Abstract
Matrix Gla protein (MGP) is a potent inhibitor of vascular calcification. The ability of MGP to inhibit calcification requires the activity of a vitamin K–dependent enzyme, which mediates MGP carboxylation. We investigated how MGP carboxylation influences the risk of calciphylaxis in adult patients receiving dialysis and examined the effects of vitamin K deficiency on MGP carboxylation. Our study included 20 patients receiving hemodialysis with calciphylaxis (cases) and 20 patients receiving hemodialysis without calciphylaxis (controls) matched for age, sex, race, and warfarin use. Cases had higher plasma levels of uncarboxylated MGP (ucMGP) and carboxylated MGP (cMGP) than controls. However, the fraction of total MGP that was carboxylated (relative cMGP concentration = cMGP/[cMGP + uncarboxylated MGP]) was lower in cases than in controls (0.58±0.02 versus 0.69±0.03, respectively; P=0.003). In patients not taking warfarin, cases had a similarly lower relative cMGP concentration. Each 0.1 unit reduction in relative cMGP concentration associated with a more than two-fold increase in calciphylaxis risk. Vitamin K deficiency associated with lower relative cMGP concentration in multivariable adjusted analyses (β=−8.99; P=0.04). In conclusion, vitamin K deficiency–mediated reduction in relative cMGP concentration may have a role in the pathogenesis of calciphylaxis. Whether vitamin K supplementation can prevent and/or treat calciphylaxis requires further study.
Keywords: calcific uremic arteriolopathy, vascular calcification, vitamin K, warfarin, calcium-binding protein, renal dialysis
Matrix Gla protein (MGP) is a potent inhibitor of vascular calcification. Mice that lack MGP develop spontaneous arterial calcification at an early age and human polymorphisms in the MGP gene have been associated with progression of coronary artery calcification.1,2 The biologic activity of MGP depends on γ-glutamylcarboxylase, an enzyme that converts inactive, uncarboxylated MGP (ucMGP) to active, carboxylated MGP (cMGP).3,4 Vitamin K serves as an essential cofactor for MGP carboxylation, similar to its role as a cofactor for carboxylation of coagulation cascade proteins. Warfarin prevents carboxylation of vitamin K–dependent proteins by depleting vitamin K reserves via competitive inhibition of enzymes involved in the vitamin K cycle.5 We and others have recently reported that treatment with warfarin is a risk factor for calciphylaxis, a rare but frequently fatal vascular calcification disorder in patients receiving dialysis.6–11 There are also case reports of calciphylaxis in patients who have conditions that predispose to vitamin K deficiency such as Crohn disease, alcoholic cirrhosis, and history of gastric bypass surgery.12–15 We conducted this study to consider the effect of vitamin K–dependent MGP carboxylation on the risk of calciphylaxis. We compared the prevalence of vitamin K deficiency in patients receiving dialysis with and without calciphylaxis and investigated the effects of vitamin K deficiency on MGP carboxylation.
In this matched case-control study, we included 20 adult (age>18 years) patients receiving hemodialysis with newly diagnosed skin biopsy–confirmed calciphylaxis (cases) and 20 patients receiving hemodialysis without calciphylaxis (controls). All patients in this study were identified using the Partners Healthcare biobank.16 Controls were matched to cases for age (±5 years), sex, race, and warfarin therapy.
