Abstract
The pathogenesis of left ventricular hypertrophy in patients with CKD is incompletely understood. Sodium intake, which is usually assessed by measuring urinary sodium excretion, has been inconsistently linked with left ventricular hypertrophy. However, tissues such as skin and muscle may store sodium. Using 23sodium-magnetic resonance imaging, a technique recently developed for the assessment of tissue sodium content in humans, we determined skin sodium content at the level of the calf in 99 patients with mild to moderate CKD (42 women; median [range] age, 65 [23–78] years). We also assessed total body overhydration (bioimpedance spectroscopy), 24-hour BP, and left ventricular mass (cardiac magnetic resonance imaging). Skin sodium content, but not total body overhydration, correlated with systolic BP (r=0.33, P=0.002). Moreover, skin sodium content correlated more strongly than total body overhydration did with left ventricular mass (r=0.56, P<0.001 versus r=0.35, P<0.001; P<0.01 between the two correlations). Linear regression analysis demonstrated that skin sodium content is a strong explanatory variable for left ventricular mass, unaffected by BP and total body overhydration. In conclusion, we found skin sodium content to be closely linked to left ventricular mass in patients with CKD. Interventions that reduce skin sodium content might improve cardiovascular outcomes in these patients.
Keywords: kidney diseases, left ventricular hypertrophy, sodium, skin, blood pressure
CKD is associated with an increased risk of cardiovascular (CV) events, specifically sudden cardiac death.1 At least in part, this is because of the high prevalence and severity of left ventricular hypertrophy (LVH).2 However, the pathogenesis of LVH in CKD is poorly understood, impeding the development of effective therapeutic interventions.
A high sodium diet was found to be a strong and BP-independent stimulus of LVH in animal models.3 Activation of the local renin-angiotensin-aldosterone system4 and of the sympathetic nervous system5 have been proposed as underlying mechanisms. Several previous studies have examined the relationship between urinary sodium excretion, in the assumption that this reflects sodium intake, and cardiac structure in humans. The Coronary Artery Risk Development In Young Adults study in 1042 young patients without apparent CV disease demonstrated a moderate relationship between urinary sodium excretion (average of three samples, each taken over 24 hours) and echocardiographically determined LVH (echo-LVH).6 Another study in a smaller number of patients with arterial hypertension (n=42) found a similar association between single 24-hour urinary sodium excretion measurements and echo-LVH (r=0.37).7 However, a cross-sectional analysis in 2260 individuals from the Framingham Heart Study was not able to demonstrate any relationship between single urinary sodium excretion measurements from spot urine samples and echo-LVH.8
These studies obviously differ with regard to sample size, patient population (normo- versus hypertensive), and methodologic details of urinary sodium measurements (24-hour versus spot urine samples). However, the assumption that urinary sodium excretion measurements truly reflect sodium intake, even when performed in 24-hour samples, has been questioned.9 Recent balance studies suggest that periodic tissue sodium storage occurs even in healthy participants, and urinary sodium excretion does not mirror sodium intake on a day-to-day basis.10 A potential explanation for these unexpected findings might be the experimental evidence that tissues, in particular skin and muscle, can store sodium.11–13 Further, excess sodium content in skin as a result of the dysregulation of skin lymphatic expansion during high sodium intake has been linked with arterial hypertension in animal models.11–13
The development of 23sodium-magnetic resonance imaging (23Na-MRI) has made investigations into the role of tissue sodium possible in humans.14,15 The first studies with 23Na-MRI have shown that skin sodium is related to the level of BP in patients with resistant hypertension,14 confirming the aforementioned experimental findings.11–13 The role of tissue sodium in target organ damage, in particular LVH, has not been examined. In the current study, we have examined the relationship of local tissue sodium in comparison with general overhydration (OH; assessed by bioimpedance spectroscopy) with LVH in patients with CKD, in whom clinical sodium retention and LVH are both common.
Results
Clinical Characteristics
The clinical characteristics of the patients enrolled are presented in Table 1. Renal function was, on average, mild to moderately impaired. Most common causes of renal disease in our cohort were diabetic nephropathy, vascular nephropathy, systemic disease affecting the kidney (e.g., rheumatologic disease) and primary glomerulopathy (e.g., IgA nephropathy). As regards comorbidities, patients were characterized by a high prevalence of diabetes (28%) and arterial hypertension (92%). Treatment of arterial hypertension consisted mainly of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β blockers, calcium channel antagonists, and diuretics.
Table 1.
Clinical characteristics of patients enrolled in CARVIDA study (n=99)
| Parameter | Value |
|---|---|
| Age, yr, median (range) | 65 (23–78) |
| Sex, men/women | 57/42 |
| Weight, kg, mean±SD | 85±17 |
| Height, cm, mean±SD | 172±9 |
| Body mass index, kg/m2, median (range) | 28 (18–41) |
| Office SBP, mmHg, mean±SD | 133±15 |
| Office DBP, mmHg, mean±SD | 81±10 |
| 24-h SBP, mmHg, mean±SD | 126±11 |
| 24-h DBP, mmHg, mean±SD | 77±8 |
| Hypertension, % | 92 |
| Treatment resistant hypertension, % | 14 |
| Number of BP medications, median (range) | 2 (0–6) |
| ACE inhibitor, % | 32 |
| ARB, % | 44 |
| β Blocker, % | 35 |
| Calcium channel antagonist, % | 38 |
| Diuretic, % | 49 |
| Thiazide diuretic, % | 27 |
| Loop diuretic, % | 22 |
| Aldosterone antagonist, % | 7 |
| Diabetes mellitus, % | 28 |
| Coronary artery disease, % | 8 |
| Cerebrovascular disease, % | 7 |
| Peripheral vascular disease, % | 3 |
| Diabetic nephropathy, % | 19 |
| Vascular nephropathy, % | 28 |
| Systemic disease affecting the kidney, % | 16 |
| Primary glomerulopathy, % | 23 |
| Serum creatinine, mg/dl, median (range) | 1.3 (0.6–4.3) |
| eGFR, ml/min per 1.73 m2, median (range) | 51 (13–127) |
| UACR, mg/g creatinine, median (range) | 432 (1–2670) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio.
