Abstract
The mammalian ureter consists of a mesenchymal wall composed of smooth muscle cells and surrounding fibrocytes of the tunica adventitia and the lamina propria and an inner epithelial lining composed of layers of basal, intermediate, and superficial cells. How these cell types arise from multipotent progenitors is poorly understood. Here, we performed marker analysis, cell proliferation assays, and genetic lineage tracing to define the lineage relations and restrictions of the mesenchymal and epithelial cell types in the developing and mature mouse ureter. At embryonic day (E) 12.5, the mesenchymal precursor pool began to subdivide into an inner and outer compartment that began to express markers of smooth muscle precursors and adventitial fibrocytes, respectively, by E13.5. Smooth muscle precursors further diversified into lamina propria cells directly adjacent to the ureteric epithelium and differentiated smooth muscle cells from E16.5 onwards. Uncommitted epithelial progenitors of the ureter differentiated into intermediate cells at E14.5. After stratification into two layers at E15.5 and three cell layers at E18.5, intermediate cells differentiated into basal cells and superficial cells. In homeostasis, proliferation of all epithelial and mesenchymal cell types remained low but intermediate cells still gave rise to basal cells, whereas basal cells divided only into basal cells. These studies provide a framework to further determine the molecular mechanisms of cell differentiation in the tissues of the developing ureter.
Keywords: ureteric bud, renal development, renal cell biology, genetics and development, kidney development, molecular genetics
The mammalian ureter has a compartmentalized tissue architecture that is functionally adapted to mediate efficient urinary drainage from the renal pelvis to the bladder. The outer mesenchymal compartment harbors a thick layer of peristaltically active smooth muscle cells (SMCs), the tunica muscularis, that is ensheathed by flexible and anchoring fibroelastic material, the tunica adventitia on the outside and the lamina propria on the inside. The inner compartment is a highly distensible epithelium that provides sealing to the luminal space. This urothelium features a single layer of cuboidal basal cells (B cells) that is attached to the lamina propria via a basement membrane, one or two layers of intermediate cells (I cells) that resemble B cells in shape and size, and a luminal layer of large squamous superficial cells (S cells) that exert a barrier function at least partly due to expression of uroplakins (UPKs) that form crystalline plaques on the apical surface.1,2
The differentiated cell types of the two ureteric tissue compartments arise from multipotent precursors during embryonic development. In the mouse, these precursor pools are established around embryonic day (E) 11.5 when the distal aspect of an epithelial diverticulum of the nephric duct, the ureteric bud, and its surrounding mesenchyme adopt a distal ureteric rather than a proximal renal fate. For the next days, the mesenchymal and epithelial progenitors multiply to support ureter elongation. At E16.5, i.e., shortly after onset of urine production in the kidney, expression of smooth muscle (SM) structural proteins and of UPKs testifies that SMC and S cell differentiation has been initiated. Around birth, the three epithelial and mesenchymal cell layers can be histologically clearly distinguished.3,4 Embryologic experiments have shown that the survival, patterning, and subsequent differentiation of the primitive ureteric epithelium and its surrounding mesenchyme depend on each other. Genetic analysis has identified some of the trans-acting signals and the downstream transcription factors that regulate these cellular programs.4 However, how the different cell types arise in time and how they relate to each other has been poorly studied.
Here, we set out to probe the developmental origin and relationship of the different epithelial and mesenchymal cell types of the mouse ureter. We describe the temporal profile of cell differentiation and proliferation in the ureter, and trace the fate of the two progenitor pools. We provide evidence that I cells are precursors for both B and S cells in development.
Results
Cell Differentiation Occurs in a Temporally Controlled and Coordinated Manner in the Epithelial and Mesenchymal Tissue Compartments of the Embryonic Ureter
Previous work reported expression of cell-type–specific genes at selected stages of ureter development but did not address the precise temporal profile of the mesenchymal and epithelial differentiation programs.3,5,6 We therefore wished to correlate histologic changes with expression profiles of cell-type–specific marker sets in either tissue compartment at all stages of embryonic ureter development.
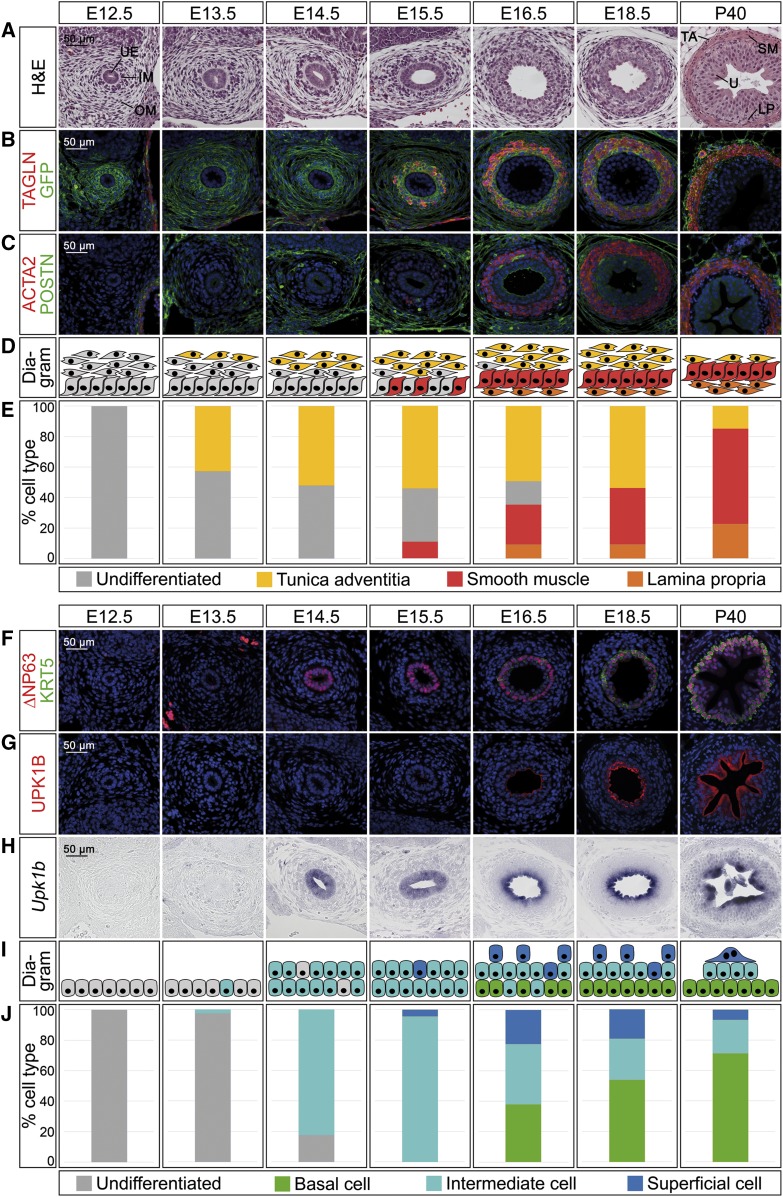
In the mature ureteric mesenchyme, adventitial fibrocytes are marked by expression of periostin (POSTN), whereas SMCs can be identified by transgelin (TAGLN) and actin, alpha 2, smooth muscle, aorta (ACTA2) expression.6,7 For the cells of the lamina propria no specific protein marker has been described, but they can be identified as mesenchymal cells negative for SMC markers adjacent to the ureteric epithelium (Figure 1, A–C, P40 panel). We have recently shown that the T-box transcription factor gene Tbx18 is expressed in the undifferentiated ureteric mesenchyme, and that the descendants of this expression domain constitute the ureteric mesenchymal wall throughout development and in adulthood.8,9 To detect and quantify cell differentiation in the ureteric mesenchyme, we therefore analyzed coexpression of cell-type–specific markers with a membrane-bound GFP reporter by immunofluorescence on proximal ureter sections in mice double heterozygous for a cre knock-in in the Tbx18 locus and the Rosa26mTmG reporter line (Tbx18cre/+;R26mTmG/+) (Figure 1, A–E).10,11
Figure 1.
