Abstract
Hyperphosphatemia is common among patients with CKD stage 5D and is associated with morbidity and mortality. Current guidelines recommend lowering serum phosphate concentrations toward normal. Tenapanor is a minimally absorbed small molecule inhibitor of the sodium/hydrogen exchanger isoform 3 that functions in the gut to reduce sodium and phosphate absorption. This randomized, double-blind, placebo-controlled trial assessed the effects of tenapanor on serum phosphate concentration in patients with hyperphosphatemia receiving hemodialysis. After a 1- to 3-week washout of phosphate binders, we randomly assigned 162 eligible patients (serum phosphate =6.0 to <10.0 mg/dl and a 1.5-mg/dl increase from before washout) to one of six tenapanor regimens (3 or 30 mg once daily or 1, 3, 10, or 30 mg twice daily) or placebo for 4 weeks. The primary efficacy end point was change in serum phosphate concentration from baseline (randomization) to end of treatment. In total, 115 patients (71%) completed the study. Mean serum phosphate concentrations at baseline (after washout) were 7.32–7.92 mg/dl for tenapanor groups and 7.87 mg/dl for the placebo group. Tenapanor provided dose-dependent reductions in serum phosphate level from baseline (least squares mean change: tenapanor =0.47–1.98 mg/dl; placebo =0.54 mg/dl; P=0.01). Diarrhea was the most common adverse event (tenapanor =18%–68%; placebo =12%) and frequent at the highest tenapanor doses. In conclusion, tenapanor treatment resulted in statistically significant, dose-dependent reductions in serum phosphate concentrations in patients with hyperphosphatemia receiving hemodialysis. Additional studies are required to clarify the optimal dosing of tenapanor in patients with CKD-related hyperphosphatemia.
Keywords: tenapanor, hyperphosphatemia, hemodialysis, chronic kidney disease, NHE3, sodium–hydrogen exchanger 3
Disorders of mineral metabolism are common among persons with CKD.1 Impaired kidney function reduces urinary phosphate excretion, the principal mechanism by which normal phosphate balance is maintained.2 Modulation of tubular reabsorption of phosphate, mediated in large part by parathyroid hormone (PTH) and the phosphatonin fibroblast growth factor 23 (FGF23), allows for maintenance of serum phosphate concentrations within a physiologic range, despite wide variation in phosphate intake on a day to day basis. However, in advanced CKD, dietary phosphate intake generally exceeds excretory capacity; for patients on dialysis, even with dietary phosphate restriction, hyperphosphatemia is almost inevitable without specific treatment.3 Among patients receiving dialysis, evidence from observational studies,1,4 retrospective database analyses,5,6 and to a lesser extent, prospective controlled trials7,8 has shown that hyperphosphatemia is associated with mortality,1,4–6 fractures,5 and cardiovascular disease, including vascular calcification8 and left ventricular hypertrophy.7
For patients with CKD stage 5D, current treatment guidelines recommend that serum phosphate concentrations are lowered toward the normal (population reference) range.9 Dietary phosphate restriction and oral phosphate binders are first-line treatments for these patients.9,10 Dietary phosphate restriction can attenuate the severity of hyperphosphatemia and secondary hyperparathyroidism, although adherence is generally poor and the effect size is modest, with achieved mean reductions in serum phosphate of approximately 0.6 mg/dl over 3 months.11 Adherence to phosphate binders is also poor, owing in part to pill burden (12 or more tablets per day are often required12 on a background of polypharmacy), timing of ingestion around mealtimes, and gastrointestinal (GI) side effects, including nausea, vomiting, constipation, and abdominal bloating. Moreover, calcium-based phosphate binders have been associated with vascular calcification and all-cause mortality.9,13,14 Importantly, the current treatment paradigm is inadequate to control hyperphosphatemia in the majority of patients receiving dialysis, because the mean serum phosphate concentration among these patients in the United States remains well above the upper end of the population reference range.9 Data from clinical trials of currently approved phosphate binders in patients receiving hemodialysis show that mean reductions in serum phosphate of 1.2–2.2 mg/dl are typically achievable over treatment periods of 2–52 weeks.15–22
To date, no approved treatment has specifically targeted the transepithelial transport of intestinal phosphate. Tenapanor (RDX5791, AZD1722) is a small molecule inhibitor of the sodium/hydrogen exchanger isoform 3 (NHE3) being developed for the control of serum phosphate in patients with CKD receiving dialysis. Tenapanor acts in the gut to reduce the absorption of sodium and phosphate, with minimal systemic drug exposure.23–26 In healthy volunteers, increases in stool phosphorus up to 14.2 mmol/d relative to placebo were observed with tenapanor dosing, with concomitant reductions in urinary phosphorus.26 The mechanism by which tenapanor reduces GI phosphate uptake is under active investigation; it does not seem to involve direct inhibition of intestinal phosphate transporters type 1 or sodium-dependent phosphate transport protein 2B (NaPi2b, also known as NPT2b).24
Changes in intestinal and urinary phosphorus elimination seen in healthy volunteers suggested that tenapanor could have a role in treating hyperphosphatemia in patients with advanced CKD. The aim of this double-blind, placebo-controlled study was to assess the short-term safety, efficacy, and dose-response of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis.
Results
Patient Disposition and Baseline Characteristics
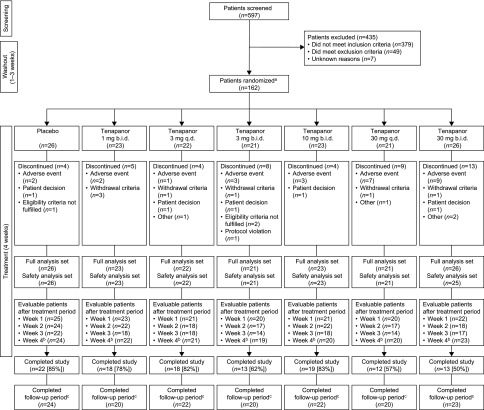
Overall, 597 patients were screened for enrollment in this seven-arm study (Figure 1), 162 of whom were randomly assigned to study treatment. The most common reason for not being randomized was serum phosphate concentration outside the required range and/or insufficient increase in serum phosphate during washout (n=242). Of the 162 patients randomly assigned to treatment, 160 were included in the analysis of the primary efficacy end point (change in serum phosphate from baseline to end of treatment/early termination); two patients were excluded owing to a lack of postbaseline serum phosphate measurements. Overall, 115 patients (71%) completed the study (Figure 1). Completion rates were 50%–83% with tenapanor compared with 85% for placebo; completion rates were lowest with tenapanor at 30 mg twice daily (50%) and 30 mg once daily (57%). Reasons for discontinuation from study treatment included adverse events (AEs; n=27; 17%), meeting study withdrawal criteria (n=7; 4%), and patient decision (n=5; 3%).
