e.g. B[a]P or TCDD) to the AHR, its translocation to the nucleus, and binding of the complex to the xenobiotic response element (XRE) (Zanger and Schwab 2013). The exact target of resveratrol's inhibition along this AHR pathway is still an active area of research; the proposed mechanisms are illustrated in Figure 4. Early work in human HepG2 cells suggested that 10 μmol/L resveratrol may act on a binding region separate from the AHR binding site (Ciolino et al. 1998). Subsequent studies with similar doses in B[a]P‐ and 7,12‐dimethylbenz[a]anthracene (DMBA)‐induced HepG2 and MCF‐7 cells suggested that resveratrol affects binding to the XRE and recruitment of RNA polymerase II (Ciolino and Yeh 1999; Beedanagari et al. 2009). A posttranscriptional mechanism of inhibition has also been proposed based on evidence that 10 μmol/L resveratrol increased the rate of CYP1A1 mRNA degradation in T47D breast cancer cells (Lee and Safe 2001). Conversely, high levels of resveratrol (50 μmol/L) weakly induced CYP1A2 mRNA expression in HepG2 cells (Koe et al. 2014). Resveratrol's effect on the AHR pathway and subsequent expression of CYP1A enzymes may depend on the dose, carcinogen, and model used.
Figure 4.
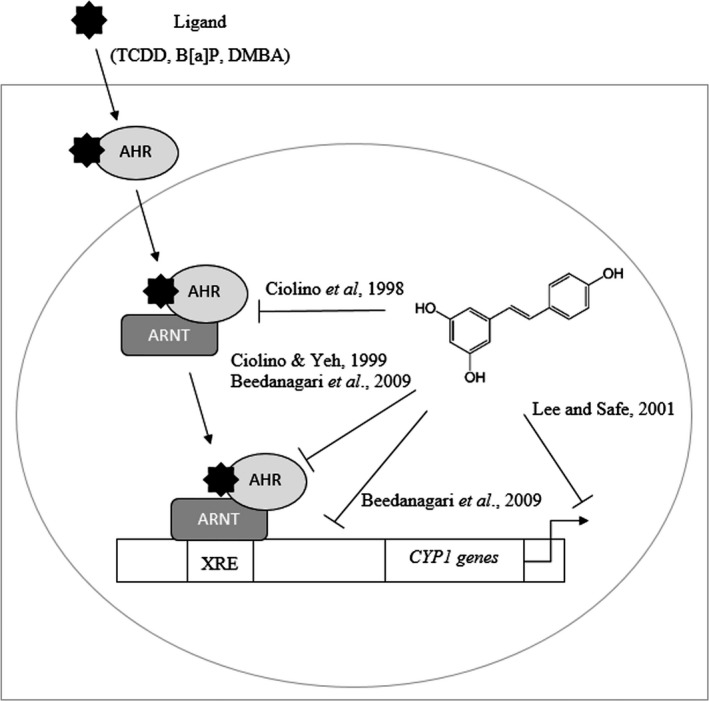
Proposed mechanisms for resveratrol's suppression of CYP1 induction. An early model showed that resveratrol inhibited the transformation of the AHR to its nuclear form by forming a complex with the ARNT. Resveratrol has also been shown to act later in the pathway, inhibiting the binding of the nuclear AHR complex to the XRE or inhibiting the recruitment of RNA polymerase II to the DNA. These mechanisms block the transcription of CYP1A1 and CYP1B1. Still, others have proposed that CYP1 inhibition occurs posttranscriptionally. AHR, aryl hydrocarbon receptor; ARNT, aryl hydrocarbon receptor nuclear translocator; CYP, cytochrome P450; XRE, xenobiotic response element.
Resveratrol has also demonstrated mechanism‐based inactivation of CYP1A2 in a microsomal assay; however, a requirement of NADPH for this inhibition indicated that a resveratrol metabolite was likely responsible for the observed inhibitory activity (Chang et al. 2001). Although a subsequent study found that RS3 did not significantly inhibit CYP1A2 in cell cultures containing the recombinant human isozyme (Yu et al. 2003), piceatannol has been shown to inhibit CYP1A activity to an extent similar to that of resveratrol in rat hepatic microsomes (Chang et al. 2007) and could affect its interactions with CYP1A enzymes.
While evidence from in vitro models demonstrated inhibition of CYP1A1 and CYP1A2 by resveratrol, there are no clinical studies to corroborate this effect. In an early phase clinical study, resveratrol‐induced CYP1A2 activity. When participants were given 100 mg caffeine after treatment with 1 g daily resveratrol for four weeks, the metabolic ratio of caffeine/paraxanthine decreased significantly compared to baseline measurements (indicative of CYP1A2 induction) (Chow et al. 2010). The observed difference between this clinical observation and previous in vitro studies could be attributed to the indirect assessment of CYP1A2 activity, or to resveratrol metabolism. The effects of resveratrol on CYP1A enzymes could also be model‐dependent. Addition of 50 μmol/L resveratrol to human medullablastoma cells resulted in upregulation of CYP1A1 as well as increased cell differentiation and apoptosis (Liu et al. 2004).
Cyp1b1
CYP1B1 is an extrahepatic enzyme overexpressed in breast, prostate, endometrial, and ovarian cancers (Gajjar et al. 2012). It has been shown to biotransform anticancer agents in vitro and may contribute to drug therapy resistance (Rochat et al. 2001). Furthermore, CYP1B1 is involved in the metabolism of 17β‐estradiol and the formation of a toxicologically active metabolite, 4‐hydroxyestradiol (Tsuchiya et al. 2004). Therefore, inhibition of CYP1B1 is an attractive target for hormonally driven cancers such as breast (Gajjar et al. 2012). Tamoxifen, a selective estrogen receptor modulator, is approved for breast cancer prevention in high‐risk women; however, its metabolites have been shown to upregulate the CYP1B1 gene, a mechanism that may explain the association between long‐term tamoxifen use and a small increased risk of endometrial cancer (Tsuchiya et al. 2004; Williams‐Brown et al. 2011).
An agent‐like resveratrol that has high favorability among the general population could potentially serve as an alternative chemoprevention option. As little as 5 μmol/L resveratrol was able to reduce the catechol estrogen formation by through inhibition of CYP1B1 in human mammary epithelial cells (Chen et al. 2004). In overweight and obese postmenopausal women, 1 g daily resveratrol for 12 weeks also had a favorable effect on estrogen metabolism (Chow et al. 2014); however, the effect on CYP1B1 was not determined. While resveratrol may be an attractive chemoprevention option, combination treatments should be avoided, particularly among breast cancer patients (see continued discussion of tamoxifen under “CYP2D6″).
