Abstract
Collectrin, encoded by the Tmem27 gene, is a transmembrane glycoprotein with approximately 50% homology with angiotensin converting enzyme 2, but without a catalytic domain. Collectrin is most abundantly expressed in the kidney proximal tubule and collecting duct epithelia, where it has an important role in amino acid transport. Collectrin is also expressed in endothelial cells throughout the vasculature, where it regulates L-arginine uptake. We previously reported that global deletion of collectrin leads to endothelial dysfunction, augmented salt sensitivity, and hypertension. Here, we performed kidney crosstransplants between wild-type (WT) and collectrin knockout (Tmem27Y/-) mice to delineate the specific contribution of renal versus extrarenal collectrin on BP regulation and salt sensitivity. On a high-salt diet, WT mice with Tmem27Y/- kidneys had the highest systolic BP and were the only group to exhibit glomerular mesangial hypercellularity. Additional studies showed that, on a high-salt diet, Tmem27Y/- mice had lower renal blood flow, higher abundance of renal sodium-hydrogen antiporter 3, and lower lithium clearance than WT mice. In WT mice, administration of angiotensin II for 2 weeks downregulated collectrin expression in a type 1 angiotensin II receptor–dependent manner. This downregulation coincided with the onset of hypertension, such that WT and Tmem27Y/- mice had similar levels of hypertension after 2 weeks of angiotensin II administration. Altogether, these data suggest that salt sensitivity is determined by intrarenal collectrin, and increasing the abundance or activity of collectrin may have therapeutic benefits in the treatment of hypertension and salt sensitivity.
Keywords: angiotensin converting enzyme, hypertension, salt sensitivity, collectrin, Tmem27
Collectrin (Tmem27 or Nx-17) is a 222–amino acid transmembrane glycoprotein with approximately 50% sequence identity with the angiotensin converting enzyme isoform 2 (ACE2). Unlike ACE2, collectrin lacks a catalytic domain.1 It is highly conserved, sharing >80% sequence identity between mouse, rat, and human. Collectrin has a discrete tissue distribution, with highest expression in the kidney, followed by pancreatic β-cells, liver, intestinal epithelial cells, retina, brain,2–7 and in endothelial cells throughout the vasculature.8 Taken together, these data suggest that collectrin may exert tissue-specific effects. For example, in vitro and in vivo studies have revealed that collectrin is involved in insulin secretion4 and islet mass in the pancreas.2 In renal proximal tubule and collecting duct epithelia, collectrin is thought to function as a chaperone for the trafficking of amino acid transporters, because deletion of collectrin results in reduced expression of neutral and cationic amino acid transporters and severe generalized urinary amino acid wasting.3,7 Similarly, in endothelial cells, collectrin regulates L-arginine (L-Arg) uptake, likely through its control of the plasma membrane expression of the L-Arg transporters CAT-1 and y+LAT1.8 Recently, collectrin was shown to bind B0-like amino acid transporters and is required for both their trafficking and catalytic activity, suggesting that collectrin is an essential subunit of this heteromultimeric secondary active transporter, rather than serving solely as a chaperone.9
The renal expression of collectrin is upregulated during the hypertrophic phase after subtotal nephrectomy10 and during exposure to high-salt diet,11 but the cause or effect was not established. Along with its homology to ACE2, these findings raised questions as to whether collectrin has a role in BP regulation. We reported that collectrin-deficient mice (Tmem27Y/- or KO) have baseline hypertension (HTN) and augmented salt sensitivity that are associated with altered states of superoxide (O2−) and nitric oxide (NO) generation.8 In addition, collectrin-deficient mice display impaired pressure natriuresis and endothelial-dependent vasorelaxation of peripheral conduit and mesenteric resistance arteries, decreased endothelial NO synthase (eNOS) dimerization, and impaired L-Arg uptake in endothelial cells.8 These findings suggest that collectrin regulates BP through both its influence on peripheral vascular resistance and renal sodium handling.
In this study, we performed kidney crosstransplantation (XTP) studies in wild-type (WT) and collectrin knockout (KO) mice to determine the relative role of renal versus extrarenal collectrin in BP homeostasis and salt sensitivity. The studies revealed that deletion of collectrin in the kidney determines salt sensitivity; WT mice with collectrin-deficient kidneys displayed a significant increase in BP after high-salt diet. Conversely, collectrin KO mice with WT kidneys exhibited similar BP as WT mice with WT kidneys and attenuated BP response to high-salt challenge. In addition, we discovered that deletion of collectrin results in reduced renal medullary neuronal NO synthase (nNOS) dimerization, increased abundance of the major proximal tubule sodium transporter Na+/H+ exchanger isoform 3 (NHE3), and reduced renal blood flow (RBF). Furthermore, we identified that the expression of collectrin is downregulated during angiotensin II (Ang II)–induced HTN, suggesting that the hypertensive effect of Ang II is at least in part mediated by suppression of collectrin.
Together, our report describes the essential role of renal collectrin in BP homeostasis and salt sensitivity through its influence on RBF and the expression of renal epithelial salt transporters.
Results
Renal Collectrin Protects against Salt Sensitivity
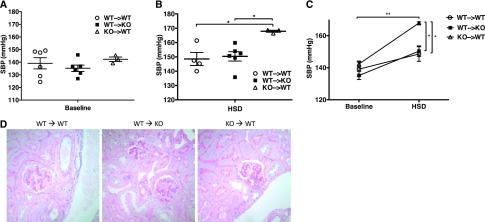
Our previous work showed that collectrin KO mice exhibit HTN at baseline and augmented salt sensitivity after high-salt diet.8 These phenotypes were associated with increased O2− generation, and decreased NO production in the kidney. Because collectrin is expressed in the renal epithelia, endothelium, and brain, we assessed the relative role of renal versus extrarenal collectrin in BP regulation using the kidney XTP approach, yielding the following groups: (1) WT mice with WT kidney (WT → WT), (2) collectrin KO mice with WT kidney (WT → KO), and (3) WT mice with collectrin KO kidney (KO → WT). BP was measured by radiotelemetry. As shown in Table 1, diurnal variation was intact across groups, with day-time BP being lower than night-time. Within each group, overall there was an increase in BP after 2 weeks on high-salt diet, compared with baseline, consistent with the known salt sensitivity of the 129 strain, and an appropriate compensatory decrease in heart rate. Across groups, the KO → WT had the highest systolic BP with high-salt feeding (P=0.01 by ANOVA; Figure 1, Table 1), a trend toward significant difference in mean arterial BP (P=0.06 by one-way ANOVA), and no differences in diastolic BP (Table 1). By light microscopy and periodic acid–Schiff staining of the kidneys, one KO → WT mouse had a focal area of perivascular and interstitial inflammation (data not shown). Otherwise, there were no significant differences among groups except for notable increased glomerular mesangial hypercellularity in the KO → WT group (Figure 1D). We previously reported similar mesangial hypercellularity in the global KO mice after 2 weeks on high-salt diet.8 These data demonstrate that renal collectrin is protective against the rise in systolic BP and the associated renal injury after high-salt feeding.
Table 1.