Figure 1.
Patient flow diagram. b.i.d., Twice daily; q.d., once daily. aSerum phosphate was monitored weekly during washout, and patients were randomized after 1–3 weeks after eligibility criteria were met; bpatients who attended the end of treatment period visit irrespective of treatment duration; cafter the end of treatment or early termination, patients resumed their prestudy phosphate binder medication and returned for a follow-up visit after 1–2 weeks.
Patient demographics and baseline characteristics were well balanced between the groups (Table 1). Overall, the mean (SD) age was 59.1 (13.7) years old, and 104 patients (64%) were men. The mean serum phosphate concentration after washout of phosphate binders was 7.32–7.92 mg/dl in the tenapanor groups and 7.87 mg/dl in the placebo group. Adherence to treatment (assessed by pill count) was generally high (range of means =85%–96%).
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Placebo, n=26 | Tenapanor | |||||
|---|---|---|---|---|---|---|---|
| 1 mg Twice Daily, n=23 | 3 mg Once Daily, n=22 | 3 mg Twice Daily, n=21 | 10 mg Twice Daily, n=23 | 30 mg Once Daily, n=21 | 30 mg Twice Daily, n=26 | ||
| Men, n (%) | 16 (62) | 16 (70) | 12 (55) | 15 (71) | 15 (65) | 13 (62) | 17 (65) |
| Race, n (%) | |||||||
| Native American or Alaskan Native | 2 (8) | 0 | 2 (9) | 1 (5) | 1 (4) | 1 (5) | 1 (4) |
| Asian | 3 (12) | 1 (4) | 1 (5) | 0 | 3 (13) | 0 | 1 (4) |
| Black | 4 (15) | 2 (9) | 6 (27) | 8 (38) | 3 (13) | 3 (14) | 9 (35) |
| White | 17 (65) | 17 (74) | 13 (59) | 12 (57) | 16 (70) | 16 (76) | 15 (58) |
| Other | 0 | 1 (4) | 0 | 0 | 0 | 0 | 0 |
| Ethnicity, n (%) | |||||||
| Hispanic or Latino | 6 (23) | 7 (30) | 8 (36) | 7 (33) | 10 (43) | 6 (29) | 4 (15) |
| Age, yr | 56.1±13.1 | 57.9±14.8 | 57.6±15.8 | 61.5±11.2 | 62.7±12.5 | 58.2±15.8 | 59.7±13.0 |
| Range | 32–77 | 31–82 | 27–81 | 44–89 | 35–81 | 30–90 | 30–88 |
| Body weight, kg | 83.3±18.4 | 85.9±22.7 | 76.6±18.9 | 84.3±19.2 | 84.8±18.9 | 79.6±18.8 | 88.6±24.6 |
| Baseline serum phosphate, mg/dl | 7.87±1.49 | 7.55±1.00 | 7.73±1.28 | 7.32±1.01 | 7.92±1.06 | 7.61±0.85 | 7.76±1.18a |
| Patients taking vitamin D, its analogs, or cinacalcet, n (%)b | 21 (81) | 15 (65) | 19 (86) | 15 (71) | 19 (83) | 18 (86) | 26 (100) |
Data are mean±SD unless otherwise stated.
n=25.
No changes to treatments permitted during study.
Efficacy
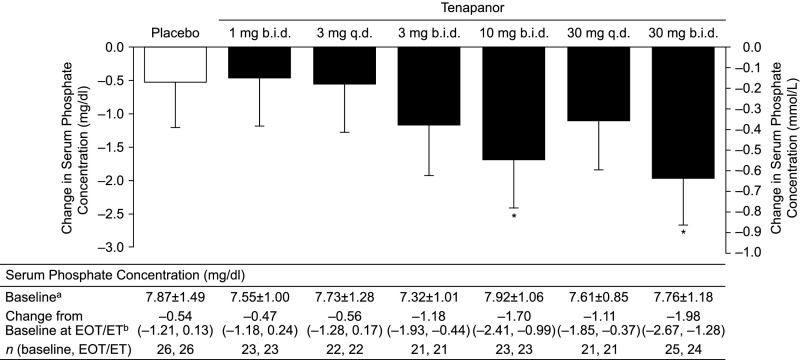
Tenapanor treatment resulted in dose-dependent reductions in serum phosphate at the end of treatment (or early termination), with least squares mean reductions in the range of 0.47–1.98 mg/dl for the tenapanor groups and a least squares mean reduction of 0.54 mg/dl in the placebo group (P=0.01) (Figure 2). The largest reductions were observed in the tenapanor 10- and 30-mg twice daily dosing groups, with a significant difference between each of these groups and placebo (P<0.05) (Figure 2). A dose–response relationship was evident with twice daily dosing of tenapanor.
Figure 2.
Tenapanor treatment resulted in dose-dependent reductions in serum phosphate from baseline to the end of treatment. Graph shows least squares mean changes at end of treatment (EOT)/early termination (ET; i.e., the last available measurement during the treatment period). Error bars show lower limits of 95% CIs. b.i.d., Twice daily; q.d., once daily. *P<0.05 versus placebo (analysis of covariance; t test); amean±SD; bleast squares mean (95% CI): P=0.01 (analysis of covariance; F test).
Serum phosphate concentrations for each group over the course of the study are shown in Supplemental Figure 1. The proportion of patients that reached the predefined serum phosphate goal (<5.5 mg/dl) at the end of treatment/early termination visit was numerically higher with tenapanor (9%–43%) than with placebo (8%) (Supplemental Table 1). The proportion of patients achieving a more stringent serum phosphate target (<4.5 mg/dl post hoc) was 5%–23% with tenapanor, with no patients reaching this target in the placebo group (Supplemental Table 1); the highest rate (23%) was achieved in patients at the highest tenapanor dose (30 mg twice daily).