Cell differentiation in ureter development occurs in a temporally and spatially controlled manner. (A–E) Time course of cell differentiation in the ureteric mesenchyme. (A) Hematoxylin and eosin (H&E) staining on transverse sections of the proximal ureter. At E12.5, the inner and outer mesenchymal region (IM and OM) and the ureteric epithelium (UE) are indicated. At P40, the three subregions of the mesenchyme, the lamina propria (LP), the smooth muscle (SM), and the tunica adventitia (TA) are marked adjacent to the urothelium (U). (B and C) Coimmunofluorescence analysis of expression of the SMC marker TAGLN and the lineage marker GFP (B) and of the SMC marker ACTA2 and the adventitial marker POSTN (C) on transverse sections of the proximal ureter in Tbx18cre/+;R26mTmG/+ mice. Nuclei are counterstained with DAPI (blue). (D) Schematic representation of cell differentiation in the ureteric mesenchyme. Fibrocytes of the tunica adventitia (yellow) are defined as POSTN+GFP+, SMCs (red) as TAGLN+ACTA2+GFP+, and lamina propria cells (orange) as TAGLN−GFP+. (E) Quantification of differentiated cell types in the ureteric mesenchyme on the basis of marker expression as explained in (D). For numbers see Supplemental Table 1A. (F–J) Time course of epithelial differentiation in the ureter. (F and G) Coimmunofluorescence analysis of expression of the B cell marker KRT5, the B and I cell marker ∆NP63 (F), and the I and S cell marker UPK1B (G) with DAPI-stained nuclei (blue), and (H) in situ hybridization for Upk1b expression on transverse sections of the proximal ureter in Tbx18cre/+;R26mTmG/+ mice. (I) Schematic representation of cell expansion and differentiation in the ureteric epithelium. B cells (green) are defined as KRT5+∆NP63+UPK1B−Upk1b−, I cells (turquois) as KRT5−∆NP63+UPK1B+(low)Upk1b+, and S cells (blue) as KRT5−∆NP63−UPK1B+(high)Upk1b+. (J) Quantification of differentiated cell types in the ureteric epithelium on the basis of marker expression as explained in (I). For numbers see Supplemental Table 1B.
At E12.5, two histologically distinct cell populations were present in the GFP+ ureteric mesenchyme. Cells adjacent to the ureteric epithelium were densely packed and spherical, whereas the outermost layers contained loosely organized, radially oriented, and spindle-shaped cells. Differentiation markers were not expressed at this stage. At E13.5, the number of GFP+ mesenchymal cells was strongly increased and the outermost spindle-shaped cells started to express POSTN. POSTN expression expanded to cover most cells of the outer mesenchymal compartment at E14.5. At E15.5, few TAGLN+ACTA2+ SMCs appeared in the dense inner mesenchyme that was surrounded by POSTN+ adventitial fibrocytes. At E16.5, a contiguous SMC layer had developed that was separated from the urothelium by a TAGLN− layer of lamina propria cells. Most of the spindle-shaped outer fibrocytes were POSTN+. At E18.5, the ureteric mesenchyme was fully patterned and differentiated in TAGLN−GFP+ lamina propria cells, a dense TAGLN+ACTA2+GFP+ SMC layer, and radially organized POSTN+GFP+ adventitial fibrocytes. The ratio of these cell types was dramatically changed at P40 by relative expansion of the lamina propria and the SMC layer and reduction of the tunica adventitia (Figure 1, A–E, Supplemental Table 1A).
Work in the bladder has shown that combinatorial expression of cytokeratin 5 (KRT5), of an isoform of the transcription factor p63 (∆NP63), and of UPKs distinguishes the three urothelial cell types in this tissue.12 We confirmed for the adult ureter that B cells are KRT5+∆NP63+UPK1B−, I cells are KRT5−∆NP63+UPK1B+, and S cells are KRT5−∆NP63−UPK1B+, with levels of UPK1B being significantly lower in I than in S cells (Figure 1, F and G, P40 panel); hence, that these markers can be used to profile urothelial differentiation in this organ (Figure 1, F–J). At E12.5, the mono-layered urothelium was negative for all of these differentiation markers. ∆NP63 expression occurred in single cells at E13.5 but was expressed in increasing cell numbers at E14.5 and labeled the majority of cells of the now double-layered urothelium at E15.5. Expression of Upk1b mRNA was weakly found throughout the urothelium at these stages as well characterizing these cells as I cells. At E15.5, single cells on the luminal side were negative for ∆NP63 which correlated with the advent of superficial UPK1B expression. At E16.5, the urothelium was composed of two to three layers. Coexpression of KRT5 and high levels of ∆NP63 indicated the presence of B cells that were intermingled with I cells (KRT5−∆NP63+UPK1B+[low]) in the basal layer. UPK1B expression was strong in the intermediate and apical layer. The abundance of B cells significantly increased from E16.5 to E18.5, and B cells constituted the majority of urothelial cells at P40. The relative number of KRT5−∆NP63+UPK1B+(low) I cells significantly decreased from E16.5 to E18.5 and remained at low abundance in the adult. KRT5−∆NP63−UPK1B+(high) S cells were clearly identifiable at E16.5 and E18.5, but decreased in abundance until P40 (Figure 1, F–J, Supplemental Table 1B). Interestingly, the relaxed adult urothelium showed a pyramidal topology on sections with large binucleated S cells at the tip, small I cells in the middle, and numerous B cells at the base (Figure 1, F–J, Supplemental Figure 1).
