Abstract
CKD appears to be a condition of soluble klotho deficiency. Despite known associations between low soluble klotho levels and conditions that promote kidney damage, such as oxidative stress and fibrosis, little information exists regarding the longitudinal association between soluble klotho levels and change in kidney function. We assayed serum soluble α-klotho in 2496 participants within the Health Aging and Body Composition study, a cohort of older adults. The associations between soluble klotho levels and decline in kidney function (relative decline: eGFR decline ≥30%; absolute decline: eGFR decline >3 ml/min per year) and incident CKD (incident eGFR <60 ml/min per 1.73 m2 and >1 ml/min per year decline) were evaluated. We adjusted models for demographics, baseline eGFR, urine albumin-to-creatinine ratio, comorbidity, and measures of mineral metabolism. Among participants, the mean (SD) age was 75 (3) years, 52% were women, and 38% were black. Median (25th, 75th percentiles) klotho level was 630 (477, 817) pg/ml. In fully adjusted models, each two-fold higher level of klotho associated with lower odds of decline in kidney function (odds ratio, 0.78 [95% confidence interval, 0.66 to 0.93] for 30% decline in eGFR, and 0.85 [95% confidence interval, 0.73 to 0.98] for >3 ml/min per year decline in eGFR), but not of incident CKD (incident rate ratio, 0.90 [95% confidence interval, 0.78 to 1.04]). Overall, a higher soluble klotho level independently associated with a lower risk of decline in kidney function. Future studies should attempt to replicate these results in other cohorts and evaluate the underlying mechanism.
Keywords: klotho, kidney dysfunction, chronic kidney disease, risk factors
Klotho is a transmembrane protein which serves as the cofactor for fibroblast growth factor 23 (FGF-23) to bind to its cognate receptor and regulate phosphorus and vitamin D metabolism.1,2 The soluble form of klotho is reported to have antiaging properties which may be mediated via multiple systemic effects including regulation of insulin signaling and prevention of vascular calcium deposits, oxidative stress, and fibrosis.3–8 The kidney has the highest levels of klotho expression and is thought to be the major source of soluble klotho,9,10 which is released through proteolytic cleavage of the transmembrane form as well as alternative gene transcription.11 CKD has been reported as a condition of klotho deficiency, with animal models of CKD demonstrating decreased klotho gene expression, lower klotho levels in kidney tissue, and lower circulating soluble klotho.7,12,13
Genetic studies have demonstrated that mutations or knockout of the klotho gene result in phenotypes prone to the development of kidney disease,6–8,14 whereas restoration of klotho in rodent models of CKD results in improvement and/or prevention of kidney damage.15,16 These findings, coupled with the potential beneficial systemic effects discussed above, raise the possibility that klotho deficiency may not just be a marker of CKD, but instead serve as a causative factor for its development.17
Few studies have examined the longitudinal association between klotho levels and change in kidney function. Health Aging and Body Composition Study (Health ABC) is a large, diverse cohort of elderly, well functioning adults with measures of soluble serum klotho and repeated measures of kidney function over 10 years of follow-up. We therefore evaluated the association of soluble serum klotho with decline in kidney function as well as the development of incident CKD in this cohort.
Results
Baseline Characteristics
Among 3075 participants, 299 did not have samples available for the klotho assay, whereas 280 were missing values for cystatin C at either baseline or at least one follow-up visit, leaving 2496 with complete data available for analysis. Fifty four percent of the remaining participants had two follow-up measures of eGFR (years 3 and 10), whereas 43% had a single follow-up eGFR measure from year 3, and 3% had a single follow-up eGFR measure from year 10. The average (SD) age of participants was 75 (3) years, with 52% women, and 38% black (Table 1). The median klotho level was 631 pg/ml (25th–75th percentile = 477–817 pg/ml), whereas the median FGF-23 level was 46 pg/ml (36.7–60.2). The mean baseline eGFR was 73 (18) ml/min per 1.73 m2 and 23% of participants had a baseline eGFR of <60 ml/min per 1.73 m2.
Table 1.