Preclinical studies have also demonstrated CYP1B1 inhibition by resveratrol. In baculovirus‐infected insect cell microsomes, the apparent Ki of heterologously expressed human CYP1B1 was found to be 0.8 μmol/L, similar to the apparent Ki of 1.2 μmol/L for CYP1A1 (Chang et al. 2001). Considering that transcription of CYP1B1 is also mediated by the AHR pathway (Go et al. 2015), inhibition by resveratrol likely occurs at the transcription level (see Fig. 4). Indeed, treatment of human MCF‐7 breast cancer cells with 10 μmol/L resveratrol inhibited TCDD‐induced expression of CYP1B1 (Beedanagari et al. 2009). In human lymphoblast microsomes, 200 μmol/L resveratrol was metabolized by CYP1B1 and produced the anticancer compound, piceatannol (Potter et al. 2002). Given that resveratrol's metabolites have a greater potential to reach mammary and other endocrine tissues, it may be more beneficial to investigate their effects on CYP1B1 than the parent compound.
Cyp2b6
CYP2B6 is a major hepatic enzyme involved in the activation of cyclophosphamide, an anticancer prodrug (Zanger and Schwab 2013) as well as bupropion, a drug used for smoking cessation and depression (Pekthong et al. 2012). Expression of CYP2B6 is regulated by xenosensing nuclear receptors, constitutive androstane receptor (CAR), and PXR (Zanger and Schwab 2013). Because resveratrol has been shown to inhibit PXR‐induced expression of CYP3A4 (Deng et al. 2014), it has also been suggested that the polyphenol may inhibit PXR‐induced expression of CYP2B6 (Smutny and Pavek 2014). Consistent with this hypothesis, liver microsomes from 6‐ to 12‐month‐old male rats incubated with high micromolar concentrations (100–250 μmol/L) resveratrol had significantly inhibited Cyp2b1, which shares a high sequence identity with human CYP2B6 (Huynh and Teel 2002). Resveratrol likewise demonstrated inhibition of CYP2B6 in human liver microsomes, but also at supraphysiological concentrations (IC50 of 100 μmol/L) (Piver et al. 2003).
There is also evidence, however, to suggest that resveratrol activates CAR and subsequently CYP2B6. In transfected HepG2 cells, 5 μmol/L resveratrol activated CAR as detected by CYP2B6 reporter activity (Yao et al. 2011). Consistent with CAR activation, multiple injections with 50 mg/kg (HED of 284 mg for a 70 kg human) resveratrol in mice induced testosterone metabolism by hepatic Cyp2b1/2 (Canistro et al. 2009). Differences in resveratrol's effect on CYP2B6 may be related to the fact that microsomal experiments tend to only observe inhibitory effects. It is also possible that CYP2B6 induction is caused by resveratrol metabolites. The effect of resveratrol on this enzyme should be elucidated in order to determine how concurrent supplementation may alter the efficacy of drugs metabolized by CYP2B6.
Cyp2e1
CYP2E1 is involved in the metabolism of various carcinogens, including volatile organic solvents like toluene and nitrosamines (Piver et al. 2001). Common carcinogens like alcohol, nicotine, and tobacco smoke increase the expression of this enzyme (Jiménez‐Garza et al. 2015) and inhibition of CYP2E1 could serve as a way to prevent carcinogenesis among those highly exposed to such compounds. For example, in rats with chemically induced hepatic cancer, 60 mg/kg resveratrol (HED of 681 mg for a 70 kg human) reduced Cyp2e1 expression and suppressed carcinogenesis (Wu et al. 2013). While expression of CYP2E1 is highest in the liver, it has also been detected in extrahepatic tissues including the brain, adrenal cortex, ovaries, testes, gastrointestinal tract, and cardiac tissue (Zanger and Schwab 2013), suggesting a potential for resveratrol to protect against cancer in multiple organs.
Resveratrol has inhibited microsomal CYP2E1, however, this mechanism is still unclear. In human and rat microsomes, 100 μmol/L resveratrol acted as a reversible, noncompetitive inhibitor of CYP2E1, as determined by kinetic studies (Piver et al. 2001). As little as 5.0 μmol/L was found to inhibit enzymatic activity in murine liver microsomes (Wang et al. 2015). Preincubation of murine liver microsomes with NADPH resulted in irreversible inhibition of CYP2E1 by similarly low doses of resveratrol (IC50 value of 18.5 μmol/L) (Mikstacka et al. 2002). The requirement of NADPH in this study suggests that resveratrol itself is metabolized by CYP2E1 and a reactive intermediate is responsible for inactivation. Mechanistic differences regarding resveratrol's inhibition of CYP2E1 could be due to differences in dose and substrate, or could be a result of the enzyme's multiple active sites (Liu et al. 2013). Nevertheless, an understanding of resveratrol's effect on this enzyme would provide additional insight regarding its anticancer properties due to the expression of CYP2E1 in multiple tissues, as well as its involvement in the metabolism of carcinogens.
Cyp2d6
CYP2D6 is found mainly in the liver, but has also been expressed in the gastrointestinal tract and brain (Zanger and Schwab 2013) and metabolizes approximately 25% of prescribed medications (Ingelman‐Sundberg 2005). CYP2D6 converts tamoxifen, a drug used for both treatment and prevention of breast cancer, to its active metabolite endoxifen (Jin et al. 2005).
In cell culture, inhibition of CYP2D6 by resveratrol and its main metabolite, R3S, was rather insignificant, with IC50 values of 87.9 μmol/L for resveratrol (Zhan et al. 2015) and > 50 μmol/L for R3S (Yu et al. 2003). In humans administered 1 g/day resveratrol for 4 weeks, however, CYP2D6 activity was decreased by 1.7‐fold, as measured by a dextromethorphan/dextrorphan molar ratio (Chow et al. 2010). While evidence is limited, these clinical results suggest that supplementation with resveratrol may significantly inhibit activation of tamoxifen, and combined treatment is likely not beneficial for breast cancer prevention and treatment.
Cyp2c9
CYP2C9 metabolizes several common drugs, including nonsteroidal anti‐inflammatory drugs, which target COX‐2 and may protect against the development of certain cancers (Ghosh et al. 2010). It also metabolizes drugs with narrow therapeutic indices, such as the anticoagulant (S)‐warfarin. Mice fed a diet containing 0.5% weight per weight resveratrol for 12 weeks experienced enhanced anticoagulant activity by warfarin, suggesting inhibition of Cyp2c9 (Chiba et al. 2016). In humans, a dose of 1 g daily resveratrol for 4 weeks was shown to inhibit CYP2C9 by 2.71‐fold using losartan as a probe drug. Blood concentrations of resveratrol reached an average of 72.7 ng/mL (0.32 μmol/L) in this study (Chow et al. 2010). One study found that R3S was not able to inhibit the recombinant CYP2C9 isozyme (Yu et al. 2003). Given the role that CYP2C9 plays in the metabolism of drugs with narrow therapeutic indices, even minor interactions could potentially impact the efficacy of these drugs.