Blood pressure and heart rate among kidney cross-transplant groups at baseline and on high salt diet.
| Variables | WT → WT (n=4) | WT → KO (n=6) | KO → WT (n=3) | |||
|---|---|---|---|---|---|---|
| Baseline | High-Salt Diet | Baseline | High-Salt Diet | Baseline | High-Salt Diet | |
| SBP | 137.4±6.2 | 148.6±4.6a,b | 135.2±2.6 | 150.4±3.3a,c | 142.3±1.8 | 167.9±0.7a,b,c |
| P value (paired t test) | NS | 0.002 | 0.01 | |||
| Day-time | 128.9±6.3 | 142.4±2.0 | 133.6±3.7 | 148.2±4.0 | 139.0±1.5 | 164.9±1.0 |
| Night-time | 138.3±5.4 | 150.8±3.8 | 139.5±3.4 | 154.4±3.5 | 144.8±1.9 | 170.5±0.8 |
| DBP | 113.2±7.2 | 122.1±6.4 | 103.4±2.5 | 120.9±1.3 | 103.8±0.8 | 126.5±3.8 |
| P value (paired t test) | 0.05 | <0.001 | 0.02 | |||
| Day-time | 109.6±6.2 | 116.2±5.7 | 99.7±3.5 | 115.8±2.0 | 101.1±2.0 | 121.9±4.8 |
| Night-time | 120.3±5.7 | 130.0±5.5 | 108.7±2.9 | 127.1±2.1 | 106.6±1.1 | 130.9±3.5 |
| MAP | 125.8±6.5 | 135.6±5.0d | 120.1±2.1 | 135.9±1.5d,e | 123.8±0.8 | 146.7±1.8d,e |
| P value (paired t test) | NS | 0.001 | 0.004 | |||
| Day-time | 118.5±8.4 | 127.1±5.8 | 114.3±3.2 | 128.8±1.9 | 120.4±1.5 | 141.1±3.3 |
| Night-time | 129.4±7.0 | 141.8±6.2 | 124.2±1.2 | 141.4±1.6 | 127.3±2.4 | 151.9±25 |
| HR | 599.3±4.9 | 567.9±17.5 | 598.6±8.8 | 563.4±6.0 | 608.3±2.1 | 574.2±1.6 |
| P value (paired t test) | NS | 0.001 | 0.01 | |||
Values are displayed as mean±SEM. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate.
P=0.01 (one-way ANOVA).
P=0.02 (unpaired t test).
P=0.01 (unpaired t test).
P=0.07 (one-way ANOVA).
P=0.003 (unpaired t test).
Figure 1.
Collectrin in the kidney protects against salt sensitivity. (A) By radiotelemetry, at baseline on normal salt diet, the collectrin KO → WT group displayed a trend toward higher baseline systolic blood pressure (SBP; mm Hg), but did not reach statistical significance (n=3–6 per group). (B) After HSD, by paired analysis, all groups had significantly higher systolic BP on HSD, compared with normal salt diet (all P<0.04). However, collectrin KO → WT group had a statistically significantly higher systolic BP under HSD compared with the other two groups (WT → WT and WT → KO; P=0.01, one way ANOVA; or P=<0.001 by two-way ANOVA). *P<0.05, **P<0.01. (C) Graph of both baseline and high-salt conditions for all three groups. (D) Representative kidney histology by periodic acid–Schiff stain (40×). KO → WT mice displayed increased glomerular mesangial hypercellularity.
Collectrin KO Mice Display Impaired Renal nNOS Dimerization
We reported that collectrin regulates amino acid reclamation in the proximal tubules7 and L-Arg uptake in endothelial cells.8 Furthermore, the hypertensive phenotype of collectrin KO mice is partially L-Arg–dependent, because L-Arg supplementation partially lowered their BP.8 This partial response to oral L-Arg could be explained by decreased intracellular L-Arg level, decreased L-citrulline (L-Cit) to L-Arg recycling pool,12 and/or that severely impaired L-Arg uptake is the rate limiting factor for NO synthesis in KO mice. Therefore, we measured plasma and kidney tissue levels of L-Arg and L-Cit in collectrin KO mice to rule out their deficiency and found that, compared with WT mice, collectrin KO mice had similar plasma concentrations and kidney tissue levels of both amino acids (Supplemental Figure 1).
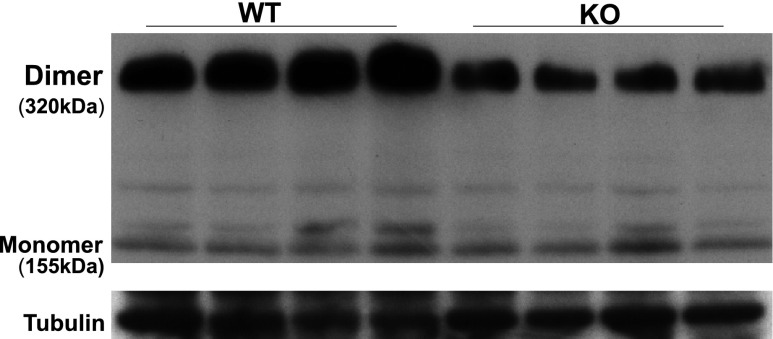
Pallone and Mattson demonstrated that infusion of L-Arg into the renal medullary interstitium of Dahl salt-sensitive (DSS) rats prevented the development of HTN during high-salt feeding.13 This BP lowering effect of L-Arg may be mediated by intrarenal NO generation, particularly by nNOS in the inner medullary collecting duct.14 Because deletion of collectrin results in decreased renal NO and diminished eNOS dimerization,8 we assessed whether the dimerization of nNOS is also altered in collectrin KO mice. As shown in Figure 2, immunoblot analysis indicated a significant decrease in nNOS dimerization in renal medullary tissues of collectrin KO mice, correlating with their impaired ability to mount an increase in renal NO production during high-salt feeding as previously reported.8
Figure 2.
Neuronal NO synthase (nNOS) dimer protein expression significantly decreased in renal medullary tissue in KO mice. nNOS monomer levels are unchanged. N=4 each.
RBF Is Reduced in Collectrin KO Mice
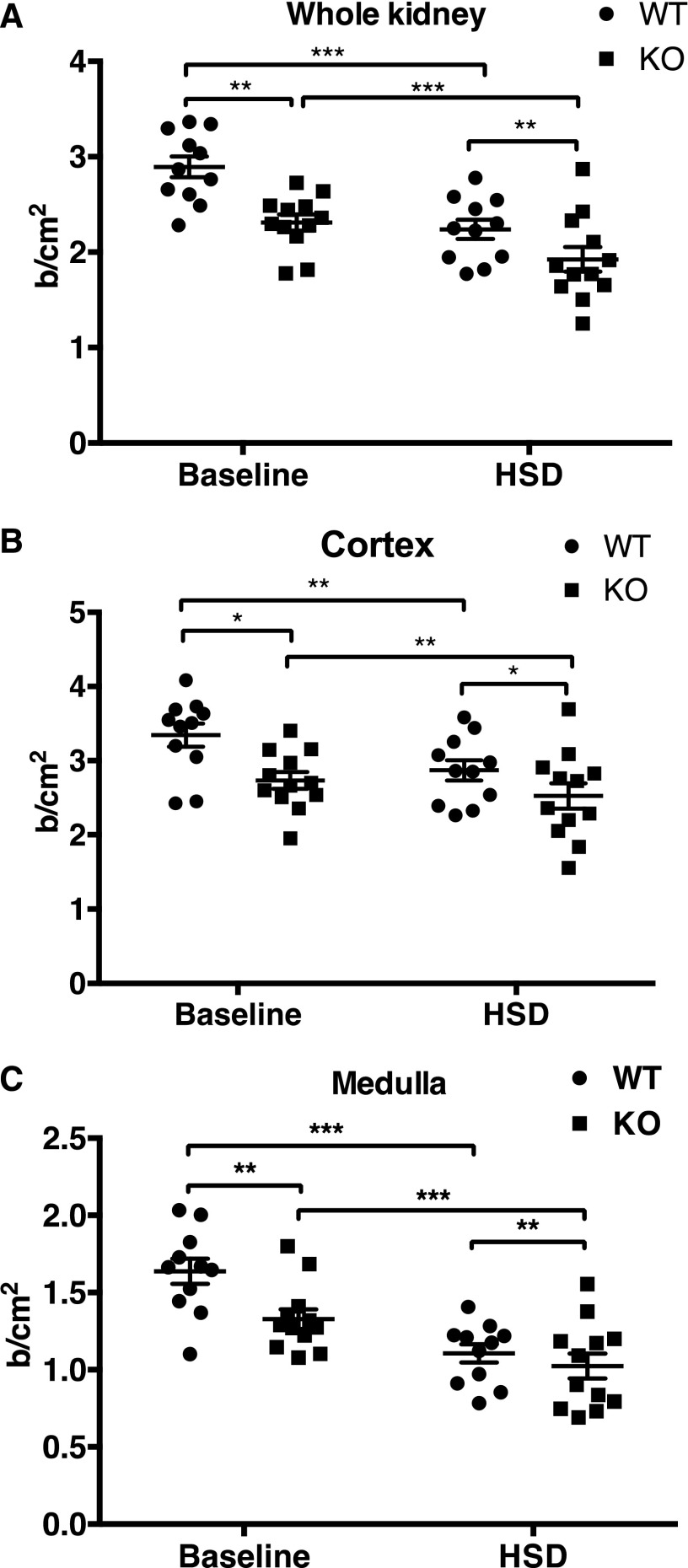