Baseline demographics and clinical characteristics by quartiles of klotho
| Variable | Full Cohort | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|---|
| N | 2496 | 610 | 632 | 632 | 622 |
| Klotho range, pg/ml | 64–5401 | 64–477 | 478–630 | 631–816 | 817–5401 |
| Age, yr | 75 (3) | 75 (3) | 75 (3) | 75 (3) | 75 (3) |
| Women | 52 | 53 | 48 | 51 | 55 |
| Black | 38 | 37 | 31 | 37 | 49 |
| Diabetes | 37 | 34 | 39 | 36 | 38 |
| Hypertension | 77 | 75 | 77 | 76 | 78 |
| Coronary artery disease | 18 | 16 | 16 | 18 | 20 |
| Heart failure | 0.9 | 0.7 | 0.8 | 1.0 | 1.1 |
| Cerebrovascular disease | 7 | 7 | 6 | 6 | 8 |
| SBP, mmHg | 134 (21) | 132 (21) | 134 (21) | 134 (21) | 134 (20) |
| DBP, mmHg | 70 (12) | 69 (11) | 71 (12) | 71 (12) | 71 (12) |
| BMI, kg/m2 | 27.3 (4.7) | 27.6 (4.9) | 27.3 (4.4) | 27.2 (4.7) | 27.0 (4.9) |
| LDL cholesterol, mg/dl | 122 (35) | 122 (36) | 120 (33) | 120 (33) | 125 (36) |
| HDL cholesterol, mg/dl | 54 (17) | 54 (17) | 54 (17) | 53 (17) | 55 (17) |
| eGFR, ml/min per 1.73 m2 | 73 (18) | 71 (19) | 71 (17) | 73 (17) | 76 (18) |
| eGFR<60 ml/min per 1.73 m2 | 23 | 28 | 25 | 23 | 17 |
| UACR, mg/g | 7.4 (3.8, 18.2) | 7.0 (3.6, 15.8) | 7.0 (3.6, 18.8) | 7.7 (4.0, 19.0) | 7.9 (4.0, 19.4) |
| UACR≥30 mg/g | 17 | 14 | 16 | 18 | 19 |
| FGF-23, pg/ml | 46.3 (36.5, 59.6) | 47.9 (37.8, 63.1) | 47.6 (37.2, 60.0) | 45.9 (36.3, 58.7) | 44.5 (35.5, 56.8) |
| Calcium, mg/dl | 8.9 (0.4) | 8.8 (0.4) | 8.8 (0.4) | 8.9 (0.4) | 8.9 (0.4) |
| Phosphorus, mg/dl | 3.6 (0.5) | 3.6 (0.5) | 3.5 (0.5) | 3.5 (0.5) | 3.6 (0.5) |
| Parathyroid hormone, pg/ml | 32.9 (24.8, 45.1) | 33.3 (25.2, 45.6) | 33.4 (24.3, 45.5) | 33.5 (25.6, 44.5) | 31.5 (24.5, 44.2) |
Presented as mean (SD), median (25th–75th percentile), or % as appropriate. eGFR on the basis of cystatin C. SBP, systolic BP; DBP, diastolic BP; BMI, body mass index.
Across quartiles of klotho, minimal differences were observed in the prevalence of comorbid conditions. Those in the lowest klotho quartile had lower prevalence of albuminuria (urine albumin-to-creatinine ratio [UACR] >30 mg/g) and modestly higher FGF-23 and C-reactive protein concentrations. Participants in the lowest quartile of klotho also had the lowest eGFR and highest cystatin C, whereas those in the highest quartile were more likely to be black. No differences were seen for age, sex, diabetes status, or for calcium, phosphorus, and parathyroid hormone.
Klotho and Change in Kidney Function
The mean annual rate of decline for all included participants was 1.77 ml/min per year (±4.76 ml/min per year). Four hundred and five participants (16%) experienced a 30% decline in kidney function, whereas 702 (28%) experienced an absolute decline in kidney function >3 ml/min per year. Each two-fold higher klotho was associated with 20% lower odds of relative decline in kidney function. This association remained significant and essentially unaltered in all multivariable models, including after adjustment for demographics, baseline kidney function, CKD and cardiovascular disease (CVD) risk factors, and measures of mineral metabolism (Table 2). Similar findings were observed when klotho was examined in quartiles, with the highest quartile demonstrating 30% lower odds of kidney function decline compared with the lowest quartile.
Table 2.