Cyp2c19
CYP2C19, a mainly hepatic enzyme, plays a major role in the metabolism of proton pump inhibitors such as omeprazole and pantoprazole, antidepressants like citalopram, and endogenous compounds like progesterone (Zanger and Schwab 2013). Resveratrol has demonstrated moderate inhibition of CYP2C19 in microsomes containing the heterologously expressed isoform (IC50 of 11.6 μmol/L) (Yu et al. 2003) and the human recombinant form (IC50 of 22.5 μmol/L) (Orsini et al. 2016). Additionally, a naturally occurring trimer of resveratrol, α‐viniferin, potently inhibited CYP2C19 activity in human liver microsomes at an IC50 of 0.93 μmol/L (Sim et al. 2014). Evidence is limited regarding resveratrol's effect on CYP2C19 and whether such interactions have implications for cancer prevention.
Resveratrol and Phase II Metabolism
In phase II metabolism, xenobiotics and endogenous compounds are primed for elimination via bile or urine. The conjugating enzymes involved in this process typically act as transferases, adding large polar groups to their substrates (Jancova et al. 2010). Such enzymes include glutathione S‐transferase, NAD(P)H hydrogenase, quinone 1, UDP‐glucuronosyl transferase, and catechol‐O‐methyl transferase. By inducing phase II enzymes, resveratrol may facilitate the removal of carcinogens from the body. The effects of resveratrol in vivo and in vitro are summarized in Tables 1 and 2, respectively.
Glutathione S‐transferase
Glutathione S‐transferase (GST) facilitates the transfer reaction between the thiol moiety of glutathione and substrates intended for elimination (Jancova et al. 2010). GST protects against exposure to oxidative stress and carcinogens, such as nitrosamines and polycyclic aromatic hydrocarbons (Pool‐Zobel et al. 2005). GST inactivates estrogen quinones, protecting against estrogen‐induced cancers (Yager 2015). GST activation may also reduce the risk of colorectal (Klusek et al. 2014) and hepatic (Shen et al. 2014) cancers as well as protect against tobacco‐induced esophageal (Zendehdel et al. 2009) and prostate carcinomas (Kidd et al. 2003). Thus, induction of GST is considered a potential strategy for cancer prevention.
A growing body of evidence strongly supports an induction effect of resveratrol on GST. In rats with nonalcoholic fatty liver disease, 10 mg/kg resveratrol (HED of 114 mg for a 70 kg human) induced total Gst activity by 84% (Ali et al. 2015). It also induced Gst activity by 63% in mouse epidermis at a topical dose of 16 μmol/L in 0.2 mL acetone, and by 20% in human keratinocyte cells (20 μmol/L) (Cichocki et al. 2014). In aged mice, administration of 16.67 mg/kg resveratrol (95 mg for a 70 kg human) for 6 months increased heart and liver Gst levels (Tung et al. 2015). Resveratrol treatment also restored overall Gst activity in spontaneously hypertensive rats (Javkhedkar et al. 2015), streptozotocin‐induced diabetic rats (Sadi et al. 2015), rats with gentamicin‐induced renal damage (Silan et al. 2007), and in mice with B[a]P‐induced lung carcinogenesis (Liu et al. 2015). Furthermore, 10 mg/kg resveratrol restored both the expression and catalytic activity of Gst after pyrogallol‐induced reduction in mouse livers (Upadhyay et al., 2008). In humans, 1 g daily dose of resveratrol for 4 weeks induced GST activity among individuals with low baseline expression, although the overall effect was not significant (Chow et al. 2010). Given the association between GST induction and reduced cancer risk (McIlwain et al. 2006), induction of this enzyme could be a significant mechanism by which resveratrol provides protection against cancer.
NAD(P)H dehydrogenase, quinone 1 and 2 (NQO1 and NQO2)
NAD(P)H dehydrogenase, quinone 1 (NQO1) plays an important role in chemoprevention due to its ability to catalyze the reduction in quinones to catechols (Lu et al. 2008). This reduction is thought to decrease quinone‐induced oxidative stress and therefore protect the cell (Zhang et al. 2012). Conversely, its homolog, NQO2, does not appear to share such benefits, as Nqo2 knockout mice demonstrated increased antioxidant and detoxifying enzyme expression (Buryanovskyy et al. 2004).
In mouse hepatoma cells, 21 μmol/L resveratrol doubled Nqo1 activity (Mayhoub et al. 2012). Furthermore, 2.0 μmol/L resveratrol induced NQO1 mRNA expression by 4‐fold in normal human bronchial epithelial cells (Tan et al. 2012). Resveratrol also inhibited NQO2 in K562 cells (Buryanovskyy et al. 2004), which is doubly favorable in terms of chemoprevention. Furthermore, R3S induced NQO1 more potently than resveratrol in murine hepatoma cells, with only 2.6 μmol/L needed to double the concentration of NQO1 (Hoshino et al. 2010).
The induction of NQO1 by resveratrol could have significant implications for breast cancer prevention. Estrogens can be metabolized into catechols and further oxidized into ortho‐quinones, which react with DNA to form adducts (Cavalieri and Rogan 2014). By inducing NQO1, resveratrol may facilitate the reduction in semiquinones to catechols and subsequent inactivation by catechol‐O‐methyl transferase (Cavalieri and Rogan 2014; Yager 2015). Rats cotreated with 3 mg estradiol daily and 50 mg resveratrol every other month for 8 months had increased tumor latency and reduced estradiol‐induced tumor development, as well as upregulation of the Nqo1 gene in mammary tissue (Singh et al. 2014), demonstrating resveratrol's potential to protect against breast cancer.
These protective benefits, however, may be age‐ or tissue‐dependent. When mice of varying ages were given 16.67 mg/kg resveratrol daily (HED of 95 mg for a 70 kg human) for 6 months, Nqo1 activity increased in young (2‐month‐old) and aged (18‐month‐old) mice but decreased in mature (12‐month‐old) mice (Tung et al. 2013). Older mice given the same resveratrol treatment as described above had increased Nqo1 activity in the brain and liver, but decreased activity in the heart (Tung et al. 2015).
Clinical evidence also supports an inhibitory effect on NQO1. In patients with nonalcoholic fatty liver disease, 3 g daily resveratrol for 8 weeks decreased NQO1 expression in peripheral blood mononuclear cells (Chachay et al. 2014). Cancer patients receiving 5 mg resveratrol had higher levels of NQO1 in colorectal mucosa than those receiving 1 g resveratrol (Cai et al. 2015), suggesting a dose‐dependent response. Resveratrol's varied effects on NQO1 further underscore the need to determine which populations may benefit from supplementation and at what dose.
