Our data demonstrate that IL-1β can impair the functional recovery of neural stem cell transplant therapy for spinal cord injury (SCI) treatment in rats. This effect is dependent on microRNA-372 (miR-372)-dependent gene repression of KIF3B and NOSIP. Therefore, specific knockdown of miR-372 may provide benefits for SCI treatments.
Keywords: KIF3B, microRNA, neural stem cell, NOSIP, spinal cord injury
Abstract
Excessive inflammation including IL-1β-initiated signaling is among the earlies reactions that can cause neuronal damage following spinal cord injury (SCI). It has been suggested that microRNAs may participate in stem cell repair to facilitate functional recovery following SCI. In this study we have shown that in cultured human neural stem cells (hNSC), IL-1β reduced the expression of both KIF3B (kinesin family member 3B) and NOSIP (nitric oxide synthase-interacting protein), two key modulators for restricting inflammation and promoting neuronal regeneration. The induction of microRNA-372 (miR-372) by IL-1β is specifically responsible for the inhibition of KIF3B and NOSIP. The 3′-untranslated regions (UTRs) of both KIF3B and NOSIP contain targeting sequences to miR-372 that directly inhibit their expression. Moreover, we found that the expression of miR-372 was stimulated in hNSC by IL-1β through an NF-κB binding site at its promoter region. Finally, stable overexpression of miR-372 inhibitor in hNSC rescued the IL-1β-induced impairment as shown by significant improvements in tissue water content, myeloperoxidase activity, and behavioral assessments in SCI rats. These findings suggest a critical role of miR-372 in inflammatory signaling and pinpoint a novel target for the treatment of acute SCI.
NEW & NOTEWORTHY Our data demonstrate that IL-1β can impair the functional recovery of neural stem cell transplant therapy for spinal cord injury (SCI) treatment in rats. This effect is dependent on microRNA-372 (miR-372)-dependent gene repression of KIF3B and NOSIP. Therefore, specific knockdown of miR-372 may provide benefits for SCI treatments.
spinal cord injury (SCI) often leads to a profound impairment in movement and sensation of patients, which may cause paralysis, disability, and even death in severe cases (Wu et al. 2012). As reported in a recent study, the incidence of SCI in mainland China is increasing annually by ~120,000 new cases per year and has now exceed 1 million cases (Liu et al. 2012). It remains a challenge to treat SCI patients despite improved medical care. Since neuron progenitor cells or neural stem cells (NSCs) contain unique properties in restoring tissue damage and restricting neuronal loss, NSC transplantation could be used as a promising therapeutic option for patients with neurological disorders including SCI. A number of preclinical studies have demonstrated the effectiveness of NSC transplant in animal models of SCI (Cummings et al. 2005; Iwanami et al. 2005; Teng et al. 2002). For instance, an implantation of the scaffold–NSC unit into an adult rat hemisection model of SCI promoted long-term improvement in neurological function (Teng et al. 2002). In immune-deficient mice, central nerve system (CNS) injury was attenuated by the engraftment of human NSCs (Cummings et al. 2005). In addition, previous studies in primates have supported the potential clinical benefits of human NSC transplantation in repairing injured spinal cords (Iwanami et al. 2005).
To effectively and safely expand the therapeutic potential of NSCs, it is important to overcome the inhibitory milieu of the injured spinal cord that significantly limits neuronal growth and formation of new relay circuits to recover CNS functions. Interleukin-1β (IL-1β) is a cytokine that is involved in proinflammatory reactions during neuronal damage. It has been shown that cell death in the spinal cord after injury may be caused in part by increased synthesis and release of IL-1β (Nesic et al. 2001). The elevation of IL-1β, along with oxidative stress elicited during traumatic or hypoxic injuries, is considered to be a major component of the inhibitory environment in the injured CNS (Hausmann 2003). In addition to apoptosis induction, the consequences of such cytokine-orchestrated inflammation following CNS trauma may include excessive nitric oxide production (Lewén et al. 2000) and disrupted motor function (Yu et al. 2013). However, it is unknown if and how IL-1β-induced signaling in the neuron may participate in these two processes. To answer these questions, we treated human neural stem cells (hNSCs) in vitro with IL-1β and then focused on two gene modulators whose expressions may be repressed in inflammation. NOSIP (nitric oxide synthase-interacting protein) is a negative regulator of nitric oxide synthases, and KIF3B (kinesin family member 3B) is a member of a motor complex responsible for intracellular transport and neuronal differentiation. Because previous reports have suggested that induction of both genes plays critical roles in functional recovery after SCI (Yu et al. 2012; Yu et al. 2013), studying the effects of IL-1β on these genes could improve our understanding toward a potential mechanism by which proinflammatory cytokines negatively impact the healing potential of NSCs in the injured spinal cord. Furthermore, a putative signaling pathway for the inhibitory role of IL-1β in hNSC was investigated.
As a class of molecular modulators, microRNAs (miR) can function as regulatory molecules that affect many biological processes, mainly by suppressing expressions of their target genes (Betel et al. 2010). In the current study, we hypothesized that miRs may serve as potential transducer implicated in IL-1β-induced gene repression, which has not been previously explored. As posttranscriptional regulators, miRs are expressed from noncoding genome regions and target the complementary sequences on the 3′-untranslated regions (UTRs) of genes (Betel et al. 2010). We utilized a nonbiased approach and identified miR-372 as a novel miR that is upregulated by IL-1β. We further demonstrated that miR-372 directly targeted the 3′-UTRs of both genes and inhibited their expression. IL-1β can directly stimulate miR-372 expression via an NF-κB-binding site on the promoter region of miR-372. Finally, inhibiting miR-372 expression in the transplanted hNSC enabled the rescue of IL-1β-induced functional impairment in a rat model of SCI. These studies suggest that a specific miR could act as a molecular suppressor of neural function recovery for SCI, which then could be used as a novel target for treating SCI patients.
MATERIALS AND METHODS
Human neural stem cell isolation, characterization, and treatment.