The augmented salt sensitivity and decreased dimerization of both eNOS and nNOS in collectrin KO mice suggest that loss of renal collectrin could result in alteration in renal hemodynamics and/or renal epithelial function. We next utilized the contrast-enhanced ultrasound (US) method to compare relative estimates of RBF in WT and collectrin KO mice. Contrast-enhanced US has been validated to provide near 100% correlation with RBF measured by the invasive transit-time flow probe technique.15 Using this technique, we have been able to detect a significant 38% increase in RBF in enalapril-treated compared with saline-treated WT male mice (see Supplemental Figure 3), similar to the effect reported in an earlier study using laser Doppler flowmetry.16 At baseline, RBF is significantly higher in the WT group (n=11) than in the KO group (n=12) in the cortex (3.34±0.16 versus 2.73±0.11 b/cm2, P<0.01), medulla (1.64±0.08 versus 1.33±0.06 b/cm2, P=0.01), and whole kidney (2.89±0.11 versus 2.31±0.08 b/cm2, P<0.001) (Figure 3). After 2 weeks of high salt diet (HSD), RBF significantly diminished in all areas measured in both groups. In the WT group, blood flow is reduced in the cortex (3.34±0.16 to 2.87±0.14 b/cm2, P<0.001), medulla (1.64±0.08 to 1.11±0.06 b/cm2, P<0.001), and whole kidney (2.89±0.11 to 2.24±0.10 b/cm2, P=0.001). Similarly, in the collectrin KO group, blood flow is also reduced in the cortex (2.73±0.11 to 2.53±0.17 b/cm2, P<0.001), medulla (1.33±0.06 to 1.02±0.08 b/cm2, P<0.001), and whole kidney (2.31±0.08 to 1.92±0.13 b/cm2, P=0.01) after HSD (Figure 3). After HSD, RBF remained significantly lower in collectrin KO mice compared with WT in the cortex (2.53±0.17 versus 2.87±0.14 b/cm2, P<0.01), medulla (1.02±0.08 versus 1.11±0.06 b/cm2, P=0.01), and whole kidney (1.92±0.13 versus 2.24±0.10 b/cm2, P=0.01) (Figure 3). By two-way repeated-measures ANOVA, the effect of genotype and diet was significant (P=0.03) on the estimated RBF. This would be consistent with the notion that collectrin KO mice have deficient NO/increased O2− that results in restriction of RBF and stimulation of sodium transport, thereby increasing the driving force for Na+ retention.
Figure 3.
RBF in the collectrin KO mice is reduced in the cortex, medulla, and whole kidney when compared with the WT mice, at both baseline and HSD. (A) Whole kidney. (B) Cortex. (C) Medulla. Comparison within group from baseline to HSD was performed by paired t test, and between groups was by un-paired t test. *P<0.05, **P<0.01, and ***P<0.001.
Expressions of Renal Sodium Transporters Are Increased in Kidneys of Collectrin KO Mice
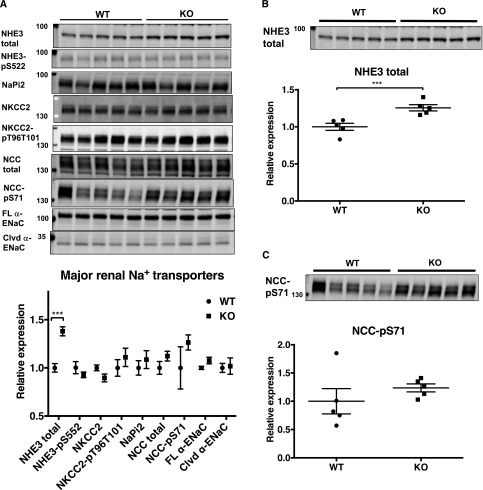
Because diminished urinary sodium excretion might result from an altered expression of sodium transporters in the kidney, we compared the protein expression profile of renal cortical sodium transporters in WT and collectrin KO mice at baseline. Five major sodium transporters along the nephron as well as their phosphorylated forms were profiled in collectrin WT and KO mice. The sodium transporters examined include total NHE3 and phosphorylated NHE3 (NHE3-pS552, associated with NHE3 inactivation) in the proximal tubule; Na+–phosphate cotransporter isoform 2 (NaPi2) in the proximal tubule; sodium-potassium-chloride cotransporter (NKCC2) and phosphorylated NKCC2 (NKCC2-p, associated with activation) in the thick ascending limb; sodium-chloride Na+/Cl− cotransporter (NCC) and phosphorylated NCC at serine 71 (NCC-pS71, associated with activation) in the distal tubule; and epithelial sodium channel α (α-ENaC), both full length and cleaved (associated with activation) forms, in the collecting duct. Shown in Figure 4, NHE3 protein is significantly more abundant in KO compared with WT kidneys (KO versus WT: 1.26 versus 1.0, P<0.004), whereas there was no difference in abundance of NHE3-pS552 (0.93 versus 1.0), NKCC2 (0.9 versus 1.0), NKCC2-pT96T101 (1.11 versus 1.0), or NaPi2 (1.04 versus 1.0) between the genotypes. Although total NCC was not significantly different between groups, (1.11 versus 1.0, P=0.36), its activated form, NCC-pS71, tended to be in higher abundance in the KO, but did not reach significance due to high phosphorylation in one WT outlier included in the analysis (1.27 versus 1.0, P=0.36) (Figure 4).
Figure 4.
Abundance of renal total NHE3 is significantly higher in collectrin KO mice. (A) The general expression profile of major Na+ transporters in collectrin WT and KO kidneys. (B) The abundance of total NHE3 is 28% higher in the KO (P<0.004). (C) There was a trend of higher expression of NCC-pS71 in the KO kidneys but it does not reach statistical significance due to one WT mouse with very high expression of NCC-pS71 (P=0.36). ***P<0.001 by t test. WT=5, KO=5.
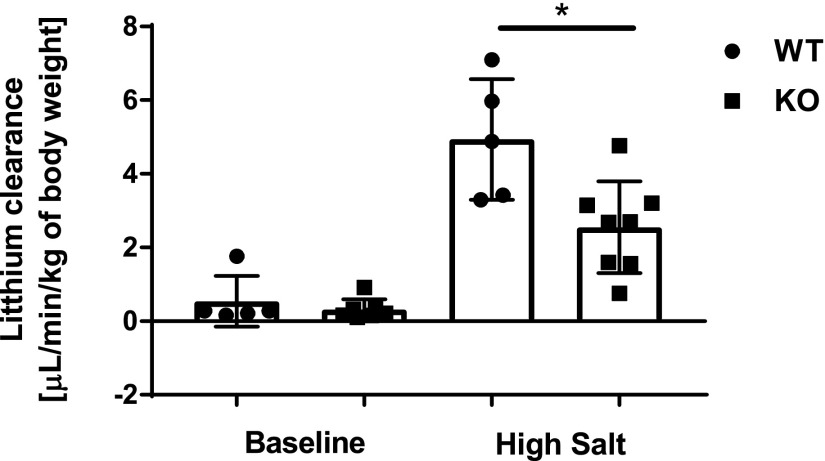
We next used lithium clearance (CLi) as a marker of renal sodium handling by the proximal tubule. CLi was measured at baseline and during the third day on high-salt diet. Shown in Figure 5, CLi was not different at baseline between groups. On high salt-diet, CLi significantly increased compared with baseline in both groups, and was significantly decreased in KO mice compared with WT. This finding suggests that there is increased NHE3 activity and therefore increased sodium reabsorption in the renal proximal tubule in collectrin KO mice, consistent with our earlier finding.8
Figure 5.