Uridine diphosphate‐ glucuronosyl transferase
UDP‐glucuronosyl transferases (UGTs) are detoxifying enzymes that facilitate the elimination of xenobiotics, such as carcinogens and pollutants, and endogenous substances, such as bile acids and hormones, through glucuronidation (Jancova et al. 2010). Increased expression of UGTs has been associated with reduced risk of various cancer types (Guillemette et al. 2014). In Caco‐2 intestinal cancer cells, 20 μmol/L resveratrol significantly induced UGT1A1 expression (Iwuchukwu et al. 2011). Hepatic Ugt activity was increased 83% in mice injected with 25 mg/kg (HED of 142 mg for a 70 kg human) (Canistro et al. 2009). In a clinical trial, 1 g daily resveratrol for 4 weeks significantly increased bilirubin clearance among subjects with low baseline UGT1A1 activity; however, the resveratrol intervention had a minimal effect on overall bilirubin clearance (Chow et al. 2010). Lack of UGT induction in this study could be due to resveratrol's low bioavailability, as resveratrol may not reach adequate concentrations in the liver to significantly induce bilirubin‐UGT.
Transport Proteins
Transport proteins play a major role in drug elimination. These transporters work in tandem with phase I and II metabolism, as various drugs are oxidized or conjugated to form substrates for these transporters (Chan et al. 2004). Transporters include ATP‐binding cassette (ABC) transport proteins like breast cancer resistance protein (BCRP, ABCG2) and multidrug resistance protein 3 (MRP3, ABCC3). They also include solute carrier transporters, such as organic anion transporting polypeptides (OATPs).
ABC transport proteins are present in the apical membrane of the small intestine, liver, and kidney, facilitating the elimination of xenobiotics by active efflux (Chan et al. 2004). Interactions with these transporters, therefore, could alter the bioavailability of xenobiotics. Supplemental doses of resveratrol (500 mg/day) have been shown to inhibit P‐glycoprotein (P‐gp, ABCB1), an ABC transporter (Bedada et al. 2014), as well as increase the bioavailability of P‐gp substrates such as doxorubicin, a first‐line breast cancer drug (Kim et al. 2014). In doxorubicin‐resistant human breast cancer cells, 12 μmol/L resveratrol was able to increase cytotoxicity and reverse doxorubicin resistance by downregulating the expression of P‐gp (Huang et al. 2014). While enhancement of P‐gp substrate bioavailability may be beneficial for cancer treatment, identifying the extent of resveratrol's activity is necessary to prevent toxicity.
Resveratrol and its metabolites are substrates for BCRP, which is responsible for their active efflux at the enterocyte and subsequent fecal excretion. In fact, efflux of glucuronidated and sulfated resveratrol metabolites was inhibited by 70% and 95%, respectively, in Bcrp knockout mice compared to wild‐type mice (Alfaras et al. 2010). MRP3 has a greater preference for glucuronidated metabolites, transporting these conjugated metabolites out of the cell for circulation and subsequent urinary excretion (van de Wetering et al. 2009). Evidence regarding interactions resveratrol and drug metabolism through these transporters, however, is lacking.
Additionally, resveratrol and its major sulfate metabolite, R3S, have been shown to interact with OATPs in hamster ovary and breast cancer cells, allowing for their uptake into enterocytes and hepatocytes (Riha et al. 2014). Competition for these transporters could result in significant interactions with drugs, including anticancer drugs like docetaxel and imatinib (Kalliokoski and Niemi 2009), or with flavonoids and other polyphenols that could potentially confer protection against cancer.
Conclusions and Future Directions
Resveratrol has received a great amount of attention over the past two decades due to its ability to inhibit cancer initiation, promotion, and progression in preclinical models (Singh et al. 2015). While these chemoprevention properties have been attributed multiple mechanisms, we have presented the available evidence describing resveratrol's effects on xenobiotic metabolism and drug transporters. Resveratrol has been shown to inhibit several important CYP enzymes, many of which are responsible for the bioactivation of carcinogens. Furthermore, resveratrol induces conjugating enzymes, facilitating the elimination of toxic substances. Since supraphysiological doses of resveratrol used in preclinical studies are not achievable in humans due to low bioavailability, it cannot be concluded that resveratrol's interaction with these enzymes is the sole mechanism by which it affords chemoprotection. However, evidence of nutrient‐drug interactions suggests that this mechanism may still contribute to resveratrol's overall anticancer properties. This evidence also suggests that resveratrol's interactions with drug metabolism are significant enough to warrant further investigation before it can be recommended clinically.
While in vitro studies provide a controlled environment for precise quantification of resveratrol's effect on phase I and II metabolism, such studies fail to capture the activity of resveratrol metabolites, which may have important clinical effects. With the limited evidence available, we have attempted to consider the activity of resveratrol's sulfated and glucuronidated metabolites to evaluate the translatability of certain animal models to humans. Future studies should investigate the anticancer and drug interaction activity of resveratrol metabolites, particularly those found in extrahepatic tissues, as they are more likely to reach these sites. Enzymes with minimal evidence available, such as CYP2C9, CYP2C19, and UGT, should perhaps receive particular attention.
Another priority for future studies should include identifying the ideal dose of resveratrol supplementation for cancer prevention. The vast majority of preclinical and clinical studies utilize very high doses. While a growing body of evidence supports higher efficacy of lower dose resveratrol for disease prevention (Cai et al. 2015; Posadino et al. 2015), lower doses could also mitigate nutrient‐drug interactions.
Caution should be taken when using supplemental doses of resveratrol for health benefits such as chemoprevention. This polyphenol has demonstrated significant interactions with phase I and II enzymes both in vitro and in vivo. While these interactions may be beneficial in terms of reduced activation and higher clearance of carcinogens, they could also result in nutrient‐drug interactions. Individuals taking drugs such as tamoxifen or warfarin, whose efficacy is highly dependent on specific CYP enzymes, could be particularly affected. Additional research is needed before recommendations can be made supporting resveratrol supplementation above dietary levels for prevention or therapeutic purposes.
Disclosure
None declared.