Adult NSCs were isolated from the spinal cords of two patients as described previously (Mothe and Tator 2015). All procedures were approved by the ethics committee of Shanghai Sixth People’s Hospital East Affiliated to Shanghai University of Medicine & Health Sciences and were in compliance with the research protocols involving human subjects. The periventricular spinal cord tissues were obtained from organ transplant donors with written informed consent. In brief, tissues were aseptically harvested and minced into pieces, followed by digestion with papain (20 U/ml) containing DNase I, 1 mM l-cysteine, and 0.5 mM EDTA. Cell suspensions were washed once and centrifuged through a discontinuous density gradient to remove membrane fragments. The cell pellets at the bottom were resuspended and filtered through a cell strainer before being plated in precoated culture plates in serum-free Neurobasal-A medium (Thermo Fisher Scientific, Waltham, MA) containing 20 ng/ml EGF, 20 ng/ml basic FGF, 2 mM l-glutamine, 100 μg/ml penicillin-streptomycin, 2% B-27, and 2 μg/ml heparin. Adherent hNSCs were harvested by trypsinization and resuspended in PBS containing 4% fetal bovine serum. After being washed, cells were stained by antibodies (eBioscience, San Diego, CA) for previously characterized hNSC surface markers (Yuan et al. 2011), including CD24 (14-0242; eBioscience) and CD184 (14-9991; eBioscience) positive and CD44 (14-0441; eBioscience) and CD271 (14-9400; eBioscience) negative. Isotype control antibody-stained cells were used to optimize photomultiplier tube (PMT) and compensation in the analysis using BD-FACScan. Flow cytometry data were analyzed with FlowJo. The cells were cultured at 37°C with 5% humidified CO2.
Drugs and treatments.
IL-1β was purchased from Sigma (I2393; St. Louis, MO) and used to treat the hNSC at a working concentration of 10 ng/ml, with PBS as vehicle control. BIX 02188 (S1530), SP600125 (S1460), and TPCA-1 (S2824) were all purchased from Selleckchem (Houston, TX) and used to treat the hNSC at working concentrations of 2, 5, and 1 μM, respectively, with DMSO as vehicle control.
MicroRNA profiling.
The differential regulation of miRs in hNSCs by IL-1β treatment was performed by a microarray-based analysis (Liu et al. 2008). The putative regulatory miRs based on 3′-UTRs of NOSIP or KIF3B human genes were analyzed by TargetScanHuman (Lewis et al. 2005). The resultant miRs were selected for miR microarray with a customized probe set containing designed oligos (Integrated DNA Technologies, IDT) based on the miR registry database (Liu et al. 2008). Total RNA including miR was isolated from cultured cells at the indicated conditions using Trizol (Invitrogen, Carlsbad, CA) and was labeled as the first-strand biotin-cDNA. The hybridization steps were performed on a hybridization station (Tecan) followed by an indirect detection of streptavidin-Alexa Fluor 647 conjugate (Invitrogen). The image analysis of microarray was carried out using GenePix Pro (Molecular Devices, Sunnyvale, CA).
MicroRNA assays.
Expression levels of miR-372 were specifically measured with a mature miR assay kit (478071_mir; Applied Biosystems, Waltham, MA) according to the manufacturer’s instructions. The miR mimic for human miR-372 (mirVana, MC10165; Applied Biosystems) was transfected into cells using Lipofectamine RNAiMAX (Invitrogen). The MISSION Lenti miR-372 inhibitor (HLTUD0530; Sigma) was used to create stable cell lines by lentiviral delivery of miR-372 inhibitory oligos.
Quantitative real-time PCR.
Total isolated RNA from cell cultures was reverse transcribed to complementary cDNAs by using SuperScript II according to the manufacturer’s instructions (Bio-Rad, Hercules, CA). Specific primers used for human gene transcripts are described as follows: KIF3B, forward 5′-TGG ATG TGG ATG TTA AGC TGG G-3′, reverse 5′-TCG GAA CGT CTC ATC GTA CAG-3′; NOSIP, forward 5′-CCC AGA ACA TTC GAC TGA GCC-3′, reverse 5′-TGA CAA CAG GAT CGT GGC AAG-3′; and GAPDH, forward 5′-ACA ACT TTG GTA TCG TGG AAG G-3′, reverse 5′-AAG TGG TCG TTG AGG GCA ATG-3′. The SYBR Green dye-based detection method was used (SYBR Green PCR Master Mix assay; Applied Biosystems). A series of dilutions of control cDNA were used to generate the standard curves and validate the melting curves for each primer set. Triplicated PCR reactions were carried out for each sample. GAPDH was used as a housekeeping gene for normalization.
Luciferase assay.
For the evaluation of miR-372 targeting potential, NSCs were transfected with pGL3 luciferase reporter constructs harboring the miR-372 target sequence (wild type or mutant) for the 3′-UTR of either KIF3B or NOSIP genes. For transcription reporter assay of miR-372, the putative promoter region was selected on the basis of the upstream sequence of human miR-372 gene from the UCSC Genome Browser. The proximal sequence of miR-372 gene (~1 kb) contains two potential binding sites for NF-κB and has been cloned into pGL3 luciferase reporter plasmid at the upstream of a luciferase (Luc) open reading frame. Mutagenesis was done by using a QuikChange site-directed mutagenesis kit (Stratagene) and confirmed by DNA sequencing. All transfections were performed using the Nucleofector system (Lonza, Basel, Switzerland), and the activities of firefly luciferase and Renilla luciferase in the cell lysates were measured with the Dual-Luciferase assay system (Promega, Madison, WI).
Western blotting.
Western blotting was performed in cultured cells following various treatments. The protein lysates (1% NP-40, 50 mM Tris, 5 mM EDTA, 1% SDS, 1% sodium deoxycholate, 1% Triton X-100, 1 mM PMSF, 10 mg/ml aprotinin, and 1 mg/ml leupeptin, pH = 7.5) were measured by Bradford assay, and the same amount of protein was resolved on SDS-PAGE followed by an electric transfer to a polyvinylidene difluoride membrane. The blots were blocked by 5% nonfat milk and incubated with primary antibodies, including KIF3B (sc-50456, 1:1,000; Santa Cruz Biotechnology, Dallas, TX), NOSIP (sc-365363, 1:1,000; Santa Cruz), p65 NF-κB (sc-514451, 1:1,000; Santa Cruz), and actin (sc-58673, 1:2,000; Santa Cruz). The blots were then incubated by appropriate horseradish peroxidase-conjugated secondary antibodies (sc-2005 or sc-2054), and signals were visualized by an enhanced chemiluminescence-based imaging system.
Chromatin immunoprecipitation.
The endogenous binding of NF-κB to the promoter of miR-372 gene in hNSC was examined using a chromatin immunoprecipitation (ChIP) assay kit (Millipore, Billerica, MA). In brief, cells were cross-linked with 1% formaldehyde, and the genomic DNA was then sheared into an average size of 400 bp (followed by an immunoprecipitation by anti-NF-κB p65; Santa Cruz). An immunoprecipitation with nonspecific IgG was also used as a negative control. Eluted DNA from the immunoprecipitation was analyzed by quantitative PCR. The ChIP-PCR primers were used to span each of two putative NF-κB binding sites in the promoter region of human miR-372. The result of binding was calculated as the fold enrichment of the ChIP sample relative to the control IgG samples.