Association of klotho with 30% kidney function decline in the health ABC study
| Exposure | N | 30% decline | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| Klotho, per doubling | 2496 | 405 | 0.82 (0.69 to 0.97) | 0.81 (0.68 to 0.97) | 0.78 (0.65 to 0.93) | 0.78 (0.66 to 0.93) |
| Klotho quartiles, pg/ml | ||||||
| <479 | 610 | 117 | 1.00(ref) | 1.00(ref) | 1.00(ref) | 1.00(ref) |
| 459–630 | 632 | 105 | 0.83 (0.62 to 1.11) | 0.84 (0.63 to 1.13) | 0.81 (0.60 to 1.09) | 0.81 (0.60 to 1.11) |
| 631–816 | 632 | 90 | 0.68 (0.50 to 0.92) | 0.69 (0.51 to 0.93) | 0.64 (0.47 to 0.88) | 0.65 (0.48 to 0.89) |
| >816 | 622 | 93 | 0.73 (0.54 to 0.99) | 0.72 (0.53 to 0.98) | 0.67 (0.49 to 0.91) | 0.68 (0.49 to 0.92) |
Model 1 = unadjusted analysis. Model 2 = adjusted for age, sex, race, study site, and baseline eGFR. Model 3 = Model 2+diabetes, cardiovascular disease, hypertension, and urine albumin-to-creatinine ratio. Model 4 = Model 3+calcium, phosphorus, parathyroid hormone, and FGF-23. Interactions: klotho × FGF-23, P=0.9; klotho × eGFR, P=0.15; klotho × phosphorus, P=0.8. OR, odds ratio; 95% CI, 95% confidence interval; ref, reference.
Each two-fold higher klotho was also associated with lower odds of absolute decline in kidney function, with the association becoming statistically significant in stepwise multivariable models, including the fully adjusted final model (Tables 3). In categoric analyses, the highest quartile of klotho was associated with lower odds of absolute decline, but did not reach statistical significance in comparison with the lowest quartile.
Table 3.
Association of klotho with rapid kidney function decline (>3 ml/min per yr)
| Exposure | N | ≥3 ml/min per yr | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| Klotho, per doubling | 2496 | 702 | 0.92 (0.80 to 1.07) | 0.87 (0.75 to 1.01) | 0.84 (0.72 to 0.97) | 0.85 (0.73 to 0.98) |
| Klotho quartiles | ||||||
| <479 | 610 | 180 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 459–630 | 632 | 177 | 0.93 (0.72 to 1.19) | 0.94 (0.73 to 1.21) | 0.91 (0.70 to 1.18) | 0.92 (0.71 to 1.19) |
| 631–816 | 632 | 168 | 0.87 (0.68 to 1.12) | 0.86 (0.67 to 1.11) | 0.82 (0.63 to 1.06) | 0.83 (0.64 to 1.08) |
| >816 | 622 | 177 | 0.93 (0.72 to 1.19) | 0.83 (0.64 to 1.07) | 0.77 (0.59 to 1.01) | 0.79 (0.61 to 1.03) |
Model 1 = unadjusted analysis. Model 2 = adjusted for age, sex, race, study site, and baseline eGFR. Model 3 = Model 2+diabetes, cardiovascular disease, hypertension, and urine albumin-to-creatinine ratio. Model 4 = Model 3+calcium, phosphorus, parathyroid hormone, and FGF-23. Interactions: klotho × FGF-23, P=0.7; klotho × eGFR, P=0.3; klotho × phosphorus, P=0.9. OR, odds ratio; 95% CI, 95% confidence interval; ref, reference.
Klotho and Incident CKD
Five hundred and eighty-two participants were excluded from analysis due to having eGFR<60 ml/min per 1.73 m2 at baseline, leaving 1914 participants available for analysis. Incident CKD was observed in 536 (25%), with an incident rate of 4.8% per year. Each two-fold higher klotho appeared to be associated with a lower rate of incident CKD, although this was not statistically significant in linear or categoric analyses (Table 4).
Table 4.
