Abstract
Vancomycin is a widely prescribed antibiotic, but the exact nature of vancomycin-associated nephrotoxicity is unclear, in particular when considering the frequent coadministration of aminoglycosides. We describe here the initial case of a 56-year-old woman with normal renal function developing unexplained ARF without hypovolemia after administration of vancomycin without coadministration of aminoglycosides. Studying the patient’s renal biopsy specimen, we ascertained that obstructive tubular casts composed of noncrystal nanospheric vancomycin aggregates entangled with uromodulin explained the vancomycin-associated ARF. We developed in parallel a new immunohistologic staining technique to detect vancomycin in renal tissue and confirmed retrospectively that deleterious vancomycin-associated casts existed in eight additional patients with acute tubular necrosis in the absence of hypovolemia. Concomitant high vancomycin trough plasma levels had been observed in each patient. We also reproduced experimentally the toxic and obstructive nature of vancomycin-associated cast nephropathy in mice, which we detected using different in vivo imaging techniques. In conclusion, the interaction of uromodulin with nanospheric vancomycin aggregates represents a new mode of tubular cast formation, revealing the hitherto unsuspected mechanism of vancomycin-associated renal injury.
Keywords: vancomycin, acute renal failure, tubular epithelium, acute tubular necrosis, tubular cast
Discovered in the 1950s, vancomycin is an old and widely prescribed drug, mostly excreted via the kidneys.1 Adverse effects of vancomycin, including nephrotoxicity, were initially imputed to the so-called “Mississippi mud,”2 a contaminant from the original formulations.3 More recently and despite the purified forms now in use, the renal toxicity of vancomycin has been suspected mostly in the clinical context of ARF, which promotes its accumulation in the blood. Given that vancomycin nephrotoxicity occurs at the same time as additional well characterized renal insults (e.g., hypovolemia and coadministration of aminoglycosides among others), evidence for vancomycin nephrotoxicity has been circumstantial and sometimes hotly debated, despite ARF having been observed in 5%–25% of patients.4
We report here a previously unsuspected mechanism of human vancomycin nephrotoxicity suggested by the study of nine patients. By also using different mouse models, we confirmed this mechanism of renal insult. We showed that rogue intratubular vancomycin noncrystal nanosphere–associated casts entangled with uromodulin explain the development of ARF and therefore, vancomycin nephrotoxicity.
A 56-year-old woman was referred to our department for ARF. The patient’s medical history included diabetes mellitus and gastric bypass surgery for morbid obesity 2 years previously. The patient was finally diagnosed with type 2 acute myelogenous leukemia. Serum electrolytes, creatinine, and BUN were normal. Serum electrophoresis and immunofixation ruled out any superimposed monoclonal gammopathy. Immediate induction chemotherapy was started with intravenous infusions of daunorubicin, cytarabin, and gemtuzumab.
Ten days later, the patient developed pancytopenia together with fever but without any hemodynamic instability. Caspofungin and intravenous broad spectrum antibiotics, including piperacillin-tazobactam at 4 g four times a day, were initiated together with a vancomycin pulse of 1.5 g followed by a 3-g daily continuous vancomycin perfusion. Three days later, fever decreased, but BP increased to 160/100 mmHg. Blood analysis showed a serum creatinine increase from 0.47 at baseline to 4.23 mg/dl, confirming ARF, whereas urine analysis did not detect proteinuria or hematuria. Seventy-two hours after the first vancomycin infusion, its concentration was still measured at 87 mg/L (expected value =15–20 mg/L) (Figure 1A). At that time, vancomycin nephrotoxicity was suspected, and the drug was immediately discontinued, whereas piperacillin-tazobactam (Tazocilin) and caspofungin were maintained for an additional 10 days. At day 17, because renal failure persisted, a kidney biopsy was performed to investigate the mechanism of renal impairment and ascertain renal prognosis. Four months later, the patient eventually recovered normal renal function (serum creatinine =0.73 mg/dl).