Rat model of spinal cord injury.
Male Sprague-Dawley rats, aged 6–8 wk and weighing 200–220 g, were used in the study with approval from the animal research review committee of Shanghai University of Medicine & Health Sciences Shanghai Sixth People’s Hospital East Campus. All methods were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. In a spinal cord contusion injury model, the anesthetized rats received dorsal laminectomy at the level of the ninth thoracic vertebra (T9) followed by contusion injury using the NYU Impactor (Gruner 1992), in which a 10-g weight impact rod was dropped from a height of 15 cm. The exposed spinal cord was then intrathecally administered through a 30-gauge needle with 4 μl of cultured hNSCs (5 × 105 cells/4 μl) at the site of the injury. Rats were assigned to the following experimental groups: 1) sham (n = 12), rats underwent sham operations; 2) SCI (n = 12), rats underwent SCI operations; 3) SCI + PBS (n = 12), rats underwent SCI operations and were injected with PBS-treated hNSC; 4) SCI + IL-1β (n = 12), rats underwent SCI operations and were injected with IL-1β-treated hNSC; 5) SCI + control (n = 12), rats underwent SCI operations and were injected with hNSC expressing negative miR inhibitor; 6) SCI + anti-miR-372 (n = 12), rats underwent SCI operations and were injected with hNSC expressing miR-372-3p inhibitor; 7) SCI + anti-miR-372 + PBS (n = 12), rats underwent SCI operations and were injected with PBS-treated hNSC expressing miR-372-3p inhibitor; and 8) SCI + anti-miR-372 + IL-1β (n = 12), rats underwent SCI operations and were injected with IL-1β-treated hNSC expressing miR-372-3p inhibitor. Skin was closed by sutures, and the animals were allowed to recover on a heating pad. Postoperative treatments included saline for rehydration and Baytril to prevent urinary tract infection. After transplant, the rats were orally administered with 100 mg/ml enrofloxacin (Bayer Animal Health) in drinking water.
Behavioral assessments.
SCI extent was evaluated at 1 day postinjury using BBB (Basso, Beattie, and Bresnahan) scores. In brief, locomotor activity was examined over a period of 4 min by independent observers in a blinded manner. The locomotor function of rats was determined on the basis of the BBB scale as described previously (Buller et al. 2010).
Spinal cord water content.
Spinal cord edema was assessed by the determination of spinal cord water content, which was calculated as %spinal cord water = (wet weight − dry weight)/wet weight × 100%. The dry weight (Havppvel method) was measured after the injured spinal cords were dried for 24 h at 110°C.
Myeloperoxidase activity.
Twenty-four hours after SCI, spinal cord tissues were harvested and weighed. The tissues were processed by using myeloperoxidase (MPO) assay kits (Sigma) for determination of the extent of polymorphonuclear leukocyte (PMN) infiltration. The MPO activity was measured as the rate of change in absorbance at 460 nm and expressed in units of MPO (the quantity of MPO enzyme to degrade 1 μmol of peroxide per minute) per gram of wet tissue.
Statistical analysis.
All data in graphs are generated from at least three independent experiments and are expressed as means ± SD as indicated. Two-tailed Student’s t-tests were used to evaluate the statistical comparisons for data in Figs. 2, 3, 6A, and 7, A–F. One-way ANOVA for data in Figs. 4, 5, and 6, B and C, or two-way ANOVA for data in Fig. 7, G–I, was also performed.
Fig. 2.
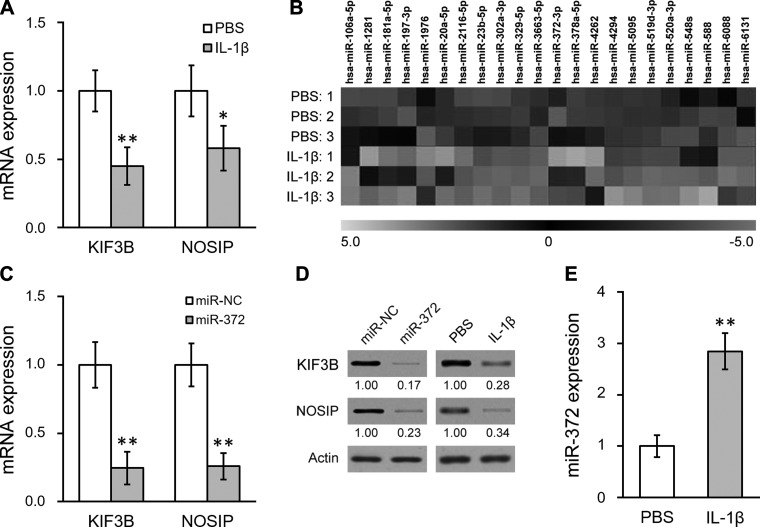
IL-1β represses KIF3B and NOSIP expression through miR-372 in hNSC. A: mRNA levels of KIF3B and NOSIP in hNSC treated with either PBS control or IL-1β. B: microRNA microarray analysis was performed in hNSC treated with either PBS control or IL-1β (in triplicate) to examine expression profiles of miRNAs predicted to target both KIF3B and NOSIP. Twenty-two miRs were found to be upregulated following IL-1β treatment. C: mRNA levels of KIF3B and NOSIP in hNSC transfected with either negative control miR (miR-NC) or miR-372 (miR-372). D: protein levels of both KIF3B and NOSIP were analyzed by Western blot in hNSC with miR-NC/miR-372 transfection or with PBS/IL-1β treatment. Numbers below the bands indicate quantification relative to miR-NC or PBS (normalized by actin loading control). E: miR-372 level was analyzed by mature miR assay in hNSC treated with either PBS control or IL-1β. Data are means ± SD from at least 3 independent experiments. *P < 0.05; **P < 0.01 compared with PBS or miR-NC control.
Fig. 3.