Association of klotho with incident CKD (eGFR<60 ml/min per 1.73 m2 and >1 ml/min per yr decline)
| Exposure | N | Incident CKD | Rate, %/yr | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Klotho, per doubling | 1914 | 536 | 4.81 | 0.90 (0.79 to 1.02) | 0.89 (0.77 to 1.01) | 0.89 (0.77 to 1.02) | 0.90 (0.78 to 1.04) |
| Klotho quartiles | |||||||
| <479 | 438 | 134 | 5.11 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 459–630 | 474 | 136 | 5.05 | 0.99 (0.79 to 1.23) | 0.86 (0.69 to 1.07) | 0.83 (0.66 to 1.03) | 0.84 (0.67 to 1.05) |
| 631–816 | 485 | 130 | 4.46 | 0.87 (0.70 to 1.09) | 0.84 (0.66 to 1.01) | 0.81 (0.65 to 1.01) | 0.84 (0.67 to 1.04) |
| >816 | 517 | 136 | 4.68 | 0.92 (0.73 to 1.14) | 0.92 (0.73 to 1.14) | 0.91 (0.73 to 1.14) | 0.93 (0.74 to 1.17) |
Excludes those with eGFR<60 ml/min per 1.73 m2 at baseline. Model 1 = unadjusted analysis. Model 2 = adjusted for age, sex, race, study site, and baseline eGFR. Model 3 = Model 2+diabetes, cardiovascular disease, hypertension, and urine albumin-to-creatinine ratio. Model 4 = Model 3+calcium, phosphorus, parathyroid hormone, and FGF-23. Interactions: klotho × FGF-23, P=0.8; klotho × eGFR, P=0.7; klotho × phosphorus, P=0.2. OR, odds ratio; 95% CI, 95% confidence interval; ref, reference.
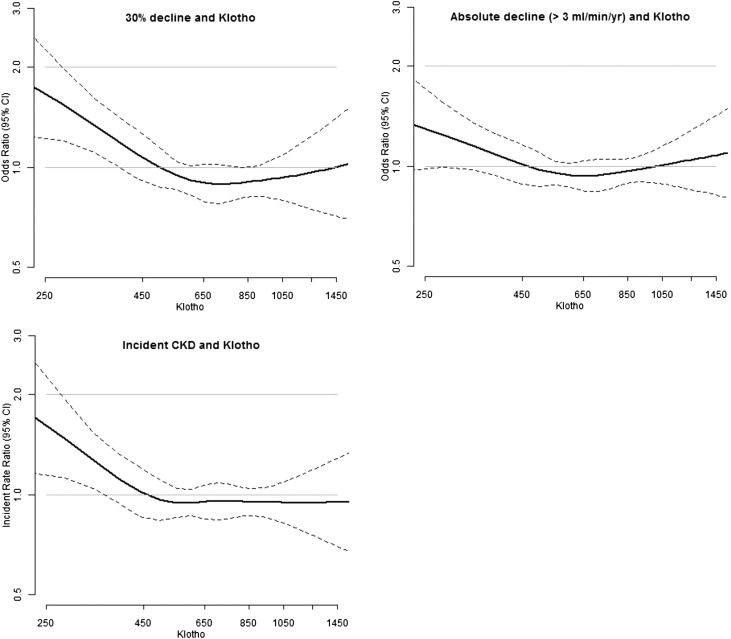
Splines
In spline analysis, we observed a consistent pattern for all three outcomes, with a linear association between low levels of klotho and higher odds of each outcome, followed by a plateau, which occurred approximately near the observed median of klotho (630 pg/ml) (Figure 1).
Figure 1.
A higher likelihood of each outcome with lower klotho levels is demonstrated. Unadjusted association between soluble klotho and 30% kidney function decline, >3 ml/min per year decline, and incident CKD. 95% confidence interval (95% CI) indicated by dotted lines.
Interactions
No significant interactions were noted between klotho and FGF-23 with relative eGFR decline, absolute decline, or incident CKD (P values of 0.9, 0.7, and 0.8, respectively). No significant interactions were noted between klotho and eGFR with relative eGFR decline, absolute decline, or incident CKD (P values of 0.15, 0.3, and 0.7, respectively). No significant interactions were noted between klotho, phosphorus, and each outcome (P values of 0.8, 0.9, and 0.2, respectively).
Discussion
In a diverse cohort of older, well functioning adults, we demonstrated an association between low soluble klotho and decline in kidney function. The relationship was robust and appears to have strengthened when adjusting for demographics, comorbidity, kidney disease risk factors, and measures of mineral metabolism. We saw a similar association between soluble klotho and incident CKD, although the results were not statistically significant.