Comparative analysis of mesenchymal and epithelial differentiation markers at proximal, medial, and distal levels of the ureter revealed that the expression patterns of differentiation markers are spatially conserved but are activated with a delay of 1–2 days at more distal levels (Supplemental Figures 2 and 3).
We conclude that differentiated cell types arise in a distinct order and in a proximal to distal wave along the ureteric tube. In the mesenchyme adventitial fibrocytes precede SMCs and lamina propria cells, whereas in the epithelial compartment I cells differentiate before B and S cells.
Proliferation Rates Explain Differential Cell-Type Expansion in the Developing Ureter
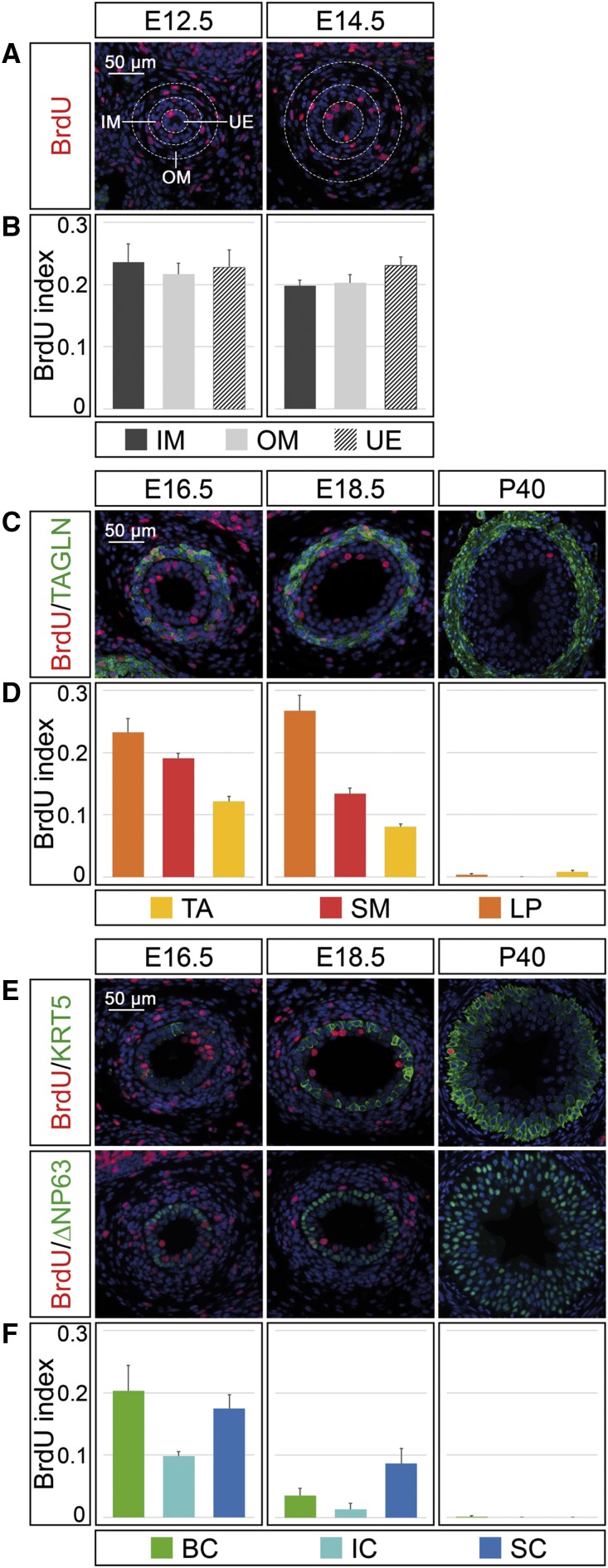
Our analysis has shown that the different epithelial and mesenchymal cell types of the ureter start to differentiate at different time points and change in abundance over time. To find out if this relates to differences in the temporal profiles of cell-cycle activity, we determined cell proliferation during ureter development and homeostasis using the BrdU incorporation assay.
At E12.5 and E14.5, proliferation rates reached 20%–23% in both the ureteric epithelium and the inner and outer region of the ureteric mesenchyme, showing that the precursor pools coordinately drive the expansion of the early organ rudiment (Figure 2, A and B, Supplemental Table 2A). From E16.5 onwards, the three mesenchymal and epithelial cell types could be molecularly distinguished. In the mesenchyme, the cells of the lamina propria maintained high proliferation rates similar to precursors at the previous stages, whereas SMCs reduced proliferation from 19% at E16.5 to 13% at E18.5, and adventitial fibrocytes from 12% to 8% in line with the relative expansion of SM and lamina cells at the expense of adventitial cells at these stages (Figure 1E). At P40, SMCs were quiescent, whereas cells of the lamina and adventitia divided at a very low rate (0.3% and 0.8%) (Figure 2, C and D, Supplemental Table 2B).
Figure 2.
Proliferation rates explain differential cell-type expansion in the developing ureter. (A) Coimmunofluorescence analysis on proximal ureter sections at E12.5 and E14.5 for BrdU and the nuclear counterstain DAPI (blue). White dotted lines demarcate the ureteric epithelium (UE), and the inner and outer mesenchymal cell populations (IM and OM). (B) Quantification of cell proliferation by the BrdU index in the areas indicated in (A). (C) Coimmunofluorescence analysis on proximal ureter sections at E16.5, E18.5, and P40 for BrdU, the nuclear counterstain DAPI (blue), and the SM marker TAGLN. (D) Quantification of cell proliferation by the BrdU index in TAGLN− outer mesenchymal cells of the tunica adventitia (TA), in TAGLN+ smooth muscle cells (SM), and in TAGLN− inner mesenchymal cells of the lamina propria (LP). (E) Coimmunofluorescence on proximal ureter sections at E16.5, E18.5, and P40 for BrdU, the nuclear counterstain DAPI (blue), and the B cell marker KRT5 (upper row) and BrdU, the nuclear counterstain DAPI (blue), and the B and I cell marker ∆NP63 (lower row). (F) Quantification of cell proliferation by the BrdU index in KRT5+ B cells (BC), in KRT5+∆NP63+ I cells (IC), and KRT5−∆NP63− S cells (SC). For numbers see Supplemental Table 2, A–C.