All patients studied participated in the CARdioVascular In Depth Assessment (CARVIDA) substudy of the German Chronic Kidney Disease (GCKD) study. Of note, anthropometric measures and level of renal function were similar in the patients enrolled in the current substudy to those of the parent GCKD study (Supplemental Table 1). However, treatment resistant hypertension, use of loop diuretics, and CV comorbidity appeared to be more prevalent in the parent study.
OH
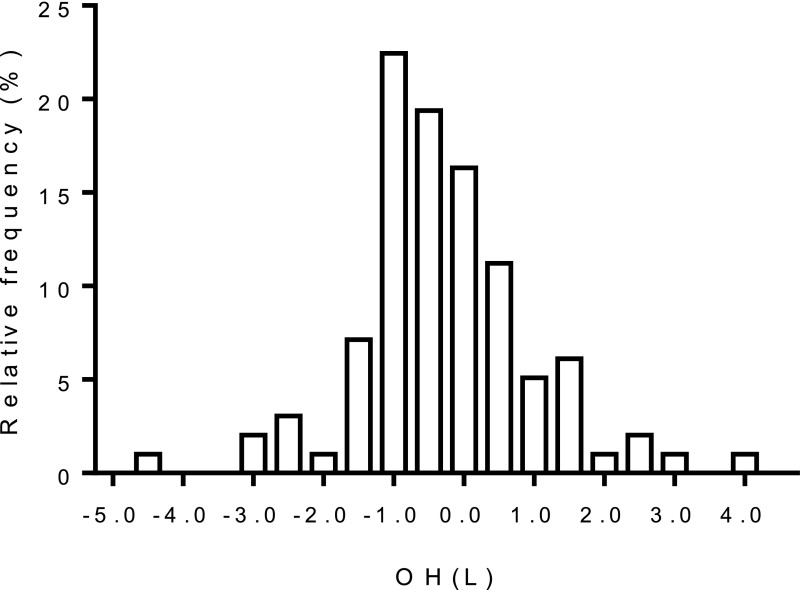
Figure 1 shows the distribution of OH, which varied widely across the cohort. In an exploratory analysis, we examined clinical characteristics according to tertiles of OH. As shown in Table 2, except for sex (men), none of the clinical parameters appeared consistently associated with OH.
Figure 1.
Distribution of OH in the cohort.
Table 2.
Clinical characteristics stratified according to OH in L (bioimpedance)
| OH | ||||
|---|---|---|---|---|
| Parameter | <−0.8 L, n=29 | −0.8 to +0.1 L, n=34 | >0.1 L, n=35 | P Value |
| Age, yr, median (range) | 63 (39–76) | 61 (25–78) | 69 (23–75) | 0.26 |
| Sex, men/women | 16/13 | 15/19 | 26/9 | 0.04 |
| Weight, kg, mean (95% CI) | 86 (81 to 92) | 82 (75 to 89) | 88 (82 to 93) | 0.34 |
| Height, cm, mean (95% CI) | 170 (166 to 175) | 170 (167 to 174) | 174 (172 to 176) | 0.15 |
| Body mass index, kg/m2, median (range) | 28 (24–41) | 28 (18–39) | 28 (20–39) | 0.30 |
| Office SBP, mmHg, mean (95% CI) | 133 (127 to 140) | 131 (126 to 135) | 135 (130 to 140) | 0.58 |
| Office DBP, mmHg, mean (95% CI) | 82 (78 to 86) | 80 (77 to 83) | 80 (76 to 83) | 0.60 |
| 24-h SBP, mmHg, mean (95% CI) | 124 (120 to 129) | 123 (120 to 127) | 129 (124 to 133) | 0.12 |
| 24-h DBP, mmHg, mean (95% CI) | 76 (72 to 79) | 76 (74 to 79) | 78 (75 to 81) | 0.51 |
| Hypertension, % | 90 | 94 | 91 | 0.81 |
| Treatment resistant hypertension, % | 10 | 15 | 17 | 0.74 |
| Number of BP medications, median (range) | 2 (0–4) | 3 (0–5) | 2 (0–6) | 0.24 |
| ACE inhibitor, % | 41 | 21 | 37 | 0.17 |
| ARB, % | 31 | 53 | 46 | 0.21 |
| β Blocker, % | 24 | 35 | 46 | 0.20 |
| Calcium channel antagonist, % | 21 | 44 | 49 | 0.05 |
| Diuretic, % | 45 | 50 | 49 | 0.92 |
| Aldosterone antagonist, % | 7 | 12 | 3 | 0.36 |
| Diabetes mellitus, % | 21 | 35 | 29 | 0.44 |
| Coronary artery disease, % | 10 | 3 | 11 | 0.38 |
| Cerebrovascular disease, % | 7 | 0 | 14 | 0.07 |
| Peripheral vascular disease, % | 0 | 6 | 3 | 0.40 |
| Diabetic nephropathy, % | 14 | 21 | 23 | 0.64 |
| Vascular nephropathy, % | 31 | 29 | 26 | 0.89 |
| Systemic disease, % | 10 | 21 | 17 | 0.54 |
| Primary glomerulopathy, % | 24 | 21 | 23 | 0.94 |
| Serum creatinine, mg/dl, median (range) | 1.4 (0.6–2.5) | 1.2 (0.8–2.0) | 1.3 (0.7–4.3) | 0.44 |
| eGFR, ml/min per 1.73 m2, median (range) | 53 (19–102) | 51 (27–100) | 49 (13–127) | 0.85 |
| UACR, mg/g creatinine, median (range) | 23 (1–1140) | 17 (2–1519) | 31 (2–1319) | 0.54 |
95% CI, 95% confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio.