Other End Points
Mean changes in serum PTH concentrations from baseline did not differ significantly between treatment groups (P=0.31). However, least squares mean changes varied widely across the groups (tenapanor =+29.2 to −71.2 ng/L; placebo =+16.9 ng/L), with large variations observed among patients across all groups (with wide 95% confidence intervals [95% CIs]) (Table 2). There was large variability among groups in geometric mean intact FGF23 concentrations at baseline (Table 2) and at end of treatment; however, tenapanor treatment resulted in significant reductions from baseline to the end of treatment in FGF23 compared with placebo (P<0.05; post hoc analysis of covariance) (Table 2).
Table 2.
Biomarker end points
| Biomarker | Placebo, n=26 | Tenapanor | |||||
|---|---|---|---|---|---|---|---|
| 1 mg Twice Daily, n=23 | 3 mg Once Daily, n=22 | 3 mg Twice Daily, n=21 | 10 mg Twice Daily, n=23 | 30 mg Once Daily, n=21 | 30 mg Twice Daily, n=26 | ||
| Serum PTH | |||||||
| Baseline serum PTH, ng/La | 438.8±288.8 | 392.3±265.4 | 472.0±297.6 | 515.8±321.5 | 435.8±195.5 | 429.3±297.3 | 386.1±277.1 |
| Least squares mean change from baseline at EOT/ET, ng/Lb | 16.9 | 23.6 | 29.2 | −32.9 | −59.1 | −71.2 | −40.9 |
| 95% CI | −53.8 to 87.6 | −50.5 to 97.7 | −46.5 to 104.9 | −111.0 to 45.2 | −131.3 to 13.2 | −150.7 to 8.3 | −113.3 to 31.5 |
| n (Baseline, EOT/ET) | 26, 24 | 23, 22 | 22, 21 | 21, 20 | 23, 23 | 21, 19 | 25, 23 |
| Serum FGF23 | |||||||
| Baseline serum FGF23, pg/mlc | 4937 (206) | 4052 (264) | 3057 (255) | 2601 (231) | 6294 (202) | 5312 (218) | 4491 (347) |
| Ratio of geometric least squares mean between EOT/ET and baselined | 1.22 | 0.91e | 0.89e | 0.76f | 0.72f | 0.73f | 0.81f |
| 95% CI | 1.00 to 1.48 | 0.74 to 1.11 | 0.72 to 1.09 | 0.62 to 0.93 | 0.59 to 0.88 | 0.57 to 0.92 | 0.66 to 0.98 |
| n (Baseline, EOT/ET) | 23, 22 | 21, 19 | 21, 20 | 21, 19 | 20, 22 | 18, 15 | 22, 21 |
EOT, end of treatment; ET, early termination.
Mean±SD.
P=0.31 (analysis of covariance; F test).
Geometric mean (coefficient of variation; %).
Analysis of covariance; post hoc.
P<0.05 for ratio of geometric least squares means (EOT/ET) to placebo (analysis of covariance; t test post hoc).
P<0.01 for ratio of geometric least squares means (EOT/ET) to placebo (analysis of covariance; t test post hoc).
Safety
Overall, 94 patients (58%) experienced at least one AE during the study. The incidence of AEs was similar with placebo (42%) and tenapanor at 1 mg twice daily (43%) and higher with the other tenapanor doses (57%–76%) (Table 3). In all, 14 patients (9%) had at least one serious AE, none of which were considered by the investigator to be treatment related. There was one fatal AE reported: one patient died of cardiac failure 23 days after the last dose of tenapanor. Twenty-eight patients (17%) discontinued the study drug because of AEs, the most frequent of which was diarrhea (n=19).
Table 3.
Summary of AEs
| AE Category | Placebo, n=26 | Tenapanor | |||||
|---|---|---|---|---|---|---|---|
| 1 mg Twice Daily, n=23 | 3 mg Once Daily, n=22 | 3 mg Twice Daily, n=21 | 10 mg Twice Daily, n=23 | 30 mg Once Daily, n=21 | 30 mg Twice Daily, n=25 | ||
| Any AE | 11 (42) | 10 (43) | 13 (59) | 12 (57) | 16 (70) | 13 (62) | 19 (76) |
| Any serious AE | 4 (15) | 2 (9)a | 1 (5) | 2 (10) | 3 (13) | 0 | 2 (8) |
| Fatal serious AE | 0 | 1 (4) | 0 | 0 | 0 | 0 | 0 |
| Any AE leading to discontinuation | 2 (8) | 3 (13) | 1 (5) | 3 (14) | 3 (13) | 7 (33) | 9 (36) |
| Diarrhea AE leading to discontinuation | 0 | 2 (9) | 0 | 2 (10) | 1 (4) | 6 (29) | 8 (32) |
| AEs by system organ classb | |||||||
| Cardiac disorders | 2 (8) | 1 (4) | 0 | 1 (5) | 0 | 1 (5) | 0 |
| Ear and labyrinth disorders | 0 | 0 | 0 | 3 (14) | 0 | 0 | 0 |
| GI disorders | 5 (19) | 7 (30) | 5 (23) | 9 (43) | 15 (65) | 12 (57) | 19 (76) |
| Diarrhea | 3 (12) | 6 (26) | 4 (18) | 6 (29) | 11 (48) | 11 (52) | 17 (68) |
| Nausea | 1 (4) | 0 | 2 (9) | 1 (5) | 1 (4) | 1 (5) | 1 (4) |
| Abdominal pain | 1 (4) | 0 | 1 (5) | 0 | 0 | 0 | 2 (8) |
| Vomiting | 0 | 0 | 1 (5) | 1 (5) | 0 | 2 (10) | 0 |
| Fecal incontinence | 0 | 0 | 0 | 1 (5) | 2 (9) | 0 | 0 |
| General disorders and administration site conditions | 0 | 2 (9) | 2 (9) | 0 | 0 | 0 | 2 (8) |
| Infections and infestations | 3 (12) | 0 | 2 (9) | 1 (5) | 1 (4) | 1 (5) | 0 |
| Injury, poisoning, and procedural complications | 0 | 2 (9) | 1 (5) | 2 (10) | 1 (4) | 0 | 2 (8) |
| Metabolism and nutrition disorders | 2 (8) | 1 (4) | 1 (5) | 1 (5) | 2 (9) | 1 (5) | 1 (4) |
| Musculoskeletal and connective tissue disorders | 2 (8) | 0 | 0 | 1 (5) | 0 | 2 (10) | 2 (8) |
| Nervous system disorders | 0 | 1 (4) | 2 (9) | 1 (5) | 1 (4) | 3 (14) | 2 (8) |
| Psychiatric disorders | 2 (8) | 0 | 1 (5) | 0 | 0 | 0 | 2 (8) |
| Skin and subcutaneous tissue disorders | 1 (4) | 0 | 2 (9) | 0 | 1 (4) | 0 | 0 |
| Vascular disorders | 2 (8) | 2 (9) | 0 | 2 (10) | 0 | 1 (5) | 1 (4) |
Values are numbers (%). AE, adverse event.