In the epithelial compartment, B and S cells maintained high proliferation at E16.5, whereas I cells proliferated at approximately half the rate (9%). At E18.5, proliferation rates were further reduced with S cells being more proliferative than B and I cells. In the adult ureter, only B cells divided occasionally, whereas S and I cell proliferation was under the detection limit (Figure 2, E and F, Supplemental Table 2C). Together, these findings suggest that mesenchymal and epithelial cell-types withdraw at different time-points from the cell cycle, explaining their changing ratios particularly at late embryonic and at postnatal stages.
Adventitial Cells are Separated Early in Mesenchymal Development Whereas Lamina Propria Cells Derive from SMC Precursors
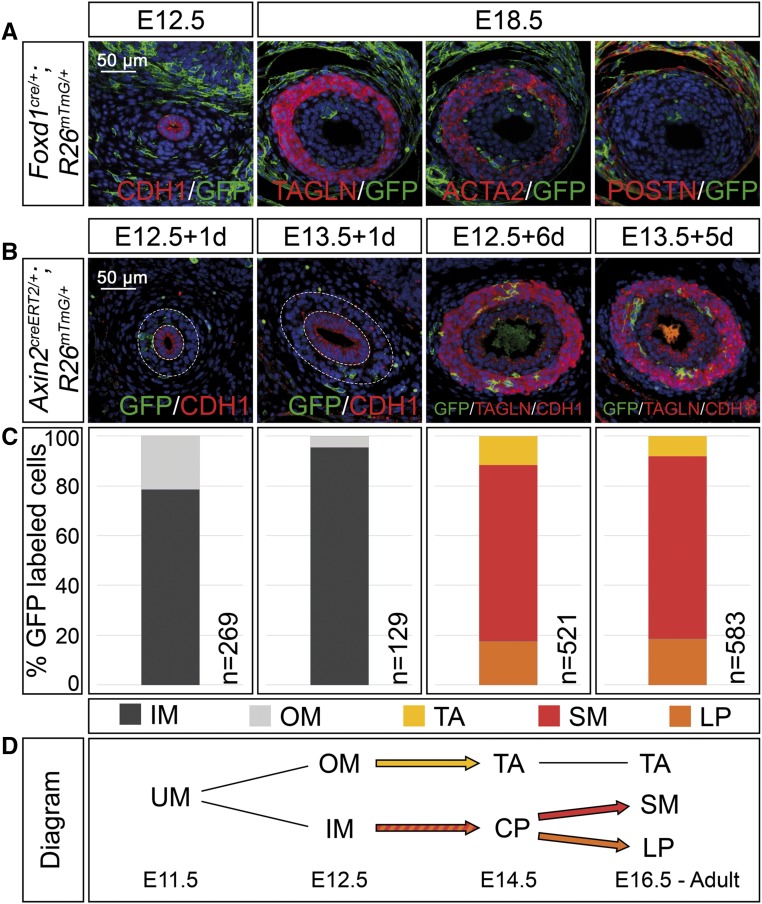
Our expression analysis showed that the homogenous mesenchymal progenitor pool is separated into a histologically and molecularly distinct inner and outer domain as early as E12.5. To determine whether this subdivision represents a compartment boundary between prospective adventitial fibrocytes and SMCs, and to define the origin of the lamina propria cells, we performed genetic lineage tracing experiments using cre lines that are specific to the outer or inner mesenchymal domain. To detect the descendants of outer mesenchymal cells, we took advantage of the fact that Foxd1 is expressed in this domain exclusively at E12.5 and E14.5 (Supplemental Figure 4A). Marker analysis on sections of proximal E12.5 and E18.5 ureters double heterozygous for a Foxd1cre allele13 and the R26mTmG reporter (Foxd1cre/+;R26mTmG/+) at E12.5 and E18.5 showed that the descendants of this expression domain localized to the outer mesenchymal ring at E12.5 and E18.5, and expressed POSTN but not the SMC markers TAGLN and ACTA2 (Figure 3A), clearly indicating that outer mesenchymal cells are lineage-restricted to become adventitial fibrocytes as early as E12.5.
Figure 3.
Adventitial cells are separated early in mesenchymal development whereas lamina propria cells derive from SMC precursors in the ureter. (A) Coimmunofluorescence analysis on transverse sections of the proximal ureter for the lineage marker GFP and the epithelial marker CDH1 at E12.5, with the SMC markers TAGLN and ACTA2, and the adventitial cell marker POSTN at E18.5 shows recombination in outer fibrocytes only in Foxd1cre/+;R26mTmG/+ embryos. (B) Coimmunofluorescence of the lineage marker GFP with the epithelial marker CDH1 and the SMC marker TAGLN on proximal sections of ureters from Axin2creERT2/+;R26mTmG/+ embryos that were tamoxifen-induced at E12.5 or E13.5 and harvested after 1 and 6 or 5 days to detect localization of recombined cells. (C) Relative distribution of GFP+ cells localized to the inner and outer ureteric mesenchyme (IM and OM), and to the tunica adventitia (TA), the smooth muscle (SM), and the lamina propria (LP) in ureters shown in (B). The number of counted GFP+ cells (n) is given. For additional numbers see Supplemental Table 3. (D) Schematic representation of the lineage relations in the ureteric mesenchyme. Abbreviations are as explained in (C). Inner mesenchymal cells contribute to the SM layer and the lamina propria but not to the tunica adventitia. UM, undifferentiated mesenchyme; CP, common precursor.