We measured plasma cMGP and ucMGP using a sandwich dual-antibody ELISA.17 Relative cMGP concentration was calculated using the following equation:
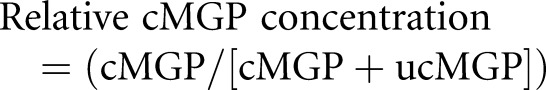 |
We measured plasma levels of circulating uncarboxylated prothrombin precursor, also known as protein induced by vitamin K absence or antagonist II (PIVKA-II), as a measure of vitamin K status and defined vitamin K deficiency as a plasma PIVKA-II level of ≥2 ng/ml, as in previous studies.18,19
We compared cMGP, ucMGP, and relative cMGP concentration between all cases and controls and between cases and controls who were not taking warfarin. Unadjusted and adjusted conditional logistic regression models (adjusted for possible confounders: diabetes mellitus, body mass index, serum phosphorous, 25-hydroxyvitamin D, vitamin D–binding protein, and parathyroid hormone [PTH]) were used to examine a potential association between relative cMGP concentration and calciphylaxis risk. Prevalence of vitamin K deficiency was compared between all cases and controls and between cases and controls who were not taking warfarin. To test how vitamin K deficiency influences MGP carboxylation in patients receiving hemodialysis, we compared cMGP, ucMGP, and relative cMGP concentration between patients with and without vitamin K deficiency and conducted unadjusted and adjusted linear regression analyses (adjusted for known possible confounders: diabetes mellitus, body mass index, and serum triglycerides) to examine the effects of vitamin K deficiency on relative cMGP concentration.20
Clinical characteristics of the patients in this study are summarized in Table 1. The average age of calciphylaxis cases and controls was 58 and 62 years, respectively; 35% were women, 90% were white, and 30% were taking warfarin. Patients who were on warfarin (six cases and six controls) were taking this medication for at least 6 months before study enrollment. Indications for warfarin included atrial fibrillation (n=8) and venous thromboembolic disease (n=4). International normalized ratios were similar between cases and controls. The frequencies of use of phosphate binder (40% versus 40%, P>.99), active vitamin D (40% versus 40%, P>.99), and cinacalcet (20% versus 15%, P=0.68) were comparable between calciphylaxis cases and controls. The use of calcium-containing (10% versus 17%, P=0.38) and noncalcium-containing phosphate binder (35% versus 30%, P=0.74) was also comparable between calciphylaxis cases and controls. There was a trend toward lower use of nutritional vitamin D in cases compared with controls (10% versus 35%, P=0.07). Serum 25-hydroxyvitamin D levels were lower in cases compared with controls likely due to the lower use of nutritional vitamin D and lower vitamin D–binding protein levels in cases compared with controls (260±21 µg/ml versus 345±20 µg/ml, P=0.01). No statistically significant differences were noted for bioavailable vitamin D (2.0±0.3 ng/ml versus 2.6±0.4, P=0.30) and for serum calcium, phosphate, and albumin. Seventy percent of calciphylaxis patients had ulcerated lesions. The mean total surface area of calciphylaxis lesions (number of lesions multiplied by surface area for each lesion) was 73±9 cm2. Six-month mortality in calciphylaxis patients was 30%.
Table 1.
Comparison of clinical characteristics between calciphylaxis cases and matched controls
| Characteristic | Calciphylaxis Cases (n=20) | Controls (n=20) | P Value |
|---|---|---|---|
| Age, yr | 58±4 | 62±3 | 0.90 |
| Women, % | 35 | 35 | 1.00 |
| White, % | 90 | 90 | 1.00 |
| Warfarin, % | 30 | 30 | 1.00 |
| Diabetes mellitus, % | 50 | 75 | 0.10 |
| Body mass index, kg/m2 | 29.9±2.0 | 31.7±1.5 | 0.47 |
| Coronary artery disease, % | 40 | 40 | 1.00 |
| Hemodialysis vintage, mo | 61±34 | 58±31 | 0.12 |
| Serum calcium (albumin-corrected), mg/dl | 9.3±0.2 | 9.3±0.2 | 0.83 |
| Serum phosphorous, mg/dl | 4.4±0.23 | 4.1±0.22 | 0.26 |
| Serum PTH, pg/ml | 275±77 | 230±42 | 0.17 |
| Serum alkaline phosphatase, U/L | 133±16 | 116±12 | 0.33 |
| Serum 25-hydroxyvitamin D, ng/ml | 18.0±2.5 | 32.4±2.9 | 0.001 |
| Serum triglycerides, mg/dl | 179±29 | 155±18 | 0.68 |
| Serum albumin, g/dl | 3.5±0.5 | 3.8±0.5 | 0.14 |
| International normalized ratio (not taking warfarin) | 1.2±0.2 (n=14) | 1.1±0.2 (n=14) | 0.42 |
| International normalized ratio (taking warfarin) | 2.1±0.4 (n=6) | 2.2±0.4 (n=6) | 0.53 |
| Single pool, Kt/V | 1.6±0.6 | 1.6±0.5 | 0.44 |
| Phosphate binder treatment, % | 40 | 40 | 1.00 |
| Active vitamin D treatment, % | 40 | 40 | 1.00 |
| Nutritional vitamin D treatment, % | 10 | 35 | 0.07 |
| Cinacalcet treatment, % | 20 | 15 | 0.68 |
Mean±SEM values are reported for continuous variables and % frequency values are reported for categoric variables.