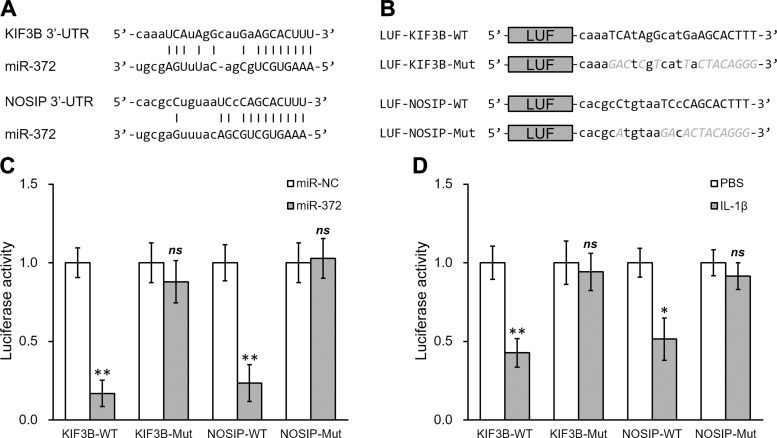
MiR-372 directly targets the 3′-UTR on the mRNA of both KIF3B and NOSIP. A: sequences of the predicted miR-372 targeting sites on the 3′-UTR of KIF3B and NOSIP mRNA. B: wild type (-WT) or mutant (-Mut) sequences from both KIF3B and NOSIP mRNA 3′-UTR were cloned downstream of the luciferase reporter gene (LUF). C and D: luciferase activities of -WT and -Mut constructs, for both KIF3B and NOSIP, were determined in hNSC with miR-NC/miR-372 transfection (C) or with PBS/IL-1β treatment (D). Data are means ± SD from at least 3 independent experiments. *P < 0.05; **P < 0.01 compared with PBS or miR-NC control; ns, not significant.
Fig. 6.
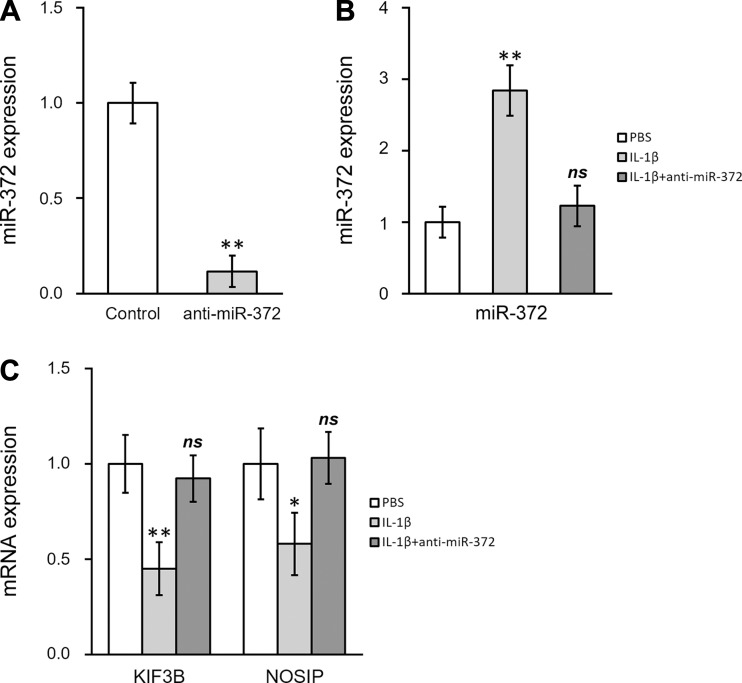
Establishing stable miR-372 inhibitor hNSC. A: miR-372 level was analyzed by mature miR assay in hNSC following transduction with miR-372 inhibitor lentivirus (anti-miR-372). B and C: miR-372 level (B) and mRNA levels of KIF3B and NOSIP (C) were analyzed in hNSC following PBS or IL-1β treatments, as well as in hNSC with stable miR-372 inhibitor simultaneously treated with IL-1β. Data are means ± SD from at least 3 independent experiments. *P < 0.05; **P < 0.01 compared with control or PBS; ns, not significant.
Fig. 7.
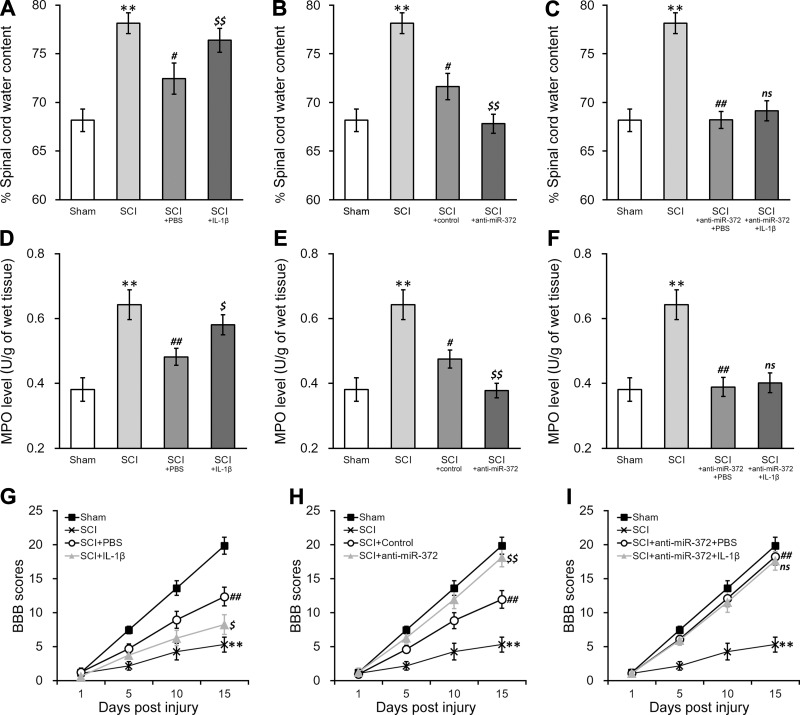
IL-1β treatment inhibits functional recovery using hNSC transplant therapy through miR-372 in rat SCI model. A–C: changes in the water content of spinal cord tissues of SCI rats 15 days after transplant with hNSC treated with PBS or IL-1β (A), control/miR-372-3p inhibitor (anti-miR-372; B), or anti-miR-372 + PBS or IL-1β (C). D–F: MPO activity of SCI rats 15 days after transplant with hNSC treated as described for A–C, respectively. G–I: BBB scores of SCI rats on days 1, 5, 10, and 15 after transplant with hNSC treated as described for A–C, respectively. Sham-operated rats (sham) and SCI rats without hNSC transplant were also plotted in all graphs as controls. Data are means ± SD from n = 12 rats in each experimental group. **P < 0.01 compared with sham rats. #P < 0.05; ##P < 0.01 compared with SCI-only rats. $P < 0.05; $$P < 0.01 compared with SCI + PBS, SCI + control, or SCI + anti-miR-372 + PBS rats; ns, not significant.
Fig. 4.
