Abstract
CKD occurs in most patients with acute intermittent porphyria (AIP). During AIP, δ-aminolevulinic acid (ALA) accumulates and promotes tubular cell death and tubulointerstitial damage. The human peptide transporter 2 (PEPT2) expressed by proximal tubular cells mediates the reabsorption of ALA, and variants of PEPT2 have different affinities for ALA. We tested the hypothesis that PEPT2 genotypes affect the severity and prognosis of porphyria-associated kidney disease. We analyzed data from 122 individuals with AIP who were followed from 2003 to 2013 and genotyped for PEPT2. At last follow-up, carriers of the PEPT2*1*1 genotype (higher affinity variant) exhibited worse renal function than carriers of the lower affinity variants PEPT2*1/*2 and PEPT2*2/*2 (mean±SD eGFR: 54.4±19.1, 66.6±23.8, and 78.1±19.9 ml/min per 1.73 m2, respectively). Change in eGFR (mean±SD) over the 10-year period was −11.0±3.3, −2.4±1.9, and 3.4±2.6 ml/min per 1.73 m2 for PEPT2*1/*1, PEPT2*1*2, and PEPT*2*2*2 carriers, respectively. At the end of follow-up, 68% of PEPT2*1*1 carriers had an eGFR<60 ml/min per 1.73 m2, compared with 37% of PEPT2*1*2 carriers and 15% of PEPT2*2*2 carriers. Multiple regression models including all confounders indicated that the PEPT2*1*1 genotype independently associated with an eGFR<60 ml/min per 1.73 m2 (odds ratio, 6.85; 95% confidence interval, 1.34 to 46.20) and an annual decrease in eGFR of >1 ml/min per 1.73 m2 (odds ratio, 3.64; 95% confidence interval, 1.37 to 9.91). Thus, a gene variant is predictive of the severity of a chronic complication of AIP. The therapeutic value of PEPT2 inhibitors in preventing porphyria-associated kidney disease warrants investigation.
Keywords: Acute Intermittent Porphyria, PEPT2, SLC15A2, chronic kidney disease, Delta-aminolevulinic acid
Mutations in the gene encoding hydroxymethylbilane synthase (HMBS), which occur in one in 1675 people in Europe1 and have a very low penetrance2, are responsible for acute intermittent porphyria (AIP), an inherited autosomal dominant disorder affecting the synthesis of heme.3,4 The acute clinical expression of AIP is a neurovisceral attack, characterized by severe abdominal pain, often complicated by agitation, peripheral neuropathy, and even coma. During AIP attacks, the porphyrin precursors δ-aminolevulinic acid (ALA) and porphobilinogen (PBG) accumulate and are massively excreted in urine as a consequence of enzymatic blockade, where their presence confirms the diagnosis.5 CKD and hepatocellular carcinoma are major long-term complications of AIP.6–9 The prevalence of porphyria associated kidney disease (PAKD) is >50% in individuals with AIP and is characterized by a chronic tubulo-interstitial nephropathy, associated with approximately half of cases with severe arteriosclerosis and fibroproliferative intimal hyperplasia.10 Experimental evidence indicates that ALA and PBG promote renal epithelial cell apoptosis and phenotypic changes suggestive of the epithelial-to-mesenchymal transition.10 The current pathophysiologic model proposes that during AIP attacks, ALA and PBG are excreted in large amounts in the urine, which promotes tubular toxicity and AKI, followed by regeneration and scarring. In support of this, the severity of PAKD is correlated with the frequency and severity of AIP attacks.10 By contrast, lower, but not strictly normal, urinary concentrations of ALA and PBG, found between crises at the basal level, could impact tubular homeostasis to an extent that remains to be established.
Condensation of the amino acid glycine and the anaplerotic compound of the Krebs cycle, succinyl CoA, mediated by ALA synthase in the mitochondria, produces ALA. ALA is a highly hydrophilic compound mainly produced in excess by the liver during AIP, and it is freely filtered by the kidneys and reabsorbed in the S2 and S3 segments of the proximal tubule by the human peptide transporter 2, PEPT2 (also referred to as SLC15A2).11 PEPT2 is a symporter that catalyzes peptide transport by coupling substrate translocation with the transmembrane electrochemical proton gradient providing the driving force.12–14 PEPT2 is involved in the reabsorption of di- and tri-peptides, peptide-like drugs (e.g., cephalosporins, angiotensin-converting enzyme inhibitors, and antiviral nucleoside prodrugs), and endogenous nondietary amino acids derivatives, such as carnitine and ALA, from the glomerular filtrate.15 The most clinically relevant function of PEPT2 in mediating ALA transport is related to its neuroprotective effect resulting from the efflux of ALA from the brain across the choroid plexus upon lead poisoning.16–18
Haplotype analyses provided evidence that PEPT2 exists in two major variants termed PEPT2*1 and PEPT2*2, which are equally distributed in the white population at approximately 44% and 47%.19 The two most abundant nonsynonymous single nucleotide polymorphisms (SNPs) are found in near complete linkage disequilibrium in a large haplotype block that begins at exon 9 and continues through exon 17. There is a C/T substitution in exon 13 termed p.Leu250Phe c.1048 C>T, and a C/T substitution in exon 15 termed p.Pro409Ser c.1225 C>T. Notably, their corresponding protein variants differ in their functional and biochemical properties. The protein variant PEPT2*1 (corresponding to exon 13C/exon 15C) has a Km value for the dipeptide Gly-Ser of 83 μM compared with a significantly higher Km value (233 μM) for the protein variant PEPT2*2 (corresponding to the genotype exon 13T/exon 15T).19 This result indicates that the PEPT2*2 variant has a lower affinity for its substrates compared with the PEPT2*1 variant, albeit with a similar Vmax. A clinical consequence of the differential affinity of these variants for ALA is that children with lead poisoning who carry the PEPT2*2 genotype have poorer motor dexterity and poorer working memory resulting from the reduced brain clearance of ALA.16,17
With this is mind, we proposed that changes in the affinity of PEPT2 variants may alter ALA tubular reabsorption and cytotoxicity and may ultimately alter the natural evolution of PAKD. To address this question, we tested whether PEPT2 variants impact the severity and prognosis of PAKD in individuals with a HMBS mutation. Because the majority of individuals with AIP have PAKD, this approach will likely allow for the identification of markers predicting the severity and evolution of the disease.