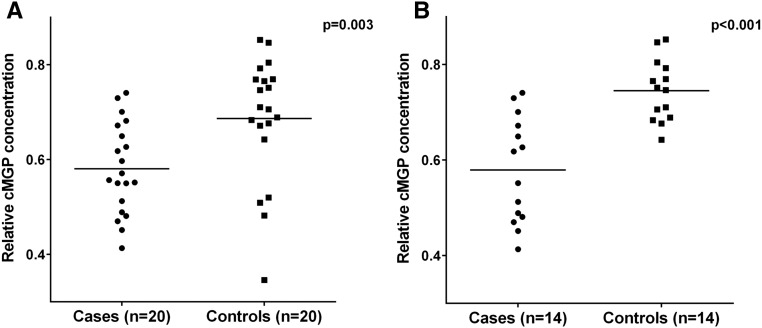
Plasma levels of both ucMGP (3076±289 pM versus 1485±436, P<0.001) and cMGP (3075±289 pM versus 2688±314 pM, P=0.002) were higher in calciphylaxis cases compared with controls; however, relative cMGP concentration was lower in cases compared with controls (0.58±0.02 versus 0.69±0.03, P=0.003; Figure 1A). Similarly, in analyses restricted to patients not taking warfarin (n=28; 14 cases and 14 controls), plasma levels of ucMGP (3042±385 pM versus 1099±172 pM, P<0.001) and cMGP (3989±277 pM versus 3024±312 pM, P=0.03) were higher in calciphylaxis cases compared with controls; however, relative cMGP concentration was lower in cases (0.58±0.03 versus 0.75±0.02, P<0.001; Figure 1B). In univariate conditional logistic regression analyses, every 0.1 point reduction in relative cMGP concentration was associated with a >2-fold increase in the odds of calciphylaxis (odds ratio, 2.25; 95% confidence interval (95% CI), 1.20 to 4.21; P=0.01). In conditional logistic regression analyses adjusted for diabetes mellitus, body mass index, serum phosphorous, 25-hydroxyvitamin D, vitamin D–binding protein, and PTH, every 0.1-point reduction in relative cMGP concentration was associated with a similar >2-fold increase in the odds of calciphylaxis (odds ratio, 2.66; 95% CI, 1.05 to 6.59; P=0.04).
Figure 1.
Relative cMGP concentration is reduced in calciphylaxis. (A) Reduced relative cMGP concentration is observed in calciphylaxis cases compared with matched controls. (B) This reduction in relative cMGP concentration was also observed in calciphylaxis cases who were not taking warfarin compared with controls who were not taking warfarin.
To obtain insights into the biologic relevance of absolute and relative cMGP concentrations in calciphylaxis, we separately examined the associations between absolute or relative cMGP concentrations with the severity and outcome of calciphylaxis. Relative cMGP concentration was significantly lower in patients with ulcerated lesions compared with patients with nonulcerated lesions (0.53±0.06 versus 0.69±0.04, P=0.03), whereas absolute cMGP concentration was comparable (3911±273 pM versus 4597±404 pM, P=0.18). Relative cMGP concentration had a significant negative correlation with lesion surface area (r=−0.54, P=0.01), whereas absolute cMGP concentration did not correlate with lesion surface area (r=−0.03, P=0.89). There was a trend toward lower relative cMGP concentration in calciphylaxis patients who died by 6 months compared with patients who were alive (0.50±0.04 versus 0.61±0.02, P=0.09), whereas absolute cMGP concentrations were comparable (4069±321 pM versus 4228±225 pM, P=0.76). Our results suggest that relative cMGP concentration is a better predictor of clinical disease severity than absolute cMGP concentration.