Twenty-four-hour CLi on high-salt diet is significantly lower in collectrin KO mice. CLi was not different between WT (n=5) and KO (n=8) mice at baseline. On high-salt diet, CLi was significantly higher than at baseline in both groups (P<0.001 by paired t test), and was significantly lower in KO compared with WT. *P=0.01 (t test).
Expression of Collectrin Is Downregulated by Ang II
The increased abundance of renal epithelial sodium transporters observed in collectrin KO mice at baseline is reminiscent of the short-term effect of nonpressor dose of Ang II.17–19 This raises the question of whether KO mice have increased renal activity of the renin-angiotensin system (RAS). We reported that collectrin KO mice have no alteration in the mRNA expression levels of Ace-2 or its Mas receptor genes in the kidney.8 Furthermore, the renal mRNA levels of AT1A receptor (Agtr1a), endothelin (ET-1, Edn1), ETA (Ednra), and ETB receptors (Ednrb) are also similar between WT and collectrin KO mice (Supplemental Figure 2).
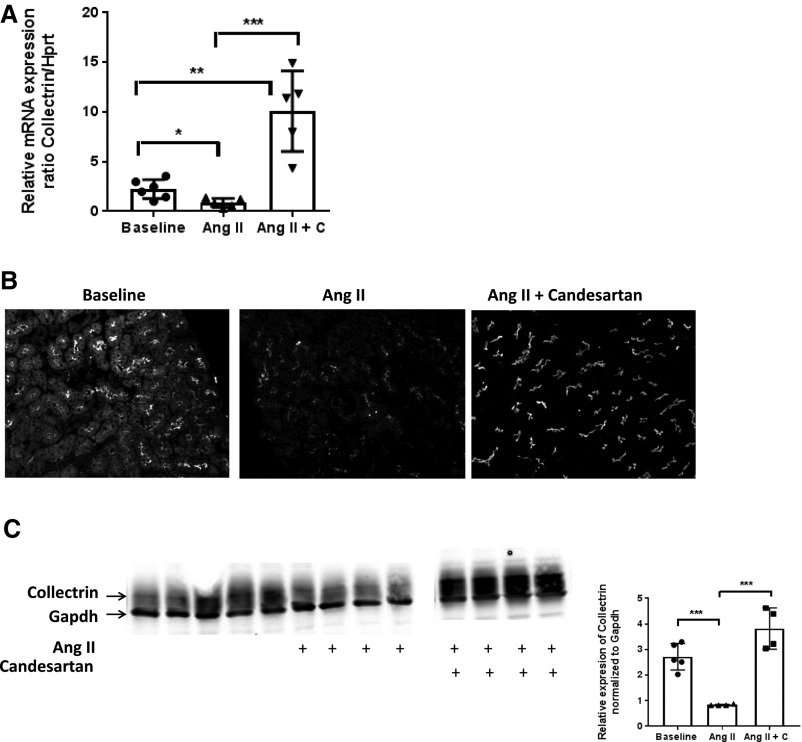
We next queried whether the expression of collectrin is regulated by Ang II, and if so, whether this is mediated through the AT1 receptor. The following aged-matched 129S6 groups were compared: (1) WT, (2) WT with Ang II osmotic minipump (600 ng/kg per minute × 2 weeks), and (3) WT with Ang II osmotic minipump (600 ng/kg per minute × 2 weeks) + candesartan (20 mg/kg per day in drinking water). Shown in Figure 6A, renal mRNA expression of collectrin is significantly reduced after 2 weeks of Ang II, but was significantly increased when treated with candesartan, even compared with baseline. By immunostaining and Western analysis, Ang II treatment significantly lowered collectrin protein expression, and this reduction was blocked by treatment with candesartan (Figure 6, B and C). Here, we show for the first time that Ang II downregulates the expression of collectrin, and that this is mediated through the AT1 receptor.
Figure 6.
Renal expression of collectrin is down-regulated by Ang II in a type 1 angiotensin-II receptor-dependent manner. (A) Renal mRNA expression of Tmem27, normalized to Hprt, is significantly downregulated during Ang II–induced HTN (*P=0.02 versus baseline). Treatment with candesartan during Ang II infusion significantly increased collectrin mRNA level (**P<0.001 versus baseline, and ***P<0.001 versus Ang II alone). (B) Immunostaining of collectrin. Kidney slides were stained with 10 μl/ml Alexa647 conjugated anti-mouse collectrin antibody and mounted in ProLong Antifade with DAPI. Images were captured with equivalent exposure time of 3.2 seconds for far red (647) and 41 milliseconds for DAPI for all slides. (C) Immublotting of collectrin and quantitation, normalized to Gapdh. (***P≤0.001).
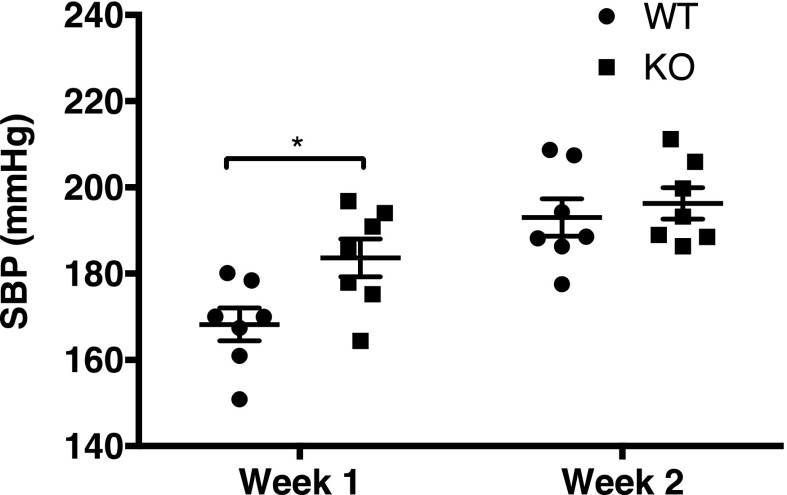
We next compared pressure response to Ang II (600 ng/kg per minute × 2 weeks via osmotic pump) between WT and KO mice (n=7 per group). By tail cuff manometry, systolic blood pressure (SBP) was significantly higher in KO mice during the first week, but was similar to that of WT during the second week of Ang II (Figure 7). This suggests that the downregulation of collectrin during the second week of Ang II in WT mice abolished the protective effect of collectrin observed during the first week of Ang II–induced HTN, resulting in HTN similar in severity to that of collectrin KO mice.
Figure 7.
BP response to Ang II differs with duration between WT and KO collectrin mice. Collectrin KO mice had significantly higher systolic BP during the first week of Ang II–induced HTN compared with the WT: WT 168.2±3.8 mmHg versus KO 183±4.4 mmHg (*P=0.02, by t test). After the second week, systolic BP was similar between the two groups: WT 193±4.3 mmHg versus KO 196.3±3.6 mmHg (P=NS). Systolic BP was measured by tail cuff.