Includes one patient with a fatal serious AE.
Data shown for system organ classes (and preferred terms for GI disorders) in which two or more patients in any group experienced an AE.
GI-related AEs were the most common AEs during the study, and they were reported by 23%–76% of patients receiving tenapanor and 19% of those receiving placebo (Table 3). Other types of AEs were uncommon, and the AE profile of tenapanor was similar to that of placebo, with the exception of the higher incidence of GI-related AEs. Diarrhea was the most frequently experienced AE, occurring in 55 patients (41%) receiving tenapanor and three patients (12%) receiving placebo. For the majority of patients, diarrhea was categorized as mild or moderate. Diarrhea categorized as severe occurred predominantly in patients receiving tenapanor doses of 30 mg once or twice daily (ten of 14).
No clinically relevant treatment-related changes in serum calcium, potassium, or sodium were observed (Table 4), and no consistent changes in vital signs related to dose or active treatment were observed.
Table 4.
Serum electrolytes
| Electrolyte | Placebo, n=26 | Tenapanor | |||||
|---|---|---|---|---|---|---|---|
| 1 mg Twice Daily, n=23 | 3 mg Once Daily, n=22 | 3 mg Twice Daily, n=21 | 10 mg Twice Daily, n=23 | 30 mg Once Daily, n=21 | 30 mg Twice Daily, n=25 | ||
| Calcium | |||||||
| Baseline, mg/dl | 8.90±0.77 | 8.57±0.65 | 8.80±0.52 | 8.81±0.72 | 8.88±0.62 | 8.64±0.62 | 8.90±0.53 |
| Change from baseline, mg/dl | 0.26±0.59 | −0.03±0.46 | 0.12±0.53 | −0.16±0.69 | 0.27±0.47 | 0.09±0.44 | 0.15±0.84 |
| n (Baseline, EOT/ET) | 26, 24 | 23, 22 | 22, 21 | 21, 20 | 23, 20 | 21, 20 | 25, 23 |
| Potassium | |||||||
| Baseline, mEq/L | 5.07±0.78 | 5.13±0.72 | 5.14±0.54 | 5.19±0.72 | 5.22±0.68 | 5.00±0.41 | 5.15±0.75 |
| Change from baseline, mEq/L | −0.01±0.68 | 0.13±0.46 | −0.01±0.60 | −0.02±0.77 | 0.06±0.70 | −0.03±0.44 | −0.09±0.61 |
| n (Baseline, EOT/ET) | 26, 24 | 23, 21 | 22, 21 | 20, 19 | 23, 19 | 19, 16 | 25, 23 |
| Sodium | |||||||
| Baseline, mEq/L | 139.7±2.99 | 140.2±2.74 | 139.2±3.21 | 139.7±3.47 | 139.0±2.80 | 138.6±3.41 | 139.2±2.55 |
| Change from baseline, mEq/L | 0.8±2.97 | 0.0±3.08 | 0.2±2.89 | 1.0±2.98 | −1.2±2.72 | 0.5±3.09 | 0.0±2.28 |
| n (Baseline, EOT/ET) | 26, 24 | 23, 22 | 22, 21 | 21, 20 | 23, 20 | 21, 20 | 25, 23 |
| Bicarbonate | |||||||
| Baseline, mEq/L | 21.1±2.32 | 21.3±2.67 | 21.5±2.34 | 21.6±2.58 | 21.3±2.24 | 21.5±2.11 | 21.6±2.80 |
| Change from baseline, mEq/L | −1.0±2.85 | −1.0±2.24 | −1.0±3.13 | −0.6±2.32 | −1.0±2.68 | −0.6±3.49 | −1.0±2.56 |
| n (Baseline, EOT/ET) | 26, 24 | 23, 22 | 22, 21 | 21, 19 | 23, 20 | 21, 20 | 25, 23 |
Data are shown as mean±SD. EOT, end of treatment; ET, early termination.
Discussion
This fixed dose, placebo-controlled, short-term randomized trial evaluated the effect of tenapanor, a small molecule inhibitor of NHE3 taken once or twice daily, on serum phosphate concentrations in patients with hyperphosphatemia receiving maintenance hemodialysis. We show that treatment with tenapanor yielded statistically significant, dose-dependent, and clinically relevant reductions in serum phosphate. Despite the short duration of the study and the use of fixed doses without titration, a higher proportion of patients treated with tenapanor reached a target serum phosphate of <5.5 mg/dl compared with those treated with placebo.