Results
PEPT2 Genotypes and Renal Expression
In 2013, we performed an observational study of a population of 136 individuals with the HMBS mutation at the French Porphyria Center to perform a comprehensive characterization of PAKD, including its evolution during a follow-up period of 10 years (started in 2003).10 For this study, individuals whose DNA was not available were contacted by mail to provide DNA either by buccal swab or blood sampling, and overall, 122 individuals were successfully genotyped for exon 13 (C/T) and exon 15 (C/T) SNPs (see Concise Methods). The distribution of T variants did not significantly diverge from the Hardy–Weinberg equilibrium (chi-squared, 0.14; P=0.69), and we measured an allelic frequency of 53%. A search for linkage disequilibrium with other gene variants in the 1000 Genome Database (100,000 Kb region) identified variants of the gene encoding ELL associated factor 2 (EAF2), a transcriptional transactivator of Transcription Elongation Factor A1 (TCEA1) activity acting in prostatic cells, which is therefore relevant in this study.
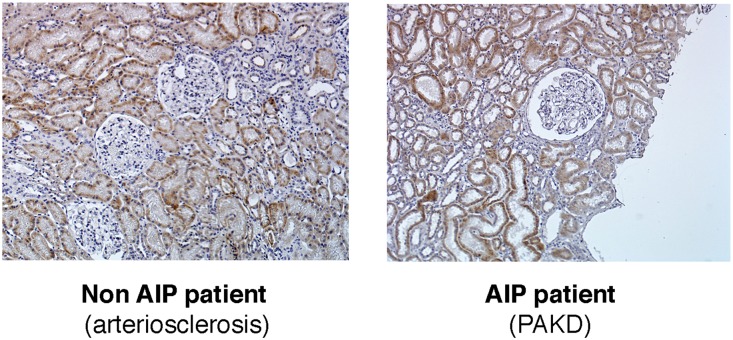
Twenty-six individuals were classified as having the PEPT2*1/*1 genotype (exon 13 CC/exon 15 CC); 62 (51%) were PEPT2*1/*2 (exon 13 CT/exon 15 CT) and 32 (26%) were PEPT2*2/*2 (exon 13 TT/exon 15 TT). Of note, two individuals were classified as PEPT2*3 genotype corresponding to an exon 13 CC/exon 15 TT haplotype, which indicates that in these cases, these two SNPs are not in linkage disequilibrium. Because the biologic relevance of this variant is unknown, we excluded these two cases from the remainder of the study. The demographic and medical characteristics of this cohort (122 individuals) are listed in Table 1. As expected, the majority of individuals were women and hypertensive, 57% of symptomatic carriers and 40% of asymptomatic carriers were under antihypertensive treatment, and basal ALA and PBG urinary concentrations were slightly elevated. Four individuals had more than four crises per year and required preventive heme arginate injections. Of note, we used immunohistochemistry to verify that PEPT2 is expressed by the proximal tubules of kidneys of patients with PAKD (Figure 1), as well as by those of individuals without PAKD.
Table 1.
Clinical and laboratory characteristics
| Characteristics | All Patients (n=122) |
|---|---|
| Age, yr | 62.7±13.5 |
| Symptomatic carriers/asymptomatic carriersa | 68/54 |
| Body mass index, kg/cm2 | 25±4.6 |
| Women, n (%) | 96 (80) |
| Hypertension, n (%) | 65 (56) |
| Diabetes, n (%) | 4 (3.5) |
| PEPT2 genotype, n (%) | |
| *1/*1 (exon 13b CC/exon 15 CC) | 26 (21.5) |
| *1/*2 (exon 13b CT/exon 15 CT) | 62 (51) |
| *2/*2 (exon 13b TT/exon 15 TT) | 32 (26) |
| *1/*3 (exon 13b CC/exon 15 TT) | 2 (1.5) |
| HMBS mutation, % null allele/missense | 71/29 |
| Urine ALA-to-creatinine ratio, μmol/mmol creatinineb | 5.1±4.7 |
| Symptomatic carriers | 6±5.3 |
| Asymptomatic carriers | 3.9±3.7 |
| Urine PBG-to-creatinine ratio, μmol/mmol creatininec | 5.8±7.1 |
| Symptomatic carriers | 7.7±7.9 |
| Asymptomatic carriers | 3.5±5 |
| eGFR, ml/min per 1.73 m2 | 67±23.7 |
| Urine protein-to-creatinine ratio, g/mmol creatinine | 0.06±0.09 |
Values are means±SD unless otherwise indicated. The GFR was estimated according to the four-variable Modification of Diet in Renal Disease formula.
“Symptomatic carrier” is defined as the association of at least one AIP attack with a typical porphyrin and heme precursor excretion profile in both urines and feces, confirmed by a 50% decrease in HMBS activity in erythrocytes and the identification of a causative mutation in the HMBS gene. “Asymptomatic carrier” status is defined after a family screening to identify those with latent disease and on the basis of a deficient HMBS enzymatic activity, and confirmed with a DNA analysis by direct sequencing to identify the causative mutation in the HMBS gene.
ALA-to-creatinine normal value <3 µmol/mmol creatinine.
PBG-to-creatinine normal value <1 µmol/mmol creatinine.
Figure 1.
PEPT2 is expressed by renal tubular cells. Analysis of the renal expression of PEPT2 by immunohistochemistry in an individual with PAKD or an individual without AIP suffering from CKD because of arteriosclerosis, showing similar expression levels. Original magnification, ×40.