Discussion
Salt sensitivity is associated with increased cardiovascular events and mortality, even in normotensive subjects who do not meet the clinical definition of HTN.20 Among hypertensive patients, the prevalence of salt sensitivity is estimated to be approximately 51%. The mechanisms mediating salt-sensitive HTN remain poorly understood. Experimental studies using genetic models of HTN in rodents have demonstrated that the genotype of the kidney primarily determines the level of BP. In this regard, Dahl and colleagues showed 40 years ago in kidney crosstransplant studies that a kidney from a Dahl salt-resistant rat strain can lower BP in the DSS rat strain, whereas a kidney from a DSS strain can increase BP in a Dahl salt-resistant strain.21 DSS rats consuming high-salt diet exhibited increased urinary excretion of 8-isoprostane, an index of oxidative stress, and significant reductions of kidney and aorta eNOS.22 The genetic basis for their phenotype is unclear. We reported that collectrin-deficient mice share several phenotypic characteristics with the DSS rats, including HTN at baseline and augmented SSH that is associated with impaired pressure natriuresis, altered states of O2− and NO generation, and decreased eNOS dimerization, suggesting a state of NOS uncoupling.8 It would be of significant interest to determine whether the DSS rat strain has an inactivating mutation of Tmem27.
In this study, using kidney XTP, we found that collectrin in the kidney affects BP response to high-salt diet. Loss of collectrin in the kidney caused significant salt-sensitive HTN in WT mice that express collectrin outside the kidney. Moreover, our transplant study also suggested that WT kidney rescued the salt sensitivity of collectrin KO mice, because the WT → KO group had lower BP on high-salt diet compared with the established HTN observed in global collectrin KO mice after exposure to a high-salt diet,8 and similar BP as the WT → WT group. There was no difference in BP at baseline among groups. Because global KO mice have HTN at baseline,8 lack of HTN in KO → WT group at baseline may suggest a contribution of vascular collectrin in the regulation of BP in normal state.
The effect of collectrin on salt sensitivity is associated with its influence on renal hemodynamics. Collectrin KO mice displayed significantly diminished RBF at baseline and during HSD. It should be noted that the WT 129S6 strain exhibits salt sensitivity23–25 and therefore it is not unexpected that even the WT mice have reduced RBF in response to a high-salt diet as compared with salt-resistant strains, which typically have increased RBF in response to HSD in many published studies. This notion and our findings are supported by observations in salt-sensitive patients who have a similar reduction of RBF in response to high sodium intake.26–29 This response has been suggested to be due to an increase in pre- and postglomerular vasoconstriction.27,28 Loss of collectrin is also associated with increased abundance of NHE3, along with decreased CLi. Taken together, the reduced RBF and increased expression (and likely activity as determined by CLi) of NHE3 favor sodium reabsorption and can explain the blunted pressure natriuresis in collectrin KO mice.8
These phenotypes, along with diminished eNOS and nNOS dimerization, could be partially explained by the role collectrin plays in the transport of amino acids, particularly the cellular uptake of L-Arg. We reported previously that collectrin KO mice displayed impaired L-Arg uptake in endothelial cells.8 Emerging evidence suggests there are several different L-Arg pools for NO synthesis by NO synthase: (1) transport of L-Arg from the extracellular space,30 (2) intracellular L-Cit to L-Arg recycling,12 and (3) lysosomal and proteasomal protein degradation.31 The requirement of L-Arg uptake for NO synthesis despite high levels of intracellular L-Arg, has led to the concept of the “L-Arg paradox.” Certain pathophysiologic conditions may determine which L-Arg pool is the rate-limiting source.31 Here, we showed that plasma and tissue levels of L-Arg and L-Cit were very similar between WT and collectrin KO mice. Taken together, these data suggest that L-Arg transport from the extracellular space may be the rate-limiting source for NO synthesis in collectrin KO mice. Furthermore, our data suggest that salt sensitivity may be determined by the efficiency of cellular L-Arg uptake.
nNOS is expressed in the inner medullary CD and is significantly increased during HSD, where it is thought to generate NO as an adaptive response.14 The renal expression of collectrin is also increased during HSD,11 suggesting collectrin is also involved in this adaptive response pathway. The diminished nNOS dimerization in collectrin KO mice would be consistent with the notion that loss of collectrin impairs the adaptive ability to appropriately increase nNOS activity, resulting in impaired NO production during HSD, and could explain the augmented SSH and altered pressure-natriuresis relationship in KO mice.8
RBF plays a critical role in the maintenance of normal kidney function, pressure-natriuresis, and BP. Under physiologic conditions, RBF is maintained by a balance of opposing vasoconstrictor and vasodilator influences.32,33 Moreover, renal vasomotor responses to vasoactive mediators are modulated by both O2− and NO.34–36 Roman and colleagues showed that medullary blood flow determines renal interstitial hydrostatic pressure and hence the natriuretic response to elevation in renal perfusion pressure.37 In this regard, cellular uptake of L-Arg in the renal medulla is critical for NO production, maintenance of medullary blood flow, and regulation of Na+ excretion and BP.38,39 It is unclear whether the source of NO regulating blood flow in the renal medulla is from eNOS or nNOS or both, and which deficient source of NO might be responsible for the reduced RBF in collectrin KO mice, because they displayed diminished dimerization of both renal medullary eNOS8 and nNOS. Although the decreased dimerization of eNOS and nNOS in collectrin KO mice can be attributable to decreased availability of L-Arg substrate,40 we cannot rule out a direct effect of collectrin on the dimerization of NOS.
Our analysis of renal mRNA levels of the components of the RAS do not support an activated renal RAS as the cause of increased levels of sodium transporters in collectrin KO mice. However, we have uncovered that collectrin is downregulated by chronic Ang II administration, and that the action of Ang II may be mediated in part through the downregulation of collectrin that is AT1 receptor–dependent. The increased renal expression of collectrin during high-salt feeding11 would be consistent with the notion of lower renin activity and hence suppression of Ang II downregulation of collectrin. Future studies will focus on delineating the signaling pathway by which AT1 transcriptionally regulates collectrin expression.
A central question is how collectrin, a chaperone of amino acid transporters, regulates the expression/abundance of NHE3 and potentially NCC phosphorylation. Future focus will include determining the direct role of collectrin on the transcriptional regulation, cellular trafficking, and covalent modification of the transporter. Higher NHE3 abundance provides a molecular mechanism for the elevated BP in the collectrin KO mice. Proximal tubule NHE3 is not only the primary Na+ reabsorption route in the proximal tubule (reabsorbing two-thirds of the filtered load even at baseline) but also the key effector of pressure natriuresis that critically influences the BP set-point. Transporter profiling in Ang II–infused rodents demonstrates that before HTN develops, abundance of sodium transporters all along the nephron, including NHE3, significantly increases.41 Then, at longer time-points after HTN develops, distal transporters (NKCC2, NCC, and ENaC) are further stimulated, whereas abundance of proximal tubule NHE3 is suppressed, which increases Na+ excretion to balance Na+ output to intake at the expense of elevated pressure.42,43 This depression of NHE3 expression is amplified, and HTN is suppressed, in Ang II–infused mice lacking proximal tubule AT1 receptors or lacking cytokine expression.19,42,44 Because both NHE3 abundance and BP are higher in the collectrin KO mice, we conclude that collectrin expression, directly or indirectly via NO production stimulation, plays a role in the pressure natriuresis regulation of NHE3 which affects the BP set-point.
The relative contribution of peripheral versus intrarenal endothelial collectrin on BP regulation has not been definitively delineated in this study. This will be a focus of future studies using endothelial-specific deletion of collectrin combined with kidney XTP. In addition, the precise tubular region through which collectrin regulates BP homeostasis, renal NO and O2− balance, NO synthase dimerization, RBF, and abundance of sodium transporters will be delineated by deletion of collectrin specifically in the proximal tubule and collecting duct.