Tissue Sodium and Water
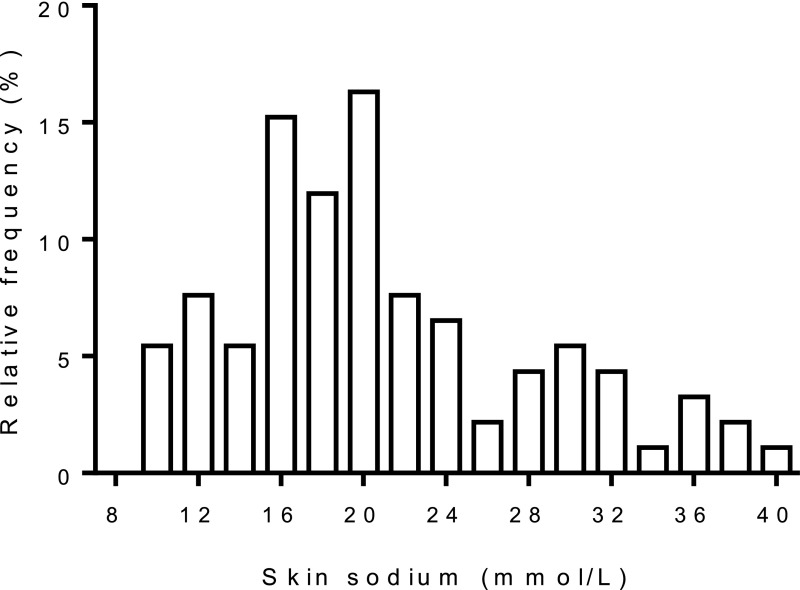
Figure 2 shows the distribution of skin sodium in our cohort. Again, we performed an exploratory analysis to study associations with clinical parameters. Table 3 shows clinical parameters according to tertiles of skin sodium. Age, men, greater weight/body mass index (BMI), higher BP, greater number of BP medications, specific BP medications (including diuretics), diabetes, target organ damage (coronary artery disease, cerebrovascular disease), and urinary albumin excretion rate were related to greater skin sodium content. Similar associations were found for skin water measurements (Supplemental Table 2).
Figure 2.
Distribution of skin sodium content in the cohort.
Table 3.
Clinical characteristics stratified according to skin sodium in mmol/L (23Na-MRI)
| Skin Sodium | ||||
|---|---|---|---|---|
| Parameter | <16.8 mmol/L, n=30 | 16.8–22.2 mmol/L, n=31 | >22.2 mmol/L, n=32 | P Value |
| Age, yr, median (range) | 54 (23–76) | 67 (46–75) | 70 (47–78) | <0.001 |
| Sex, men/women | 9/21 | 19/12 | 24/7 | 0.001 |
| Weight, kg, mean (95% CI) | 79 (73 to 86) | 85 (80 to 91) | 93 (87 to 99) | 0.005 |
| Height, cm, mean (95% CI) | 170 (167 to 173) | 172 (168 to 176) | 173 (170 to 176) | 0.40 |
| Body mass index, kg/m2, median (range) | 27 (18–37) | 28 (23–41) | 30 (23–39) | 0.02 |
| Office SBP, mmHg, mean (95% CI) | 129(123 to 135) | 133 (127 to 139) | 135 (131 to 140) | 0.22 |
| Office DBP, mmHg, mean (95% CI) | 81 (77 to 85) | 82 (79 to 86) | 78 (73 to 82) | 0.18 |
| 24 h-SBP, mmHg, mean (95% CI) | 122 (118 to 126) | 124 (121 to 127) | 132 (127 to 137) | 0.002 |
| 24 h-DBP, mmHg, mean (95% CI) | 78 (75 to 81) | 76 (74 to 79) | 77 (72 to 81) | 0.64 |
| Hypertension, % | 83 | 90 | 100 | 0.07 |
| Treatment resistant hypertension, % | 10 | 10 | 23 | 0.25 |
| Number of BP medications, median (range) | 1 (0–4) | 1 (0–5) | 3 (0–6) | <0.001 |
| ACE inhibitor, % | 37 | 19 | 42 | 0.14 |
| ARB, % | 33 | 55 | 48 | 0.23 |
| β Blocker, % | 30 | 13 | 61 | <0.001 |
| Calcium channel antagonist, % | 13 | 32 | 71 | <0.001 |
| Diuretic, % | 17 | 45 | 91 | <0.001 |
| Aldosterone antagonist, % | 3 | 3 | 10 | 0.44 |
| Diabetes mellitus, % | 0 | 42 | 45 | <0.001 |
| Coronary artery disease, % | 3 | 3 | 19 | 0.04 |
| Cerebrovascular disease, % | 0 | 0 | 13 | 0.02 |
| Peripheral vascular disease, % | 0 | 10 | 0 | 0.05 |
| Diabetic nephropathy, % | 3 | 19 | 39 | 0.003 |
| Vascular nephropathy, % | 20 | 32 | 32 | 0.47 |
| Systemic disease, % | 13 | 13 | 19 | 0.73 |
| Primary glomerulopathy, % | 30 | 16 | 23 | 0.44 |
| Serum creatinine, mg/dl, median (range) | 1.2 (0.7–2.0) | 1.3 (0.6–2.5) | 1.5 (0.7–4.3) | 0.13 |
| eGFR, ml/min per 1.73 m2, median (range) | 54 (27–127) | 53 (19–97) | 48 (13–88) | 0.24 |
| UACR, mg/g creatinine, median (range) | 42 (3–1147) | 10 (1–1519) | 67 (2–2670) | 0.03 |
95% CI, 95% confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio.