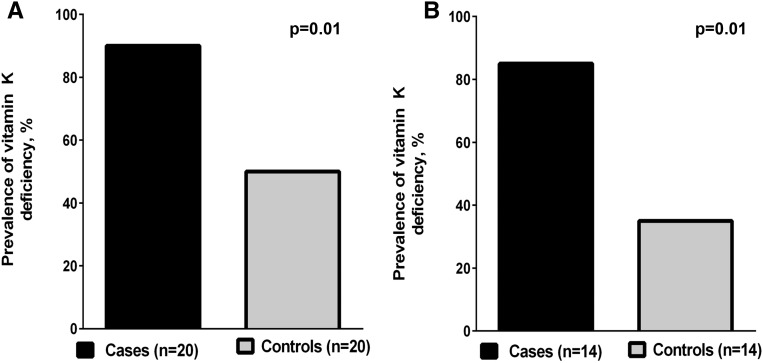
The prevalence of vitamin K deficiency was higher (90% versus 50%, P=0.01) in calciphylaxis cases compared with controls (Figure 2A), corresponding to higher PIVKA-II levels (163±37 ng/ml versus 98±35, P=0.03). In analyses restricted to patients not taking warfarin, a higher prevalence of vitamin K deficiency (85% versus 35%, P=0.01) was noted in calciphylaxis cases compared with controls (Figure 2B). Plasma levels of ucMGP (2639±376 pM versus 1444±280, P=0.03) were higher in patients with vitamin K deficiency compared with patients without vitamin K deficiency. There was no difference in cMGP levels in patients with vitamin K deficiency compared with patients without vitamin K deficiency (3511±289 pM versus 3148±326 pM, P=0.26). However, relative cMGP concentration was lower in patients with vitamin K deficiency compared with patients without vitamin K deficiency (0.60±0.02 versus 0.71±0.03, P=0.01). In unadjusted linear regression models, vitamin K deficiency was associated with reduced relative cMGP concentration (β=−11.32; 95% CI, −19.36 to −3.28; P=0.01). In analyses that were adjusted for diabetes mellitus, body mass index, and serum triglycerides, vitamin K deficiency remained associated with reduced relative cMGP concentration (β=−8.99; 95% CI, −17.53 to −0.46; P=0.04).
Figure 2.
Vitamin K deficiency is more prevalent in calciphylaxis cases compared with matched controls. Vitamin K deficiency was defined as a plasma PIVKA-II of ≥2 ng/ml. (A) A comparison of the prevalence of vitamin K deficiency in all calciphylaxis cases and controls. (B) A comparison of the prevalence of vitamin K deficiency in cases and controls who were not taking warfarin.
In receiver operating characteristic analyses conducted to examine whether vitamin K deficiency predicts relative cMGP concentration status (dichotomized at the median level of 0.63), we noted a c-statistic of 0.77 (±0.08) in univariate and 0.84 (±0.06) in multivariable analyses adjusted for diabetes mellitus, body mass index, and serum triglyceride levels. The optimal cut-off for relative cMGP concentration that maximized sensitivity and specificity was 0.6, as this cut-off provided a c-statistic of 0.81 (±0.07) in univariate and 0.85 (±0.06) in multivariable analyses adjusted for diabetes mellitus, body mass index, and serum triglyceride levels.
The carboxylated fraction of total MGP plays a critical role in determining the biologic activity of MGP.21,22 In our study, patients with calciphylaxis had reduced relative cMGP concentration and a high prevalence of vitamin K deficiency relative to matched controls. In a previous study describing semiquantitative immunohistochemical analysis of skin tissue from patients with calciphylaxis compared with controls (n=7 each), both ucMGP and cMGP protein expression levels were increased in the skin from patients with calciphylaxis.23 This previous study noted that the increase in ucMGP in the skin from patients with calciphylaxis was more than the increase in cMGP. These tissue data are consistent with the observations in our study: both plasma ucMGP and cMGP levels were higher in patients with calciphylaxis; however, the increase in ucMGP was greater than the increase in cMGP resulting in lower relative cMGP concentration. Furthermore, the difference in relative cMGP concentration between calciphylaxis cases and controls that we observed is similar to that observed in human dermal fibroblasts isolated from patients with pseudoxanthoma elasticum (another calcification disorder) and controls.24 The similar findings in two independent calcification disorders are suggestive of a pathophysiologic significance of relative cMGP concentration.