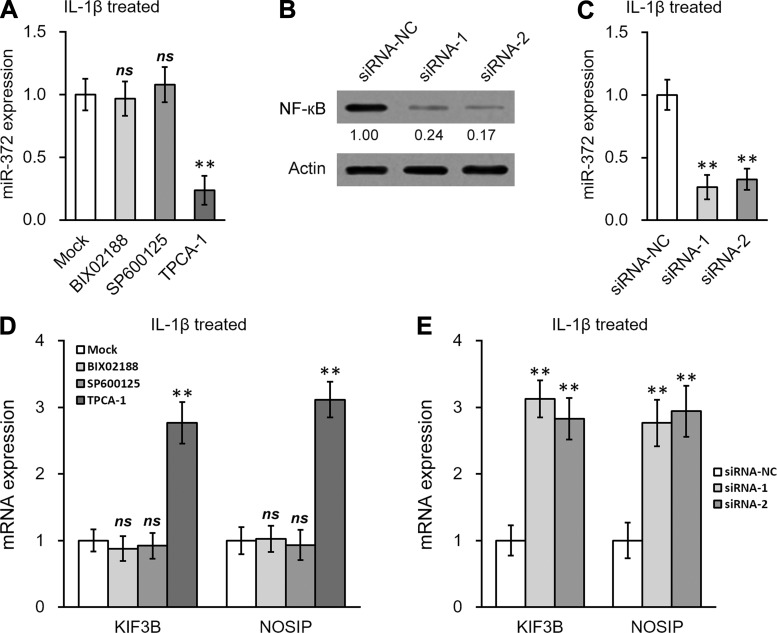
NF-кB activation is required for IL-1β-induced upregulation of miR-372 in hNSC. A: expression of miR-372 in IL-1β-treated hNSC was examined after mock treatment or treatment with the p38 MAPK inhibitor BIX 02188, the JNK inhibitor SP600125, or the IKK inhibitor TPCA-1, respectively. B and C: NF-кB (B) and miR-372 (C) were examined in IL-1β-treated hNSC, following siRNA knockdown of NF-кB. D and E: expression of KIF3B and NOSIP in IL-1β-treated hNSC was examined following treatment with small inhibitors (D) or NF-кB siRNAs (E). Data are means ± SD from at least 3 independent experiments. **P < 0.01 compared with mock or siRNA-NC; ns, not significant.
Fig. 5.
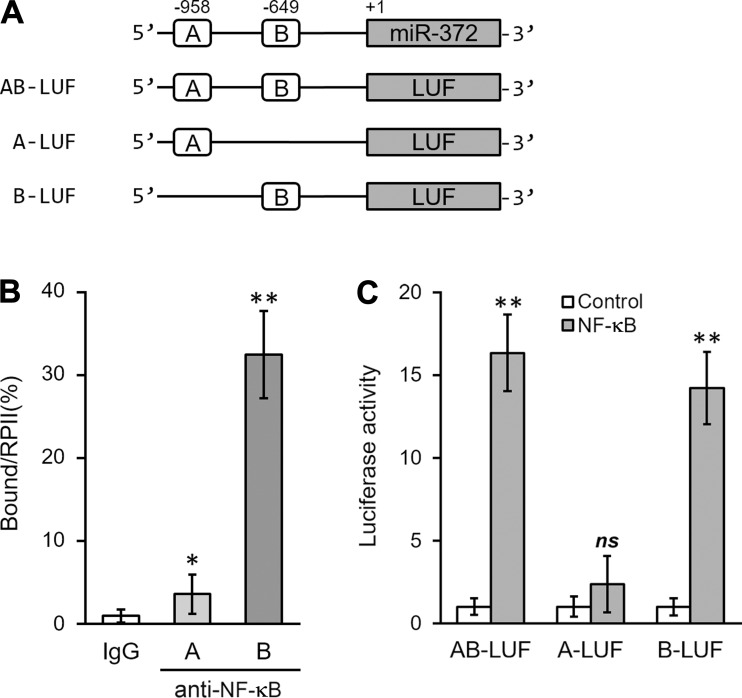
NF-κB directly binds to promoter region of miR-372. A: 2 putative NF-κB binding sites in the promoter region of human miR-372 were cloned to the upstream of a luciferase (LUF) reporter gene (AB-LUF, A-LUF, and B-LUF, respectively). B: ChIP assay was performed using IgG- or NF-κB-specific antibody to analyze NF-κB binding to the promoter of miR-372 in hNSC. C: luciferase activities of AB-LUF, A-LUF, and B-LUF constructs were measured in hNSC following control or NF-κB treatment. Data are means ± SD from at least 3 independent experiments. *P < 0.05; **P < 0.01 compared with IgG or control; ns, not significant.
RESULTS
IL-1β increases miR-372 that can repress KIF3B and NOSIP expressions in hNSC.
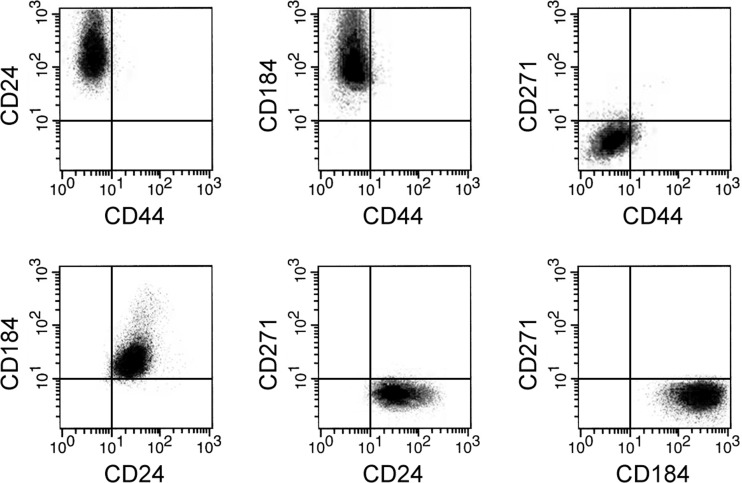
The hNSC derived from the spinal cord was obtained from human patients according to a previously characterized approach (Mothe and Tator 2015). NSC identity was then confirmed in the isolated cells by flow cytometry analysis of surface markers (Yuan et al. 2011), which was positive for CD24 and CD184 but negative for CD44 and CD271 (Fig. 1). To study the inflammation-induced NSC dysfunction, we treated NSC with the proinflammatory cytokine IL-1β and examined the expression levels of KIF3B and NOSIP, two important modulators of neural cell response and repair during SCI. As shown in Fig. 2A, both KIF3B and NOSIP gene expression was reduced in hNSC following IL-1β treatment (10 ng/ml for 24 h), suggesting a potential mechanism through which IL-1β could impair the functional recovery of hNSC. Because miRs could function to suppress expression of target genes, we hypothesized that IL-1β might induce a microRNA that could target KIF3B and NOSIP in hNSC. It is commonly observed that a complementary sequence of miRs resides at the 3′-UTR of the target gene. Using TargetScanHuman (Lewis et al. 2005), we selected ~100 candidate miRs that have complementary sites on 3′-UTRs of both KIF3B and NOSIP in a customized microarray screen to examine miR expression profiles. As shown in Fig. 2B, 22 miRs are significantly increased by 10 ng/ml IL-1β treatment in hNSC for 24 h, including miR-372. We then tested these candidates by overexpression study in hNSC. To study their potential roles in repressing KIF3B and NOSIP, gene expression of hNSC following individual miR overexpression was assessed compared with control transfection (miR-NC). Among the 22 candidates, only miR-372 overexpression resulted in the decreased mRNA expression of both KIF3B and NOSIP (Fig. 2C; data not shown for other miRs). Consistent with the findings on miR expression, we also observed that protein levels of KIF3B and NOSIP were downregulated by miR-372 in hNSC (Fig. 2D). In addition, we confirmed that miR-372 expression in hNSC was indeed stimulated by IL-1β compared with PBS control (Fig. 2E). Together, these data suggested that miR-372 might mediate the suppression of KIF3B and NOSIP gene expression by IL-1β in hNSC.