CKD Is Highly Prevalent among PEPT2*1/*1 Individuals
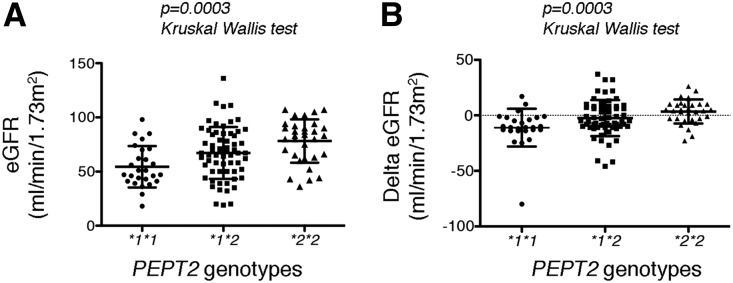
Whereas the three genotype groups have similar demographic and medical characteristics (Table 2) at the time of the evaluation (2013), individuals with the PEPT2*1/*1 genotype had significantly more severe CKD compared with those carrying a PEPT2*2 allele (Table 3). The vast majority (65%) of PEPT2*1/*1 carriers have a Kidney Disease-Improving Global Outcomes (KDIGO) CKD stage 3–5 compared with 29% and 22% for the PEPT2*1/*2 and PEPT2*2/*2 genotypes, respectively, and proteinuria is two-fold higher in the PEPT2*1/*1 group compared with carriers of the PEPT2*2 genotype (Figure 2A, Table 3). In addition, CKD tends to progress faster in the PEPT2*1/*1 group compared with the PEPT2*2 group. Indeed, the mean loss of the GFR over the 10 years of follow-up was −11±3.3 ml/min per 1.73 m2 for PEPT2*1/*1 individuals compared with −2.4±1.9 ml/min per 1.73 m2 for PEPT2*1/*2 and +3.4±2.6 ml/min per 1.73 m2 for PEPT2*2/*2 individuals (Figure 2B, Table 3). Consistent with this, the proportion of individuals reaching KDIGO CKD stage 3 or higher during the follow-up period was 20% in the PEPT2*1/*1 group compared with 9% in the PEPT2*1/*2 and none in the PEPT2*2/*2 group. Together, these results indicate that PAKD is more severe and progresses faster in individuals carrying the PEPT2*1/*1 genotype, encoding the high affinity transporter, compared with individuals carrying the PEPT2*2 genotype, encoding the low affinity transporter. Notably, PEPT2 genotypes also impacted CKD severity in the subgroup of patients with symptomatic disease. The mean eGFR at the time of evaluation was 50.5±18 ml/min per 1.73 m2 in the group of individuals carrying the PEPT2*1/*1 genotype compared with 64.8±18.3 ml/min per 1.73 m2 in the group of individuals with the PEPT2*2/*2 genotype (P=0.02, Wilcoxon test). Consistent with this result, the eGFR decline over time was −13.3±18 ml/min per 1.73 m2 in carriers of the PEPT2*1/*1 genotype compared with −1.3±11 ml/min per 1.73 m2 in carriers of the PEPT2*2/*2 genotype (P=0.01, Wilcoxon test).
Table 2.
Characteristics of the cohort according to the PEPT2 genotype
| Characteristics | PEPT2*1/*1 (n=26) | PEPT2*1/*2 (n=62) | PEPT2*2/*2 (n=32) | P Value |
|---|---|---|---|---|
| Age, yr | 65±2.6 | 61±1.7 | 62.7±2.5 | 0.50 |
| Symptomatic carriers, n (%)a | 19 (73) | 33 (53) | 16 (50) | 0.14 |
| Body mass index, kg/cm2 | 23.7±1 | 25.2±0.6 | 25.6±0.9 | 0.33 |
| Women, n (%) | 23 (88) | 48 (80) | 23 (71) | 0.28 |
| Hypertension, n (%) | 15 (62) | 31 (53) | 18 (58) | 0.73 |
| Diabetes, n (%) | 2 (4) | 2 (3.5) | 1 (3.2) | 0.98 |
| Urine ALA-to-creatinine ratio, μmol/mmol creatinineb | 4.7±0.9 | 5.4±0.6 | 4.8±0.9 | 0.77 |
| Urine PBG-to-creatinine ratio, µmol/mmol creatininec | 5.6±1.5 | 6.4±0.9 | 4.7±1.3 | 0.57 |
Values are means±SD unless otherwise indicated. The three groups were compared using one-way ANOVA.
Symptomatic carrier is an association of at least one AIP attack with a typical porphyrin and heme precursor excretion profile in both urines and feces, confirmed by a 50% decrease in HMBS activity in erythrocytes and the identification of a causative mutation in HMBS gene.
ALA-to-creatinine normal value <3 µmol/mmol creatinine.
PBG-to-creatinine normal value <1 µmol/mmol creatinine.
Table 3.
Renal parameters of the cohort according to the PEPT2 genotype
| Parameters | PEPT2*1/*1 (n=26) | PEPT2*1/*2 (n=62) | PEPT2*2/*2 (n=32) | P Value |
|---|---|---|---|---|
| eGFR-1, ml/min per 1.73 m2 | 65±23 | 69±19 | 74±18 | 0.2 |
| eGFR-2, ml/min per 1.73 m2 | 54.4±19.1 | 66.6±23.8 | 78.1±19.9 | 0.004 |
| eGFR-1<60 ml/min per 1.73 m2, n (%) | 13 (50) | 18 (29) | 7 (22) | 0.06 |
| eGFR-2<60 ml/min per 1.73 m2, n (%) | 17 (65) | 23 (37) | 5 (15) | 0.002 |
| Progression to stage 3 CKD, n (%)a | 5 (20) | 9 (14) | 0 (0) | 0.01 |
| ΔeGFR, 2013–2003 ml/min per 1.73 m2 | −11±3.3 | −2.4±1.9 | +3.4±2.6 | 0.001 |
| Urine protein-to-creatinine ratio, g/mmol creatinine | 0.1±0.02 | 0.05±0.01 | 0.04±0.01 | 0.003 |
Values are means±SD unless otherwise indicated. The GFR was estimated according to the four-variable Modification of Diet in Renal Disease formula. eGFR-1 denotes eGFR measured in the cohort in 2003; eGFR-2 denotes eGFR measured in the cohort in 2013. The three groups were compared using one-way ANOVA.
Individuals who reached KDIGO CKD stage 3 during the follow-up period. KDIGO CKD stage 3: 45–30 ml/min per 1.73 m2.
Figure 2.