Previous studies showed that patients receiving hemodialysis have subclinical vitamin K deficiency, possibly a result of overall low dietary intake or poor intake of vitamin K–rich foods.18,25,26 Demonstration of lower relative cMGP concentration in nonwarfarin-treated patients with calciphylaxis compared with controls implicates nonwarfarin-mediated mechanisms of vitamin K deficiency in the pathogenesis of calciphylaxis. We do not have information concerning the diets of the patients in this study, but three of the nonwarfarin-exposed patients had conditions known to cause vitamin K deficiency (two patients had cirrhosis and one had a history of gastric bypass surgery). These three patients corresponded to lower relative cMGP concentration among the nonwarfarin-exposed cases. None of the nonwarfarin-exposed control patients had clinical predispositions like hepatobiliary disease, inflammatory bowel disease, gastric bypass, or antibiotic exposure to explain the vitamin K deficiency.13,14 Future studies will be needed to better understand nonwarfarin-mediated causes of vitamin K deficiency (including nutritional deficits, alterations in gastrointestinal microbiome, malabsorption, and hepatic disorders) and the activity of γ-glutamylcarboxylase in patients with calciphylaxis.
Considering the limited sample size of this study, confirmation of our novel findings in larger case-control studies is needed. Methodologic differences may account for differences in characteristics such as warfarin use and PTH level between this study and a previous report by our group.6 In this study, data were collected at the time of calciphylaxis diagnosis, whereas in a previous report by our group data were collected at the time that dialysis was initiated, and median duration between dialysis initiation and subsequent calciphylaxis development was 925 days (interquartile range, 273–2185 days).6 Despite limited sample size, a number of clinical characteristics of our patients with calciphylaxis are representative of previously described calciphylaxis cohorts and thus are generalizable.6,9 The exact reasons for the relatively lower levels of serum phosphorous and PTH at the time of calciphylaxis diagnosis, although described in previous studies, remain unclear and need future investigation.9,27
We are also limited by the cross-sectional assessment of exposure and outcomes and acknowledge the need for future longitudinal studies and clinical trials targeting MGP carboxylation to further define the biologic relevance of relative cMGP concentration in the pathogenesis of calciphylaxis. We recently started a proof-of-concept randomized, placebo-controlled clinical trial to examine whether vitamin K supplementation can increase relative cMGP concentration in calciphylaxis patients (CT.gov: NCT02278692).28 Future studies are needed to examine the effects of vitamin K supplementation on clinical outcomes related to calciphylaxis prevention and treatment. Reduced activity of the γ-glutamylcarboxylase enzyme has been reported in a uremic animal model of calcification and could further accentuate reduction in relative vitamin K–dependent MGP carboxylation.29 We did not specifically measure this enzyme activity; however, dialysis adequacy as measured by single pool Kt/V was comparable between cases and controls, suggesting similar uremic burden. Although our data on circulating MGP are consistent with a previous study that reported tissue MGP expression, studies to provide further insights into the relationship between the tissue and circulating MGP in calciphylaxis and to address mechanisms of circulating MGP elevation in calciphylaxis are needed.23
In conclusion, vitamin K deficiency–mediated reduction in relative cMGP concentration may play a role in the pathogenesis of calciphylaxis. Our novel findings motivate examination of vitamin K supplementation to optimize MGP carboxylation as a potential preventive and/or therapeutic approach for calciphylaxis.
Concise Methods
Our Institutional Review Board approved the study protocol. All procedures performed in this study were conducted in accordance with the Good Clinical Practice principles and Declaration of Helsinki ethical standards. Written informed consent was obtained from all patients included in this study.