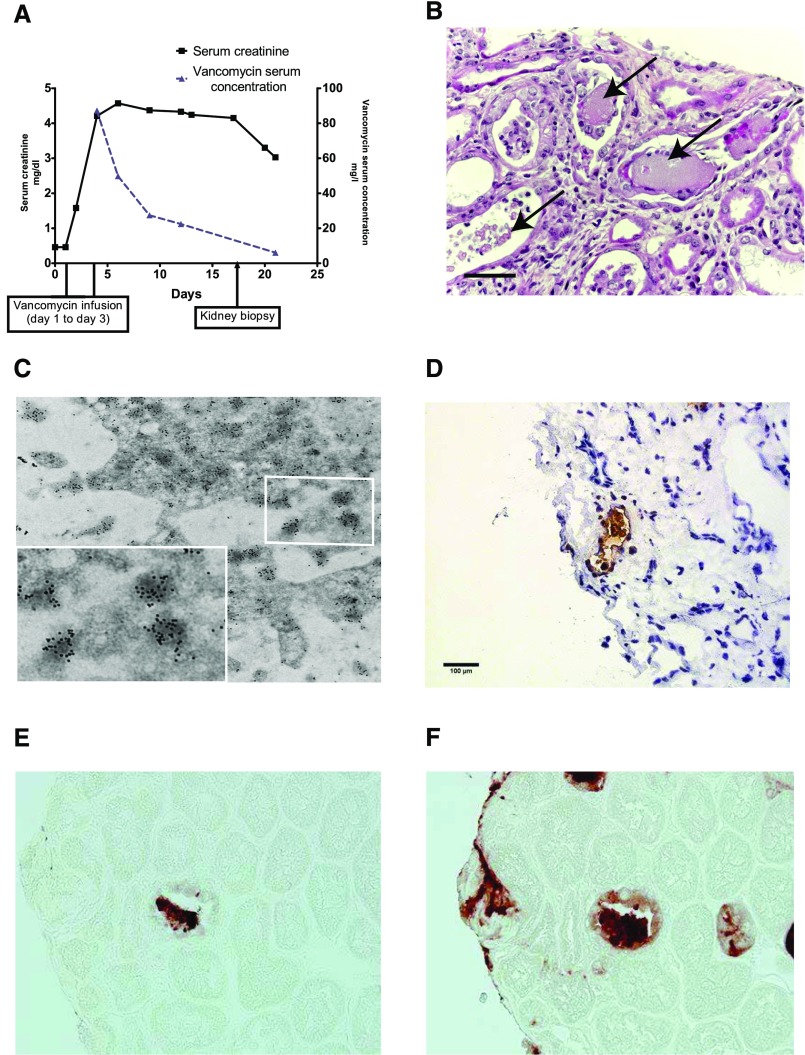
Figure 1.
ARF associated with nanospheric vancomycin tubular casts entangled with uromodulin. (A) Graph showing the evolution of the patient’s renal function with vancomycin plasma concentrations over time. (B) Human kidney biopsy (17 days after vancomycin injection) analysis revealed nonspecific granular tubular and proteinaceous cast formation (arrows) and ATN lesions (hematoxylin and eosin staining). Scale bar, 50 μm. (C) Transmission electron microscopy analysis with immunogold labeling detecting vancomycin nanospheres in a tubular cast formation (original, magnification, ×18,500), with detail on nanospheric vancomycin (labeled with 20-nm gold particles). (D) Staining with antivancomycin antibody (frozen section) revealed the specific accumulation of vancomycin in the tubular lumen. Note the absence of vancomycin in the surrounding tissue. Original magnification, ×400. (E) Serial biopsy (3-μm thick) showing one cast colocalizing vancomycin and (F) uromodulin showing the obstructive nature of vancomycin-associated casts. Horseradish peroxidase staining. Original magnification, ×600.