Renal function is preserved in PEPT2*2 carriers. (A) Scatter dot plots showing distribution of the relative frequencies of eGFR values according to PEPT2 genotypes. The lines represent the mean and SD. Distributions were compared using the Kruskal–Wallis test. (B) Scatter dot plots showing distribution of the relative frequencies of the ΔeGFR values between 2013 and 2003 according to PEPT2 genotypes. The lines represent the mean and SD. Distributions were compared using the Kruskal–Wallis test.
PEPT2*1/*1 Independently Predicts PAKD Severity
To demonstrate that the PEPT2*1/*1 genotype contributes to the severity of PAKD, we characterized determinants of the severity of renal disease. As expected, age, hypertension, AIP activity (symptomatic carriers compared with asymptomatic carriers, see Concise Methods), renal function eGFR in 2003, and urine PBG concentrations were significantly associated with CKD stage 3 or higher (Table 4). In addition, the distribution of PEPT2 genotypes in the group of individuals with an eGFR<60 ml/min per 1.73 m2 was skewed toward PEPT*1/*1 compared with an inverse distribution in the group of individuals with an eGFR>60 ml/min per 1.73 m2 (Table 4). To test the independence of the PEPT2 genotype in the association with eGFR levels, we performed a multivariate analysis using a nominal logistic regression including all parameters associated with eGFR levels (> or <60 ml/min per 1.73 m2) in the univariate analysis with P<0.1 (Table 5). The three following parameters were significantly associated with an eGFR<60 ml/min per 1.73 m2: (1) symptomatic carriers, with an odds ratio (OR) of 18.8 (95% confidence interval [95% CI], 2.96 to 200); (2) renal function in 2003 (eGFR-1) with an OR of 0.91 per increase of 1 ml/min per 1.73 m2 (95% CI, 0.85 to 0.96); (3) and PEPT2 genotype (PEPT2*1/*1), with an OR of 6.85 (95% CI, 1.34 to 46.2), indicating that the PEPT2*1/*1 genotype is an independent explanatory variable for the severity of CKD (eGFR<60 ml/min per 1.73 m2).
Table 4.
Determinants of the severity of PAKD
| Parameters | eGFR-2<60 ml/min per 1.73 m2 (n=45) | eGFR-2>60 ml/min per 1.73 m2 (n=75) | P Value |
|---|---|---|---|
| eGFR-2, ml/min per 1.73 m2 | 42.9±1.5 | 81.6±1.7 | <0.001 |
| Age, yr | 67±10 | 60±14 | 0.002 |
| Women, n (%) | 39 (88) | 55 (75) | 0.1 |
| Hypertension, n (%) | 33 (76) | 31 (44) | 0.001 |
| Diabetes, n (%) | 2 (4) | 2 (3) | 0.6 |
| Body mass index, kg/cm2 | 24±5 | 25.3±4 | 0.4 |
| Symptomatic carriers, n (%) | 40 (88) | 28 (37) | <0.001 |
| eGFR-1, ml/min per 1.73 m2 | 53.8±2.3 | 79.4±1.8 | <0.001 |
| Urine ALA-to-creatinine ratio, μmol/mmol creatinine | 5.3±0.8 | 5±0.5 | 0.6 |
| Urine PBG-to-creatinine ratio, μmol/mmol creatinine | 7.4±8.8 | 4.8±5.6 | 0.04 |
| Urine protein-to-creatinine ratio, g/mmol creatinine | 0.09±0.01 | 0.04±0.05 | 0.01 |
| PEPT2 genotype | 0.002 | ||
| *1/*1 | 17 (37) | 9 (12) | |
| *1/*2 | 23 (51) | 39 (52) | |
| *2/*2 | 5 (11) | 27 (36) |
Values are means±SD unless otherwise indicated. The GFR was estimated according to the four-variable Modification of Diet in Renal Disease formula. eGFR-1 denotes the eGFR measured in the cohort in 2003; eGFR-2 denotes the eGFR measured in the cohort in 2013. The two groups were compared using paired t test or a likelihood ratio test.
Table 5.
Nominal multivariate analysis of parameters associated with eGFR levels
| Parameters | Adjusted OR (95% CI) | P Value |
|---|---|---|
| Age, per increase of 1 yr | 1 (0.96 to 1.11) | 0.28 |
| Hypertension, yes versus no | 1 (0.19 to 5.04) | 0.99 |
| Symptomatic carrier versus asymptomatic carrier | 18.8 (2.96 to 200) | 0.001 |
| eGFR-1, per increase of 1 ml/min per 1.73 m2 | 0.9 (0.85 to 0.96) | <0.001 |
| Urine protein-to-creatinine ratio, per increase of 1 g/mmol | 2 (0.78 to 5.74) | 0.14 |
| Urine PBG-to-creatinine ratio, per increase of 1 μmol/mmol creatinine | 0.9 (0.87 to 1.1) | 0.67 |
| PEPT2 genotype | ||
| *1/*1 versus *1/*2 | 4.7 (0.8 to 35.4) | 0.08 |
| *1/*1 versus *2/*2 | 18.5 (2.38 to 232) | 0.004 |
| *1/*1 versus *2 | 6.8 (1.34 to 46.2) | 0.01 |
Adjusted ORs were calculated using a multivariate model incorporating all covariates listed in Table 4, which were associated (P<0.1) with an eGFR<60 ml/min per 1.73 m2 in the univariate analysis.
PEPT2*1/*1 Contributes to Renal Function Decline during PAKD
Because carriers of the PEPT2*1/*1 genotype had a mean decline of eGFR between 2003 and 2013 of −11 ml/min per m2 compared with no decline in PEPT2*2/*2 during the same period of time, we asked whether the PEPT2 genotype was predictive of the decline of renal function. The univariate analysis of the parameters that may be involved in renal function degradation is detailed in Table 6, using 1 ml/min per 1.73 m2 per year as a cutoff between a physiologic and pathologic slope.20,21 Only symptomatic carriers, proteinuria, and PEPT2 genotypes were significantly associated with an annual loss in the eGFR of at least 1 ml/min per 1.73 m2 (Table 6). In a model generated using nominal logistic regression including the three parameters (symptomatic carriers, proteinuria, and PEPT2 genotypes; Table 7), only symptomatic carriers and PEPT2 genotypes remained significantly correlated with the eGFR decline rate (symptomatic carriers: OR, 2.57; 95% CI, 1.04 to 6.76; PEPT2*2 allele: OR, 3.64; 95% CI, 1.37 to 9.91), indicating that the PEPT2 genotype is an independent risk factor for eGFR degradation over time.