Study Patients and Data
Calciphylaxis Research Initiative of Partners Healthcare biobank is a prospective registry of patients with calciphylaxis designed to collect clinical data and biospecimens from patients with calciphylaxis.16 For this study, we identified 20 adult (age>18 years) patients receiving hemodialysis with newly diagnosed biopsy-confirmed calciphylaxis that were enrolled into the Calciphylaxis Research Initiative of our institute’s biobank between January of 2013 and December of 2014.These cases were matched for age (±5 years), sex, race, and warfarin therapy to 20 patients receiving hemodialysis without calciphylaxis that were enrolled over the contemporaneous period into our institute’s biobank (controls). Data on demographics, body weight, height, comorbidities (diabetes mellitus, coronary artery disease, indications for warfarin therapy, liver disease, malabsorption disorders, gastric bypass surgery), medications, and laboratory parameters were collected. For patients with calciphylaxis, data on calciphylaxis lesion characteristics (ulcerated versus nonulcerated, surface area, and number of lesions) and 6-month mortality were also collected. Total surface area of calciphylaxis lesions for each case was calculated by multiplying the number of lesions by the surface area of each lesion. Nonfasting blood samples were drawn predialysis at the time of study enrollment. For cases, the time of enrollment coincided with the time of new diagnosis of calciphylaxis. Serum calcium levels were corrected for albumin using the following formula: albumin-corrected serum calcium = measured serum calcium + 0.8 × (4 − measured serum albumin).30
Study Measurements
All laboratory examinations were performed in series from dedicated aliquots stored at −80°C. Serum calcium, phosphate, alkaline phosphatase, intact PTH, albumin, 25-hydroxyvitamin D, triglycerides, and plasma international normalized ratio were measured using standard laboratory procedures. Serum vitamin D–binding protein levels were measured by commercial ELISA (Catalog Number DVDBP0; R&D Systems, Minneapolis, MN) and a previously described equation was applied for determination of bioavailable 25-hydroxyvitamin D.31
Plasma levels of MGP isoforms (cMGP and ucMGP) were measured using a sandwich dual-antibody ELISA (VitaK; Maastricht University, The Netherlands).17 According to the manufacturer, assays for cMGP and ucMGP use a monoclonal antibody specifically targeting the nonphosphorylated amino acid sequence 3–15 as the capture antibody, with the detection monoclonal antibody directed against the noncarboxylated amino acid sequence 35–49 in human MGP for ucMGP measurement, and the detection monoclonal antibody directed against the carboxylated amino acid sequence 35–53 in human MGP for cMGP measurement. The MGP isoforms are stable upon storage at −80°C.32 Intra-assay variability is 5.6% and 3.7% and interassay variability is 9.9% and 11.5% for ucMGP and cMGP, respectively. We determined relative cMGP concentration using the following equation:
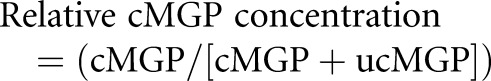 |
Vitamin K deficiency was assessed by measurement of plasma PIVKA-II levels using an ELISA assay (Stago). Plasma PIVKA-II levels provide a highly sensitive determination of vitamin K deficiency and are unaffected by renal function.18,19
Statistical Analyses
For categoric variables, we reported frequency, and for continuous variables, we reported mean and SEM. Categoric variables were compared between cases and controls using the Fisher exact test. Continuous variables were compared between cases and controls using the Mann–Whitney U test. Separate comparisons were also made between cases and control patients not taking warfarin. Additionally, we compared characteristics of patients with and without vitamin K deficiency using the Fisher exact test (for categoric variables) and the Mann–Whitney U test (for continuous variables).
Unadjusted and adjusted (adjusted for possible confounders: diabetes mellitus, body mass index, serum phosphorous, 25-hydroxyvitamin D, vitamin D–binding protein, and PTH) conditional logistic regression models were used to compute odds ratios to describe an association between relative cMGP concentration and calciphylaxis risk. To obtain insights into the biologic relevance of absolute and relative cMGP concentrations in calciphylaxis, we separately examined the associations between absolute and relative cMGP concentrations with the severity and outcome of calciphylaxis.
To investigate the effects of vitamin K deficiency on relative cMGP concentration, we conducted unadjusted and adjusted (adjusted for known possible confounders: diabetes mellitus, body mass index, and serum triglycerides) linear regression analyses. We constructed receiver operating characteristic curves and calculated c-statistics to examine whether vitamin K deficiency predicts relative cMGP concentration status.
All analyses were performed using the SAS program (version 9.4, Cary, NC). Statistical significance was set at P<0.05.
Disclosures
S.U.N. reports receiving speaker honoraria from Sanofi-Aventis and has served as a consultant to Ardelyx. R.I.T. is a consultant to Fresenius Medical Care North America and Celgene, and has received a research grant from Abbott Laboratories. R.M. receives consultant fees from Mallinckrodt Pharmaceuticals.