Greater muscle sodium was associated with age, greater weight/BMI, higher office systolic BP, greater number of BP medications, diabetes, calcium channel blockers, diuretics, and peripheral vascular disease (Supplemental Table 3). In contrast to the relationship between sex and skin sodium, sex was not related to muscle sodium. Muscle water only related to lower BMI but not consistently to any of the other clinical parameters (Supplemental Table 4).
Cardiac Structure
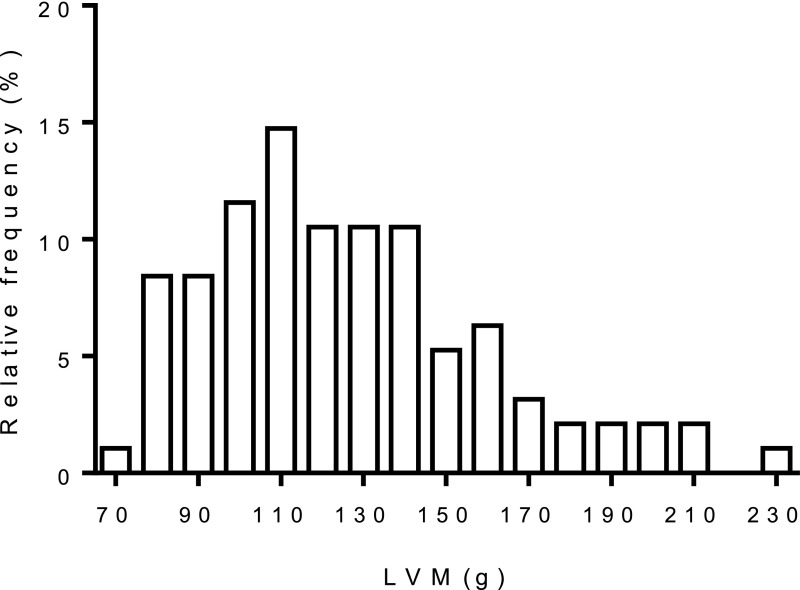
The distribution of left ventricular mass (LVM) is shown in Figure 3. LVH was present in 30% of men and 26% of women.
Figure 3.
Distribution of LVM in the cohort.
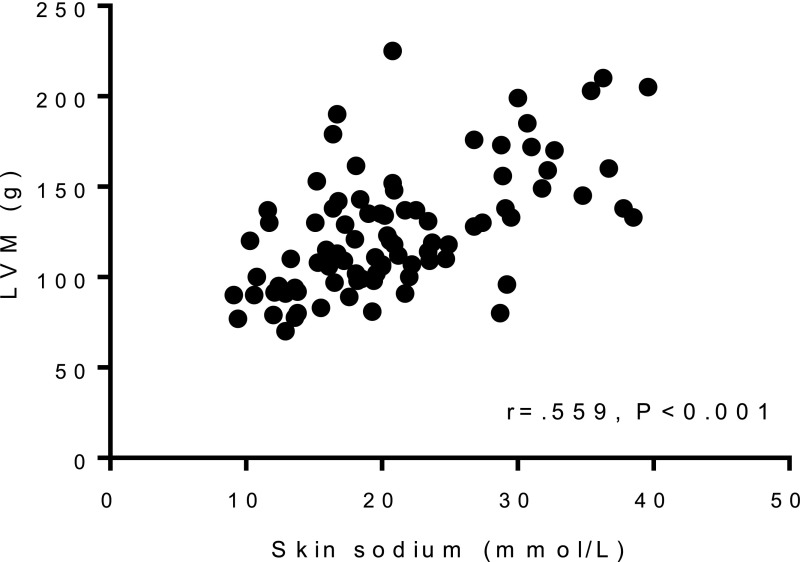
We then performed multiple linear regression analyses to study the value of the available variables for explaining ln LVM (LVM was logarithmically transformed to achieve normal distribution). The basic model consisted of the predefined clinical variables of sex, height, and 24-hour systolic BP (SBP; corrected R2=0.55, Table 4). Skin sodium correlated closely with LVM (Figure 4), and adding skin sodium as an explanatory variable substantially improved the model (corrected R2=0.64, Table 4, P<0.001 for change in F-statistic). Of note, the standardized β-value of 24-hour SBP decreased by almost 50% from 0.228 to 0.130 after skin sodium was added to the model, and the P value of 24-hour SBP increased from 0.003 to 0.08. Skin water closely correlated with skin sodium (r=0.95, P<0.001), and thus adding skin water instead of skin sodium improved the model in a very similar way (corrected R2=0.63, P<0.001 for change in F-statistic). The standardized β-values of skin sodium (standardized β=0.345) and skin water (standardized β=0.331) were also similar in the two models. In contrast, adding muscle sodium or muscle water did not substantially improve the model (corrected R2 values of 0.56 and 0.54 almost unchanged, changes in F-statistic P=0.07 and P=0.60, respectively).
Table 4.
Linear regression models for explanation of ln LVM
| Basic Model | +Skin Sodium | +Skin Water | +Muscle Sodium | +Muscle Water | +OH | +Skin Sodium +OH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corr. R2=0.55 | Corr. R2=0.64 | Corr. R2=0.63 | Corr. R2=0.56 | Corr. R2=0.54 | Corr. R2=0.58 | Corr. R2=0.63 | ||||||||
| Parameter | Stand. β-Value | P Value | Stand. β-Value | P Value | Stand. β-Value | P Value | Stand. β-Value | P Value | Stand. β-Value | P Value | Stand. β-Value | P Value | Stand. β-Value | P Value |
| Sex | −0.442 | <0.001 | −0.293 | 0.003 | −0.293 | 0.003 | −0.433 | <0.001 | −0.422 | <0.001 | −0.438 | <0.001 | −0.301 | 0.003 |
| Height | 0.303 | 0.002 | 0.341 | <0.001 | 0.370 | <0.001 | 0.291 | 0.004 | 0.312 | 0.002 | 0.268 | 0.004 | 0.333 | <0.001 |
| 24-h SBP | 0.228 | 0.003 | 0.130 | 0.08 | 0.129 | 0.09 | 0.205 | 0.01 | 0.232 | 0.004 | 0.204 | 0.01 | 0.130 | 0.08 |
| Skin sodium | 0.345 | <0.001 | 0.326 | 0.001 | ||||||||||
| Skin water | 0.331 | <0.001 | ||||||||||||
| Muscle sodium | 0.123 | 0.11 | ||||||||||||
| Muscle water | 0.019 | 0.80 | ||||||||||||
| OH | 0.185 | 0.01 | 0.023 | 0.78 | ||||||||||
Corr. R2, corrected R2; Stand. β-value, Standardized β-value.