Table 6.
Univariate analysis of parameters associated with a decline in renal function
| Parameters | eGFR loss >1 ml/min per 1.73 m2 per yr (n=37) | eGFR loss <1 ml/min per 1.73 m2 per yr (n=83) | P Value |
|---|---|---|---|
| ΔeGFR 2013–2003, ml/min per 1.73 m2 | −19±1.8 | 4.5±1.2 | <0.001 |
| Age, yr | 63.5±2.2 | 62.3±1.4 | 0.64 |
| Women, n (%) | 30 (81) | 64 (74) | 0.79 |
| Hypertension, n (%) | 21 (58) | 43 (55) | 0.17 |
| Diabetes, n (%) | 1 (3) | 3 (4) | 0.75 |
| Body mass index, kg/cm2 | 24.3±0.8 | 24.4±0.5 | 0.89 |
| Symptomatic carriers, n (%) | 27 (72) | 41 (49) | 0.001 |
| eGFR-1, ml/min per 1.73 m2 | 70.2±2.5 | 69.3±2.6 | 0.79 |
| Urine ALA-to-creatinine ratio, μmol/mmol creatinine | 5.1±0.8 | 5.1±0.5 | 0.95 |
| Urine PBG-to-creatinine ratio, µmol/mmol creatinine | 5.5±1.2 | 6±0.8 | 0.72 |
| Urinary protein-to-creatinine ratio, g/mmol creatinine | 0.09±0.01 | 0.05±0.01 | 0.03 |
| PEPT2 genotype | <0.001 | ||
| *1/*1 | 16 (43) | 10 (12) | |
| *1/*2 | 18 (48) | 44 (53) | |
| *2/*2 | 3 (8) | 29 (35) |
Values are means±SD unless otherwise indicated. The GFR was estimated according to the four-variable Modification of Diet in Renal Disease formula. eGFR-1 denotes the eGFR measured in the cohort in 2003; eGFR-2 denotes the eGFR measured in the cohort in 2013. The two groups were compared using paired t test or a likelihood ratio test.
Table 7.
Nominal multivariate analysis of parameters associated with renal function degradation
| Parameters | Adjusted OR (95% CI) | P Value |
|---|---|---|
| Symptomatic carrier versus asymptomatic carrier | 2.5 (1.04 to 6.76) | 0.04 |
| Urinary protein-to-creatinine ratio per increase of 1 g/mmol creatinine | 8.7 (0.46 to 289) | 0.15 |
| PEPT2 genotype | ||
| *1/*1 versus *1/*2 | 2.6 (0.98 to 7.59) | 0.05 |
| *1/*1 versus *2/*2 | 9.4 (2.36 to 48.91) | 0.001 |
| *1/*1 versus *2 | 3.6 (1.37 to 9.91) | 0.01 |
Adjusted ORs were calculated using a multivariate model incorporating all covariates listed in Table 6, which were associated (P<0.1) with an eGFR loss of at least 1 ml/min per 1.73 m2 per year in the univariate analysis.
PEPT2 Genotype Impacts Renal Function in Asymptomatic Carriers
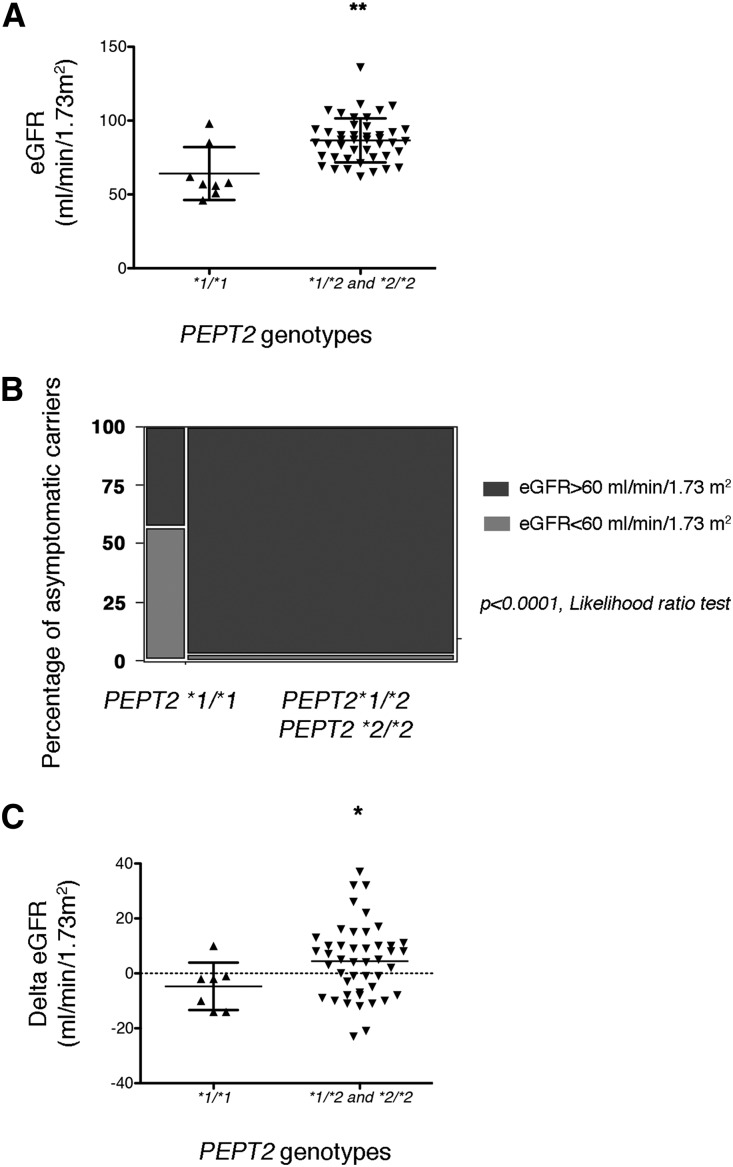
By definition, asymptomatic carriers experience no AIP attack and are usually less exposed to the risk of chronic complications of AIP. In a sensitivity analysis, we asked whether PEPT2 genotypes impact renal function in this group of patients. The mean eGFR in asymptomatic carriers carrying the PEPT2*1/*1 genotype was 20 ml/min per 1.73 m2 lower compared with those carrying a PEPT2*2 allele (65±5.9 ml/min per 1.73 m2 compared with 85.9±2.3 ml/min per 1.73 m2; P=0.01, Wilcoxon test) (Figure 3A). Whereas a majority (54%) of asymptomatic carriers with the PEPT2*1/*1 genotype had CKD stage 3 or higher (Figure 3B), only 2% of those with the PEPT2*2 genotype reached these severity stages. Finally, the decline of the eGFR over the follow-up period was much higher in the PEPT2*1/*1 genotype group compared with those carrying one or two PEPT2*2 alleles (−13±18 ml/min per 1.73 m2 versus −4.8±14.8 ml/min per 1.73 m2; P=0.05, Wilcoxon test) (Figure 3C). Together, these results indicate that PEPT2 variants impact the severity and evolution of CKD individuals with HMBS mutation even if they do not have clinically overt disease.