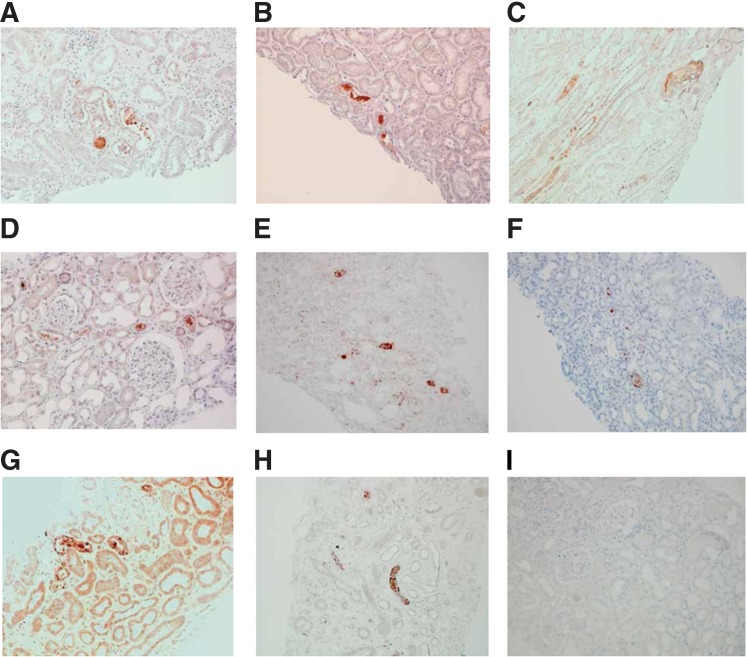
Histologic analysis showed the absence of immune deposits or glomerular injury but severe acute tubular necrosis (ATN). Some tubular lumens contained nonspecific proteinaceous casts on light microscopy (Figure 1B). These casts were initially described as “light-voided black ovoid structures or atypical proteinaceous casts.” These original formations did not show any birefringence fringe after polarization. We eventually examined the composition of these casts by scanning electron microscopy (Supplemental Figure 1, B, D, and F) and infrared microspectroscopy (Supplemental Figure 1, A, C, and E), suspecting that vancomycin might be associated with cast genesis. Nano- to microspherical formations that corresponded with the vancomycin spectral signature (i.e., similar to a desiccated vancomycin solution deposited on control slides) were found within the casts. This signal was not detected in the surrounding tissue (despite a 6.25-μm spatial resolution) (Supplemental Figure 1). Additional analysis by transmission electron microscopy with immunogold labeling confirmed that intratubular casts are formed in the part of vancomycin aggregates that are noncrystalline spherical formations, mainly nanometric in size (100–900 nm) (Figure 1C). We developed the immunohistologic detection of vancomycin on frozen kidney (Figure 1D) and paraffin-embedded sections (Figure 1E). Moreover, vancomycin deposits colocalized with uromodulin inside the casts (Figure 1F compared with Figure 1E). Uromodulin was also found in the Bowman’s space, suggesting the obstructive nature of vancomycin-associated casts (Supplemental Figure 2A). A CD68+ macrophagic infiltrate was also observed surrounding the casts and within the kidney’s interstitium, suggesting that pathologic casts might induce an inflammatory process (Supplemental Figure 2B). To further confirm the pathogenicity of vancomycin-associated casts, we retrospectively examined eight additional renal biopsies with ATN that had been performed in the clinical context of high-vancomycin trough levels preceding ARF (Table 1). To do so, we developed the staining of vancomycin from paraffin-embedded sections (Figure 2, Supplemental Figure 3). Using this technique, in each patient, we were able to detect vancomycin-associated casts in a similar location as our index patient (Figure 2, A–H). One patient who developed typical acute myeloma cast nephropathy and received intravenous vancomycin at the same time was used as a genuine negative control; vancomycin trough level was normal. His renal biopsy did not show any vancomycin staining (Figure 2I, Supplemental Figure 3), the same has been observed in other forms of ATN.