Current treatment for hyperphosphatemia in patients with advanced CKD relies on dietary phosphate restriction and oral phosphate binders.9 Calcium-based phosphate binders (calcium carbonate and calcium acetate) are widely used, although recent years have seen increased use of the noncalcium-based agents: sevelamer carbonate, lanthanum carbonate, sucroferric oxyhydroxide, and ferric citrate, all of which were developed in part because of concerns about positive calcium balance, hypercalcemia, and the risk of vascular calcification with calcium salts.27 Studies show that all phosphate binders are only modestly effective in reducing serum phosphate concentrations in patients with CKD, although some seem to be slightly more potent than others.28 In a placebo-controlled trial in patients on dialysis, 2 weeks of treatment with sevelamer hydrochloride (mean =5.8 g/d) resulted in reductions in serum phosphate of 1.2 mg/dl compared with an increase in serum phosphate of 0.2 mg/dl in patients receiving placebo.16 In studies comparing sevelamer with other commonly used phosphate binders but without placebo groups included, mean reductions in serum phosphate of 1.4 and 1.7 mg/dl were observed after 4 weeks of treatment with sevelamer hydrochloride (4.8–6.4 g/d) and lanthanum carbonate (2.25–3 g/d), respectively,22 whereas reductions of 2.0 and 2.1 mg/dl were achieved when sevelamer hydrochloride (mean =3.4–4.9 g/d) and calcium acetate (mean =3.4–5.0 g/d), respectively, were each administered over 8 weeks.15 The largest reductions in serum phosphate after 4 weeks of treatment with fixed doses of tenapanor in our study (1.70 and 1.98 mg/dl for tenapanor at 10 mg twice daily and 30 mg twice daily, respectively) are of similar magnitude to those achieved in trials of commonly used phosphate binders where individual dose titration was allowed.15,16,22
A large proportion of patients with advanced CKD treated with phosphate binders does not achieve the targets for serum phosphate directed by clinical practice guidelines.9,29,30 One potential explanation for this is that the passive binding of phosphate to cations in the intestinal lumen has limited ability to affect net phosphate transport. The relative contribution of active phosphate transport to net phosphate absorption in humans is unclear, and phosphate transport may be affected by multiple mechanisms, including active phosphate transport mediated by NPT2b, binding of phosphate by cations in the intestinal lumen, or modifying passive paracellular phosphate transport. Recent data show that nicotinamide can reduce serum phosphate concentrations in persons with normal or near-normal kidney function and patients with CKD by reducing the intestinal absorption of phosphate,31 a process thought to be related to inhibition of intestinal NPT2b expression.32 However, frequent AEs seen in clinical trials with nicotinamide could lessen enthusiasm for this approach.
Tenapanor is not a phosphate binder but rather, a small molecule inhibitor of the sodium/hydrogen exchanger NHE3, which plays an important role in sodium and fluid homeostasis. However, tenapanor is able to effectively reduce serum phosphate concentration, despite having no direct effect on NPT2b23,24 and without directly binding intestinal phosphate. Studies in healthy volunteers and patients with CKD stage 5D have shown that tenapanor treatment increased stool sodium23,25,26 and phosphorus26 content, and concomitantly, it reduced urinary sodium23,26 and phosphorus content,26 consistent with reduced sodium and phosphate absorption. The precise mechanism by which tenapanor reduces intestinal phosphate absorption is currently under investigation and may allow for a multifaceted approach to reducing phosphate absorption in the future.
Poor treatment adherence with phosphate binders is a concern, with studies reporting nonadherence rates of 22%–74%.33 The high pill burden associated with these agents is one factor that likely affects adherence. Tablets must be taken with each meal, and the overall number required each day can be large (e.g., 6–12 tablets daily for calcium acetate and sevelamer, with total daily doses of up to 8 and 9.6 g, respectively).3 A study among 233 patients on maintenance hemodialysis in the United States showed that patients took an average of 11 medications per day, with a median daily pill count of 19.12 On average, phosphate binders accounted for approximately one half of the total pill burden. Adherence with phosphate binders was low (38%) and decreased with higher pill count; a higher pill burden was also associated with lower health-related quality of life.12 Furthermore, a recent report describing data from the Dialysis Outcomes and Practice Patterns Study of 5262 patients on hemodialysis showed a trend toward greater nonadherence to phosphate binders and a higher number of prescribed phosphate binder pills per day. Nonadherence to phosphate binder prescription was associated with serum phosphate concentrations exceeding 5.5 mg/dl and serum PTH concentrations exceeding 600 pg/ml.30 Studies have also reported improved satisfaction among patients with CKD stage 5 when switched from their existing phosphate binder to one with a lower pill burden.34,35 Tenapanor, administered once or twice daily in milligram quantities, may provide a means to reduce pill burden and mass in patients with hyperphosphatemia, thereby improving adherence to treatment. As is the case with phosphate binders, patients took the study drug (tenapanor or placebo) with meals in our study. In a three-way crossover trial in healthy volunteers, the effects of tenapanor on phosphate absorption were slightly greater when tenapanor was administered before meals compared with in a fasting state, suggesting that tenapanor should be administered with meals to maximize effect. However, there was no significant difference in phosphate absorption when tenapanor was administered shortly before or after meals, which may support flexibility in the timing of administration of tenapanor with regard to food consumption.36
In our study, GI-related AEs, particularly diarrhea, were the most common AEs associated with tenapanor treatment, with GI-related AEs reported by 23%–76% of patients receiving tenapanor and 19% of patients on placebo. Similar rates of GI-related AEs are reported with phosphate binders.3 In a recent trial in 1059 patients on dialysis comparing the iron-based phosphate binder PA21 (sucroferric oxyhydroxide) with sevelamer over 24 weeks, GI-related AEs were reported in 45% of patients receiving PA21 compared with 34% of patients receiving sevelamer.17 Diarrhea as a common AE in our study is consistent with the mechanism of action of tenapanor, which increases stool sodium and water content, and it is in line with changes seen in studies in healthy volunteers, in which tenapanor increased stool frequency and affected stool consistency.23,26 Relevant to an understanding of the net clinical effect observed with tenapanor is recognition that GI symptoms are quite common in patients on hemodialysis. Colonic transit time is significantly prolonged in patients on hemodialysis,37 and constipation has been reported in 12%–70% of patients on dialysis depending on the population and constipation definition used.38–41 Therefore, in addition to serum phosphate lowering, the pharmacodynamic effects of tenapanor on increasing stool frequency and softening stool consistency might be beneficial to a substantial proportion of patients on dialysis and should be evaluated in future clinical trials. It will thus be important to determine whether the effect of tenapanor on stool consistency observed in our short-term study persists or reduces over longer treatment exposure and with individually optimized dosing. It will also be important to investigate any potential drug–drug interactions with tenapanor. Two studies in healthy volunteers have investigated whether there is an effect on the absorption of midazolam42 or cefadroxil43 when coadministered with tenapanor, whereas another healthy volunteer trial showed that the pharmacodynamic effects of tenapanor were not affected when coadministered with sevelamer.44
Our study has a number of limitations. It was of short duration, with patients receiving only 4 weeks of treatment, making it difficult to draw conclusions about the longer-term effects that tenapanor may have on dialysis-related outcomes. Furthermore, patients received a fixed treatment dose, with no dose titration. Although the overall sample size was relatively large, the number of patients per group was modest, and there were some slight imbalances in demographic characteristics. There was also a higher proportion of men than women in all treatment groups. These factors may reduce the precision in determining dose-specific safety and efficacy in the entire dialysis population. Another double-blind, randomized, three-arm registration study (ClinicalTrials.gov identifier NCT02675998) in patients with hyperphosphatemia in CKD stage 5D is investigating the effect of tenapanor for 8 weeks: two treatment arms will receive tenapanor at fixed doses of either 3 or 10 mg twice daily, whereas the third arm will follow a weekly downtitration scheme according to GI tolerability, with a starting dose of 30 mg twice daily.45
In conclusion, treatment with once or twice daily tenapanor, an oral, minimally systemic, intraluminal inhibitor of NHE3, resulted in statistically significant reductions in serum phosphate in patients with hyperphosphatemia receiving hemodialysis. Significant reductions in serum FGF23 concentrations were also observed. The ability to manage hyperphosphatemia with a single pill given once or twice daily offers great potential to improve the management of CKD-related bone mineral disorders. Additional trials are needed to clarify the optimal dosing of tenapanor in the management of hyperphosphatemia and explore the potential effects of tenapanor on intermediate, nonlaboratory-based end points.