Guthrie A. R., Sherry Chow H.‐H., Martinez J. A.. Effects of resveratrol on drug‐ and carcinogen‐metabolizing enzymes, implications for cancer prevention, Pharma Res Per, 5(1), 2017, e00294, doi: 10.1002/prp2.294
References
- Aires V, Limagne E, Cotte AK, Latruffe N, Ghiringhelli F, Delmas D (2013). Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol Nutr Food Res 57: 1170–1181. [DOI] [PubMed] [Google Scholar]
- Alfaras I, Pérez M, Juan ME, Merino G, Prieto JG, Planas JM, et al. (2010). Involvement of breast cancer resistance protein (BCRP1/ABCG2) in the bioavailability and tissue distribution of trans‐ resveratrol in knockout mice. J Agric Food Chem 58: 4523–4528. [DOI] [PubMed] [Google Scholar]
- Ali MHH, Messiha BAS, Abdel‐Latif HA‐T (2015). Protective effect of ursodeoxycholic acid, resveratrol, and N‐acetylcysteine on nonalcoholic fatty liver disease in rats. Pharm Biol 54: 1198–1208. Taylor & Francis. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Ménard C, Quirion R (2015). Neuroprotective action of resveratrol. BBA Mol Basis Dis 1852: 1195–1201.Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Bedada SK, Nearati P (2015). Effect of resveratrol on the pharmacokinetics of carbamazepine in healthy human volunteers. Phyther Res 29: 701–706. [DOI] [PubMed] [Google Scholar]
- Bedada SK, Yakkanti SA, Neerati P (2014). Resveratrol enhances the bioavailability of fexofenadine in healthy human male volunteers : involvement of P‐glycoprotein inhibition. J Bioequiv Availab 6: 158–163. [Google Scholar]
- Beedanagari SR, Bebenek I, Bui P, Hankinson O (2009). Resveratrol inhibits dioxin‐induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci 110: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt JK, Thomas S, Nanjan MJ (2012). Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res 32: 537–541, Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Bitterman JL, Chung JH (2015). Metabolic effects of resveratrol: addressing the controversies. Cell Mol Life Sci 72: 1473–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode LM, Bunzel D, Huch M, Cho G‐S, Ruhland D, Bunzel M, et al. (2013). In vivo and in vitro metabolism of trans‐resveratrol by human gut microbiota. Am J Clin Nutr 97: 295–309. [DOI] [PubMed] [Google Scholar]
- Boocock D, Faust G, Patel K, Schinas A, Brown V, Ducharme M, Booth T, Crowell J, Perloff M, Gescher A, Steward W, Brenner D (2007). Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol biomarkers Prev 16: 1246–1252. [DOI] [PubMed] [Google Scholar]
- Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, et al. (2011). Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr 106: 383–389. [DOI] [PubMed] [Google Scholar]
- Brill SS, Furimsky AM, Ho MN, Furniss MJ, Li Y, Green AG, et al. (2006). Glucuronidation of trans‐resveratrol by human liver and intestinal microsomes and UGT isoforms. J Pharm Pharmacol 58: 469–479. [DOI] [PubMed] [Google Scholar]
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, et al. (2010). Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin‐like growth factor axis. Cancer Res 70: 9003–9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buryanovskyy L, Fu Y, Boyd M, Ma Y, Hsieh TC, Wu JM, et al. (2004). Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry 43: 11417–11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Scott E, Kholghi A, Andreadi C, Rufini A, Karmokar A, et al. (2015) Cancer chemoprevention: evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci Transl Med 7:298ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canistro D, Bonamassa B, Pozzetti L, Sapone A, Abdel‐Rahman SZ, Biagi GL, et al. (2009). Alteration of xenobiotic metabolizing enzymes by resveratrol in liver and lung of CD1 mice. Food Chem Toxicol 47: 454–461. [DOI] [PubMed] [Google Scholar]
- Carter LG, D'Orazio JA, Pearson KJ (2014). Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer 21: R209–R225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri E, Rogan E (2014). The molecular etiology and prevention of estrogen‐initiated cancers: Ockham's Razor: pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Mol Aspects Med 36: 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachay VS, Kirkpatrick CMJ, Hickman IJ, Ferguson M, Prins JB, Martin JH (2011). Resveratrol ‐ pills to replace a healthy diet? Br J Clin Pharmacol 72: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachay VS, Macdonald GA., Martin JH, Whitehead JP, O'Moore–Sullivan TM, Lee P, et al. (2014) Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 12:2092–2103.e6, Elsevier, Inc. [DOI] [PubMed] [Google Scholar]
- Chan WK, Delucchi AB (2000). Resveratrol, a red wine constituent, is a mechanism‐based inactivator of cytochrome P450 3A4. Life Sci 67: 3103–3112. [DOI] [PubMed] [Google Scholar]
- Chan LM, Lowes S, Hirst BH (2004). The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharm Sci 21: 25–51. [DOI] [PubMed] [Google Scholar]
- Chang TK, Chen J, Lee WB (2001). Differential inhibition and inactivation of human CYP1 enzymes by trans‐resveratrol: evidence for mechanism‐based inactivation of CYP1A2. J Pharmacol Exp Ther 299: 874–882. [PubMed] [Google Scholar]
- Chang TKH, Chen J, Yu C‐T (2007). In vitro inhibition of rat CYP1A1 and CYP1A2 by piceatannol, a hydroxylated metabolite of trans‐resveratrol. Drug Metab Lett 1: 13–16. [DOI] [PubMed] [Google Scholar]
- Chen Z‐H, Hurh Y‐J, Na H‐K, Kim J‐H, Chun Y‐J, Kim D‐H, et al. (2004). Resveratrol inhibits TCDD‐induced expression of CYP1A1 and CYP1B1 and catechol estrogen‐mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis 25: 2005–13. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kimura Y, Suzuki S, Tatefuji T, Umegaki K (2016). Trans ‐Resveratrol Enhances the Anticoagulant Activity of Warfarin. J Atheroscler Thromb 23: 1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Choi BC, Kang KW (2009). Effect of resveratrol on the pharmacokinetics of oral and intravenous nicardipine in rats: possible role of P‐glycoprotein inhibition by resveratrol. Pharmazie 64: 49–52. [PubMed] [Google Scholar]
- Chow HHS, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, et al. (2010). Resveratrol modulates drug‐ and carcinogen‐metabolizing enzymes in a healthy volunteer study. Cancer Prev Res 3: 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow H‐H, Garland LL, Heckman‐Stoddard BM, Hsu C‐H, Butler VD, Cordova CA, et al. (2014). A pilot clinical study of resveratrol in postmenopausal women with high body mass index: effects on systemic sex steroid hormones. J Transl Med 12: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichocki M, Szaefer H, Krajka‐Kuźniak V, Baer‐Dubowska W (2014). The effect of resveratrol and its methylthio‐derivatives on EGFR and Stat3 activation in human HaCaT and A431 cells. Mol Cell Biochem 396: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolino HP, Yeh GC (1999). Inhibition of aryl hydrocarbon‐induced cytochrome P‐450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol 56: 760–767. [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Yeh GC (1998). Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res 58: 5707–5712. [PubMed] [Google Scholar]