We are not aware of any previously published studies that have evaluated the relationship between serum klotho and change in kidney function over time. Multiple previous studies have demonstrated cross-sectional associations between lower eGFR and lower soluble klotho levels, with the lowest levels observed in patients requiring hemodialysis.18–20 The interpretation of many of these studies is that the development of CKD may result in physiologic or cellular conditions, which lead to decreased klotho expression and production.9,10,12 However, there is also evidence which suggests that low klotho may be a risk factor for the development of kidney disease, rather than simply a marker of kidney disease.20
Klotho is reported to be an antiaging gene4 whose transcription results in a transmembrane protein that acts as a coreceptor necessary for FGF-23 function within the kidney.11,21 Klotho is also found in soluble form in serum, CSF, and urine11,18 and is formed by two distinct processes: (1) proteolytic degradation of the membrane-bound form and (2) an alternative gene transcription product.11,22,23 Although the exact mechanism of action of soluble klotho remains to be identified at this time, soluble klotho has been shown to influence multiple cellular and endocrine pathways including insulin release and the renin-angiotensin system,24–26 with higher expression associated with less pathologic evidence of aging such as kidney fibrosis, systemic markers of oxidative stress, and vascular calcification.6–8,27,28 Each of these effects has been hypothesized to have kidney protective effects.20 Indeed, restoration of exogenous klotho in klotho-deficient rodents results in both the prevention and reduction of kidney damage.14–16 We acknowledge, however, that low klotho could serve as a surrogate for comorbidity or an early indicator of kidney function decline, and thus simply be a marker for those at risk of future kidney decline. Arguing against this possibility is the lack of attenuation in our analyses after adjusting for age, multiple comorbidities, and key laboratory findings that are associated with kidney function decline.
The strengths of this study include a large and diverse cohort, accurate ascertainment of risk factors, and repeated measure of kidney function over a moderate follow-up period. There are also several limitations to the this study. First, concern has been raised as to the accuracy of the commercially available assay which we have also used in this study29–31 Specifically, the assay has been reported to detect high levels of soluble klotho in rodents with CKD,32 raising questions as to whether the current assay is specific for soluble klotho, and a recent study demonstrated that the commercial ELISA may experience a decrease in accuracy after samples have undergone multiple freeze-thaw cycles.18 Although the commercial assay we used may be less specific for klotho when compared with highly labor intensive immunoprecipitant immune blot assay methods,18 our results are in agreement with the majority of previously published cross-sectional studies.18–20 Furthermore, inaccuracy in the assay could be hypothesized to dilute results and bias toward the null, rather than produce a false positive finding. In addition, our samples did not undergo multiple freeze-thaw cycles, eliminating this potential source of inaccuracy. We did not have simultaneous assessment of both the exposure (year 2) and baseline kidney function, although the measures were within 1 year of each other, and there is little evidence that klotho levels change substantially over time in stable patients.33 Importantly, we also did not have follow-up measures of kidney function outside of year 3 or 10 which results in imprecision in slope estimates and differentiation between relatively short-term and chronic changes in eGFR challenging. In addition, use of a relatively short interval for outcomes on the basis of year 3 eGFR may not be reflective of longer-term outcomes. We also note that we did not have other measures of kidney disease such as albuminuria in follow-up. Finally, our cohort did not include any participants with advanced CKD, so we are unable to generalize our results to this higher-risk population.
In summary, we found an independent association between low klotho levels and decline in kidney function. These results need to be confirmed in additional cohorts, incorporating other measures of klotho, and should include individuals with more advanced CKD. In addition, potential mechanisms which may explain the observed associations need to be further evaluated.
Concise Methods
Study Population
Health ABC is a prospective cohort initiated in 1997 with a goal of assessing how health conditions affect age-related physiologic and functional status. The study population consists of 3075 persons aged 70–79 years at baseline with equal numbers of men and women and approximately one third black. All persons included were determined to be free of disability in activities of daily living and free of functional limitation at baseline. All participants who had measures of klotho and kidney function at baseline, and at least one repeat measure of kidney function (n=2496), were included in this study.