Concise Methods
Study Design
This randomized, double-blind, placebo-controlled, multicenter study (EudraCT no. 2013–004319–33; ClinicalTrials.gov identifier NCT02081534) was conducted at 47 centers in the United States, Poland, Slovakia, and the United Kingdom. The study was performed in accordance with the Declaration of Helsinki as well as International Conference on Harmonization and Good Clinical Practice guidelines. The study protocol and its amendments were approved by an independent ethics committee or institutional review board. All study participants provided written informed consent before undergoing any study procedure.
After screening, eligible patients underwent a 1- to 3-week washout of phosphate binders. We assessed patients weekly during washout to assess eligibility for randomization (see below). We randomized patients after they met eligibility criteria (i.e., there was no requirement to complete all 3 weeks of washout); patients who did not meet eligibility criteria after 3 weeks of washout were excluded from additional participation.
We randomly assigned patients to one of six tenapanor regimens (3 or 30 mg once daily or 1, 3, 10, or 30 mg twice daily) or placebo for 4 weeks (Figure 1). Tenapanor was formulated as round 9-mm-diameter plain white film–coated tablets irrespective of dose. To ensure blinding, all patients took one tablet in the morning and one in the evening just before breakfast and dinner, respectively. No dose adjustments were permitted. At the end of the treatment period, patients resumed their prestudy dose of phosphate binders and underwent a follow-up visit 1–2 weeks after the last dose of study drug. Separate balanced randomization schemes were generated for the United States and the European Union regions using a computer-based random number generator.
Patients were withdrawn from study treatment if they met any of the following criteria: hypophosphatemia (serum phosphate ≤2.5 mg/dl at any time), severe hyperphosphatemia (serum phosphate ≥10.0 mg/dl at week 2 postrandomization or later), metabolic acidosis (serum bicarbonate <14 mmol/L), hyponatremia (sodium <125 mmol/L in two consecutive predialysis samples), or hypotension.
Patients were not permitted to use antacids during the treatment period. Changes in other concomitant medication were avoided during study participation. However, medication considered necessary for patients’ safety and wellbeing was allowed at the discretion of the investigator.
Patients
Adults (age ≥18 years old) with CKD stage 5D and hyperphosphatemia were eligible for participation. For inclusion at screening, patients had to be receiving maintenance hemodialysis three times per week for at least 3 months; show evidence of dialysis adequacy defined as Kt/V of at least 1.2 or a urea removal rate of at least 65% for the last month; be prescribed a stable daily dose of phosphate binder (defined as ≥800 mg sevelamer, ≥250 mg calcium carbonate/acetate, or ≥500 mg lanthanum over the past 3 weeks); and have serum phosphate =3.5–8.0 mg/dl, normal serum calcium, and no evidence of severe hyperphosphatemia (≥10 mg/dl) in the previous 3 months. Women of childbearing potential were required to have a negative pregnancy test at screening and randomization and not to be lactating and had to agree to use effective methods of contraception during the study period.
After the 1- to 3-week washout of phosphate binders, to be eligible for randomization, patients had to have a serum phosphate level of at least 6.0 mg/dl but <10 mg/dl and show an increase of at least 1.5 mg/dl from screening.
Key exclusion criteria were inflammatory bowel disease, GI bleeding or other GI disease, serum PTH exceeding 1200 pg/ml (on the basis of the last available value before screening), severe metabolic acidosis (serum bicarbonate <18 mmol/L) at the last visit before randomization, and clinical signs of hypovolemia at randomization.
Study Assessments
Patients underwent visits at weekly intervals throughout the study period, with serum phosphate monitoring at each visit. All study visits occurred (including blood sampling) after a short dialysis interval (≤48 hours), and all assessments carried out at the visits were performed before dialysis. Adherence to medication was assessed by pill count determined as (pills dispensed − pills returned)/(expected number of pills taken), where the denominator was the (date of last dose − date of first dose +1) × (expected number of pills per day).
The primary efficacy end point was the change in serum phosphate concentration from baseline (randomization) to the end of treatment or early termination (i.e., last available measurement during treatment). Other efficacy measures included changes in serum PTH, calcium, and calcium × phosphate product from baseline to the end of treatment/early termination. Safety assessments included physical examination, 12-lead electrocardiogram, measurement of BP and heart rate, and clinical laboratory evaluations (clinical chemistry and hematology). AEs were assessed at all study visits.