Axin2 is expressed at E12.5 and subsequent stages in the inner ring of mesenchymal cells in the ureter as a response to WNT signals emitted from the adjacent epithelium (Supplemental Figure 4B).6 To trace the descendants of this Axin2+ domain, we crossed mice with a creERT2 knock-in in the Axin2 locus14 with the R26mTmG reporter line and induced recombination by a single oral administration of 4 mg tamoxifen. To confirm the region-specificity of the labeling reaction, we treated Axin2creERT2/+;R26mTmG/+ embryos at E12.5 and E13.5 and analyzed the distribution of GFP+ cells in the inner and outer mesenchymal compartment 24 hours later. In embryos injected at E12.5, 23% of the GFP+ cells were detected in the outer compartment, 77% in the inner compartment; in E13.5 embryos, the relation was 6%–94%, indicating that the labeling reaction in E13.5 ureters almost exclusively occurred in the inner ring (Figure 3, B and C, Supplemental Table 3). When Axin2creERT2/+;R26mTmG/+ embryos were analyzed at E18.5, GFP+ descendants localized to 71% to the SMC layer, to 18% to the lamina propria, and to 11% to the tunica adventitia when tamoxifen administration was done at E12.5. For embryos tamoxifen-treated at E13.5 the numbers were 73% and 17%, whereas only 10% of labeled cells were detected in the tunica adventitia (Figure 3, B and C, Supplemental Table 3). We conclude that Axin2+ inner mesenchymal cells are precursors of SMCs, and that lineage restriction to adventitial fibrocytes occurs around E12.5 to E13.5. Lamina propria cells derive from inner mesenchymal SMC progenitors by switching off the SMC program at E14.5 to E16.5.
I Cells are Progenitors of B and S Cells in Development
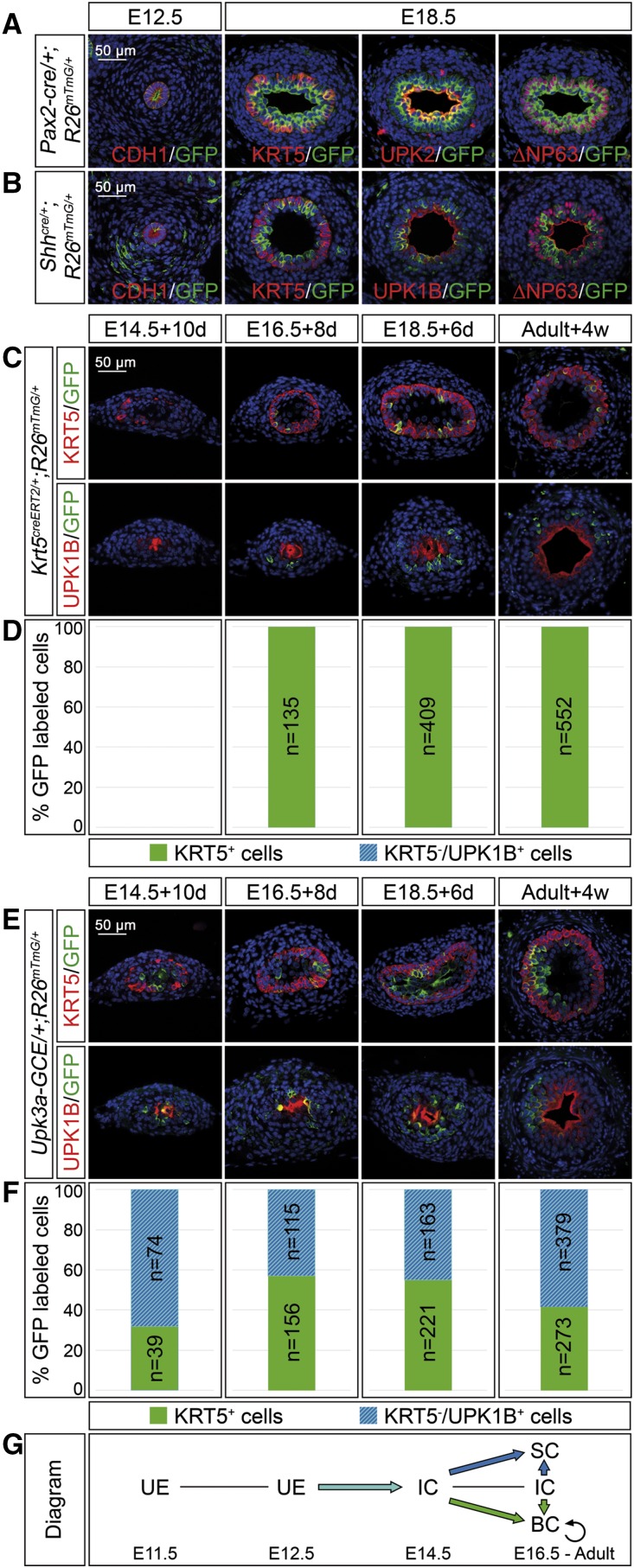
The ureteric epithelium derives from the distal aspect of the ureter bud that emerges as an outgrowth of the nephric duct around E10.5. To confirm that the distal ureter stalk indeed harbors precursors for all epithelial cell types of the mature ureter, we performed genetic lineage tracing on the basis of cre lines mediating recombination in all of these precursors. Pax2 and Shh are genes that are expressed throughout the ureteric epithelium at E12.5 and at subsequent stages (Supplemental Figure 4, C and D). A transgenic line, in which cre was expressed under Pax2 regulatory elements (Pax2-cre),15,16 mediated full recombination of the R26mTmG reporter allele in the ureteric epithelium at E12.5; a line harboring a cre knock-in into the Shh locus17 did so partially (Figure 4, A and B). In Pax2-cre/+;R26mTmG/+ and Shhcre/+;R26mTmG/+ ureters analyzed at E18.5, GFP+ cells populated all three urothelial cell layers, whereas a contribution to the ureteric mesenchyme was never observed (Figure 4, A and B).
Figure 4.
I cells are progenitors of B and S cells in ureter development and homeostasis. (A and B) Coimmunofluorescence analysis on transverse sections of the proximal ureter of the lineage marker GFP with the epithelial marker CDH1 at E12.5, and the B cell marker KRT5, the B and I cell marker ∆NP63, and the S cell markers UPK1B/UPK2 in Pax2-cre/+;R26mTmG/+ embryos (A) and in Shhcre/+;R26mTmG/+ embryos (B) shows that Pax2+ and Shh+ cells of the early ureteric bud contribute to all urothelial cell types in the ureter but not the surrounding mesenchyme. (C and E) Coimmunofluorescence analysis of the lineage marker GFP with the B cell marker KRT5 (upper panel) and the S cell marker UPK1B (lower panel) on transverse sections of ureters isolated at E14.5, E16.5, and E18.5, induced with 500 nM 4-hydroxytamoxifen for the first 24 hours and cultured for 10, 8, and 6 days; and in adult ureters 4 weeks after tamoxifen administration in Krt5creERT2/+;R26mTmG/+ mice (C) and Upk3a-GCE/+;R26mTmG/+ mice (E). (D and F) The bar diagrams display the percentage of lineage-labeled (GFP+) cells contributing to KRT5+UPK1B− B cells or to KRT5−UPK1B+ I and S cells in the ureter of Krt5creERT2/+;R26mTmG/+ mice (D) and of Upk3a-GCE/+;R26mTmG/+ mice (F) at the indicated stages and conditions. The number of counted GFP+ cells (n) is given. For additional numbers see Supplemental Table 4, A and B. (G) Schematic representation of the lineage relations of urothelial cell types. KRT5+ cells only give rise to B cells whereas UPK3A+ cells give rise to B, I, and S cells. BC, basal cell; IC, intermediate cell; SC, superficial cell; UE, undifferentiated epithelium.