Exposure
Klotho was assayed using a commercially available sandwich ELISA test (IBL-International, Japan) from never-thawed frozen serum stored at −70°C and obtained at the year 2 visit, approximately 1 year after the baseline visit. This assay is reported to have a sensitivity of 6.15 pg/ml,34 and demonstrated an interassay coefficient of 18%. The assay has been used in multiple peer-reviewed publications19,30,31,35 and demonstrates reasonable correlation with more labor-intensive assay methods using synthetic antibodies and immunoprecipitation-immunoblots, particularly when the number of free-thaw cycles is low.
Covariates
All covariates were obtained at baseline enrollment (year 1 visit) with the exception of measures of mineral metabolism, which were obtained at the year 2 visit. Demographics (age, sex, and race) were obtained by self-report at study enrollment. CVD status was defined as a prior history of coronary artery disease, stroke, or heart failure. Diabetes was defined as use of hypoglycemic agents, self-reported history, fasting plasma glucose level ≥126 mg/dl, or 2-hour oral glucose tolerance test result ≥200 mg/dl. Systolic and diastolic BP were obtained by trained and certified clinical staff from the right arm using a conventional mercury sphygmomanometer with the participant in a seated position. Hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or current use of antihypertensive medications and a self-reported physician’s diagnosis of hypertension. UACR was determined at baseline. Urine albumin was measured using a particle-enhanced turbidimetric inhibition immunoassay allowing for direct albumin quantification (Siemens), whereas urine creatinine was measured by a modified Jaffé method on a clinical chemistry analyzer (Siemens). C-reactive protein was measured using an ELISA kit from R&D Systems (Minneapolis, MN). Measures of mineral metabolism including calcium, phosphorus, parathyroid hormone, and FGF-23 were measured at year 2, concurrent with klotho measurement, from frozen stored samples. In particular, FGF-23 was measured in never-thawed plasma EDTA using a commercial ELISA that measures the full-length peptide (Kainos Laboratories, Japan). This assay is widely used and may be advantageous in individuals with milder degrees of CKD, as it may provide more accurate results at low levels of FGF-23 compared with the C-terminal assay. For this study, the intra-assay coefficient of variation was 5.0% and the interassay coefficient of variation was 10.7% (mean 42 pg/ml).
Outcomes
Cystatin C was measured at baseline, as well as years 3 and 10, from stored frozen serum samples at the Health ABC core laboratory (University of Vermont, Burlington, VT) using a BNII nephelometer (Dade Behring Inc., Deerfield, IL) and a particle-enhanced immunonephelometric assay (N Latex Cystatin C).36 As in prior Health ABC studies,37,38 serum cystatin C was used as the primary measure of kidney function rather than serum creatinine for two reasons. First, cystatin C measures were calibrated across all samples, whereas there was a shift in the creatinine assay from non-Isotope Dilution Mass Spectrometry–traceable (baseline) to Isotope Dilution Mass Spectrometry-traceable during the study (years 3 and 10). Second, cystatin C is less influenced by age, sex, and race,39 and in particular muscle mass, and has been shown to be more strongly associated with adverse outcomes in the elderly population.40,41 Among 61 healthy individuals with three cystatin C measurements over a 6-month period, the intraindividual coefficient of variation was 7.7%, reflecting long-term stability of the measurement. eGFR was calculated using a validated cystatin C–based estimating equation.39,42 Using eGFR, three kidney outcomes were defined as follows:
Change in Kidney Function
(1) Relative decline in kidney function was defined as an eGFR decline of 30% or greater. When two follow-up measures of eGFR were available, the last available value (year 10) was used. Recent publications and a recent workshop between the US Food and Drug Administration and the National Kidney Foundation have argued that a 30% decline in GFR is an acceptable clinical endpoint for clinical trials, as it predicts the development of ESRD.43,44
(2) Absolute decline in kidney function was defined as a decline in eGFR of >3 ml/min per year.45 Rate of decline per year was determined by creating a slope using either two (single follow-up eGFR) or three (two follow-up eGFR) data points. This definition has been used in prior publications37,38 and has been shown to be associated with increased mortality in older adults.45
Incident CKD
Incident CKD was defined as eGFR of <60 ml/min per 1.73 m2 at any point during follow-up and an annual absolute decline >1 ml/min per 1.73 m2 per year, with the latter requirement in place to avoid inclusion of participants with minor changes in eGFR. Participants with an eGFR<60 ml/min per 1.73 m2 at baseline were excluded from this analysis.