In conclusion, our study identifies that renal collectrin provides a protective effect against salt sensitivity. Furthermore, collectrin is a novel molecular target of Ang II and may regulate pressure natriuresis through its influence on RBF and expression of NHE3. Future work will focus on delineating the precise renal mechanism—endothelial versus epithelial—through which collectrin regulates BP homeostasis and salt sensitivity. Our findings suggest that increasing the abundance or activity of collectrin may be a novel therapeutic approach in the treatment of HTN and salt sensitivity.
Concise Methods
Animals
Tmem27Y/− mice were generated via electroporation of a targeting construct into 129/SvEv (129S6) strain embryonic stem cells, which contained a thymidine kinase and a neomycin cassette flanked by 3 kb 5′ and 3′ homology regions disrupting exon 4, intron 4, and exon 5 of Tmem27 as previously described.7 The Tmem27 null mutation was backcrossed onto the 129S6 background for >20 generations. WT (Tmem27Y/+) and KO (Tmem27Y/−) male littermates of approximately 3 months of age were used for the studies and housed in a pathogen-free facility under protocols approved by the Institutional Animal Care and Use Committee at the University of Virginia (Charlottesville, VA), and in accordance with National Institutes of Health guidelines.
Mouse Kidney XTP
Kidney XTP was performed through the service at the Duke O’Brien Center (Durham, NC) described previously,45 and BP was recorded under normal and high-salt conditions in the following groups: (1) WT mice with WT kidney (WT → WT), (2) collectrin KO mice with WT kidney (WT → KO), and (3) WT mice with collectrin KO kidney (KO → WT). Briefly, animals were anesthetized with isoflurane, and the donor kidney, ureter, and bladder were harvested en bloc, including the renal artery with a small aortic cuff and the renal vein with a small caval cuff. These vascular cuffs were anastomosed to the recipient abdominal aorta and vena cava, respectively, below the level of the native renal vessels. Total ischemic time averaged 35–40 minutes. Donor and recipient bladders were attached dome to dome. The right native kidney was removed at the time of transplant, and the left native kidney was removed through a flank incision 1–3 days later; care was taken to preserve the recipient adrenal glands intact with their normal blood supply. The overall surgical mortality was approximately 5%. Data collection was initiated 2 weeks after transplantation to allow resolution of any ischemic injury associated with the transplant procedure. In addition, at the end of the experiments, sections of kidney grafts were examined to confirm normal renal structure, including the absence of ischemic tubular damage.
Radiotelemetric BP Monitoring In Vivo
For kidney XTP studies, BPs were measured in conscious mice under unrestrained conditions by radiotelemetry. The implantation of radiotelemetric units was as reported previously with modification.8 Briefly, mice were anesthetized with 2% isoflurane mixed with 95% O2 and 5% CO2. A catheter with sensor (TA11PA-C10; Data Sciences International [DSI]) was implanted in the left carotid artery, and the radiotransmitter was placed in a subcutaneous pouch along the flank. Buprenorphine (0.1 mg/kg body wt, sc.) and bupivacaine (0.25%, 0.02–0.05 mg/kg, sc.) were given postoperatively for pain control. Mice were allowed to recover for 7 days after surgery to regain their normal circadian rhythms before BP measurements and experiments were initiated. While BP was being monitored, mice were housed in a quiet room in individual cages placed above the telemetric receivers with an output to a computer. BP values were recorded every hour for 5 minutes. Data were processed and analyzed using Dataquest A.R.T. 20 software (DSI).
Immunoblotting of nNOS and Collectrin
Renal medullary tissue was dissected and immediately placed into ice-cold isolation buffer (10 mM Tris, 250 mM sucrose, and 5 mM EDTA, pH 7.4) with protease inhibitor cocktail (Sigma-Aldrich). Homogenates were then rapidly processed as previously described.8 Briefly, tissues or cells were homogenized for 10 seconds and lysates were spun at 3000 g for 10 minutes at 4°C. The supernatant was saved on ice, and the pellet was resuspended, homogenized, and centrifuged. The two supernatants were combined and spun at 16,000 g for 30 minutes at 4°C. The resulting supernatant represented the intracellular fraction. Pellets were resuspended in isolation buffer and represented the plasma membrane fraction. Protein concentrations of each fraction were determined by the BCA assay (Bioassay Systems). Thirty micrograms of total protein were loaded onto 12% SDS-PAGE gels and then transferred to nitrocellulose membranes per manufacturer instructions (X-Cell Blot Module; Invitrogen). The membrane was blocked using Odyssey Blocking Buffer (LI-COR) for 1 hour at room temperature. The membrane was then incubated at 4°C overnight with rabbit anti-nNOS (Cell Signaling) and rabbit anti-Collectrin (Covance) primary antibody diluted (1:1000) in blocking buffer. Secondary antibody incubation was performed for 1 hour at room temperature with anti-rabbit and anti-mouse conjugated with IRDye 700 or 800 (Rockland Immunochemicals) at 1:10,000. Blots were visualized by LI-COR Odyssey CLx Imager.
Renal Hemodynamic Study Using Contrast-Enhanced Ultrasonography
Mice were anesthetized by ketamine (90 mg/kg) and xylazine (9 mg/kg). Fur was then removed by shaving and a depilatory. Mice were then positioned on a modified microscope stage under a US transducer held in place with a ring clamp. Prewarmed US gel was placed on the depilated skin for US application. Mouse body temperature was monitored via rectal probe (Fine Science Tools) and maintained at 36±0.5°C with a heating pad and heat lamp. A Sequoia 512 ultrasound machine with a 15L8w transducer (Acuson) was used for ultrasound studies. Once the animal’s body temperature was stabilized, the right kidney was localized in real-time using conventional B-mode imaging with a frequency of 14 MHz and an on-screen imaging mechanical index (MI) of 0.99. Once the kidney was identified, the contrast function was initiated with a frequency of 7 MHz and imaging MI of 0.16. A 60 µl bolus injection of the microbubble contrast agent (1.5 × 105 microbubbles per ml) was then administered via retro-orbital plexus. Once steady-state was obtained and no evidence of acoustic shadowing was present, 5-second videos of microbubble destruction and replenishment were recorded. Microbubbles were destroyed using the BURST function and were 1 second in duration with an MI of 1.9. These parameters were derived from preliminary experiments in our laboratory and were sufficient to generate a wash-in curve with an initial exponential return followed by a saturating plateau. After a minimum of three videos were recorded for each animal, the two-dimensional area of the kidney image was estimated by measuring the length and thickness of the kidney (using the CALIBER application). For data acquisition, the resultant images were analyzed using the Sequoia analysis software (SYNGO). Tissue regions of interest were drawn and the destruction replenishment curve was fitted to the function: PI = A(1-e−βt), where PI is pixel intensity at time t, A refers to the maximum pixel signal during replenishment (estimate of total perfusion), and β refers to the rate constant (estimate of blood velocity). (β/kidney weight [g]) has been shown to correlate well with RBF measurements by Doppler.15 We corrected β by the estimated two-dimensional kidney area to remove the need for euthanasia or kidney removal. The two-dimensional kidney area was calculated on the basis of the equation for an ellipse: area (cm2) = π × (kidney length [cm]/2) × (kidney thickness [cm]/2). The estimated two-dimensional area and tissue weight after euthanasia were highly correlated (R2=0.67). An estimate of RBF was then calculated (β/kidney area [square cm]) for each animal at baseline and again after high-salt diet.
Renal Sodium Transporter Expression at Baseline
Homogenate Preparation.