Our expression analysis further demonstrated that the epithelial compartment of the ureter remains undifferentiated until E13.5 and expresses markers for I cells from E14.5 onwards. S cells and B cells were only detected from E15.5 and E16.5 onwards, respectively, suggesting that I cells are precursors for both S and B cells in development. To validate this hypothesis, we used cell-type–specific creERT2 lines and traced their descendants in ureter explant cultures after 4-hydroxytamoxifen–induced stage-specific recombination of the R26mTmG reporter allele.
To trace the fate of B cells we used a creERT2 lineage tracing system on the basis of the B cell–specific expression of the Krt5 gene.12,18 When ureters from Krt5creERT2/+;R26mTmG/+ embryos were explanted at E14.5, treated with 4-hydroxytamoxifen, and analyzed after 10 days GFP+ cells were not detected, compatible with the fact that KRT5 expression only occurs around E16.5. When ureters from E16.5 and E18.5 embryos were treated and analyzed after 8 and 6 days, respectively, GFP+ cells were present and unambiguously expressed the B cell marker KRT5, suggesting a lineage restriction of the B cell population (Figure 4, C and D, Supplemental Table 4A).
Because cre lines specific for I cells are not established, we used a Upk3a-GCE/+ line,12,19 that labels I and S cells but not B cells (Supplemental Figure 5), to trace I cell descendants. Irrespective of whether ureters of Upk3a-GCE/+;R26mTmG/+ embryos were explanted at E14.5 and analyzed after 10 days, explanted at E16.5 and analyzed after 8 days, or explanted at E18.5 and analyzed after 6 days, GFP+ cells differentiated in roughly equal ratio into KRT5+ B cells and KRT5−UPK1B+ I and S cells, suggesting that I cells are B cell progenitors in development (Figure 4, E and F, Supplemental Table 4B).
To detect the fate of Krt5- and Upk3a-expressing cells under homeostatic conditions, we injected Krt5creERT2/+;R26mTmG/+ and Upk3a-GCE/+;R26mTmG/+ mice at P100 with three doses of 5 mg tamoxifen on alternating days and analyzed for GFP expression after 4 weeks. In Krt5creERT2/+;R26mTmG/+ ureters, all lineage-labeled cells coexpressed KRT5, indicating that B cells give only rise to B cells. In Upk3a-GCE/+;R26mTmG/+ ureters, lineage-labeled cells expressed in roughly equal ratio KRT5 or UPK1B, indicating that I cells still give rise to B cells (and probably S cells) in homeostasis (Figure 4, C–F, Supplemental Table 4, A and B).
Discussion
Early Lineage Restriction in the Ureteric Mesenchyme Separates Adventitial Fibrocytes from a Common Precursor of SMCs and Subepithelial Fibrocytes
Our previous work has shown that all mesenchymal cell types of the ureter derive from a Tbx18+ mesenchymal progenitor pool that surrounds the distal portion of the ureter bud and separates the metanephric blastema from the nephric duct around E11.5.9 Shortly after, at E12.5, this homogenous cell population is subdivided into an inner compartment of densely packed, spherical cells and an outer compartment of loosely organized, spindle-shaped, and radially oriented cells, implying that mesenchymal cells of the two domains have acquired different fates. Our findings support such an early lineage restriction in the ureteric mesenchyme. First, differential gene expression occurs around E12.5 in the two mesenchymal subregions. The outer domain expresses POSTN and Foxd1, whereas the inner region is positive for Axin2. Second, and more importantly, lineage tracing of Foxd1+ cells showed that these cells exclusively contribute to adventitial fibrocytes at E18.5, whereas Axin2+ cells differentiate into cells of the lamina propria and the SM layer. Our marker and proliferation analyses argue that adventitial fibrocytes arise first and become terminally differentiated before the other mesenchymal cell-types around E16.5 to E18.5. In contrast, Axin2+ cells exist as bipotent precursors from E12.5 to E15.5, and differentiate into SMCs and subepithelial fibrocytes around E16.5. The descent of lamina propria and SMCs from a common precursor is also supported by the observation that lamina cells initially also express ACTA2 but downregulate it afterward. Lamina cells and SMCs maintain high proliferation rates at least until E18.5, arguing that they only terminally differentiate postnatally. Given the maintenance of cell division at low rates in both fibrocyte populations in adults, it is tempting to speculate that adventitial fibrocytes replenish themselves whereas lamina cells serve as precursors for themselves and for SMCs.
Tissue separation experiments provided compelling evidence that SMC differentiation in the ureter, and in many other visceral tubular organs, depends on signals from the adjacent epithelium.20 Our own work has recently shown that canonical WNT signaling is required to initiate SM precursor development and to suppress an adventitial fate in the ureter.6 Because Axin2 is a direct transcriptional target of this signaling pathway,21 this suggests that only cells adjacent to the ureteric epithelium that perceive a WNT signal escape from the default program to become adventitial fibrocytes, and differentiate into SM progenitors instead. It is interesting to note that Axin2 expression persists after E14.5 only in cells directly in contact with the ureteric epithelium, i.e., in lamina propria cells, supporting our idea that these cells represent a persisting pool of SM progenitors. Whether downregulation of WNT signaling is a prerequisite for SM progenitors to differentiate into SMCs is an attractive possibility that can be experimentally tested.