Statistical Analysis
We examined baseline characteristics of participants across quartiles of klotho. These were summarized with means and SD, or medians and interquartile ranges for highly skewed variables, or proportions for categoric variables. For eGFR and UACR, we also included the proportion of participants in each quartile below or above clinically relevant cut points (eGFR<60 ml/min per1.73 m2, UACR>30 mg/g). To examine the functional form of the association between klotho and each outcome, we fitted unadjusted natural cubic splines with knots placed at the quartiles and extreme values were excluded so as to avoid implausible extrapolation of shapes of the association.
Klotho and Change in Kidney Function
Multivariable logistic regression models were used to assess the relationship between klotho and decline in eGFR. Klotho was examined as both a continuous variable (log base 2, so that interpretation would be per doubling of the exposure) and categorized as quartiles. Model 1 was unadjusted. Multivariable models were then sequentially constructed through a series of nested models using prespecified variables as follows: model 2: adjusted for age, sex, race, study site, and baseline eGFR; model 3: additionally adjusted for diabetes, CVD, hypertension, and UACR; model 4: additionally adjusted for calcium, phosphorus, parathyroid hormone, and FGF-23. On the basis of a priori hypotheses that a synergistic relationship could potentially exist between klotho and either FGF-23 or baseline eGFR, we then evaluated if the association between klotho and decline in GFR was modified by FGF-23 or baseline eGFR by including interaction terms for each in the final multivariable models.
Klotho and Incident CKD
Poisson (log-link) regression was used to model the incidence rate ratio of CKD as a function of klotho level with robust variance estimation and an offset for follow-up time. Exposure variables were again examined as both linear terms and by category (quartiles) to assess for nonlinear relationships. Identical multivariable models were constructed as described above, including interaction terms.
Analyses were conducted using SPSS (IBM SPSS Statistics for Windows, Version 23.0. Released 2015. IBM Corp, Armonk, NY) and Stata (Stata Statistical Software: Release 13. 2013. StataCorp LP, College Station, TX.) A two-sided P value of <0.05 was considered statistically significant for all analyses including interaction terms.
Disclosures
None.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, NIA grant R01-AG028050, and National Institue of Nursing Research grant R01-NR012459, and has been approved for submission by the NIA. M.Shlipak, M.Sarnak, J.I., and R.K. were supported by NIA grant 5R01AG027002-07. R.D.S. was supported by R01 AG27012, R01 HL111271, and R21 HL112662. A.H. was supported by Nutrition Obesity Research Center grant P30 DK035816.
The study sponsors had no role in study design; collection, analysis, and interpretation of the data; writing the report; and the decision to submit the report for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu M-C, Moe OW, Kuro-o M: Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Nabeshima Y: Klotho: A fundamental regulator of aging. Ageing Res Rev 1: 627–638, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M: Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286: 8655–8665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S: Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes 60: 1907–1916, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiura H, Yoshida T, Shiohira S, Kohei J, Mitobe M, Kurosu H, Kuro-o M, Nitta K, Tsuchiya K: Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol 302: F1252–F1264, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Lindberg K, Amin R, Moe OW, Hu M-C, Erben RG, Wernerson AÖ, Lanske B, Olauson H, Larsson TE: The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 25: 2169–2175, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, Ravikumar P, Gillings N, Bian A, Sidhu SS, Kuro-o M, Moe OW: Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol 27: 79–90, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y: Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y: Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int 81: 539–547, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Sakan H, Nakatani K, Asai O, Imura A, Tanaka T, Yoshimoto S, Iwamoto N, Kurumatani N, Iwano M, Nabeshima Y, Konishi N, Saito Y: Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One 9: e86301, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu M-C, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW: Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Sun Z: Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54: 810–817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, Fujimori T, Xie P, Kanwar YS: Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci USA 104: 2331–2336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MC, Kuro-o M, Moe OW: The emerging role of Klotho in clinical nephrology. Nephrol Dial Transplant 27: 2650–2657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker SL, Pastor J, Carranza