Table 1.
Clinical characteristics of patients with the retrospective diagnosis of vancomycin-associated cast nephropathy on the basis of their renal biopsies
| Characteristic | Patient | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | |
| Sex | W | M | M | M | M | W | W | M | W |
| Age, yr | 48 | 45 | 19 | 69 | 46 | 73 | 69 | 66 | 46 |
| Initial Clinical Context | Meningitis | Sepsis | Septic arthritis | Septic arthritis | Pneumonia | Sepsis | Fever and neutropenia | Septic arthritis | Fever and neutropenia |
| Circulatory Shock | No | No | No | No | No | No | No | No | No |
| SC at Renal Biopsy, mg/dl | 6.2 | 3.8 | Dialysis | 13 | Dialysis | 3.2 | 5.7 | 4.7 | 4.2 |
| SV Levels, mg/L | 42 | 35 | 106 | 18.9 | 57.6 | 51.5 | 50 | 51 | 87 |
| Vancomycin Therapy Duration (Dosage) | 19 d (4 g/d) | 3 d (NA) | 10 d (NA) | 3 d (2 g/d) | 14 d (1.5 g/d) | 8 d (2 g/d) | 7 d (NA) | 12 d (NA) | 3 d (3 g/d) |
| Vancomycin Withdrawal to Renal Biopsy, d | 7 | 7 | 20 | 10 | 27 | 17 | 15 | 10 | 17 |
| Other Nephrotoxic Drugsa | No | No | G | G | G,C | G | No | No | No |
| Dialysis | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Outcome | RRF | Death | RRF | Death | Death | RRF | RRF | Death | RRF |
Patient I corresponds to the patient described in Figure 1. W, woman; M, man; SC, serum creatinine; SV, serum vancomycin; NA, not available; G, gentamicin; C, cisplatin; RRF, recovery of renal function.
Drugs known to be nephrotoxic and administered before renal biopsy during the hospital stay.
Figure 2.
Vancomycin detection in a suspected case of vancomycin-associated cast nephropathy (A to H) Vancomycin staining on paraffin-embedded renal biopsy samples from 8 patients with unexplained acute renal failure occurring in the clinical context of high trough vancomycin plasma levels. Patients did not develop circulatory shock. Concomitant high trough vancomycin plasma levels were identified retrospectively from our center's database (1999-2016). Patients' clinical characteristics (A to H) are shown in Table 1. Anti-vancomycin mouse monoclonal antibody (1:1000, Abbot, 6E44-21) has been used on 4 μm dewaxed kidney sections. Nuclei are stained with hematoxylin. A strong staining has been detected in all cases in tubular lumens, with a patchy distribution among the renal cortex. Negative control has been shown in (I). Magnification × 200.