Figure 3.
PEPT2*2 is protective in asymptomatic carriers. (A) Scatter dot plots representing the distribution of eGFR according to the PEPT2 genotype in asymptomatic carriers compared with a Wilcoxon test. The lines represent the mean and SD. **P<0.01. (B). Graph representing the proportion of individuals with an eGFR<60 ml/min per 1.73 m2 according to PEPT2 genotype. Frequencies were compared with a likelihood ratio test. (C) Scatter dot plots representing the distribution of the difference of eGFRs between 2013 and 2003 according to the PEPT2 genotype in asymptomatic carriers compared with a Wilcoxon test. The lines represent the mean and SD. *P<0.05.
Discussion
As AIP is a rare disease, and the symptoms of attacks are nonspecific, clinicians may be unaware of the possibility of AIP attack when managing an unusual acute abdominal syndrome with agitation. The prevalence of AIP, based on acute symptoms, is likely largely underestimated because the prevalence of symptomatic disease in Europe and the United States is 100 times less frequent than22 that of the prevalence of HMBS mutations in the general population,1 with the consideration that estimating HMBS mutation and AIP crisis frequencies is very difficult. Because of this low penetrance, 95% of individuals with HMBS mutations are asymptomatic. Among the 5% of symptomatic carriers, 90% have only one inaugural crisis in their life and stay in remission, and half of the remaining individuals have more than four crises per year and are referred to as recurrent, and in these cases, heme arginate is prescribed for the prevention of crisis. That PEPT2 variants impact the severity of CKD in individuals with HMBS mutation without overt clinical disease raises interesting hypotheses on the pathophysiology of CKD associated with HMBS mutations. This observation supports a model in which basal urinary concentrations of porphyrin precursors, including ALA, which can be slightly elevated in asymptomatic carriers, can promote tubular injury. Individuals who carry the high affinity transporter may have more severe CKD because ALA is more efficiently reabsorbed by the tubule, and consequently, is more nephrotoxic. Moreover, it is possible that many people in the general population carry undiagnosed HMBS mutations because of the lack of an identified symptomatic proband. However, PEPT2 variants have not been reported in genome-wide association studies of CKD-defining traits,23 albeit numerous members of the solute carrier family have been identified as impacting the eGFR in large populations,24 indicating the variations of the activity or affinity of transporters can participate in the predisposition to CKD. The fact that the severity of renal disease associated with AIP is usually mild may explain why PEPT2 variants can be overlooked in these studies.
We performed a proof-of-concept for the existence of an inherited disorder of metabolism affecting the kidneys, the natural evolution of which can be influenced by another gene variant. The risk of symptomatic disease is higher within families of HMBS carriers compared with the general population of individuals carrying a mutation of HMBS (20% versus 5%; C. Schmitt, unpublished observations), highlighting the importance of predisposing genes and modifiers that modulate the clinical activity of AIP. Consistent with this, we found five pairs of siblings in our cohort, with one pair carrying the PEPT2*1/*1_PEPT2*1/*1 genotypes and the others carrying the PEPT2*1/*2_PEPT2*2/*2 genotypes, indicating that some families could have different risk levels of CKD severity. The type of mutation (70% nonsense and 30% missense in our cohort) was not associated with eGFR levels and eGFR decrease between 2003 and 2013, but the type of HMBS mutation could impact eGFR decline rates. For example, patients with AIP carrying the c.291delG nonsense mutation have an eGFR decline more severe compared with patients with AIP carrying the c.517C>T missense mutation. However, we found 46 different mutations of HMBS in the cohort, which makes a systematic analysis of the impact of specific HMBS mutations difficult to do. Together, these results indicate that some specific mutation and their interactions with PEPT2 variants may be associated with a worse renal prognosis, but the groups of individuals are too small to draw firm conclusions. In addition to variants that affect the Km of PEPT2, cis-acting polymorphisms can impact the expression levels of the transporter,19 which adds a level of complexity to the possible biologic and clinical impacts of PEPT2 genetic variants.
The relevance of PEPT2 variants in the phenotypic expression of ALA neurotoxicity during lead poisoning is well established on the basis of a mechanism that mirrors the expected mechanism that occurs upon PAKD. High affinity PEPT2 (PEPT2*1/*1) variants are more potent transporters that promote ALA efflux from the brain through the blood–brain barrier and are therefore associated with less severe neurologic impairment.16,17 This finding raises the hypothesis that PEPT2 variants can impact the severity of CKD upon lead poisoning. If we anticipate that ALA is involved in the pathophysiology of renal disease upon lead poisoning, it is possible that individuals carrying PEPT2*2 variants with low affinity may have lower ALA tubular reabsorption and therefore could be protected with CKD of a lower severity.