Statistical Analyses
The safety analysis set included all patients who received at least one dose of randomized treatment and had postdose data. Efficacy analyses were performed on the full analysis set, which included all randomized patients (intention to treat). For the primary efficacy measure, we used an analysis of covariance model, with treatment group as a fixed factor and baseline serum phosphate as a covariate. An F test was used to evaluate the null hypothesis of equal means at the 5% significance level followed by pairwise comparisons between each tenapanor treatment group and placebo using a pairwise t test. Least squares mean estimates of change from baseline in serum phosphate and associated 95% CIs were determined for each treatment group. We undertook a similar approach in analyzing changes in FGF23 and PTH, except that the log-transformed FGF23 data were analyzed (conducted post hoc) due to highly skewed data on the original scale; therefore, the ratio of least squares geometric means between the end of treatment (or early termination) and baseline with associated 95% CI was reported for each treatment group.
Assuming a placebo-corrected reduction in serum phosphate of 2.0 mg/dl after tenapanor at 30 mg twice daily and an SD of 2.0 mg/dl, a sample size of 23 patients in each group would have 90% power for a pairwise comparison between tenapanor at 30 mg twice daily and placebo using a one-sided test at the 2.5% significance level. The study, therefore, aimed to randomize 25 patients to each of the tenapanor at 30 mg twice daily and placebo groups plus 20 patients to each of the other five groups.
All statistical analyses were conducted with SAS (version 9.1.3 or higher; SAS Institute, Inc., Cary, NC).
Disclosures
G.A.B. serves as a consultant to Ardelyx Inc. (Fremont, CA), and he and his practice have received equity ownership interest in Ardelyx Inc. D.P.R. is an employee of and has ownership interests in Ardelyx Inc. M.L.-Z., M.Å., M.K., and A.M.L. are employees of AstraZeneca Gothenburg (Mölndal, Sweden). S.J. is an employee of and has ownership interests in AstraZeneca Gothenburg. G.M.C. is a consultant to and has received equity ownership interest in Ardelyx Inc.
Supplementary Material
Acknowledgments
We thank Andrew Yan of Ardelyx Inc. (Fremont, CA) for critical review of statistical content. Medical writing support was provided by Steven Inglis of PharmaGenesis London (London, United Kingdom) and funded by AstraZeneca Gothenburg (Mölndal, Sweden) and Ardelyx Inc.
This study was funded by AstraZeneca Gothenburg.
Data in this manuscript have been presented in part at the American Society of Nephrology Kidney Week, San Diego, California, November 3–8, 2015 (Block et al., J Am Soc Nephrol 26: 28A [TH-OR114], 2015).
The authors take full responsibility for the content of the manuscript. G.A.B. and G.M.C. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016080855/-/DCSupplemental.
References
- 1.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Hruska KA, Mathew S, Lund R, Qiu P, Pratt R: Hyperphosphatemia of chronic kidney disease. Kidney Int 74: 148–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonelli M, Pannu N, Manns B: Oral phosphate binders in patients with kidney failure. N Engl J Med 362: 1312–1324, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler DC; ARO Investigators : Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 26: 1948–1955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayus JC, Mizani MR, Achinger SG, Thadhani R, Go AS, Lee S: Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: A prospective, controlled study. J Am Soc Nephrol 16: 2778–2788, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Burke SK, Raggi P; Treat to Goal Working Group : Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Cannata-Andía JB, Fernández-Martín JL, Locatelli F, London G, Gorriz JL, Floege J, Ketteler M, Ferreira A, Covic A, Rutkowski B, Memmos D, Bos WJ, Teplan V, Nagy J, Tielemans C, Verbeelen D, Goldsmith D, Kramar R, Martin PY, Wüthrich RP, Pavlovic D, Benedik M, Sánchez JE, Martínez-Camblor P, Naves-Díaz M, Carrero JJ, Zoccali C: Use of phosphate-binding agents is associated with a lower risk of mortality. Kidney Int 84: 998–1008, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Sullivan C, Sayre SS, Leon JB, Machekano R, Love TE, Porter D, Marbury M, Sehgal AR: Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: A randomized controlled trial. JAMA 301: 629–635, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R: Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 4: 1089–1096, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, Lok CE, Fitchett D, Tsuyuki RT: Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: An updated systematic review and meta-analysis. Lancet 382: 1268–1277, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Patel L, Bernard LM, Elder GJ: Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: A meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 11: 232–244, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleyer AJ, Burke SK, Dillon M, Garrett B, Kant KS, Lynch D, Rahman SN, Schoenfeld P, Teitelbaum I, Zeig S, Slatopolsky E: A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis 33: 694–701, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Chertow GM, Burke SK, Lazarus JM, Stenzel KH, Wombolt D, Goldberg D, Bonventre JV, Slatopolsky E: Poly[allylamine hydrochloride] (RenaGel): A noncalcemic phosphate binder for the treatment of hyperphosphatemia in chronic renal failure. Am J Kidney Dis 29: 66–71, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Floege J, Covic AC, Ketteler M, Rastogi A, Chong EM, Gaillard S, Lisk LJ, Sprague SM; PA21 Study Group : A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 86: 638–647, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg DI, Dillon MA, Slatopolsky EA, Garrett B, Gray JR, Marbury T, Weinberg M, Wombolt D, Burke SK: Effect of RenaGel, a non-absorbed, calcium- and aluminium-free phosphate binder, on serum phosphorus, calcium, and intact parathyroid hormone in end-stage renal disease patients. Nephrol Dial Transplant 13: 2303–2310, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Joy MS, Finn WF; LAM-302 Study Group : Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: A new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis 42: 96–107, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Lewis JB, Sika M, Koury MJ, Chuang P, Schulman G, Smith MT, Whittier FC, Linfert DR, Galphin CM, Athreya BP, Nossuli AK, Chang IJ, Blumenthal SS, Manley J, Zeig S, Kant KS, Olivero JJ, Greene T, Dwyer JP; Collaborative Study Group : Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 26: 493–503, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qunibi WY, Hootkins