Figure 4.
Relationship between skin sodium content and LVM.
OH correlated moderately with skin sodium (r=0.39, P<0.001, n=91). To examine the role of total body water as an explanatory variable for ln LVM, we added OH to the basic model. This resulted only in a moderate improvement of the model (corrected R2=0.58, Table 4, P<0.01 for change in F-statistic). Of note, when we added OH to the model that already contained skin sodium, the model was not further improved (corrected R2=0.63 almost unchanged, P=0.99 for change in F-statistic). In that case, the standardized β-value of OH decreased by almost 90% (from 0.185 to 0.023), with the respective P value for OH increasing from 0.01 to 0.78, whereas skin sodium was retained as a strong explanatory variable. In line with these results, the relationship between skin sodium and LVM (r=0.56, P<0.001, n=89, Figure 4) was stronger than the relationship between OH and LVM (r=0.35, P<0.001, n=94; P<0.01 for Hotelling t test between the two correlations).
Overall, these data suggest that skin sodium is a strong explanatory variable of ln LVM that is essentially unaffected by BP or OH. We emphasize that skin sodium had a very strong effect in all considered models (standardized β-value >0.3, P<0.001), regardless of the combination with other covariables. Finally, the fact that both BP and OH essentially lost their explanatory value after skin sodium was added to the model suggests that at least some of the information of BP and OH as regards explanation of ln LVM is already contained in the information on skin sodium.
Discussion
As the main novel finding of this study, we found that skin sodium was closely linked to LVH in patients with CKD, and that this appeared independent of BP and OH.
An extensive set of experimental data has shown that skin stores sodium in an actively regulated fashion. In rats and mice, a high salt diet leads to increased tonicity of the skin interstitium.11–13 Further, there is profound hyperplasia of the lymphatic capillary network, mediated by secretion of vascular endothelial growth factor-C (VEGF-C) from cells of the monocyte-macrophage system. Blocking VEGF-C secretion impairs lymphatic capillary hyperplasia in response to a high salt diet, resulting in an exaggerated increase in skin interstitial tonicity and BP.11–13 Of note, the increase in interstitial tonicity correlates closely with the increase of BP. Hyperplasia of the skin lymphatic capillaries is thus considered to provide a buffering mechanism against the development of salt-sensitive hypertension through facilitating the clearance of interstitial skin electrolytes. The precise mechanism linking skin sodium with BP remains to be elucidated, albeit downregulation of vascular endothelial nitric oxide synthase appears to be involved.11–13
With further developments of magnetic resonance imaging (MRI), such as sodium MRI (23Na-MRI), skin sodium can now be quantified in humans.15 In addition, standard hydrogen MRI (1H-MRI) enables the measurement of tissue water.16 First, in vivo studies demonstrated increased skin and muscle sodium in patients with arterial hypertension associated with hyperaldosteronism.15 Further, surgical or medical treatment of hyperaldosteronism led to a substantial decrease in tissue sodium content and decrease of BP.15 In subsequent studies, tissue sodium content was found to be increased in patients with treatment-resistant hypertension and in subgroups of patients on hemodialysis compared with healthy control participants.14,17 In extension to the aforementioned experimental findings,11–13 these data suggest that skin sodium content is also related to BP in humans. The consequences of this new concept of water and electrolyte metabolism were further tested in a long-term space simulation study (Mars500).10 Twelve young and healthy volunteers spent up to 520 days in an enclosed habitat in Moscow, living and working like cosmonauts on an international space station. As part of a metabolic/nutritional project, daily salt intake was fixed for 30–60 days at 6, 9, and 12 g/d. At constant salt intake, urinary sodium excretion varied with a weekly rhythm. These data indicate that (1) the body stores sodium periodically, and (2) single urinary sodium excretion measurements do not provide a reliable estimate of sodium intake or sodium content of the body.9
The potential relationship of skin sodium with target organ damage has not been studied so far. To this end, we enrolled patients with CKD, in whom sodium retention and LVH are particularly common. The average value of skin sodium content in patients with CKD (20.9±7.4 mmol/L overall, 23.6±7.2 mmol/L in men and 17.5±6.1 mmol/L in women) did not appear to differ from the average skin sodium content in healthy control participants enrolled in previous studies.14,17 However, values of skin sodium content exhibited a wide range in patients with CKD and are partly overlapping even with those of patients on hemodialysis.17 We also found associations with clinical parameters. Confirming the results of our previous studies, older age and male gender were associated with greater skin sodium content.14,15 A new and particularly interesting observation was that greater weight and diabetes appear to be related to greater skin sodium content. A more detailed analysis of molecular mechanisms of diabetes-related sodium retention may improve our understanding of the well known clinical association between diabetes and arterial hypertension.
For comparison with the tissue magnetic resonance imaging measurements, we used bioimpedance measurements to determine total body water. Similarly, a wide range of total body water content was found in patients with CKD, in keeping with clinical experience that not all, but some patients with CKD are clinically overhydrated. Type and stage of kidney disease, concomitant heart disease, sodium and water intake, and intensity of diuretic therapy are likely to be key determinants.