From a therapeutic point of view, the most potent inhibitor of PEPT2 is the angiotensin II receptor antagonist losartan,25 and inhibiting the tubular reabsorption of the cytotoxic compound ALA with this drug may be a credible nephroprotective strategy in AIP. Whether other disorders in which ALA is excreted in excess and can participate in tubulo-interstitial structural deterioration, such as lead poisoning, could be targeted and slowed by the use of PEPT2 inhibitors is a promising preventive strategy that remains to be tested. In addition to endogenous molecules, such as ALA, many compounds are reabsorbed by PEPT2 in the proximal tubule, such as antiviral nucleoside prodrugs. In this setting, variations in PEPT2 affinity can be instrumental for modulating the tissue response to nephrotoxic drugs because it constitutes a therapeutic target. Other than nephrotoxicity, any factor impairing the reabsorption of bioactive compounds, such as cephalosporins, is likely to impact their bioavailability and therefore their clinical efficacy.26,27
In conclusion, we demonstrated that variants in the gene encoding PEPT2, which decrease the affinity of the transporter for ALA, are associated with a reduced severity and a slower progression of PAKD. For the first time, a susceptibility gene has been identified that impacts the severity of a chronic complication of AIP and is a therapeutic target. This study provides a basis for the deeper exploration of the clinical relevance of the genetic variability of PEPT2 affecting the natural history of renal diseases other than AIP. Whether PEPT2 variants impact renal function even in the absence of overt (clinical) AIP may help identify some cases of chronic tubulo-interstitial kidney diseases of unknown origin.
Concise Methods
AIP Diagnosis
The criteria for AIP diagnosis followed the European Porphyria Network guidelines.3,28 AIP diagnosis and treatments were performed in the French Porphyria Center, at the Louis Mourier hospital. The “symptomatic carrier” status is defined by the association of at least one AIP attack with a typical porphyrin and heme precursor excretion profile in urine, plasma, and feces. The diagnosis is confirmed by a 50% decrease in HMBS activity in erythrocytes, and by the identification of a causative mutation in the HMBS gene by direct sequencing as previously described.29 Urinary ALA and PBG were measured using the Bio-Rad ALA/PBG column kit (Bio-Rad, Marnes la Coquette, France), according to the manufacturer’s protocol. The “asymptomatic carrier” status is defined after a family screening to identify those with latent disease and on the basis of deficient HMBS enzymatic activity, and is confirmed using DNA analysis by direct sequencing to identify the causative mutation in the HMBS gene, which requires prior identification of the mutation in a related affected family member.
Cohort
A cohort of 136 individuals with an HMBS mutation was established and analyzed in 2013 to provide a comprehensive characterization of PAKD. These patients were contacted by mail to provide detailed information, including AIP symptoms and presence of hypertension or diabetes. Biologic analysis included serum creatinine, eGFR evaluation using the four-variable Modification of Diet in Renal Disease formula, ALA and PBG urinary concentrations, and measurements of the protein-to-creatinine ratio in a morning urine sample. These patients were those for whom biologic data from 2003 were available and included an estimation of GFR. DNA was available for 40 patients and the remaining (n=96) individuals were contacted by mail to provide a DNA sample using a buccal swab or a blood sample. Eighty answered and a total of 122 samples were available for PEPT2 genotyping. Hypertension was defined by a systolic BP >140 mmHg and/or a diastolic BP >90 mmHg. Diabetes was defined according to the American Diabetes Association criteria. The institutional review board of Louis Mourier Hospital approved the study, written informed consent was obtained from all patients, and the database was deposited in the Commission Nationale Informatique et Libertés (agreement number 1187326).
PEPT2 Genotyping
DNA was prepared from buccal swab or peripheral blood leukocytes using the QIAmp DNA Blood Mini Kit (Qiagen). PEPT2 gene exons 13 and 15 were amplified by PCR using the following primers:
PEPT2 exon 13 forward: 5′-TGTGAGACAGAGTGTAAACAAGCA-3′; PEPT2 exon 13 reverse: 5′-AAAACCAATCCTCACTAAGAAAAA-3′; PEPT2 exon 15 forward: 5′ATTTTTCAGAGGGAGAAAACTATGTC-3′; and PEPT2 exon 13 reverse: 5′-AATTCATTCAGGTACACACCTCAC-3′.
The two strands of PCR products were sequenced using the BigDye Terminator Cycle and Sequencing Ready Reaction kit on an ABI PRISM 3130xl sequencer (Applied Biosystems).
Immunochemistry
Immunohistochemistry for PEPT2 was performed on paraffin-embedded kidney biopsy tissues. Kidney biopsies were performed for diagnostic purposes (i.e., exploration of CKD with an eGFR<60 ml/min per 1.73 m2), independent of the present study. Target retrieval was performed by heating the tissue in citrate buffer (DakoCytomation, Les Ulis, France) at pH 6. Endogenous peroxidase was inactivated by incubating the specimen for 10 minutes at room temperature in 0.3% H2O2. The sections were incubated overnight at 4°C with PBS containing 1:50 of anti-SLC15A2 rabbit polyclonal antibody orb253362 (Biorbyt, Cambridge, UK). The immunoreactive proteins were visualized using the Envision + HRP system (DakoCytomation). Finally, tissue sections were counterstained with hematoxylin and mounted using an aqueous mounting medium (DakoCytomation). The primary antibodies were replaced by equal concentrations of rabbit or mouse IgG (DakoCytomation) as negative controls.
Statistical Analyses
The results for the categorical variables are expressed as frequencies and percentages, and as the mean±SD for continuous variables. Associations between the continuous clinical and biologic features of patients were tested using paired t test or Wilcoxon test, or by one-way ANOVA when appropriate. Frequencies were compared for calculating the chi-squared value using the likelihood ratio test. Multivariate analyses were performed using a nominal multiple regression. All tests were two-sided, and a P value <0.05 was considered statically significant. The statistical analyses were performed using JMP 10.0.0 (SAS France).
Disclosures
None.