- Cottart CH, Nivet‐Antoine V, Beaudeux JL (2014). Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol Nutr Food Res 58: 7–21. [DOI] [PubMed] [Google Scholar]
- Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, et al. (2012). Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci 67: 1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS (2004). Resveratrol‐associated renal toxicity. Toxicol Sci 82: 614–619. [DOI] [PubMed] [Google Scholar]
- Dash S, Xiao C, Morgantini C, Szeto L, Lewis GF (2013). High‐dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler Thromb Vasc Biol 33: 2895–901. [DOI] [PubMed] [Google Scholar]
- Deng R, Xu C, Chen X, Chen P, Wang Y, Zhou X, et al. (2014). Resveratrol Suppresses the Inducible Expression of CYP3A4 Through the Pregnane X Receptor. J Pharmacol Sci 126: 146–154. [DOI] [PubMed] [Google Scholar]
- Detampel P, Beck M, Krähenbühl S, Huwyler J (2012). Drug interaction potential of resveratrol. Drug Metab Rev 44: 253–265. [DOI] [PubMed] [Google Scholar]
- Ding Y, Ward J, Hammond D, Watson C (2014). Mouth‐Level Intake of Benzo[a]pyrene from Reduced Nicotine Cigarettes. Int J Environ Res Public Health 11: 11898–11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay JR, Steele V, Crowell JA (2009). Energy homeostasis and cancer prevention: the AMP‐activated protein kinase. Cancer Prev Res (Phila) 2: 301–309. [DOI] [PubMed] [Google Scholar]
- Gajjar K, Martin‐Hirsch PL, Martin FL (2012). CYP1B1 and hormone‐induced cancer. Cancer Lett 324: 13–30, Elsevier Ireland Ltd. [DOI] [PubMed] [Google Scholar]
- Ghosh N, Chaki R, Mandal V, Mandal SC (2010). COX‐2 as a target for cancer chemotherapy. Pharmacol Rep 62: 233–44. [DOI] [PubMed] [Google Scholar]
- Go R‐E, Hwang K‐A, Choi K‐C (2015). Cytochrome P450 1 family and cancers. J Steroid Biochem Mol Biol 147: 24–30,Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Guillemette C, Lévesque É, Rouleau M (2014). Pharmacogenomics of human uridine diphospho‐glucuronosyltransferases and clinical implications. Clin Pharmacol Ther 96: 324–339. [DOI] [PubMed] [Google Scholar]
- Hong SP, Choi DH, Choi JS (2008). Effects of resveratrol on the pharmacokinetics of diltiazem and its major metabolite, desacetyldiltiazem, in rats. Cardiovasc Ther 26: 269–275. [DOI] [PubMed] [Google Scholar]
- Hoshino J, Park EJ, Kondratyuk TP, Marler L, Pezzuto JM, Van Breemen RB, et al. (2010). Selective synthesis and biological evaluation of sulfate‐conjugated resveratrol metabolites. J Med Chem 53: 5033–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Wu XN, Chen J, Wang WX, Lu ZF (2014). Resveratrol reverses multidrug resistance in human breast cancer doxorubicin‐resistant cells. Exp Ther Med 7: 1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh HT, Teel RW (2002). Effects of plant‐derived phenols on rat liver cytochrome P450 2B1 activity. Anticancer Res 22: 1699–1703. [PubMed] [Google Scholar]
- Ingelman‐Sundberg M (2005). Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J 5: 6–13. [DOI] [PubMed] [Google Scholar]
- Iwuchukwu OF, Tallarida RJ, Nagar S (2011). Resveratrol in combination with other dietary polyphenols concomitantly enhances antiproliferation and UGT1A1 induction in Caco‐2 cells. Life Sci 88: 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancova P, Anzenbacher P, Anzenbacherova E (2010). Phase II drug metabolizing enzymes. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 154: 103–116. [DOI] [PubMed] [Google Scholar]
- Javkhedkar AA, Quiroz Y, Rodriguez‐Iturbe B, Vaziri ND, Lokhandwala MF, Banday AA (2015). Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 308: R840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez‐Garza O, Baccarelli AA., Byun H‐M, Márquez‐Gamiño S, Barrón‐Vivanco BS, Albores A (2015) CYP2E1 epigenetic regulation in chronic, low‐level toluene exposure: relationship with oxidative stress and smoking habit. Toxicol Appl Pharmacol 286:207–215, Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, et al. (2005). CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97: 30–39. [DOI] [PubMed] [Google Scholar]
- Kalash R, Berhane H, Au J, Rhieu BH, Epperly MW, Goff J, et al. (2014). Differences in irradiated lung gene transcription between fibrosis‐prone C57BL/6NHsd and fibrosis‐resistant C3H/HeNHsd mice. In Vivo 28: 147–171. [PMC free article] [PubMed] [Google Scholar]
- Kalliokoski A, Niemi M (2009). Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 158: 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd LCR, Woodson K, Taylor PR, Albanes D, Virtamo J, Tangrea JA (2003). Polymorphisms in glutathione‐S‐transferase genes (GST‐M1, GST‐T1 and GST‐P1) and susceptibility to prostate cancer among male smokers of the ATBC cancer prevention study. Eur J cancer Prev Off J Eur Cancer Prev Organ ECP 12: 317–320. [DOI] [PubMed] [Google Scholar]
- Kim TH, Shin YJ, Won AJ, Lee BM, Choi WS, Jung JH, et al. (2014). Resveratrol enhances chemosensitivity of doxorubicin in multidrug‐resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim Biophys Acta ‐ Gen Subj 1840: 615–625, Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Klusek J, Głuszek S, Klusek J (2014) GST gene polymorphisms and the risk of colorectal cancer development. Contemp Oncol (Poznań, Poland) 18:219–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe XF, Tengku Muhammad TS, Chong AS‐C, Wahab HA, Tan ML (2014). Cytochrome P450 induction properties of food and herbal‐derived compounds using a novel multiplex RT‐qPCR in vitro assay, a drug‐food interaction prediction tool. Food Sci Nutr 2: 500–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SS, Cantó C (2014). The molecular targets of resveratrol. Biochim Biophys Acta ‐ Mol Basis Dis 1852: 1114–1123, Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Lee JE, Safe S (2001). Involvement of a post‐transcriptional mechanism in the inhibition of CYP1A1 expression by resveratrol in breast cancer cells. Biochem Pharmacol 62: 1113–1124. [DOI] [PubMed] [Google Scholar]
- de Ligt M, Timmers S, Schrauwen P (2015). Resveratrol and obesity: can resveratrol relieve metabolic disturbances? Biochim Biophys Acta ‐ Mol Basis Dis 1852: 1137–1144, Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Q, Wu DC, Wang XW, Sun Y, Chen XY, et al. (2004). Differential regulation of CYP1A1 and CYP1B1 expression in resveratrol‐treated human medulloblastoma cells. Neurosci Lett 363: 257–261. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu BY, Hao P, Li X, Li YX, Wang JF (2013). Pi‐Pi Stacking mediated drug‐drug interactions in human CYP2E1. Proteins Struct Funct Bioinforma 81: 945–954. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu Y, Yu Y, Cao C, Zhang J, Li K, et al. (2015). Curcumin and resveratrol in combination modulate drug‐metabolizing enzymes as well as antioxidant indices during lung carcinogenesis in mice. Hum Exp Toxicol 34: 620–627. [DOI] [PubMed] [Google Scholar]