Age-matched WT (n=5) and KO (n=5) mice were fasted for 5 hours (water supplied) before euthanasia in order to avoid dietary K+ affecting the expression of NCC-pS71.46 After fasting, left kidneys were collected, cut in half, and snap-frozen. Kidney cortices were dissected and homogenized in 1 ml of isolation buffer (5% sorbitol, 0.5 mM disodium EDTA, and 5 mM histidine-imidazole buffer, pH = 7.5, with the addition of 0.2 mM PMSF, 9 µg/ml aprotinin, and 5 µl/ml phosphatase inhibitor cocktail [Sigma]). Each sample was homogenized for 5 minutes at a low-speed setting with an Ultra-Turrax T25 (IKA-Labortechnik) and then centrifuged at 2000 g for 10 minutes. Supernatants were retained and the pellets were rehomogenized in another 1 ml of isolation buffer, recentrifuged, and pooled with the first supernatants. The 2000 g supernatant (So) protein concentrations were determined using the BCA assay (Pierce Thermo Scientific). The samples were aliquoted and stored at −80°C.
Quantitative Immunoblotting.
Homogenates were denatured in SDS-PAGE sample buffer for 20 minutes at 60°C. To verify uniform protein concentration, 5 µg of protein from each sample was resolved by SDS-PAGE, stained with Coomassie blue, and multiple random bands quantified and determined to be uniform as in Supplemental Figure 4.47 Each sample was run at both one and one-half amounts to verify linearity of the detection system as in Supplemental Figure 5. Specific protein abundance and phosphorylation was assessed using antibodies as described in detail elsewhere.43 Primary antibodies used included the following: anti-NHE3 (1:2000; McDonough lab), anti–NHE3 phosphorylated at Ser552 (anti-NHE3pS552, 1:1000; Santa Cruz), anti-NaPi2 (1:1000; J. Biber, Zurich), anti-NCC (1:5000; McDonough lab), anti–NCC phosphorylated at Ser71 (NCCpS71, 1:5000; J. Loffing, Zurich), and anti-αENaC (1:5000; J. Loffing, Zurich). Secondary antibodies were tagged with either Alexa Fluor 680 (Invitrogen) or IRDye 800 (LI-COR). Signals were detected with Odyssey Infrared Imaging System (LI-COR) and quantified by accompanying software. Arbitrary density units were normalized to mean intensity of control group, defined as 1.0. Because the samples were run twice (at one and one-half), the normalized values were averaged and mean values compiled for statistical analysis.
CLi
Mice were housed individually in metabolic cages to allow for urine collection for lithium excretion/clearance experiments. Experiments were performed first on regular diet and later repeated on high-salt diet. Mice were weighed and plasma was collected after 72 hours in metabolic cages. Urine output was quantified in microliters. Plasma and urine lithium levels were measured using an IL943 Flame photometer with sensitivity range 1.0–3.0 mmol/L (±0.15 mmol/L). To ensure accuracy of collection and measurement, mice were allowed to acclimate in metabolic cages for 3 days and only mice with daily urine sample volumes exceeding 250 and 1200 µl for the regular and high-salt diet collections, respectively, were included in the analyses. Endogenous CLi was determined for 24 hours using urine collected on day 3 and was calculated as V X ULi/PLi, where V is urine volume, ULi is urine lithium concentration, and PLi is plasma lithium concentration, corrected by kg body weight.
Ang II–Induced Hypertension
Ang II was delivered via osmotic minipumps at 600 ng/kg per minute for 2 weeks. Systolic BP was measured at the same time daily for 2 weeks using tail cuff manometer after a daily training period of 2 weeks as previously reported.48
Collectrin Expression in Ang II–HTN
Collectrin gene expression in the kidney was examined using quantitative RT-PCR (see Supplemental Material) using the following primers: forward primer: CTGTGCCCGTCTGGATTATT; reverse primer: TCATGTCCAAGGGATCACAA.
Immunostaining of collectrin was performed using paraffin-embedded kidney tissues. Briefly, 3–5 μm sections were deparaffinized and rehydrated through decreasing ethanol solutions in water. Antigen capture was performed using a commercially available unmasking solution (Vector Laboratories, CA). Slides were then incubated in a blocking solution (2.4G2 in 10% horse serum, 0.1% triton, PBS solution) and then incubated with AlexaFluor-647 conjugated anti-mouse polyclonal collectrin antibody overnight (5 μg/ml in 10% horse serum, 0.1% triton, PBS solution). Slides were sealed with Prolong Diamond Antifade with DAPI (Invitrogen) and visualized using a Carl Zeiss Axiovert 200M microscope with ApoTome imaging and AxioVision software (Carl Zeiss).
Statistical Analyses
All data are presented as mean±SEM. All mean and SEM values were calculated from at least triplicates of a representative experiment. Statistical calculations were done with commercially available software packages (Minitab, Inc. and NCSS). The t test was used for comparisons between two groups unless otherwise stated. Differences between matched samples were analyzed by a paired t test. Two-way ANOVA was performed to test for significant overall group differences when there were greater than two groups. A P value <0.05 is considered statistically significant. P values were two sided. *P<0.05, **P<0.01, and *** P<0.001.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Thomas Coffman, Dr. Susan Gurley, and Mr. Robert Griffiths of the Duke O’Brien Center for Kidney Research for the kidney crosstransplantation work which was supported by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under award number P30DK096493.
This work was supported by NIH/NIDDK T32 DK072922 Training Grant, Division of Nephrology, University of Virginia to P.-L.C. and G. B.-K.; the Ben J. Lipps Research Fellow Award from the American Society of Nephrology to P.-L.C.; NIH/NIDDK DK083785 to A.A.M.; and NIH/NIDDK DK094907 and divisional support to T.H.L.
P.-L.C. and T.H.L. conceived and designed the research; P.-L.C., J.C.G., S.C., G.B.-K., F.C., and D.L.R. performed the experiments; K.K. and A.L.K. provided technical expertise and material support for Contrast-enhanced ultrasound study for renal blood flow measurements; N.H. and R.M.C. provided technical support for measurement of lithium clearance; P.-L.C., J.C.G., S.C., G. B.-K., D.L.R., and T.H.L. analyzed the data; P.-L.C., J.C.G., S.C., A.A.M., and T.H.L. interpreted the results of the experiments; P.-L.C. and T.H.L. prepared the figures; P.-L.C. drafted the manuscript; P.-L.C., J.C.G., A.A.M., and T.H.L. edited and revised the manuscript. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016060675/-/DCSupplemental.