Acknowledgments
N.P. is funded by grants from the Agence Nationale pour le Recherche (ANR-16-CE14-0019-01), la Fédération Nationale pour l’Aide aux Insuffisants Rénaux, la Fondation du Rein, and l’Agence de la Biomédecine.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Nordmann Y, Puy H, Da Silva V, Simonin S, Robreau AM, Bonaiti C, Phung LN, Deybach JC: Acute intermittent porphyria: Prevalence of mutations in the porphobilinogen deaminase gene in blood donors in France. J Intern Med 242: 213–217, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Chen B, Solis-Villa C, Hakenberg J, Qiao W, Srinivasan RR, Yasuda M, Balwani M, Doheny D, Peter I, Chen R, Desnick RJ: Acute intermittent porphyria: Predicted pathogenicity of HMBS variants indicates extremely low penetrance of the autosomal dominant disease. Hum Mutat 37: 1215–1222, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puy H, Gouya L, Deybach JC: Porphyrias. Lancet 375: 924–937, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Siegesmund M, van Tuyll van Serooskerken AM, Poblete-Gutiérrez P, Frank J: The acute hepatic porphyrias: Current status and future challenges. Best Pract Res Clin Gastroenterol 24: 593–605, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Herrick AL, McColl KE: Acute intermittent porphyria. Best Pract Res Clin Gastroenterol 19: 235–249, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Andersson C, Floderus Y, Wikberg A, Lithner F: The W198X and R173W mutations in the porphobilinogen deaminase gene in acute intermittent porphyria have higher clinical penetrance than R167W. A population-based study. Scand J Clin Lab Invest 60: 643–648, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Marsden JT, Chowdhury P, Wang J, Deacon A, Dutt N, Peters TJ, Macdougall IC: Acute intermittent porphyria and chronic renal failure. Clin Nephrol 69: 339–346, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Andersson C, Lithner F: Hypertension and renal disease in patients with acute intermittent porphyria. J Intern Med 236: 169–175, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Sardh E, Andersson DE, Henrichson A, Harper P: Porphyrin precursors and porphyrins in three patients with acute intermittent porphyria and end-stage renal disease under different therapy regimes. Cell Mol Biol 55: 66–71, 2009 [PubMed] [Google Scholar]
- 10.Pallet N, Mami I, Schmitt C, Karim Z, François A, Rabant M, Nochy D, Gouya L, Deybach JC, Xu-Dubois Y, Thervet E, Puy H, Karras A: High prevalence of and potential mechanisms for chronic kidney disease in patients with acute intermittent porphyria. Kidney Int 88: 386–395, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Döring F, Walter J, Will J, Föcking M, Boll M, Amasheh S, Clauss W, Daniel H: Delta-aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J Clin Invest 101: 2761–2767, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irie M, Terada T, Sawada K, Saito H, Inui K: Recognition and transport characteristics of nonpeptidic compounds by basolateral peptide transporter in Caco-2 cells. J Pharmacol Exp Ther 298: 711–717, 2001 [PubMed] [Google Scholar]
- 13.Bravo SA, Nielsen CU, Frokjaer S, Brodin B: Characterization of rPEPT2-mediated Gly-Sar transport parameters in the rat kidney proximal tubule cell line SKPT-0193 cl.2 cultured in basic growth media. Mol Pharm 2: 98–108, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Sala-Rabanal M, Loo DD, Hirayama BA, Wright EM: Molecular mechanism of dipeptide and drug transport by the human renal H+/oligopeptide cotransporter hPEPT2. Am J Physiol Renal Physiol 294: F1422–F1432, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kamal MA, Keep RF, Smith DE: Role and relevance of PEPT2 in drug disposition, dynamics, and toxicity. Drug Metab Pharmacokinet 23: 236–242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobin C, Flores-Montoya MG, Gutierrez M, Parisi N, Schaub T: δ-Aminolevulinic acid dehydratase single nucleotide polymorphism 2 (ALAD2) and peptide transporter 2*2 haplotype (hPEPT2*2) differently influence neurobehavior in low-level lead exposed children. Neurotoxicol Teratol 47: 137–145, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobin C, Gutierrez M, Alterio H: Polymorphisms of delta-aminolevulinic acid dehydratase (ALAD) and peptide transporter 2 (PEPT2) genes in children with low-level lead exposure. Neurotoxicology 30: 881–887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Shen H, Keep RF, Smith DE: Peptide transporter 2 (PEPT2) expression in brain protects against 5-aminolevulinic acid neurotoxicity. J Neurochem 103: 2058–2065, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Pinsonneault J, Nielsen CU, Sadée W: Genetic variants of the human H+/dipeptide transporter PEPT2: Analysis of haplotype functions. J Pharmacol Exp Ther 311: 1088–1096, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Weinstein JR, Anderson S: The aging kidney: Physiological changes. Adv Chronic Kidney Dis 17: 302–307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies DF, Shock NW: Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 29: 496–507, 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elder G, Harper P, Badminton M, Sandberg S, Deybach JC: The incidence of inherited porphyrias in Europe. J Inherit Metab Dis 36: 849–857, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Wuttke M, Köttgen A: Insights into kidney diseases from genome-wide association studies. Nat Rev Nephrol 12: 549–562, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Pattaro C, Teumer A, Gorski M, Chu AY, Li M, Mijatovic V, Garnaas M, Tin A, Sorice R, Li Y, et al.: Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun 7: 10023, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knütter I, Kottra G, Fischer W, Daniel H, Brandsch M: High-affinity interaction of sartans with H+/peptide transporters. Drug Metab Dispos 37: 143–149, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Lee YS, Kim BH, Kim BC, Shin A, Kim JS, Hong SH, Hwang JA, Lee JA, Nam S, Lee SH, Bhak J, Park JW: SLC15A2 genomic variation is associated with the extraordinary response of sorafenib treatment: Whole-genome analysis in patients with hepatocellular carcinoma. Oncotarget 6: 16449–16460, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R, Tang AM, Tan YL, Limenta LM, Lee EJ: Interethnic differences of PEPT2 (SLC15A2) polymorphism distribution and associations with cephalexin pharmacokinetics in healthy Asian subjects. Eur J Clin Pharmacol 65: 65–70, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Tollånes MC, Aarsand AK, Villanger JH, Støle E, Deybach JC, Marsden J, To-Figueras J, Sandberg S; European Porphyria Network (EPNET) : Establishing a network of specialist Porphyria centres - effects on diagnostic activities and services. Orphanet J Rare Dis 7: 93, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puy H, Deybach JC, Lamoril J, Robreau AM, Da Silva V, Gouya L, Grandchamp B, Nordmann Y: Molecular epidemiology and diagnosis of PBG deaminase gene defects in acute intermittent porphyria. Am J Hum Genet 60: 1373–1383, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]