I Cells are Progenitors for B and S Cells in Urothelial Development and Homeostasis
Here, we have shown that all cell types of the mature urothelium derive from a common Pax2+Shh+ precursor in the ureter bud. Differentiated cell-types arise from this precursor in a strict temporal sequence. At E14.5, all epithelial cells bear the molecular signature of an I cell. With the onset of stratification at E15.5, single apical cells express S cell markers. At E16.5, KRT5+ cells appear in the basal layer but subsequently expand and constitute the main cell-type of the mature urothelium. The sequential appearance of differentiated cell types suggests that I cells are a common precursor for S and B cells. This hypothesis is further supported by our genetic lineage tracing experiments. Whereas Upk3a+ cells contributed to all cell-types throughout development and in adult tissue homeostasis, Krt5+ cells only replicated themselves. Interestingly, proliferation rates of I cells were low compared with that of B and S cells, suggesting that they first differentiate along their respective lineages and then expand. Of course, our analysis cannot exclude that I cells are heterogeneous and harbor distinct subpopulations that give rise to B and S cells, respectively. Identification of additional biomarkers, possibly by single cell transcriptome analysis, may help to resolve such an issue in the future.
So far, urothelial progenitors have only been studied in the bladder, mostly under conditions of regeneration. Even though the urothelium of the bladder and the ureter are derived from different germ layers, our study and a recent report suggest that stratification and differentiation follow a similar time course in both organs, thus, might be controlled by a common molecular program.12 However, the nature of the urothelial progenitors in the bladder has been controversially discussed. Although initially B cells were thought to be progenitors for the other urothelial cell types,22 Gandhi and colleagues suggested I cells to be S cell progenitors only and defined B cells as a unipotent, self-renewing population of unknown descent.12 A recent work identified a KRT14+ subpopulation of B cells to give rise to all urothelial cell types after injury.23 Whether the injury conditions ablate S and I cells so much that B cells are reprogrammed to compensate for their loss remains to be seen. Unlike the bladder, where KRT14 expression occurs in a large fraction of KRT5+ B cells both in the embryonic and adult urothelium, KRT14 is not expressed in the embryonic ureter and is found at very low levels in few B cells in the mature ureteric urothelium (Supplemental Figure 6). This indicates that B cells in both organs are of different heterogeneity, and that a KRT14+ subpopulation of B cells might be of less relevance for urothelial homeostasis and regeneration in the ureter.
The molecular pathways that regulate the specification, differentiation, and maintenance of the urothelium remain obscure. Retinoic acid signaling has been implied in the specification of the bladder urothelium from an endodermal precursor,12 whereas BMP4 was reported to be involved in the maintenance of basal cell differentiation.24 Whether these signaling pathways are involved in urothelial development of the ureter as well should be a focus of future research.
Concise Methods
Animals
Gt(ROSA)26Sortm4(ACTB-tdTomato-EGFP)Luo (synonym: R26mTmG),10 Krt5tm1.1(cre/ERT2)Blh (synonym: Krt5creERT2),18 Tg(Upk3a-GFP/cre/ERT2)26Amc (synonym: Upk3a-GCE),19 Foxd1tm1(GFP/cre)Amc (synonym: Foxd1cre),13 Shhtm1(EGFP/cre)Cjt (synonym: Shhcre),17 and Axin2tm1(cre/ERT2)Rnu (synonym: Axin2creERT2)14 mouse lines were all obtained from the Jackson Laboratory. Tbx18tm4(cre)Akis (synonym: Tbx18cre)11 and Tg(Pax2-cre)1AKis (synonym: Pax2-cre)15,16 were previously generated in the laboratory. All lines were maintained on an NMRI outbred background. Embryos for gene expression and proliferation assays were derived from matings of NMRI wildtype mice, or from matings of males heterozygous for the cre driver with R26mTmG/mTmG females. For timed pregnancies, vaginal plugs were checked in the morning after mating, and noon was defined as E0.5. Embryos and urogenital systems were dissected in PBS. Ureters for explant cultures were dissected in L-15 Leibovitz medium (Biochrom). Specimens were fixed in 4% PFA/PBS, transferred to methanol, and stored at −20°C before immunofluorescence or in situ hybridization analyses. PCR genotyping was performed on genomic DNA prepared from yolk sac or tail biopsies. For conditional lineage tracing experiments, tamoxifen (Sigma) was dissolved in pure ethanol at 100 mg/ml and then emulsified in corn oil (Sigma) to a final concentration of 12.5 mg/ml. Within 1 week, three times 5 mg of tamoxifen were given to 14-week-old mice via oral gavage. Urogenital systems were harvested 4 weeks thereafter. All animal work conducted for this study was performed according to European and German legislation.
Histologic Analysis
Embryos, urogenital systems, and ureters were paraffin-embedded and sectioned to 5 µm. Hematoxylin and eosin staining was performed according to standard procedures.
In Situ Hybridization Analysis
Nonradioactive in situ hybridization analysis of gene expression was performed on 10 µm paraffin sections of the proximal ureter with digoxigenin-labeled antisense riboprobes.25 At least three embryos of each genotype were used for each analysis.
Immunofluorescence Detection of Antigens
For immunofluorescence analysis on 5 µm paraffin sections polyclonal rabbit–anti-TAGLN (1:250, ab14106; Abcam), monoclonal mouse–anti-GFP (1:250, 11814460001; Roche), polyclonal rabbit–anti-GFP (1:250, sc-8334; Santa Cruz), monoclonal mouse–anti-ACTA2 (1:250, A5228; Sigma-Aldrich), polyclonal rabbit–anti-∆NP63 (1:250, 619001; BioLegend), polyclonal rabbit–anti-KRT5 (1:250, PRB-160P; Covance), monoclonal mouse–anti-KRT14 (1:250, ab7800; Abcam), monoclonal mouse–anti-UPK1B (1:250, WH0007348M2; Sigma-Aldrich), polyclonal rabbit–anti-POSTN (1:250, ab14041; Abcam), monoclonal mouse–anti-BrdU (1:250, 1170376; Roche), or polyclonal rabbit–anti-CDH1 (1:250, gift from Rolf Kemler) were used as primary antibodies. Biotinylated goat–anti-rabbit IgG (1:250, 111065033; Dianova), Alexa488-conjugated goat–anti-rabbit IgG (1:500, A11034; Molecular Probes), and Alexa555-conjugated goat–anti-mouse IgG (1:500, A21422; Molecular Probes) were used as secondary antibodies. The signals of BrdU, ∆NP63 and POSTN primary antibodies were amplified using the Tyramide Signal Amplification system (NEL702001KT; Perkin Elmer). For coimmunofluorescence with primary antibodies from the same host (∆NP63 and KRT5) detection was performed sequentially and epitopes of the first antibody were blocked with goat–anti-rabbit Fab (1:250, 111007003; Dianova). Before staining, paraffin sections were deparaffinized and cooked for 15 minutes in antigen unmasking solution (H-3300; Vector Laboratories). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). At least three embryos of each genotype were used for each analysis.