Fig. 1.
Isolated hNSC were analyzed by flow cytometry for the presence of surface markers characteristic of hNSC. CD24 is a cell adhesion molecule, and CD184 is a G protein-coupled receptor, and both are positive markers of hNSC. CD44 and CD271 are negative markers of hNSC.
IL-1β-stimulated miR-372 targets 3′-UTR of KIF3B and NOSIP to repress their expression.
We next utilized a luciferase reporter assay to test the specific recognition of miR-372 by its complementary sequences on the 3′-UTR of target genes. The potential binding pairs between miR-372 with respective 3′-UTRs of KIF3B or NOSIP were found using computational analysis (Fig. 3A), and wild type or mutated sequences of 3′-UTRs were constructed into luciferase reporters (Fig. 3B). As shown in Fig. 3C, cotransfection of miR-372 greatly decreased the luciferase activity of the reporter containing the wild-type sequence of KIF3B 3′-UTR. In contrast, the reduction was absent if the 3′-UTR binding sequence to miR-372 was mutated. Similar modulation was found in NOSIP 3′-UTR, as well. miR-372 decreased the luciferase activity of the wild-type but not the mutant 3′-UTR of NOSIP. We then performed similar experiments in cells treated with IL-1β. In response to IL-1β, the activities of 3′-UTRs of both KIF3B and NOSIP genes were downregulated. Importantly, the repression by IL-1β was dependent on the complementary sites for miR-372 (Fig. 3D). These results demonstrated that IL-1β-induced miR-372 could directly bind the 3′-UTR of KIF3B and NOSIP mRNAs and repress their expression.
NF-кB activation is required for IL-1β-induced miR-372 upregulation.
Given the strong association of the IKK (IκB kinase)/NF-κB pathway with inflammation and IL-1β signaling (Napetschnig and Wu 2013), we sought to examine NF-κB activation and its involvement in miR-372 induction by IL-1β. First, an inhibitor of IKK, TPCA-1, significantly blocked the stimulated miR-372 expression by IL-1β in hNSC (Fig. 4A). As controls, inhibitors of p38 MAP kinase (BIX 02188) or JNK (SP600125) pathways had no significant effects. Second, the induction of miR-372 by IL-1β was also abolished when NF-κB was knocked down by two different small-interference RNA (siRNA) oligos (Fig. 4, B and C). Consistent with our above-described results showing that the gene repression by IL-1β was likely mediated through miR-372, diminishing miR-372 by blocking NF-κB signaling, with either chemical inhibition (Fig. 4D) or genetic approaches (Fig. 4E), upregulated KIF3B and NOSIP levels in hNSC in the presence of IL-1β.
We further investigated direct binding of NF-κB to the promoter region of miR-372. We have found two potential NF-κB-responsive DNA-binding sites within the upstream region of human miR-372 gene. We named these portions, bearing the consensus sequences GGRNNYYC (R, purine; Y, pyrimidine; N, any base), region A (-958) and region B (-649), respectively (Fig. 5A). We next performed chromatin immunoprecipitation assays in IL-1β-treated hNSC and found that NF-κB was highly bound to region B and to a much smaller extent to region A, as well (Fig. 5B). In comparison, the promoter activity of miR-372 gene in hNSC was stimulated upon cotransfection of NF-κB, which occurred only in the presence of intact region B (Fig. 5C). Thus NF-κB signaling, through a specific binding element in miR-372 gene promoter (-649), appeared to play an essential role in the induction of miR-372 expression in hNSC by IL-1β treatment.
Functional recovery of SCI by hNSC transplantation is inhibited by IL-1β pretreatment, which is dependent on miR-372.
To further study the potential role of miR-372 in hNSC, we established an hNSC line with stable expression of the miR-372 inhibitor by taking advantage of a lentiviral transduction system. The miR-372 level was significantly decreased in this cell line (miR-372 inhibitor as anti-miR-372; Fig. 6A). We then examined the in vitro IL-1β effects on these cells. Compared with that in control cells, the induction of miR-372 by IL-1β was completely abolished in anti-miR-372-expressing cells (Fig. 6B). In addition, gene repression of both KIF3B and NOSIP by IL-1β was rescued in anti-miR-372 cells (Fig. 6C), further confirming that miR-372 is required for the IL-1β-induced suppression of KIF3B and NOSIP in hNSC.
The suppression of KIF3B and NOSIP in hNSC by IL-1β could be correlated with a negative impact on the tissue repair following SCI in vivo. The rescuing effects of miR-372 inhibitor in reversing gene repression of both KIF3B and NOSIP may thus imply a possible strategy to improve healing. To test these hypotheses, we employed a rat model of SCI (Fig. 7, sham vs. SCI). SCI-operated rats received transplantation of hNSC with various in vitro pretreatments. Compared with that in the control pretreatment, spinal cord water content after the transplantation was significantly increased (Fig. 7A), suggesting a detrimental effect of IL-1β on the ability of hNSC to restrict tissue edema after SCI. In contrast, the tissue water content was alleviated when hNSC contained miR-372 inhibitor (Fig. 7B). We then compared the effects in rats with transplanted anti-miR-372 hNSC in the absence or presence of IL-1β pretreatment. Remarkably, the edema-inducing effects of IL-1β on SCI rats receiving transplantation were largely ablated by miR-372 inhibitor (Fig. 7C). Similarly, IL-1β pretreatment stimulated, whereas the inhibition of miR-372 decreased, the levels of MPO activity in SCI rats after hNSC transplantation (Fig. 7, D and E). In addition, IL-1β-stimulated MPO levels in SCI rats were absent when hNSC expressing anti-miR-372 were used for the transplantation (Fig. 7, D–F). On separate cohorts, we also assessed the nerve function recovery by monitoring behavioral scores in rats with similar experimental settings. The measurements of the BBB scores show that the SCI rats receiving transplantation of IL-1β-pretreated hNSC had significant decreases in nerve functions, compared with the PBS-treated control group (Fig. 7G). Again, the inhibition of miR-372 in transplanted hNSC enhanced the function recovery of SCI rats and rescued the reduction of postinjury motor activity induced by IL-1β (Fig. 7, H and I).