References
- 1.Zhang H, Wada J, Hida K, Tsuchiyama Y, Hiragushi K, Shikata K, Wang H, Lin S, Kanwar YS, Makino H: Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J Biol Chem 276: 17132–17139, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Akpinar P, Kuwajima S, Krützfeldt J, Stoffel M: Tmem27: A cleaved and shed plasma membrane protein that stimulates pancreatic beta cell proliferation. Cell Metab 2: 385–397, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Danilczyk U, Sarao R, Remy C, Benabbas C, Stange G, Richter A, Arya S, Pospisilik JA, Singer D, Camargo SM, Makrides V, Ramadan T, Verrey F, Wagner CA, Penninger JM: Essential role for collectrin in renal amino acid transport. Nature 444: 1088–1091, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Fukui K, Yang Q, Cao Y, Takahashi N, Hatakeyama H, Wang H, Wada J, Zhang Y, Marselli L, Nammo T, Yoneda K, Onishi M, Higashiyama S, Matsuzawa Y, Gonzalez FJ, Weir GC, Kasai H, Shimomura I, Miyagawa J, Wollheim CB, Yamagata K: The HNF-1 target collectrin controls insulin exocytosis by SNARE complex formation. Cell Metab 2: 373–384, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Gu X, Neric NJ, Crabb JS, Crabb JW, Bhattacharya SK, Rayborn ME, Hollyfield JG, Bonilha VL: Age-related changes in the retinal pigment epithelium (RPE). PLoS One 7: e38673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malakauskas SM, Kourany WM, Zhang XY, Lu D, Stevens RD, Koves TR, Hohmeier HE, Muoio DM, Newgard CB, Le TH: Increased insulin sensitivity in mice lacking collectrin, a downstream target of HNF-1alpha. Mol Endocrinol 23: 881–892, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malakauskas SM, Quan H, Fields TA, McCall SJ, Yu MJ, Kourany WM, Frey CW, Le TH: Aminoaciduria and altered renal expression of luminal amino acid transporters in mice lacking novel gene collectrin. Am J Physiol Renal Physiol 292: F533–F544, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cechova S, Zeng Q, Billaud M, Mutchler S, Rudy CK, Straub AC, Chi L, Chan FR, Hu J, Griffiths R, Howell NL, Madsen K, Jensen BL, Palmer LA, Carey RM, Sung SS, Malakauskas SM, Isakson BE, Le TH: Loss of collectrin, an angiotensin-converting enzyme 2 homolog, uncouples endothelial nitric oxide synthase and causes hypertension and vascular dysfunction. Circulation 128: 1770–1780, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Fairweather SJ, Bröer A, Subramanian N, Tumer E, Cheng Q, Schmoll D, O’Mara ML, Bröer S: Molecular basis for the interaction of the mammalian amino acid transporters B0AT1 and B0AT3 with their ancillary protein collectrin. J Biol Chem 290: 24308–24325, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Wada J, Kanwar YS, Tsuchiyama Y, Hiragushi K, Hida K, Shikata K, Makino H: Screening for genes up-regulated in 5/6 nephrectomized mouse kidney. Kidney Int 56: 549–558, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Yasuhara A, Wada J, Malakauskas SM, Zhang Y, Eguchi J, Nakatsuka A, Murakami K, Kanzaki M, Teshigawara S, Yamagata K, Le TH, Makino H: Collectrin is involved in the development of salt-sensitive hypertension by facilitating the membrane trafficking of apical membrane proteins via interaction with soluble N-ethylmaleiamide-sensitive factor attachment protein receptor complex. Circulation 118: 2146–2155, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Hecker M, Sessa WC, Harris HJ, Anggård EE, Vane JR: The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: Cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A 87: 8612–8616, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pallone TL, Mattson DL: Role of nitric oxide in regulation of the renal medulla in normal and hypertensive kidneys. Curr Opin Nephrol Hypertens 11: 93–98, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Roczniak A, Zimpelmann J, Burns KD: Effect of dietary salt on neuronal nitric oxide synthase in the inner medullary collecting duct. Am J Physiol 275: F46–F54, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Kogan P, Johnson KA, Feingold S, Garrett N, Guracar I, Arendshorst WJ, Dayton PA: Validation of dynamic contrast-enhanced ultrasound in rodent kidneys as an absolute quantitative method for measuring blood perfusion. Ultrasound Med Biol 37: 900–908, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dukacz SA, Adams MA, Kline RL: Short- and long-term enalapril affect renal medullary hemodynamics in the spontaneously hypertensive rat. Am J Physiol 276: R10–R16, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Dixit MP, Xu L, Xu H, Bai L, Collins JF, Ghishan FK: Effect of angiotensin-II on renal Na+/H+ exchanger-NHE3 and NHE2. Biochim Biophys Acta 1664: 38–44, 2004 [DOI] [PubMed] [Google Scholar]
- 18.He P, Klein J, Yun CC: Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 285: 27869–27878, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA: Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ-/- and interleukin-17A-/- mice. Hypertension 65: 569–576, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M: Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Dahl LK, Heine M: Primary role of renal homografts in setting chronic blood pressure levels in rats. Circ Res 36: 692–696, 1975 [DOI] [PubMed] [Google Scholar]
- 22.Ni Z, Oveisi F, Vaziri ND: Nitric oxide synthase isotype expression in salt-sensitive and salt-resistant Dahl rats. Hypertension 34: 552–557, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Francois H, Athirakul K, Howell D, Dash R, Mao L, Kim HS, Rockman HA, Fitzgerald GA, Koller BH, Coffman TM: Prostacyclin protects against elevated blood pressure and cardiac fibrosis. Cell Metab 2: 201–207, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Ryan MJ, Didion SP, Davis DR, Faraci FM, Sigmund CD: Endothelial dysfunction and blood pressure variability in selected inbred mouse strains. Arterioscler Thromb Vasc Biol 22: 42–48, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Hummler E, Nussberger J, Clément S, Gabbiani G, Brunner HR, Burnier M: Blood pressure, cardiac, and renal responses to salt and deoxycorticosterone acetate in mice: Role of Renin genes. J Am Soc Nephrol 13: 1509–1516, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Campese VM, Parise M, Karubian F, Bigazzi R: Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension 18: 805–812, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Chiolero A, Würzner G, Burnier M: Renal determinants of the salt sensitivity of blood pressure. Nephrol Dial Transplant 16: 452–458, 2001 [DOI] [PubMed] [Google Scholar]
- 28.van Paassen P, de Zeeuw D, Navis G, de Jong PE: Does the renin-angiotensin system determine the renal and systemic hemodynamic response to sodium in patients with essential hypertension? Hypertension 27: 202–208, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Williams GH, Hollenberg NK: Non-modulating hypertension. A subset of sodium-sensitive hypertension. Hypertension 17[Suppl]: I81–I85, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Closs EI, Scheld JS, Sharafi M, Förstermann U: Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: Role of cationic amino acid transporters. Mol Pharmacol 57: 68–74, 2000 [PubMed] [Google Scholar]
- 31.Simon A, Plies L, Habermeier A, Martiné U, Reining M, Closs EI: Role of neutral amino acid transport and protein breakdown for substrate supply of nitric oxide synthase in human endothelial cells. Circ Res 93: 813–820, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Arendshorst WJ, Navar LG: Renal Circulation and Glomerular Hemodynamics, Philadelphia, PA, Lipincott Williams and Wilkins, 2007 [Google Scholar]
- 33.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD: Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Just A, Olson AJ, Whitten CL, Arendshorst WJ: Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol 292: H83–H92, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Mattson DL, Meister CJ: Renal cortical and medullary blood flow responses to L-NAME and ANG II in wild-type, nNOS null mutant, and eNOS null mutant mice. Am J Physiol Regul Integr Comp Physiol 289: R991–R997, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Berthold H, Just A, Kirchheim HR, Ehmke H: Interaction between nitric oxide and endogenous vasoconstrictors in control of renal blood flow. Hypertension 34: 1254–1258, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Roman RJ, Zou AP: Influence of the renal medullary circulation on the control of sodium excretion. Am J Physiol 265: R963–R973, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Kakoki M, Kim HS, Arendshorst WJ, Mattson DL: L-Arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am J Physiol Regul Integr Comp Physiol 287: R1478–R1485, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Kakoki M, Wang W, Mattson DL: Cationic amino acid transport in the renal medulla and blood pressure regulation. Hypertension 39: 287–292, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Balligand JL, Feron O, Dessy C: eNOS activation by physical forces: From short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89: 481–534, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Nguyen MT, Han J, Ralph DL, Veiras LC, McDonough AA: Short-term nonpressor angiotensin II infusion stimulates sodium transporters in proximal tubule and distal nephron. Physiol Rep 3: e12496 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonough AA, Nguyen MT: Maintaining balance under pressure: Integrated regulation of renal transporters during hypertension. Hypertension 66: 450–455, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen MT, Lee DH, Delpire E, McDonough AA: Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: Distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol 305: F510–F519, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM: AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM: Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 47.McDonough AA, Veiras LC, Minas JN, Ralph DL: Considerations when quantitating protein abundance by immunoblot. Am J Physiol Cell Physiol 308: C426–C433, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salzler HR, Griffiths R, Ruiz P, Chi L, Frey C, Marchuk DA, Rockman HA, Le TH: Hypertension and albuminuria in chronic kidney disease mapped to a mouse chromosome 11 locus. Kidney Int 72: 1226–1232, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.