RE, McDowell LL, Meyer MS, Simon M, Garza RO, Pelham RW, Cleveland MV, Muenz LR, He DY, Nolan CR: Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study). Kidney Int 65: 1914–1926, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Sprague SM, Ross EA, Nath SD, Zhang P, Pratt RD, Krause R: Lanthanum carbonate vs. sevelamer hydrochloride for the reduction of serum phosphorus in hemodialysis patients: A crossover study. Clin Nephrol 72: 252–258, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, Bell N, Tabora J, Joly KM, Navre M, Jacobs JW, Charmot D: Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 6: 227ra36, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Labonté ED, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, Dy E, Black D, Zhong Z, Langsetmo I, Spencer AG, Bell N, Deshpande D, Navre M, Lewis JG, Jacobs JW, Charmot D: Gastrointestinal inhibition of sodium–hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 26: 1138–1149, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Block GA, Rosenbaum DP, Leonsson-Zachrisson M, Stefansson BV, Rydén-Bergsten T, Greasley PJ, Johansson SA, Knutsson M, Carlsson BC: Effect of tenapanor on interdialytic weight gain in patients on hemodialysis. Clin J Am Soc Nephrol 11: 1597–1605, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson S, Rosenbaum DP, Knutsson M, Leonsson-Zachrisson M: A phase 1 study of the safety, tolerability, pharmacodynamics, and pharmacokinetics of tenapanor in healthy Japanese volunteers [published online ahead of print July 1, 2016]. Clin Exp Nephrol doi:10.1007/s10157-016-1302-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ketteler M, Wüthrich RP, Floege J: Management of hyperphosphataemia in chronic kidney disease-challenges and solutions. Clin Kidney J 6: 128–136, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navaneethan SD, Palmer SC, Craig JC, Elder GJ, Strippoli GF: Benefits and harms of phosphate binders in CKD: A systematic review of randomized controlled trials. Am J Kidney Dis 54: 619–637, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Farrand KF, Copley JB, Heise J, Fridman M, Keith MS, Poole L: Analysis of serum phosphate control and phosphate binder utilization in incident hemodialysis patients. Int J Nephrol Renovasc Dis 7: 261–269, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fissell RB, Karaboyas A, Bieber BA, Sen A, Li Y, Lopes AA, Akiba T, Bommer J, Ethier J, Jadoul M, Pisoni RL, Robinson BM, Tentori F: Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: Findings from the DOPPS. Hemodial Int 20: 38–49, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenglet A, Liabeuf S, El Esper N, Brisset S, Mansour J, Lemaire-Hurtel AS, Mary A, Brazier M, Kamel S, Mentaverri R, Choukroun G, Fournier A, Massy ZA: Efficacy and safety of nicotinamide in haemodialysis patients: The NICOREN study [published online ahead of print May 4, 2016]. Nephrol Dial Transplant doi:10.1093/ndt/gfw1042 [DOI] [PubMed] [Google Scholar]
- 32.Eto N, Miyata Y, Ohno H, Yamashita T: Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant 20: 1378–1384, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Karamanidou C, Clatworthy J, Weinman J, Horne R: A systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end-stage renal disease. BMC Nephrol 9: 2, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehrotra R, Martin KJ, Fishbane S, Sprague SM, Zeig S, Anger M; Fosrenol Overview Research Evaluation Study for Early Experience Study Group : Higher strength lanthanum carbonate provides serum phosphorus control with a low tablet burden and is preferred by patients and physicians: A multicenter study. Clin J Am Soc Nephrol 3: 1437–1445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vemuri N, Michelis MF, Matalon A: Conversion to lanthanum carbonate monotherapy effectively controls serum phosphorus with a reduced tablet burden: A multicenter open-label study. BMC Nephrol 12: 49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbaum DP, Knutsson M, Leonsson-Zachrisson M, Johansson S: The pharmacodynamic effect of tenapanor is most pronounced when administered before food in healthy volunteers [Abstract]. J Am Soc Nephrol 26: 749A–750A, 2015 [Google Scholar]
- 37.Wu MJ, Chang CS, Cheng CH, Chen CH, Lee WC, Hsu YH, Shu KH, Tang MJ: Colonic transit time in long-term dialysis patients. Am J Kidney Dis 44: 322–327, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Murtagh FE, Addington-Hall J, Higginson IJ: The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis 14: 82–99, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Yasuda G, Shibata K, Takizawa T, Ikeda Y, Tokita Y, Umemura S, Tochikubo O: Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis 39: 1292–1299, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Odaka K, Inamoto H, Sata K, Kunitou K, Wada T, Saruta T: Constipation and dietary fiber in dialysis patients 2. Incidence and causative factors of constipation (in Japanese). J Jpn Soc Dial Ther 22: 995–998, 1989 [Google Scholar]
- 41.Lee A, Lambert K, Byrne P, Lonergan M: Prevalence of constipation in patients with advanced kidney disease. J Ren Care 42: 144–149, 2016 [DOI] [PubMed] [Google Scholar]
- 42.ClinicalTrials.gov: Ardelyx: A Phase 1, Single-Center, Fixed-Sequence, Open Label Study to Evaluate the Effect of Oral Repeated Doses of AZD1722 on the Pharmacokinetics of Oral Midazolam in Healthy Volunteers. ClinicalTrials.gov Identifier: NCT02140268, 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT02140268. Accessed November 9, 2016
- 43.ClinicalTrials.gov: Ardelyx: A Phase 1, Single-Center, Randomized, 2-Way Cross-Over, Open-Label Study to Evaluate the Effect of Oral Repeated Doses of AZD1722 on the Pharmacokinetics of Oral Cefadroxil in Healthy Volunteers. ClinicalTrials.gov Identifier: NCT02140281, 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT02140281. Accessed November 9, 2016
- 44.Johansson S, Leonsson-Zachrisson M, Knutsson M, Spencer AG, Labonte ED, Deshpande D, Kohler J, Kozuka K, Charmot D, Rosenbaum DP: Preclinical and healthy volunteer studies of potential drug-drug interactions between tenapanor and phosphate binders [published online ahead of print September 22, 2016]. Clin Pharmacol Drug Dev doi:10.1002/cpdd.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ClinicalTrials.gov: Ardelyx: An 8-Week, Multicenter, Randomized, Double-Blind, Parallel Group Study with a 4-Week, Placebo-Controlled, Randomized Withdrawal Period to Evaluate the Efficacy, Safety, and Tolerability of Tenapanor to Treat Hyperphosphatemia in End-Stage Renal Disease Patients on Hemodialysis (ESRD-HD). ClinicalTrials.gov Identifier: NCT02675998, 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT02675998. Accessed April 19, 2016
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.