Three-Dimensional Reconstruction of S cells
For three-dimensional reconstruction of S cells in the adult ureter 28 consecutive 5 µm transverse paraffin sections were stained for CDH1 and DAPI as described above. CDH1 and DAPI signals were used to highlight cell boundaries and nuclei that were reconstructed with the Amira 5.3.3 software (FEI).
Cell Proliferation Assay
Cell proliferation rates in wildtype ureters (n=3 per stage) were investigated by the detection of incorporated BrdU on 5 µm paraffin sections similar to published protocols.26 For embryonic stages the BrdU incorporation time was 1 hour; for adult ureters incorporation time was 10 hours due to low cell division rates. For each specimen 12 sections of the proximal ureter were assessed. The BrdU-labeling index was defined as the number of BrdU-positive nuclei relative to the total number of nuclei as detected by DAPI counterstaining in arbitrarily or molecularly defined compartments of the ureter. Data were expressed as mean±SD.
Organ Cultures
Ureters were dissected from the embryo, explanted on 0.4 µm polyester membrane Transwell supports (Corning), and cultured at the air-liquid interface for 24 hours with DMEM/F12 (Gibco) supplemented with 10% FCS (Biochrom), 1× penicillin/streptomycin (Gibco), 1× pyruvate (Gibco), and 1× Glutamax (Gibco). 4-Hydroxytamoxifen (H7904; Sigma-Aldrich) was added to the medium at a final concentration of 500 nM for the first 24 hours of culture to induce recombination. Culture medium was replaced every day.
Image Analysis
Sections were photographed using a Leica DM5000 microscope with Leica DFC300FX digital camera or a Leica DM6000 microscope with Leica DFC350FX digital camera and afterward processed in Adobe Photoshop CS4.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Rolf Kemler for the anti-CDH1 antiserum.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG KI728/9-1) to A.K.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016080849/-/DCSupplemental.
References
- 1.Velardo JT: Histology of the ureter. In: The ureter, 2nd Ed., edited by Bergman H, New York, Springer-Verlag, 1981 [Google Scholar]
- 2.Yu J, Manabe M, Wu XR, Xu C, Surya B, Sun TT: Uroplakin I: A 27-kD protein associated with the asymmetric unit membrane of mammalian urothelium. J Cell Biol 111: 1207–1216, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss RM, Guo S, Shan A, Shi H, Romano RA, Sinha S, Cantley LG, Guo JK: Brg1 determines urothelial cell fate during ureter development. J Am Soc Nephrol 24: 618–626, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohnenpoll T, Kispert A: Ureter growth and differentiation. Semin Cell Dev Biol 36: 21–30, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Airik R, Trowe MO, Foik A, Farin HF, Petry M, Schuster-Gossler K, Schweizer M, Scherer G, Kist R, Kispert A: Hydroureternephrosis due to loss of Sox9-regulated smooth muscle cell differentiation of the ureteric mesenchyme. Hum Mol Genet 19: 4918–4929, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Trowe MO, Airik R, Weiss AC, Farin HF, Foik AB, Bettenhausen E, Schuster-Gossler K, Taketo MM, Kispert A: Canonical Wnt signaling regulates smooth muscle precursor development in the mouse ureter. Development 139: 3099–3108, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Sorocos K, Kostoulias X, Cullen-McEwen L, Hart AH, Bertram JF, Caruana G: Expression patterns and roles of periostin during kidney and ureter development. J Urol 186: 1537–1544, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Airik R, Bussen M, Singh MK, Petry M, Kispert A: Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest 116: 663–674, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohnenpoll T, Bettenhausen E, Weiss AC, Foik AB, Trowe MO, Blank P, Airik R, Kispert A: Tbx18 expression demarcates multipotent precursor populations in the developing urogenital system but is exclusively required within the ureteric mesenchymal lineage to suppress a renal stromal fate. Dev Biol 380: 25–36, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Trowe MO, Shah S, Petry M, Airik R, Schuster-Gossler K, Kist R, Kispert A: Loss of Sox9 in the periotic mesenchyme affects mesenchymal expansion and differentiation, and epithelial morphogenesis during cochlea development in the mouse. Dev Biol 342: 51–62, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, Laufer E, Metzger D, Liang F, Liao Y, Sun TT, Aronow B, Rosen R, Mauney J, Adam R, Rosselot C, Van Batavia J, McMahon A, McMahon J, Guo JJ, Mendelsohn C: Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell 26: 469–482, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Amerongen R, Bowman AN, Nusse R: Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 11: 387–400, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Kuschert S, Rowitch DH, Haenig B, McMahon AP, Kispert A: Characterization of Pax-2 regulatory sequences that direct transgene expression in the Wolffian duct and its derivatives. Dev Biol 229: 128–140, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Trowe MO, Maier H, Petry M, Schweizer M, Schuster-Gossler K, Kispert A: Impaired stria vascularis integrity upon loss of E-cadherin in basal cells. Dev Biol 359: 95–107, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ: Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118: 517–528, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C: Distinct stem cells contribute to mammary gland development and maintenance. Nature 479: 189–193, 2011 [DOI] [PubMed] [Google Scholar]
- 19.McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, Zhang P, . GUDMAP project: GUDMAP: the genitourinary developmental molecular anatomy project. J Am Soc Nephrol 19: 667–671, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Cunha GR: Epithelial-stromal interactions in development of the urogenital tract. Int Rev Cytol 47: 137–194, 1976 [DOI] [PubMed] [Google Scholar]
- 21.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F: Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22: 1172–1183, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA: Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472: 110–114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papafotiou G, Paraskevopoulou V, Vasilaki E, Kanaki Z, Paschalidis N, Klinakis A: KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun 7: 11914, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI: Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem 277: 7412–7419, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Moorman AF, Houweling AC, de Boer PA, Christoffels VM: Sensitive nonradioactive detection of mRNA in tissue sections: Novel application of the whole-mount in situ hybridization protocol. J Histochem Cytochem 49: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Bussen M, Petry M, Schuster-Gossler K, Leitges M, Gossler A, Kispert A: The T-box transcription factor Tbx18 maintains the separation of anterior and posterior somite compartments. Genes Dev 18: 1209–1221, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.