DISCUSSION
MicroRNAs, or miRs, a class of 21- to 23-nucleotide-long RNAs expressed from noncoding genome, have emerged as endogenous modulators to repress mRNA stability and/or translation efficiency (Li and Rana 2014). Although their physiological functions are still largely unknown, the best characterized molecular processes of miRs are often identified as complementary sequences with the 3′-UTRs of their target mRNAs (He and Hannon 2004; Krol et al. 2010). Through such conserved mechanisms for targeting specific genes, the small noncoding miR species are believed to have critical functions across almost every biological process. In the present study, we identified miR-372 as a novel mediator of gene suppression in hNSC. Induced by IL-1β via a newly identified NF-κB-binding site on its promoter, miR-372 was shown to be indispensable for the repression of NOSIP and KIF3B. The binding sites for miR-372 on the 3′-UTRs of NOSIP and KIF3B were both characterized and confirmed by mutagenesis studies. Thus miR-372 appears to play a modulating function in NSC response during inflammation.
The potential involvement of miR-372 in stem cell biology has been documented. In fact, the miR-372 cluster (or miR-290 cluster in mice) consists of a group of miRs, known as an embryonic stem cell cycle family, to antagonize the differentiation of embryonic stem cells (ESC) (Melton et al. 2010). The differential expression of miR-372 has been reported in various states of ESC (Qi et al. 2009; Stadler et al. 2010), and overexpression of miR-372 is able to enhance reprogramming efficiency of somatic cells during the generation of induced pluripotent stem cells (Subramanyam et al. 2011). The role of miR-372 in NSCs, however, has not been specifically investigated, and little is known about its role in neuronal cells. During a previous study involving miR profiling for in vitro neurotoxicity testing (Pallocca et al. 2013), it was found that miR-372 was significantly increased in neuronal/glial culture after exposure to methyl mercury chloride. These data suggested that miR-372 may participate in the regulation of cellular stress response in neural cells. Nevertheless, the detailed mechanism and gene targets of miR-372 were not investigated. Similar to this finding, our current study identified the induction of miR-372 in NSCs after the challenge of proinflammatory cytokine IL-1β. Taking these findings together, we speculate that miR-372 may be an important player in the defensive pathway of neuronal lineage cells, as well as an operative target of progenitors to enhance cell tolerance or acquire protective properties against detrimental cellular environment.
Mechanistically, two miR-372 target genes were recognized during our investigation. NOSIP is a negative regulator of nitric oxide synthases, which play key roles not only in vascular function but also in secondary damage to the CNS (Dedio et al. 2001; Dreyer et al. 2003; Yu et al. 2012). High levels of nitric oxide immediately following primary SCI may stimulate the free radical generation of reactive nitrogen species, damage the mitochondrial electron transport system and the citric acid cycle, interfere with DNA transcription and translation, or potentiate neuron apoptosis induced by excitatory amino acid toxicity (Conti et al. 2007). Decreasing NOSIP expression by miR-372, therefore, may worsen the SCI through unrestricted nitric oxide production. In addition, our study also found that miR-372 could inhibit KIF3B, a motor protein that is abundantly expressed in neurons (Nonaka et al. 1998). Yu et al. (2013) found that KIF3B expression exhibited a marked increase in rats after SCI, particularly in proliferating astrocytes and microglia. Since KIF3 family members form motor complexes responsible for intracellular transport and neuronal differentiation (Hirokawa et al. 2010; Nishimura et al. 2004), the attenuation of KIF3B expressions by miR-372 may hurdle the specific axonal regeneration required for functional recovery of SCI. Consistently, our study has shown that IL-1β treatment, which represses both NOSIP and KIF3B in hNSC, resulted in a significant decline of healing in a rat model of SCI. Furthermore, inhibiting miR-372 rescued the IL-1β-induced functional impairment. In line with the in vitro observations, our current data acquired from a rat model of SCI clearly suggest that enhancing expression of NOSIP and KIF3B in transplanted hNSC exerted protective effects against the excess inflammation following SCI and improved the repair efficiency. The physiological relevance of this finding may be further validated by directly evaluating SCI in animals deficient in NOSIP or KIF3B.
As a promising therapeutic option to reconstruct the complex structured neural network following CNS injury, NSC needs to be fine-tuned to improve the safety and efficacy of the transplantation therapy. Although underlying mechanisms have not been fully elucidated, the persistent activation of inflammatory signaling normally inhibits the stem cell-mediated healing process. Significant outcomes of CNS injury have been associated with inflammation damage, which may set the stage for neural dysfunction and chronic deficits. Partly due to such challenges, only 42.1% patients could partially recover from SCI as suggested by multicenter prospective studies (Sale et al. 2012). In the current study we show that proinflammatory cytokine IL-1β may reduce the efficiency of NSC-mediated spinal cord repair, likely through downregulation of NOSIP and KIF3B expression. Importantly, these actions of IL-1β are mediated through a miR pathway. Thus the differential regulation of miRs by IL-1β may play an essential role in the inflammatory response elicited during SCI. Indeed, NF-κB signaling, a major proinflammation pathway, has been shown to activate expression of various miRs (Ma et al. 2011). Our current study has identified an NF-κB binding site at the miR-372 promoter region that directly mediates IL-1β-induced miR-372 expression in hNSC. Furthermore, there are multiple miRs revealed by our microarray screen for IL-1β signaling in hNSC. The mechanistic insights and downstream targets of these microRNAs await further characterization. An NF-κB-dependent, miR-mediated signaling cascade may act as a general scheme in modulating different functions of IL-1β signaling. For instance, as a pleiotropic cytokine, IL-1β can also promote neural tissue remodeling (David and Kroner 2011). Investigations into the potential roles of miR in IL-1β signaling will likely provide novel insights for better understanding of inflammation responses following SCI. In this context, our study may pave the way for a new strategy against inflammation during SCI treatment. Finally, because tumor cells often harbor ESC-like signatures, and miR-372 has been implicated as an oncogene in a variety of cancers, our finding may also indicate a putative switch of the IL-1β/miR-372 signaling axis during tumorigenesis.
Conclusion.