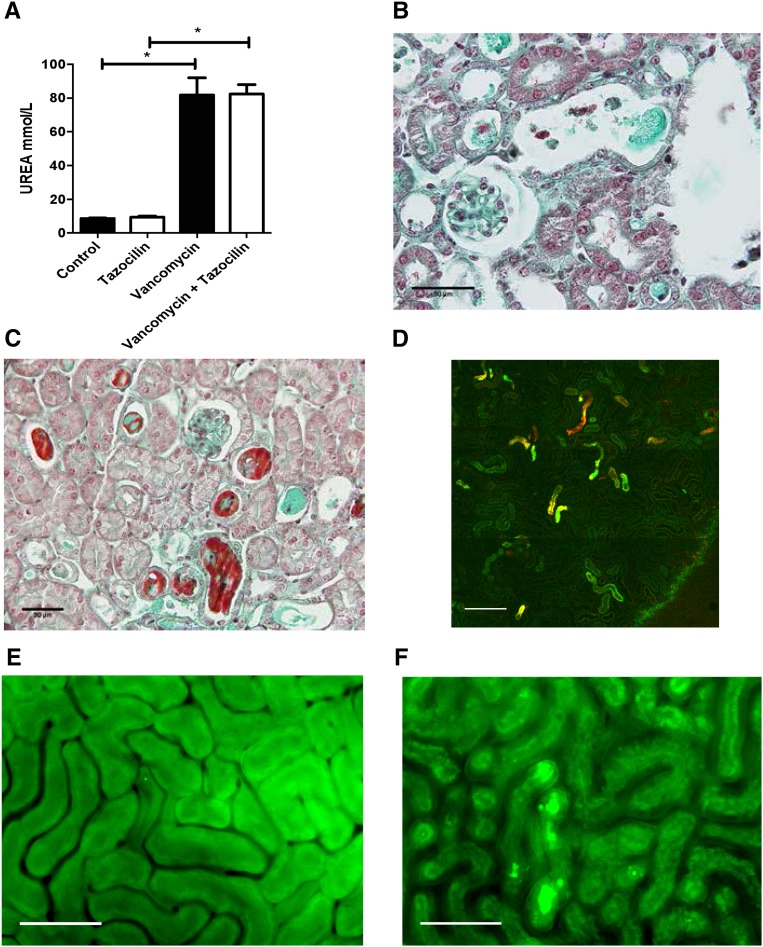
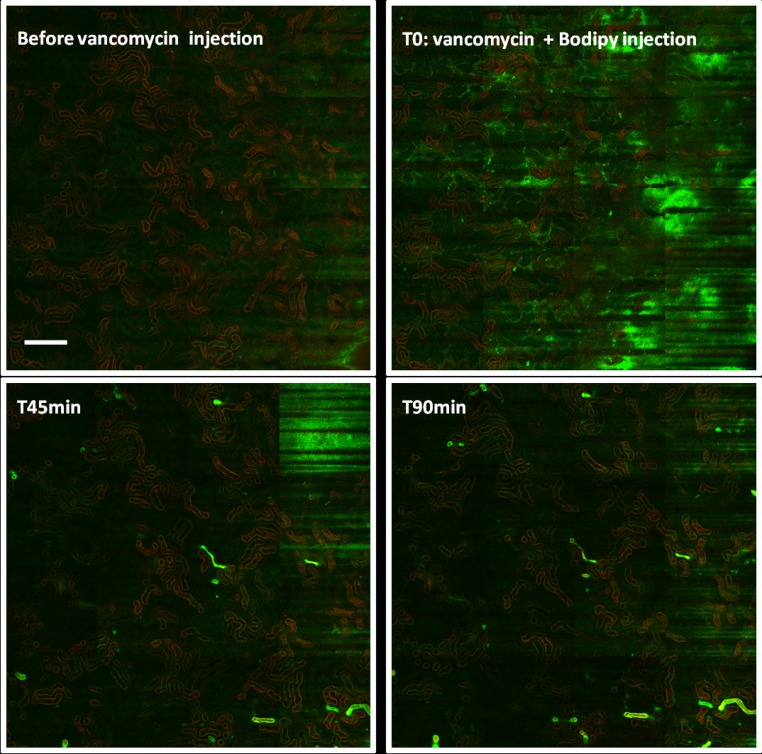
To further understand the kinetics of vancomycin-associated cast formation, we injected vancomycin into mice. Observations were made between 2 hours and 2 days after the injection of vancomycin. We confirmed experimentally the nephrotoxicity of vancomycin and ruled out any pathologic role of piperacillin-tazobactam (Figure 3A). Mouse kidney sections were examined by either the aforementioned techniques or in vivo imaging (Figure 3, B–F). A spectral vancomycin signature was detected in pathologic tubular casts in the kidneys of vancomycin-treated mice (Figure 3, B and C, Supplemental Figure 1, E and F). We also examined the formation of vancomycin casts by in vivo imaging at day 2 (Supplemental Movies 1 and 2) and after 2 hours (Figure 3, E and F). Casts have been highlighted using a vancomycin-boron-dipyrromethene–associated dye. Vancomycin obstructive casts appear very early after the injection of vancomycin and are easily detected as early as 40 minutes postinjection. Cast distribution appears very patchy across the renal cortex (Figures 3D and 4). Furthermore the staining of uromodulin was eventually found in the glomeruli (Supplemental Figure 4), confirming the obstructive nature of the casts.