D, Quiñones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS: The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 30: 223–233, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y: Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 16: 722–729, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Hu MC, Kuro-o M, Moe OW: Klotho and chronic kidney disease. Contrib Nephrol 180: 47–63, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, Iwano A, Imura A, Fujimori T, Kuro-o M, Hanai N, Takeshige K, Nabeshima Y: Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun 267: 597–602, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y: Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424: 6–10, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Chen C-D, Podvin S, Gillespie E, Leeman SE, Abraham CR: Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA 104: 19796–19801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Utsugi T, Ohno T, Ohyama Y, Uchiyama T, Saito Y, Matsumura Y, Aizawa H, Itoh H, Kurabayashi M, Kawazu S, Tomono S, Oka Y, Suga T, Kuro-o M, Nabeshima Y, Nagai R: Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism 49: 1118–1123, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y: Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24: 771–785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Borst MH, Vervloet MG, ter Wee PM, Navis G: Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 22: 1603–1609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugiura H, Yoshida T, Tsuchiya K, Mitobe M, Nishimura S, Shirota S, Akiba T, Nihei H: Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 20: 2636–2645, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen L, Pedersen SM, Brasen CL, Rasmussen LM: Soluble serum Klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem 46: 1079–1083, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Heijboer AC, Blankenstein MA, Hoenderop J, de Borst MH, Vervloet MG; NIGRAM consortium : Laboratory aspects of circulating α-Klotho. Nephrol Dial Transplant 28: 2283–2287, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Seiler S, Wen M, Roth HJ, Fehrenz M, Flügge F, Herath E, Weihrauch A, Fliser D, Heine GH: Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int 83: 121–128, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Fang Y, Ginsberg C, Sugatani T, Monier-Faugere M-C, Malluche H, Hruska KA: Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int 85: 142–150, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cano FJ, Freundlich M, Ceballos ML, Rojo AP, Azocar MA, Delgado IO, Ibacache MJ, Delucchi MA, Lillo AM, Irarrázabal CE, Ugarte MF: Longitudinal FGF23 and Klotho axis characterization in children treated with chronic peritoneal dialysis. Clin Kidney J 7: 457–463, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima Y: Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun 398: 513–518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubal DB, Yokoyama JS, Zhu L, Broestl L, Worden K, Wang D, Sturm VE, Kim D, Klein E, Yu G-Q, Ho K, Eilertson KE, Yu L, Kuro-o M, De Jager PL, Coppola G, Small GW, Bennett DA, Kramer JH, Abraham CR, Miller BL, Mucke L: Life extension factor klotho enhances cognition. Cell Rep. 7: 1065–1076, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD 3rd , Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldenstein L, Driver TH, Fried LF, Rifkin DE, Patel KV, Yenchek RH, Harris TB, Kritchevsky SB, Newman AB, Sarnak MJ, Shlipak MG, Ix JH; Health ABC Study Investigators : Serum bicarbonate concentrations and kidney disease progression in community-living elders: The Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis 64: 542–549, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madero M, Peralta C, Katz R, Canada R, Fried L, Najjar S, Shlipak M, Simonsick E, Lakatta E, Patel K, Rifkin D, Hawkins M, Newman A, Sarnak M; Health ABC Study : Association of arterial rigidity with incident kidney disease and kidney function decline: The Health ABC study. Clin J Am Soc Nephrol 8: 424–433, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shastri S, Katz R, Rifkin DE, Fried LF, Odden MC, Peralta CA, Chonchol M, Siscovick D, Shlipak MG, Newman AB, Sarnak MJ: Kidney function and mortality in octogenarians: cardiovascular health study All Stars. J Am Geriatr Soc 60: 1201–1207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deo R, Fyr CL, Fried LF, Newman AB, Harris TB, Angleman S, Green C, Kritchevsky SB, Chertow GM, Cummings SR, Shlipak MG; Health ABC study : Kidney dysfunction and fatal cardiovascular disease--an association independent of atherosclerotic events: Results from the Health, Aging, and Body Composition (Health ABC) study. Am Heart J 155: 62–68, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Shastri S, Tighiouart H, Katz R, Rifkin DE, Fried LF, Shlipak MG, Newman AB, Sarnak MJ: Chronic kidney disease in octogenarians. Clin J Am Soc Nephrol 6: 1410–1417, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS; CKD Prognosis Consortium : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J: GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 64: 821–835, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]