Acknowledgments
The authors would like to acknowledge Kenneth D. Bloch for his inspiration. The authors would like to thank Anders Berg for measurements of vitamin D–binding protein and bioavailable 25-hydroxyvitamin D.
S.U.N. is supported by the American Heart Association’s National Center for Research Program Winter 2015 Fellow-to-Faculty Transition Award (15FTF25980003) and by the KL2/Catalyst Medical Research Investigator Training award (an appointed KL2 award) from Harvard Catalyst, the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award KL2 TR001100). This work also received support from the National Kidney Foundation’s Young Investigator Award, the Fund for Medical Discovery Award from Massachusetts General Hospital’s Executive Committee on Research (R00000000007190), and the American Heart Association’s National Center for Research Program Summer 2014 Mentored Clinical and Population Research Award (15CRP22900008) to S.U.N., and by the United States Department of Agriculture Agricultural Research Service cooperative agreement 58-1950-7-707 to S.L.B. D. B.B. is supported by the Leducq Foundation and by National Institute of Diabetes and Digestive and Kidney Diseases grant DK082971. R.I.T. is supported by National Institutes of Health grants DK094872 and DK094486. R.M. was supported by the Fellow-to-Faculty Transition Award 11FTF7290032 from the American Heart Association, the Wild Family Foundation, and the K08HL111210 grant from the National Heart, Lung, and Blood Institute.
Preliminary findings of this manuscript were presented as an oral abstract at the American Society of Nephrology Kidney Week 2015 on November 6, 2015 in San Diego, California.
The content is solely the responsibility of the authors and does not represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, the National Institutes of Health, or the United States Department of Agriculture.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “K-alcification Protection in Dialysis Patients: The Underestimated Phenomenon of Vitamin K Deficiency,” on pages 1667–1668.
References
- 1.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G: Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386: 78–81, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Cassidy-Bushrow AE, Bielak LF, Levin AM, Sheedy PF 2nd, Turner ST, Boerwinkle E, Lin X, Kardia SL, Peyser PA: Matrix gla protein gene polymorphism is associated with increased coronary artery calcification progression. Arterioscler Thromb Vasc Biol 33: 645–651, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price PA, Williamson MK: Primary structure of bovine matrix Gla protein, a new vitamin K-dependent bone protein. J Biol Chem 260: 14971–14975, 1985 [PubMed] [Google Scholar]
- 4.Schurgers LJ, Uitto J, Reutelingsperger CP: Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol Med 19: 217–226, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Suttie JW: The biochemical basis of warfarin therapy. Adv Exp Med Biol 214: 3–16, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Nigwekar SU, Zhao S, Wenger J, Hymes JL, Maddux FW, Thadhani RI, Chan KE: A Nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol 27: 3421–3429, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigwekar SU, Bhan I, Turchin A, Skentzos SC, Hajhosseiny R, Steele D, Nazarian RM, Wenger J, Parikh S, Karumanchi A, Thadhani R: Statin use and calcific uremic arteriolopathy: A matched case-control study. Am J Nephrol 37: 325–332, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, Sato Y; Japanese Calciphylaxis Study Group : A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant 27: 1580–1584, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Brandenburg VM, Kramann R, Rothe H, Kaesler N, Korbiel J, Specht P, Schmitz S, Krüger T, Floege J, Ketteler M: Calcific uraemic arteriolopathy (calciphylaxis): Data from a large nationwide registry. [published online ahead of print January 29, 2016] Nephrol Dial Transplant : 10.1093/ndt/gfv438 [DOI] [PubMed] [Google Scholar]