Figure 3.
Vancomycin-associated cast nephropathy confirmed in mice. (A). Following vancomycin injection (n=4 per group) mice developed acute renal failure (observation made at day 2) that is not aggravated by concomitant Tazocilin injection. *P<0.05. (B) Kidney injuries have been visible as early as two days after vancomycin injection on Masson's trichrome. Acute tubular necrosis is visible with granular material found in the tubular lumen. Scale bar: 50 μm. (C) Lower magnification showing the patchy distribution of vancomycin-associated casts. Scale bar: 50 μm. (D) Intravital confocal microscopy (enlarged field reconstructed) showing the patchy distribution of vancomycin-associated tubular casts (green) magnified by a vancomycin-linked dye (boron-dipyrromethene) at 2 hours following vancomycin injection. Scale bar: 200 μm. Control mice (E) compared to vancomycin-injected mice at day 2 post-vancomycin injection (F) observed with intravital wide-field microscopy. Tubular cast formation magnified by a vancomycin-linked dye (boron-dipyrromethene) is easily visible in (F). *P<.05. Scale bar: 100 μm.
Figure 4.
In vivo formation of obstructive vancomycin-associated tubular casts in mice. Intravital confocal microscopy analyses (enlarged field reconstructed) show that intratubular cast formation occurs nearly 40 minutes after vancomycin injection. The same kidney cortex area has been observed sequentially at different time points. Tubular casts are not observed before vancomycin injection (upper left panel). When vancomycin and vancomycin-linked green fluorescent dye (boron-dipyrromethene) are injected intravenously, a green fluorescence appears (upper right panel) in capillary vessels, reflecting the intravascular circulation of vancomycin. At 45 minutes (lower left panel), the first vancomycin-associated intratubular casts (green) are now visible, with their number increasing further at 90 minutes (lower right panel). Scale bar, 200 μm.
We describe a previously unsuspected mechanism of vancomycin-induced ARF due to vancomycin-associated tubular cast formation. Pathologic obstructive casts are made of vancomycin nanospheres entangled with uromodulin. We showed the toxicity of vancomycin from the study of paradigmatic patients in whom serum creatinine elevation and ARF appeared immediately after infusion of vancomycin. All patients were normovolemic, and one half received vancomycin in the absence of any concomitant nephrotoxic agent. They previously had normal renal function. Kidney biopsies performed as long as 17 days after vancomycin discontinuation showed ATN and tubular casts containing vancomycin. The detection of vancomycin within the casts was confirmed by several independent methods: infrared spectroscopy, immunohistochemistry, and electron microscopy with immunogold labeling. Lastly, we replicated in mice vancomycin-induced cast formation and determined intratubular obstruction by in vivo imaging. These data were also confirmed by the pathologic detection of uromodulin on glomeruli.
Published animal data suggest that vancomycin induces direct oxidative stress on proximal tubular cells. However, we evidenced a similar cast formation when examining published pictures from these studies.5–7 However, the obstructive mechanism described in our study cannot exclude an additional and direct toxicity of vancomycin on tubular cells that has been suggested before.5–7 Intratubular crystal–associated drug nephropathy is a well known cause of AKI and related to the intratubular precipitation of antibiotics,8 but it has never been described with noncrystal formations, such as vancomycin.