Despite a moderate correlation between skin sodium and total body water measurements, we found that skin sodium correlated much more strongly than total body water with BP and LVH. Several potential explanations need to be considered. Local skin and water content and total body water content could biologically be regulated in the same way, but skin MRI measurements may simply give a more accurate/sensitive indication of sodium retention. Alternatively, skin sodium and total body water may be regulated independently from each other, and there is a specific biologic link of skin sodium but not total body water with BP and LVH. The above cited experimental data would favor the latter explanation.11–13 In contrast to our study demonstrating no relationship between total body water and BP, an association of total body water with BP has been reported in patients with more advanced CKD.18 Whether this is simply because of a greater range in total body water in patients with more advanced CKD, or whether body water plays a more important role for BP regulation in advanced compared with less advanced CKD, requires further study. Clearly, our studies of the role of sodium in LVH should be extended to stages of more advanced CKD in future.
The fact that the relationship between skin sodium and LVH was unaffected by BP may provide indirect evidence for BP-independent effects of sodium on the heart, as suggested by experimental studies.4,19 Whether skin sodium reflects sodium content of the heart, or whether there are other, perhaps hormonal, and BP-independent mechanisms linking skin sodium with LVH is unknown at present. Direct measurements of cardiac sodium content by MRI would be extremely interesting, but are technically challenging at present.
Our study has several strengths and limitations. As a strength, sodium content of skin was measured with the 23Na-MRI technique, available in only a few centers around the world. Further, LVM in the current study was measured by MRI, unlike previous investigations, which have used echocardiography. Cardiac MRI is much more sensitive and precise for LVM quantification.20 Important limitations of the current study are its cross-sectional design, the relatively small sample size, and the absence of an assessment of sodium intake or excretion. Because of the observational nature of our study, any causal relationship between sodium stores and LVH can only be hypothesized. Longitudinal studies are required to determine whether increased sodium content in skin precedes the development of LVH. Further, our results should not be interpreted in a sense that skin sodium is generally more important than BP for LVH development. We can only state that at the time the patients were investigated, current skin sodium content was a stronger explanatory variable of LVM than current “on-treatment” BP.
As compared with the parent GCKD study, patients enrolled in the CARVIDA substudy appeared to have less treatment resistant hypertension, perhaps suggesting that a slightly healthier subpopulation agreed to undergo more extensive CV phenotyping investigations. Thus, we believe that the degree of skin sodium retention and the prevalence of LVH are likely to be similar, if not even greater, in the parent GCKD study population. However, we do not know to which extent these observations can be extrapolated to other patient populations, including those with other ethnicities.
In summary, the novel finding of this study was that skin sodium is tightly linked with LVH, a prognostically important complication in patients with CKD. The first studies in patients with decompensated heart failure suggest that removal of skin sodium is possible with appropriate diuretic therapy.21 Whether therapy with diuretics or nonpharmacologic approaches (e.g., sodium restriction) can lead to reductions in skin sodium with concomitant improvements of LVH and clinical outcomes merits further investigation in patients with CKD.
Concise Methods
Study Design
CARVIDA is a substudy of the GCKD study. The methodology and baseline data of GCKD have been reported in detail.22,23 In brief, 5127 white patients with CKD were included on the basis of two inclusion strata: estimated glomerular filtration rate (eGFR) of 30–60 ml/min per 1.73 m2 or overt proteinuria (urinary albumin-to-creatinine >300 mg/g, albuminuria >300 mg/d, urinary protein-to-creatinine >500 mg/g, or proteinuria >500 mg/d) in the presence of eGFR>60 ml/min per 1.73 m2. Patients after solid organ or bone marrow transplantation, with active malignancy within 24 months before screening, heart failure New York Heart Association class IV, representation by a legal guardian, or inability to provide consent were excluded. The GCKD study has been approved by local ethics committees and is registered in the national registry for clinical studies (Deutsches Register Klinischer Studien 00003971).
CARVIDA is conducted at three of nine regional GCKD centers in Germany (Würzburg, Aachen, and Erlangen). The aim of the CARVIDA substudy is to provide detailed CV phenotyping in order to examine the pathophysiologic basis underlying the increased CV risk observed in patients with CKD. Patients were approached in writing and had to provide written informed consent to participate in the CARVIDA substudy. A total of n=322 patients were enrolled in CARVIDA across the three study sites. However, the specific MRI techniques for investigation of tissue sodium and water were only available in Erlangen. From the 100 patients enrolled in Erlangen, one patient had to be excluded from further statistical analysis because of marked OH from right ventricular failure and chronic venous insufficiency. From the remaining 99 patients, some did not undergo or complete the MRI measurements, most commonly because of anxiety immediately before or during the MRI scans. Thus, cardiac MRI scans were completed in 96 patients, skin sodium measurements in 93 patients, and skin water measurements in 92 patients. Investigators assessing BP and investigators reporting cardiac MRI scans were blinded to the results of the tissue sodium/water measurements.
Biochemical Measurements
Plasma, serum, blood, and spot urine samples were collected, processed, and shipped frozen to a central laboratory for routine clinical chemistry of a core set of parameters (Synlab, Heidelberg, Germany). Serum creatinine was analyzed using an isotope dilution mass spectrometry–traceable methodology. GFR values were estimated using the Chronic Kidney Disease Epidemiology Collaboration formula.24 The biochemical data presented were taken from the GCKD study baseline visit.