- Lu F, Zahid M, Wang C, Saeed M, Cavalieri EL, Rogan EG (2008). Resveratrol prevents estrogen‐DNA adduct formation and neoplastic transformation in MCF‐10F cells. Cancer Prev Res 1: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhoub AS, Marler L, Kondratyuk TP, Park EJ, Pezzuto JM, Cushman M (2012). Optimization of thiazole analogues of resveratrol for induction of NAD(P)H:quinone reductase 1 (QR1). Bioorganic Med Chem 20: 7030–7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain CC, Townsend DM, Tew KD (2006). Glutathione S‐transferase polymorphisms: cancer incidence and therapy. Oncogene 25: 1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez‐del Villar M, González‐Ortiz M, Martínez‐Abundis E, Pérez‐Rubio KG, Lizárraga‐Valdez R (2014). Effect of Resveratrol Administration on Metabolic Syndrome, Insulin Sensitivity, and Insulin Secretion. Metab Syndr Relat Disord 12: 497–501, Mary Ann Liebert, Inc. [DOI] [PubMed] [Google Scholar]
- Miksits M, Maier‐Salamon A, Aust S, Thalhammer T, Reznicek G, Kunert O, et al. (2005). Sulfation of resveratrol in human liver: evidence of a major role for the sulfotransferases SULT1A1 and SULT1E1. Xenobiotica 35: 1101–1119. [DOI] [PubMed] [Google Scholar]
- Mikstacka R, Gnojkowski J, Baer‐Dubowska W (2002). Effect of natural phenols on the catalytic activity of cytochrome P450 2E1. Acta Biochim Pol 49: 917–925. [PubMed] [Google Scholar]
- Omiecinski CJ, Vanden Heuvel JP, Perdew GH, Peters JM (2011). Xenobiotic metabolism, disposition, and regulation by receptors: from biochemical phenomenon to predictors of major toxicities. Toxicol Sci 120: 49–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini F, Verotta L, Klimo K, Gerhäuser C (2016). Synthesis of Resveratrol Derivatives and in Vitro Screening for Potential Cancer Chemopreventive Activities. Arch Pharm (Weinheim) 349: 414–427. [DOI] [PubMed] [Google Scholar]
- Patel KR, Andreadi C, Britton RG, Horner‐Glister E, Karmokar A, Sale S, et al. (2013) Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci Transl Med 5:205ra133. [DOI] [PubMed] [Google Scholar]
- Pekthong D, Desbans C, Martin H, Richert L (2012). Bupropion hydroxylation as a selective marker of rat CYP2B1 catalytic activity. Drug Metab Dispos 40: 32–38. [DOI] [PubMed] [Google Scholar]
- Piver B, Berthou F, Dreano Y, Lucas D (2001). Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol Lett 125: 83–91. [DOI] [PubMed] [Google Scholar]
- Piver B, Berthou F, Dreano Y, Lucas D (2003). Differential inhibition of human cytochrome P450 enzymes by ε‐viniferin, the dimer of resveratrol: comparison with resveratrol and polyphenols from alcoholized beverages. Life Sci 73: 1199–1213. [DOI] [PubMed] [Google Scholar]
- Pool‐Zobel B, Veeriah S, Bohmer FD (2005). Modulation of xenobiotic metabolising enzymes by anticarcinogens—focus on glutathione S‐transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res ‐ Fundam Mol Mech Mutagen 591: 74–92. [DOI] [PubMed] [Google Scholar]
- Posadino AM, Cossu A, Giordo R, Zinellu A, Sotgia S, Vardeu A, et al. (2015). Resveratrol alters human endothelial cells redox state and causes mitochondrial‐dependent cell death. Food Chem Toxicol 78: 10–6, Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Potter GA, Patterson LH, Wanogho E, Perry PJ, Butler PC, Ijaz T, et al. (2002). The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br J Cancer 86: 774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde‐Jørgensen H, et al. (2013). High‐dose resveratrol supplementation in obese men: an investigator‐initiated, randomized, placebo‐controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 62: 1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Sun J, Xia S, Tang X, Shi Y, Le G (2014). Effects of resveratrol on gut microbiota and fat storage in a mouse model with high‐fat‐induced obesity. Food Funct 5: 1241. [DOI] [PubMed] [Google Scholar]
- Ramis MR, Esteban S, Miralles A, Tan D‐X, Reiter RJ (2015) Caloric restriction, resveratrol and melatonin: role of SIRT1 and implications for aging and related‐diseases. Mech Ageing Dev 146–148:28–41, Elsevier Ireland Ltd. [DOI] [PubMed] [Google Scholar]
- Riha J, Brenner S, Böhmdorfer M, Giessrigl B, Pignitter M, Schueller K, et al. (2014). Resveratrol and its major sulfated conjugates are substrates of organic anion transporting polypeptides (OATPs): impact on growth of ZR‐75‐1 breast cancer cells. Mol Nutr Food Res 58: 1830–1842. [DOI] [PubMed] [Google Scholar]
- Rochat B, Morsman JM, Murray GI, Figg WD, McLeod HL (2001). Human CYP1B1 and anticancer agent metabolism: mechanism for tumor‐specific drug inactivation? J Pharmacol Exp Ther 296: 537–541. [PubMed] [Google Scholar]
- Rose M, Holland J, Dowding A, Petch SRG, White S, Fernandes A, et al. (2015). Investigation into the formation of PAHs in foods prepared in the home to determine the effects of frying, grilling, barbecuing, toasting and roasting. Food Chem Toxicol 78: 1–9. [DOI] [PubMed] [Google Scholar]
- Ruotolo R, Calani L, Fietta E, Brighenti F, Crozier A, Meda C , et al. (2013). Anti‐estrogenic activity of a human resveratrol metabolite. Nutr Metab Cardiovasc Dis 23: 1086–1092. [DOI] [PubMed] [Google Scholar]
- Saberi Hosnijeh F, Boers D, Portengen L, Bueno‐de‐Mesquita HB, Heederik D, Vermeulen R (2012). Plasma Cytokine Concentrations in Workers Exposed to 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD). Front Oncol 2: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadi G, Baloğlu MC, Pektaş MB (2015). Differential Gene Expression in Liver Tissues of Streptozotocin‐Induced Diabetic Rats in Response to Resveratrol Treatment. PLoS ONE 10: e0124968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried TN, Shelton LM (2010). Cancer as a metabolic disease. Nutr Metab (Lond) 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y‐H, Chen S, Peng Y‐F, Shi Y‐H, Huang X‐W, Yang G‐H, et al. (2014). Quantitative assessment of the effect of glutathione S‐transferase genes GSTM1 and GSTT1 on hepatocellular carcinoma risk. Tumor Biol 35: 4007–4015. [DOI] [PubMed] [Google Scholar]
- Silan C, Uzun O, Comunoğlu NU, Gokçen S, Bedirhan S, Cengiz M (2007). Gentamicin‐induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol Pharm Bull 30: 79–83. [DOI] [PubMed] [Google Scholar]