Our data demonstrate that IL-1β can impair the efficiency of hNSC transplant therapy for SCI treatment in rats. This effect is dependent on miR-372, which suppresses KIF3B and NOSIP gene expression in hNSC. Therefore, specific knockdown of miR-372 may provide benefits for SCI treatments.
GRANTS
This work was supported by Shanghai University of Medicine & Health Sciences Seed Foundation Project No. HMSF-16-21-018.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.Z. and T.Y. performed experiments; W.Z., T.Y., Y.G., and P.Y. analyzed data; W.Z., T.Y., and X.Y. drafted manuscript; W.Z., T.Y., Y.G., P.Y., W.L., C.P., Y.L., and X.Y. approved final version of manuscript; Y.G., P.Y., W.L., C.P., and Y.L. prepared figures; X.Y. conceived and designed research; X.Y. interpreted results of experiments; X.Y. edited and revised manuscript.
REFERENCES
- Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol 11: R90, 2010. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska-Solgot A, Chopp M, Zhang ZG. MicroRNA-21 protects neurons from ischemic death. FEBS J 277: 4299–4307, 2010. doi: 10.1111/j.1742-4658.2010.07818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti A, Miscusi M, Cardali S, Germanò A, Suzuki H, Cuzzocrea S, Tomasello F. Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res Brain Res Rev 54: 205–218, 2007. doi: 10.1016/j.brainresrev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci USA 102: 14069–14074, 2005. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12: 388–399, 2011. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- Dedio J, König P, Wohlfart P, Schroeder C, Kummer W, Müller-Esterl W. NOSIP, a novel modulator of endothelial nitric oxide synthase activity. FASEB J 15: 79–89, 2001. doi: 10.1096/fj.00-0078com. [DOI] [PubMed] [Google Scholar]
- Dreyer J, Hirlinger D, Müller-Esterl W, Oess S, Kuner R. Spinal upregulation of the nitric oxide synthase-interacting protein NOSIP in a rat model of inflammatory pain. Neurosci Lett 350: 13–16, 2003. doi: 10.1016/S0304-3940(03)00771-7. [DOI] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma 9: 123–128, 1992. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord 41: 369–378, 2003. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5: 522–531, 2004. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68: 610–638, 2010. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Iwanami A, Kaneko S, Nakamura M, Kanemura Y, Mori H, Kobayashi S, Yamasaki M, Momoshima S, Ishii H, Ando K, Tanioka Y, Tamaoki N, Nomura T, Toyama Y, Okano H. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res 80: 182–190, 2005. doi: 10.1002/jnr.20436. [DOI] [PubMed] [Google Scholar]
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11: 597–610, 2010. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Lewén A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma 17: 871–890, 2000. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov 13: 622–638, 2014. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- Liu CG, Calin GA, Volinia S, Croce CM. MicroRNA expression profiling using microarrays. Nat Protoc 3: 563–578, 2008. doi: 10.1038/nprot.2008.14. [DOI] [PubMed] [Google Scholar]
- Liu P, Yao Y, Liu MY, Fan WL, Chao R, Wang ZG, Liu YC, Zhou JH, Zhao JH. Spinal trauma in mainland China from 2001 to 2007: an epidemiological study based on a nationwide database. Spine 37: 1310–1315, 2012. doi: 10.1097/BRS.0b013e3182474d8b. [DOI] [PubMed] [Google Scholar]
- Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-κB signaling. J Mol Cell Biol 3: 159–166, 2011. doi: 10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463: 621–626, 2010. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe A, Tator CH. Isolation of neural stem/progenitor cells from the periventricular region of the adult rat and human spinal cord. J Vis Exp 99: e52732, 2015. doi: 10.3791/52732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys 42: 443–468, 2013. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R. IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma 18: 947–956, 2001. doi: 10.1089/089771501750451857. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol 6: 328–334, 2004. doi: 10.1038/ncb1118. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95: 829–837, 1998. doi: 10.1016/S0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Pallocca G, Fabbri M, Sacco MG, Gribaldo L, Pamies D, Laurenza I, Bal-Price A. miRNA expression profiling in a human stem cell-based model as a tool for developmental neurotoxicity testing. Cell Biol Toxicol 29: 239–257, 2013. doi: 10.1007/s10565-013-9250-5. [DOI] [PubMed] [Google Scholar]
- Qi J, Yu JY, Shcherbata HR, Mathieu J, Wang AJ, Seal S, Zhou W, Stadler BM, Bourgin D, Wang L, Nelson A, Ware C, Raymond C, Lim LP, Magnus J, Ivanovska I, Diaz R, Ball A, Cleary MA, Ruohola-Baker H. microRNAs regulate human embryonic stem cell division. Cell Cycle 8: 3729–3741, 2009. doi: 10.4161/cc.8.22.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale P, Mazzarella F, Pagliacci MC, Aito S, Agosti M, Franceschini M. Sport, free time and hobbies in people with spinal cord injury. Spinal Cord 50: 452–456, 2012. doi: 10.1038/sc.2011.161. [DOI] [PubMed] [Google Scholar]
- Stadler B, Ivanovska I, Mehta K, Song S, Nelson A, Tan Y, Mathieu J, Darby C, Blau CA, Ware C, Peters G, Miller DG, Shen L, Cleary MA, Ruohola-Baker H. Characterization of microRNAs involved in embryonic stem cell states. Stem Cells Dev 19: 935–950, 2010. doi: 10.1089/scd.2009.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanyam D, Lamouille S, Judson RL, Liu JY, Bucay N, Derynck R, Blelloch R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol 29: 443–448, 2011. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA 99: 3024–3029, 2002. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Chen YC, Liu L, Chen TJ, Huang WC, Cheng H, Tung-Ping S. Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology 78: 1051–1057, 2012. doi: 10.1212/WNL.0b013e31824e8eaa. [DOI] [PubMed] [Google Scholar]
- Yu X, Wen H, Cao J, Sun B, Ding T, Li M, Wu H, Long L, Cheng X, Xu G, Zhang F. Temporal and spatial expression of KIF3B after acute spinal cord injury in adult rats. J Mol Neurosci 49: 387–394, 2013. doi: 10.1007/s12031-012-9901-7. [DOI] [PubMed] [Google Scholar]
- Yu X, Zhong Y, Zhu Z, Wu T, Shen A, Huang Y. Increased expression of nitric oxide synthase interacting protein (NOSIP) following traumatic spinal cord injury in rats. J Mol Histol 43: 661–668, 2012. doi: 10.1007/s10735-012-9460-9. [DOI] [PubMed] [Google Scholar]
- Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA, Emre N, Marsala S, Marsala M, Gage FH, Goldstein LS, Carson CT. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One 6: e17540, 2011. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]