Body Composition Measurements
Fluid status was measured with a portable whole-body bioimpedance spectroscopy device (body composition monitor [BCM]; Fresenius Medical Care, Bad Homburg, Germany). Electrodes are attached to the hand and foot on the nondominant side of the body and electrical responses at 50 different frequencies between 5 and 1000 kHz are measured. From the resulting impedance data and additional clinical parameters, extracellular water, intracellular water, and total body water are calculated by the equations proposed by Moissl et al.25
Fluid status assessed by the BCM is represented as OH (in Liter), on the basis of a three-compartment model developed by Chamney et al.26 The three compartments are lean tissue mass, adipose tissue mass, and OH. OH is the difference between the amount of extracellular water in the tissue actually detected by the BCM and the amount of water present in tissue predicted using physiologic models under normal (euvolemic) conditions.
Tissue Sodium (23Na-MRI) and Water (1H-MRI) Measurements
The methodology for quantitative tissue sodium analysis by 23Na-MRI has been previously published.15 In brief, we measured sodium content in muscle and skin of the lower leg with a 23Na volume coil (Stark-Contrast, Erlangen, Germany) at 3.0 T with an MRI scanner (Magnetom-Verio; Siemens Healthcare, Erlangen, Germany) using a 2D-FLASH sequence (total acquisition time =13.7 minutes; time to echo [TE] =2.07 ms; time to repetition [TR] =100 ms; flip angle [FA] =90°; 128 averages, resolution: 3×3×30 mm3). Four tubes containing aqueous solutions with 10, 20, 30, and 40 mmol/L sodium chloride served as calibration standards by relating intensity to a concentration in a linear trend analysis. In parallel, we quantified tissue water content by 1H-MRI, using a fat-saturated inversion recovery sequence with spin density contrast (total inversion time =210 ms; total acquisition time =6.29 minutes; TE=12 ms; TR=3 seconds; FA 1/2=90/180°; 128 averages, resolution: 1.5×1.5×5 mm3), as described by other investigators.16 The 10 mmol/L sodium chloride tube served as a calibration standard for tissue water. The average coefficient of variation for the analysis of same images between seven different readers was found to be 2.1% for skin sodium, 0.5% for muscle sodium, 7.6% for skin water, and 0.5% for muscle water (inter-reader variability). In the current study, image analysis was performed by a single reader (A.B.). The coefficient of variation for day-to-day measurements was found to be 8.1% for skin sodium and 5.5% for muscle sodium (five consecutive measurements in n=2 individuals). For week-to-week measurements, we found a coefficient of variation of 7.2% for skin sodium and 8.6% for muscle sodium (four consecutive measurements in n=5 individuals).
BP Measurements
Office BP was measured as the mean of three oscillometric measurements in the supine position after 5 minutes of rest (Infinity Gamma XL; Draeger, Lübeck, Germany). 24-hour BP was measured with the Mobil-O-Graph device (IEM Healthcare, Stolberg, Germany). All BP measurements (office and ambulatory blood pressure monitoring) were done on the same day as all other investigations. Treatment-resistant arterial hypertension was defined as an office systolic BP of ≥140 mmHg or an office diastolic BP ≥90 mmHg, despite treatment with three or more antihypertensive medications including a diuretic.
Cardiac MRI
MR examinations were performed on a 1.5 Tesla MR scanner equipped with high-performance gradients (Magnetom Aera; Siemens AG, Erlangen, Germany). The imaging protocol included balanced steady-state free precession cine sequences for functional and volumetric analysis. Retrospectively gated electrocardiographically triggered balanced steady-state free precession cine images were acquired during breath holding in standard four-chamber, three-chamber, and two-chamber long- as well as short-axis views, covering the entire left ventricle, with a 10 % slice gap. Scan parameters were as follows: slice thickness =8 mm, in-plane resolution =2.5 × 1.8 mm, TE=1.1 ms, TR=42 ms, and FA=50°.
Quantitative image data analysis was performed using dedicated commercially available software that enables postprocessing of cardiac MRI data (syngo.via; Siemens AG, Erlangen, Germany). The left ventricle was judged to be successfully detected by the software if the left ventricle rather than a different anatomic structure was marked. LVM was measured at end diastole by multiplying the myocardial volume by the specific gravity of the myocardium (1.05 g/ml). LVH was defined according to data from the Framingham Heart Study by a cut-off value of >75 g/m2 in men and >60 g/m2 in women.27
Statistical Analyses
IBM SPSS Statistics version 21 was used for statistical analysis. Data are presented as means with SD or means with 95% confidence intervals for normally distributed variables, medians with ranges for non-normally distributed variables, and distributions or percentages for categorical variables. Comparisons between tertiles were made by one-way ANOVA for normally distributed variables, Kruskal–Wallis tests were used for non-normally distributed variables, and chi-squared tests for categorical variables. Correlation was assessed by calculating Pearson (r) and Spearman (rho) correlation coefficients as appropriate. Hotelling t tests were used for comparison of correlations. Linear regression analyses were performed to study the effects of explanatory variables on LVM. LVM was not normally distributed and was therefore logarithmically transformed for linear regression analysis. The basic model consisted of the predefined clinical variables sex, height, and 24-hour SBP. Other variables of interest were then added to the basic model. In each case, the explanatory variables were entered simultaneously into the model. To compare models, we analyzed changes in the corrected R2 coefficient and the F-statistic.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Gabi Ruschkowski-Fritz, Beatrix Wiesenegger, Sabine Ernst, Stefan Lipski, and Gabriele Hartner for their expert technical assistance. The contribution of all CARdioVascular In Depth Assessment (CARVIDA) and German Chronic Kidney Disease (GCKD) study collaborators is also gratefully acknowledged.
The CARVIDA study was supported by Fresenius Medical Care, Bad Homburg, Germany. The GCKD study is supported by the Bundesministerium für Bildung und Forschung and the Kuratorium für Heimdialyse und Nierentransplantation e.V.– Stiftung Präventivmedizin.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016060662/-/DCSupplemental.