- Sim J, Jang HW, Song M, Kim JH, Lee SH, Lee S (2014). Potent inhibitory effect of alpha‐viniferin on human cytochrome P450. Food Chem Toxicol 69: 276–280, Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Singh B, Shoulson R, Chatterjee A, Ronghe A, Bhat NK, Dim DC, et al. (2014). Resveratrol inhibits estrogen‐induced breast carcinogenesis through induction of NRF2‐mediated protective pathways. Carcinogenesis 35: 1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CK, Ndiaye M A., Ahmad N (2015) Resveratrol and cancer: challenges for clinical translation. Biochim Biophys Acta ‐ Mol Basis Dis 1852:1178–1185, Elsevier B.V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny T, Pavek P (2014). Resveratrol as an Inhibitor of Pregnane X Receptor (PXR): another Lesson in PXR Antagonism. J Pharmacol Sci 126: 177–178. [DOI] [PubMed] [Google Scholar]
- Szkudelski T, Szkudelska K (2015). Resveratrol and diabetes: from animal to human studies. Biochim Biophys Acta ‐ Mol Basis Dis 1852: 1145–1154, Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Tan XL, Marquardt G, Massimi AB, Shi M, Han W, Spivack SD (2012). High‐throughput library screening identifies two novel NQO1 inducers in human lung cells. Am J Respir Cell Mol Biol 46: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thazhath SS, Wu T, Bound MJ, Checklin HL, Standfield S, Jones KL, et al. (2016). Administration of resveratrol for 5 wk has no effect on glucagon‐like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: a randomized controlled trial. Am J Clin Nutr 103: 66–70. [DOI] [PubMed] [Google Scholar]
- Tian D, Hu Z (2014). CYP3A4‐mediated Pharmacokinetic Interactions in Cancer Therapy. Curr Drug Metab 15: 808–817. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. (2011). Calorie restriction‐like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14: 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y, Nakajima M, Kyo S, Kanaya T, Inoue M, Yokoi T (2004). Human CYP1B1 Is Regulated by Estradiol via Estrogen Receptor. Cancer Res 64: 3119–3125. [DOI] [PubMed] [Google Scholar]
- Tung BT, Rodríguez‐Bies E, Ballesteros‐Simarro M, Motilva V, Navas P, López‐Lluch G (2013). Modulation of endogenous antioxidant activity by resveratrol and exercise in mouse liver is age dependent. J Gerontol A Biol Sci Med Sci 9: 1–12. [DOI] [PubMed] [Google Scholar]
- Tung BT, Rodriguez‐Bies E, Thanh HN, Le‐Thi‐Thu H, Navas P, Sanchez VM, et al. (2015). Organ and tissue‐dependent effect of resveratrol and exercise on antioxidant defenses of old mice. Aging Clin Exp Res. doi:10.1007/s40520‐015‐0366‐8. [DOI] [PubMed] [Google Scholar]
- Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. (2015). A randomized, double‐blind, placebo‐controlled trial of resveratrol for Alzheimer disease. Neurology 85: 1383–91, Lippincott Williams & Wilkins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay G, Singh AK, Kumar A, Prakash O, Singh MP (2008). Resveratrol modulates pyrogallol‐induced changes in hepatic toxicity markers, xenobiotic metabolizing enzymes and oxidative stress. Eur J Pharmacol 596: 146–152. [DOI] [PubMed] [Google Scholar]
- Uzunlulu M, Telci Caklili O, Oguz A (2016). Association between Metabolic Syndrome and Cancer. Ann Nutr Metab 68: 173–179. [DOI] [PubMed] [Google Scholar]
- Walle T, Hsieh F, Delegge MH, Oatis JE, Walle UK (2004). High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab disposition 32: 1377–1382. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang Y, Fan X, Tan H, Zeng H, Wang Y, et al. (2015). Hepato‐protective effect of resveratrol against acetaminophen‐induced liver injury is associated with inhibition of CYP‐mediated bioactivation and regulation of SIRT1‐p53 signaling pathways. Toxicol Lett 236: 82–89, Elsevier Ireland Ltd. [DOI] [PubMed] [Google Scholar]
- Wenzel E, Somoza V (2005). Metabolism and bioavailability of trans‐resveratrol. Mol Nutr Food Res 49: 472–481. [DOI] [PubMed] [Google Scholar]
- van de Wetering K, Burkon A, Feddema W, Bot A, De Jonge H, Somoza V, et al. (2009). Intestinal Breast Cancer Resistance Protein (BCRP)/Bcrp1 and Multidrug Resistance Protein 3 (MRP3)/Mrp3 Are Involved in the Pharmacokinetics of Resveratrol. Mol Pharmacol 75: 876–885. [DOI] [PubMed] [Google Scholar]
- Williams‐Brown MY, Salih SM, Xu X, Veenstra TD, Saeed M, Theiler SK, et al. (2011). The effect of tamoxifen and raloxifene on estrogen metabolism and endometrial cancer risk. J Steroid Biochem Mol Biol 126: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Kerti L, Margulies DS, Floel A (2014). Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J Neurosci 34: 7862–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Li C, Xing G, Qi X, Ren J (2013). Resveratrol Downregulates Cyp2e1 and Attenuates Chemically Induced Hepatocarcinogenesis in SD Rats. J Toxicol Pathol 26: 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JD (2015). Mechanisms of estrogen carcinogenesis: the role of E2/E1–quinone metabolites suggests new approaches to preventive intervention – A review. Steroids 99: 56–60, Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Yasuoka A, Kamei A, Kitagawa Y, Rogi T, Taieishi N, et al. (2011). Polyphenols in Alcoholic Beverages Activating Constitutive Androstane Receptor CAR. Biosci Biotechnol Biochem 75: 1635–1637. [DOI] [PubMed] [Google Scholar]
- Yu C, Shin YG, Kosmeder JW, Pezzuto JM, van Breemen RB (2003). Liquid chromatography/tandem mass spectrometric determination of inhibition of human cytochrome P450 isozymes by resveratrol and resveratrol‐3‐sulfate. Rapid Commun Mass Spectrom 17: 307–313. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Schwab M (2013). Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of gentic variation. Pharmacol Ther 138: 103–141, Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Zendehdel K, Bahmanyar S, McCarthy S, Nyren O, Andersson B, Ye W (2009). Genetic polymorphisms of glutathione S‐transferase genes GSTP1, GSTM1, and GSTT1 and risk of esophageal and gastric cardia cancers. Cancer Causes Control 20: 2031–2038. [DOI] [PubMed] [Google Scholar]
- Zhan Y‐Y, Liang B‐Q, Li X‐Y, Gu E‐M, Dai D‐P, Cai J‐P, et al. (2015). The effect of resveratrol on pharmacokinetics of aripiprazole in vivo and in vitro. Xenobiotica 46: 439–444, Taylor & Francis. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jin Y, Huang M, Penning TM (2012). The role of human aldo‐keto reductases in the metabolic activation and detoxication of polycyclic aromatic hydrocarbons: interconversion of PAH catechols and PAH o‐quinones. Front Pharmacol NOV3: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Chan SY, Goh BC, Chan E, Duan W, Huang M, et al. (2005). Mechanism‐based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet 44: 279–304. [DOI] [PubMed] [Google Scholar]
- Zhou S‐F, Wang B, Yang L‐P, Liu J‐P (2010). Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metab Rev 42: 268–354. [DOI] [PubMed] [Google Scholar]
- Zordoky BNM, Robertson IM, Dyck JRB (2015). Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta ‐ Mol Basis Dis 1852: 1155–1177, Elsevier B.V. [DOI] [PubMed] [Google Scholar]