Our patients had several risk factors that are typically associated with vancomycin nephrotoxicity: high trough concentrations,9,10 obesity,11 concomitant administration of piperacillin-tazobactam, and in a few patients, aminoglycosides.12,13 A recent study has suggested that piperacillin-tazobactam increases vancomycin-induced nephrotoxicity, but we failed to reproduce this finding experimentally in mice.12 All of our patients had normal renal function at baseline, and their vancomycin dosage was under 4 g/d, a threshold usually associated with increased vancomycin toxicity.14 Finally, our experimental models showed that vancomycin alone could be nephrotoxic by precipitating within tubular lumens independent of all other known risk factors, except high-trough vancomycin level, which is a prerequisite. Indeed, blood vancomycin concentration should be closely monitored.
Concise Methods
More details are in Supplemental Material.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Prof. Pierre Ronco (Assistance Publique – Hôpitaux de Paris, Hôpital Tenon, Néphrologie et Dialyses, Paris, France) as well as Prof. Patrice Callard, Dr. David Buob, and Prof. Isabelle Brocheriou (Assistance Publique – Hôpitaux de Paris, Hôpital Tenon, Anatomie et cytologie pathologiques, Paris, France) for their constructive comments. We also thank the teams of the Plate-forme d'Imagerie Cellulaire Pitié Salpêtrière (Paris, France) and the Plate-forme d'Imagerie et de Cytométrie de Tenon (Paris, France) and are particularly grateful to Aurélien Dauphin for the generation of confocal microscopy images.
Y.L. and L.M. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Vancomycin in the Kidney—A Novel Cast Nephropathy,” on pages 1669–1670.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016080867/-/DCSupplemental.
References
- 1.Rodvold KA, Blum RA, Fischer JH, Zokufa HZ, Rotschafer JC, Crossley KB, Riff LJ: Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother 32: 848–852, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith RS: Introduction to vancomycin. Rev Infect Dis 3[Suppl]: S200–S204, 1981 [PubMed] [Google Scholar]
- 3.Farber BF, Moellering RC Jr.: Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother 23: 138–141, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine DP: Vancomycin: A history. Clin Infect Dis 42[Suppl 1]: S5–S12, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Oktem F, Arslan MK, Ozguner F, Candir O, Yilmaz HR, Ciris M, Uz E: In vivo evidences Suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: Protection by erdosteine. Toxicology 215: 227–233, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Sabler IM, Berkovitch M, Sandbank J, Kozer E, Dagan Z, Goldman M, Bahat H, Stav K, Zisman A, Klin B, Abu-Kishk I: Exposure to hyperbaric oxygen intensified vancomycin-induced nephrotoxicity in rats. PLoS One 11: e0152554, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celik I, Cihangiroglu M, Ilhan N, Akpolat N, Akbulut HH: Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic Clin Pharmacol Toxicol 97: 325–332, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Verdesca S, Fogazzi GB, Garigali G, Messa P, Daudon M: Crystalluria: Prevalence, different types of crystals and the role of infrared spectroscopy. Clin Chem Lab Med 49: 515–520, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL: Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49: 507–514, 2009 [DOI] [PubMed] [Google Scholar]
- 10.van Hal SJ, Paterson DL, Lodise TP: Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 57: 734–744, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall RG 2nd, Hazlewood KA, Brouse SD, Giuliano CA, Haase KK, Frei CR, Forcade NA, Bell T, Bedimo RJ, Alvarez CA: Empiric guideline-recommended weight-based vancomycin dosing and nephrotoxicity rates in patients with methicillin-resistant Staphylococcus aureus bacteremia: A retrospective cohort study. BMC Pharmacol Toxicol 14: 12, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgess LD, Drew RH: Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy 34: 670–676, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Rybak MJ, Albrecht LM, Boike SC, Chandrasekar PH: Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J Antimicrob Chemother 25: 679–687, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Lodise TP, Lomaestro B, Graves J, Drusano GL: Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52: 1330–1336, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.