References
- 1.Pun PH: The interplay between CKD, sudden cardiac death, and ventricular arrhythmias. Adv Chronic Kidney Dis 21: 480–488, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paoletti E, Specchia C, Di Maio G, Bellino D, Damasio B, Cassottana P, Cannella G: The worsening of left ventricular hypertrophy is the strongest predictor of sudden cardiac death in haemodialysis patients: A 10 year survey. Nephrol Dial Transplant 19: 1829–1834, 2004 [DOI] [PubMed] [Google Scholar]
- 3.De Simone G, Devereux RB, Volpe M, Camargo MJ, Wallerson DC, Laragh JH: Midwall LV mechanics in rats with or without renovascular hypertension: Effect of different Na+ intakes. Am J Physiol 270: H628–H637, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Zhao X, White R, Van Huysse J, Leenen FH: Cardiac hypertrophy and cardiac renin-angiotensin system in Dahl rats on high salt intake. J Hypertens 18: 1319–1326, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD: Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation 108: 560–565, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez CJ, Bibbins-Domingo K, Jin Z, Daviglus ML, Goff DC Jr, Jacobs DR Jr: Association of sodium and potassium intake with left ventricular mass: Coronary artery risk development in young adults. Hypertension 58: 410–416, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmieder RE, Messerli FH, Garavaglia GE, Nunez BD: Dietary salt intake. A determinant of cardiac involvement in essential hypertension. Circulation 78: 951–956, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Dhingra R, Pencina MJ, Benjamin EJ, Levy D, Larson MG, Meigs JB, Rifai N, D’Agostino RB Sr, Vasan RS: Cross-sectional relations of urinary sodium excretion to cardiac structure and hypertrophy. The Framingham Heart Study. Am J Hypertens 17: 891–896, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Lerchl K, Rakova N, Dahlmann A, Rauh M, Goller U, Basner M, Dinges DF, Beck L, Agureev A, Larina I, Baranov V, Morukov B, Eckardt KU, Vassilieva G, Wabel P, Vienken J, Kirsch K, Johannes B, Krannich A, Luft FC, Titze J: Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension 66: 850–857, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakova N, Jüttner K, Dahlmann A, Schröder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, Vassilieva G, Lenkova L, Johannes B, Wabel P, Moissl U, Vienken J, Gerzer R, Eckardt KU, Müller DN, Kirsch K, Morukov B, Luft FC, Titze J: Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab 17: 125–131, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Machnik A, Dahlmann A, Kopp C, Goss J, Wagner H, van Rooijen N, Eckardt KU, Müller DN, Park JK, Luft FC, Kerjaschki D, Titze J: Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension 55: 755–761, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J: Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15: 545–552, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Müller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J: Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 123: 2803–2815, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J: 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 61: 635–640, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schöfl C, Renz W, Santoro D, Niendorf T, Müller DN, Neininger M, Cavallaro A, Eckardt KU, Schmieder RE, Luft FC, Uder M, Titze J: (23)Na magnetic resonance imaging of tissue sodium. Hypertension 59: 167–172, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Bley TA, Wieben O, François CJ, Brittain JH, Reeder SB: Fat and water magnetic resonance imaging. J Magn Reson Imaging 31: 4–18, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Dahlmann A, Dörfelt K, Eicher F, Linz P, Kopp C, Mössinger I, Horn S, Büschges-Seraphin B, Wabel P, Hammon M, Cavallaro A, Eckardt KU, Kotanko P, Levin NW, Johannes B, Uder M, Luft FC, Müller DN, Titze JM: Magnetic resonance-determined sodium removal from tissue stores in hemodialysis patients. Kidney Int 87: 434–441, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung SC, Kuo KL, Peng CH, Wu CH, Lien YC, Wang YC, Tarng DC: Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int 85: 703–709, 2014 [DOI] [PubMed] [Google Scholar]
- 19.de Simone G, Devereux RB, Camargo MJ, Wallerson DC, Laragh JH: Influence of sodium intake on in vivo left ventricular anatomy in experimental renovascular hypertension. Am J Physiol 264: H2103–H2110, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ: Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2: 271–278, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Hammon M, Grossmann S, Linz P, Kopp C, Dahlmann A, Garlichs C, Janka R, Cavallaro A, Luft FC, Uder M, Titze J: 23Na magnetic resonance imaging of the lower leg of acute heart failure patients during diuretic treatment. PLoS One 10: e0141336, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckardt KU, Bärthlein B, Baid-Agrawal S, Beck A, Busch M, Eitner F, Ekici AB, Floege J, Gefeller O, Haller H, Hilge R, Hilgers KF, Kielstein JT, Krane V, Köttgen A, Kronenberg F, Oefner P, Prokosch HU, Reis A, Schmid M, Schaeffner E, Schultheiss UT, Seuchter SA, Sitter T, Sommerer C, Walz G, Wanner C, Wolf G, Zeier M, Titze S: The German Chronic Kidney Disease (GCKD) study: Design and methods. Nephrol Dial Transplant 27: 1454–1460, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Titze S, Schmid M, Köttgen A, Busch M, Floege J, Wanner C, Kronenberg F, Eckardt KU; GCKD study investigators : Disease burden and risk profile in referred patients with moderate chronic kidney disease: Composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant 30: 441–451, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, Korth O, Müller MJ, Ellegård L, Malmros V, Kaitwatcharachai C, Kuhlmann MK, Zhu F, Fuller NJ: Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 27: 921–933, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Chamney PW, Wabel P, Moissl UM, Müller MJ, Bosy-Westphal A, Korth O, Fuller NJ: A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr 85: 80–89, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Yeon SB, Salton CJ, Gona P, Chuang ML, Blease SJ, Han Y, Tsao CW, Danias PG, Levy D, O’Donnell CJ, Manning WJ: Impact of age, sex, and indexation method on MR left ventricular reference values in the Framingham Heart Study offspring cohort. J Magn Reson Imaging 41: 1038–1045, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.