- 10.Nigwekar SU, Kroshinsky D, Nazarian RM, Goverman J, Malhotra R, Jackson VA, Kamdar MM, Steele DJ, Thadhani RI: Calciphylaxis: Risk factors, diagnosis, and treatment. Am J Kidney Dis 66: 133–146, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandenburg VM, Cozzolino M, Ketteler M: Calciphylaxis: A still unmet challenge. J Nephrol 24: 142–148, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Barri YM, Graves GS, Knochel JP: Calciphylaxis in a patient with Crohn’s disease in the absence of end-stage renal disease. Am J Kidney Dis 29: 773–776, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Sermijn E, Strobbe T, Vandekerckhove L, Libbrecht L, Colle I, Schoonjans R, Vogelaers D: Calciphylaxis: A rare complication in alcoholic liver disease. Acta Clin Belg 68: 116–119, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Allegretti AS, Nazarian RM, Goverman J, Nigwekar SU: Calciphylaxis: A rare but fatal delayed complication of Roux-en-Y gastric bypass surgery. Am J Kidney Dis 64: 274–277, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Nigwekar SU, Wolf M, Sterns RH, Hix JK: Calciphylaxis from nonuremic causes: A systematic review. Clin J Am Soc Nephrol 3: 1139–1143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A study of calciphylaxis: Partners biobank research initiative. Available at https://biobank.partners.org/research-initiatives. Accessed March 4, 2016
- 17.Dalmeijer GW, van der Schouw YT, Vermeer C, Magdeleyns EJ, Schurgers LJ, Beulens JW: Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem 24: 624–628, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Elliott MJ, Booth SL, Hopman WM, Holden RM: Assessment of potential biomarkers of subclinical vitamin K deficiency in patients with end-stage kidney disease. Can J Kidney Health Dis 1: 13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosler K, von Kries R, Vermeer C, Saupe J, Schmitz T, Schuster A: Assessment of vitamin K deficiency in CF--how much sophistication is useful? J Cyst Fibros 2: 91–96, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Shea MK, O’Donnell CJ, Vermeer C, Magdeleyns EJ, Crosier MD, Gundberg CM, Ordovas JM, Kritchevsky SB, Booth SL: Circulating uncarboxylated matrix gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J Nutr 141: 1529–1534, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shea MK, Booth SL: Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients 8: 1–25, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danziger J: Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol 3: 1504–1510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramann R, Brandenburg VM, Schurgers LJ, Ketteler M, Westphal S, Leisten I, Bovi M, Jahnen-Dechent W, Knüchel R, Floege J, Schneider RK: Novel insights into osteogenesis and matrix remodelling associated with calcific uraemic arteriolopathy. Nephrol Dial Transplant 28: 856–868, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Gheduzzi D, Boraldi F, Annovi G, DeVincenzi CP, Schurgers LJ, Vermeer C, Quaglino D, Ronchetti IP: Matrix Gla protein is involved in elastic fiber calcification in the dermis of pseudoxanthoma elasticum patients. Lab Invest 87: 998–1008, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Fusaro M, D’Alessandro C, Noale M, Tripepi G, Plebani M, Veronese N, Iervasi G, Giannini S, Rossini M, Tarroni G, Lucatello S, Vianello A, Santinello I, Bonfante L, Fabris F, Sella S, Piccoli A, Naso A, Ciurlino D, Aghi A, Gallieni M, Cupisti A: Low vitamin K1 intake in haemodialysis patients [published online ahead of print April 28, 2016]. Clin Nutr doi: 10.1016/j.clnu.2016.04.024 [DOI] [PubMed] [Google Scholar]
- 26.Schlieper G, Westenfeld R, Krüger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, Djuric Z, Damjanovic T, Ketteler M, Vermeer C, Dimkovic N, Floege J, Schurgers LJ: Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol 22: 387–395, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo D, Capuano A, Cozzolino M, Napolitano P, Mosella F, Russo L, Saviano C, Zoccali C: Multimodal treatment of calcific uraemic arteriolopathy (calciphylaxis): A case series. Clin Kidney J 9: 108–112, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evaluation of vitamin K supplementation for Calcific Uremic Arteriolopathy (VitK-CUA). Available at: https://clinicaltrials.gov/ct2/show/NCT02278692. Accessed March 4, 2016.
- 29.Kaesler N, Magdeleyns E, Herfs M, Schettgen T, Brandenburg V, Fliser D, Vermeer C, Floege J, Schlieper G, Krüger T: Impaired vitamin K recycling in uremia is rescued by vitamin K supplementation. Kidney Int 86: 286–293, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Payne RB, Little AJ, Williams RB, Milner JR: Interpretation of serum calcium in patients with abnormal serum proteins. BMJ 4: 643–646, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI: Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int 82: 84–89, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewé RB, Brandenburg VM, Bekers O, Vermeer C: Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost 104: 811–822, 2010 [DOI] [PubMed] [Google Scholar]