Abstract
The mammalian distal convoluted tubule (DCT) makes an important contribution to potassium homeostasis by modulating NaCl transport. The thiazide-sensitive Na+/Cl− cotransporter (NCC) is activated by low potassium intake and by hypokalemia. Coupled with suppression of aldosterone secretion, activation of NCC helps to retain potassium by increasing electroneutral NaCl reabsorption, therefore reducing Na+/K+ exchange. Yet the mechanisms by which DCT cells sense plasma potassium concentration and transmit the information to the apical membrane are not clear. Here, we tested the hypothesis that the potassium channel Kir4.1 is the potassium sensor of DCT cells. We generated mice in which Kir4.1 could be deleted in the kidney after the mice are fully developed. Deletion of Kir4.1 in these mice led to moderate salt wasting, low BP, and profound potassium wasting. Basolateral membranes of DCT cells were depolarized, nearly devoid of conductive potassium transport, and unresponsive to plasma potassium concentration. Although renal WNK4 abundance increased after Kir4.1 deletion, NCC abundance and function decreased, suggesting that membrane depolarization uncouples WNK kinases from NCC. Together, these results indicate that Kir4.1 mediates potassium sensing by DCT cells and couples this signal to apical transport processes.
Keywords: slc12a3, thiazide, potassium channels, diuretics, wnk4, spak
Homeostasis requires that cells sense, and respond to, changes in the composition of the fluid bathing them. For epithelia, changes typically occur predominantly on one surface; this requires that basolateral signals are transmitted to apical membranes to permit transport rates across the two membranes to be similar.1 Calcium, for example, is sensed via the G-protein–coupled calcium-sensing receptor that is expressed at the cell surface. At the time this receptor was identified,2 the authors suggested that similar receptors might also exist for other ions such as potassium. Yet in the ensuing quarter century, no similar potassium sensor has been identified. This is likely for two reasons; first, because the difference between intracellular and extracellular potassium concentration, [K+], is much smaller than that for calcium, and second, because K+ dominates the resting membrane conductance of most mammalian cells. For these reasons, the ratio of extra- to intracellular [K+] typically “sets” the interior-negative baseline cell voltage. Further, as extracellular [K+] is present at low concentrations, small changes in extracellular [K+] have substantial effects on membrane voltage. This means that the membrane voltage could act as a “second messenger,” connecting extra- and intracellular events.
In mammals, a single meal may contain more K+ than is present in the extracellular fluid, presenting a substantial physiologic challenge. Rapid movement of K+ from outside to inside cells comprises the first adaptation to such loads; yet the definitive resolution to K+ challenge requires renal K+ excretion, driven by a delicate interplay between the adrenal gland and the kidney distal nephron. When extracellular fluid [K+] is high, aldosterone is secreted by adrenal zona glomerulosa cells. Aldosterone then activates the epithelial Na+ channel, ENaC, which is expressed along the aldosterone-sensitive distal nephron; this stimulates K+ secretion via the potassium channels, ROMK and BK.
Although this scenario is well established, it has become clear recently that the thiazide-sensitive NaCl cotransporter (NCC), expressed exclusively along the apical membrane of distal convoluted tubule (DCT) cells, also plays an essential and nonredundant role in K+ homeostasis. States of high plasma [K+], whether induced by dietary excess,3,4 by intravenous infusion,5 or by blocking3 or deleting6 ENaC, reduce the abundance of phosphorylated (activated) NCC. Conversely, hypokalemia strikingly increases NCC abundance and activity.7,8 That regulation of NCC is required for normal K+ homeostasis is further indicated by the [K+] derangements that occur in two monogenic human diseases, Gitelman syndrome (GS) and familial hyperkalemic hypertension (FHHt, also called pseudohypoaldosteronism type 2). GS is characterized by profound hypokalemia, resulting exclusively from massive renal K+ wasting owing to NCC processing abnormalities. FHHt is characterized, conversely, by hyperkalemia from K+ retention, resulting largely from excess NCC activity. Clearly, mammals cannot maintain normal K+ balance if NCC activity cannot be modulated normally; on the basis of such findings, we recently proposed that the primary physiologic role of NCC is in K+ homeostasis.3
We postulated that NCC activity in DCT cells is regulated directly by plasma [K+], acting through intracellular chloride activity.3 Chloride activity, known to modulate WNK kinases,8,9 is believed to be sensitive to membrane potential, because it moves across the basolateral membrane of DCT cells at least in part via chloride channels.3 A 40 pS K+ channel that is believed to comprise Kir4.1 (encoded by kcnj10)/kir5.1 (encoded by kcnj16) heteromers appears to be the predominant K+ conductive pathway along the DCT.10,11 Mutations in one component of this pathway, KCNJ10, cause EAST/SeSAME syndrome in humans, with a renal phenotype resembling GS.12,13 Thus, converging evidence suggests that Kir4.1 might play a role in renal K+-sensing. Previous attempts to study Kir4.1 function in rodents have been limited, because deletion of kcnj10 causes neurologic dysfunction and neonatal death,12,14 preventing thorough physiologic characterization. Nevertheless, previous work, carried out in neonatal mice, suggested that deletion of kcnj10 does affect basolateral membrane voltage and NCC abundance in the DCT, but these experiments may have been confounded by developmental changes in nephron structure and function. Here, to test the hypothesis that Kir4.1 (likely with Kir5.1) acts as the DCT’s K+-sensor, and is essential for modulating NCC activity and salt transport along the DCT, we developed an inducible kidney-specific (KS) kcnj10 knockout mouse.
Results
Kir4.1 Deletion in Adult Mouse Kidney
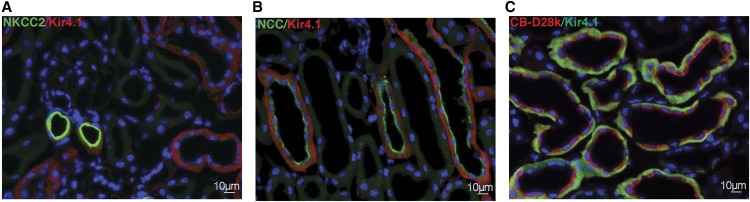
Kir4.1 is expressed along some, but not all, thick ascending limb segments (Figure 1A), as well as along the entire DCT (Figure 1B) and into more distal segments (Figure 1D, Supplemental Figure 1), in a basolateral orientation.12,15 The physical appearance and body weight of mice after deletion of kcnj10 with doxycycline were identical to kcnj10fl/fl mice (Supplemental Figure 1, Table 1). KS-kcnj10−/− mice live for at least 5 months after the doxycycline treatment.
Figure 1.
NCC and Kir4.1 are co-expressed along the DCT. Kir4.1 localized at the basolateral membrane of cTAL, DCT, and connecting tubule/collecting duct in Kcnj10fl/fl mice. Colocalization of Kir4.1 and NKCC2 (A). Kir4.1 (red) was localized in cTAL (NKCC2, green). Colocalization of Kir4.1 and NCC (B). Kir4.1 (red) colocalized in DCT (NCC, green). Colocalization of Kir4.1 and CB-D28k in Kcnj10fl/fl (C). Kcnj10 (green) was localized in DCT2 and connecting tubule, both of which express calbindin D 28K (CB D28k, red). Original magnification, ×400.
Table 1.
Effects of KS-Kcnj10 deletion on biochemical parameters in adult mice fed with a normal diet
| Parameter | Kcnj10fl/fl | KS-Kcnj10−/− |
|---|---|---|
| Body weight, g | 24.75±0.8 | 22.94±1.1 |
| Na, mmol/L | 143.3±0.5 | 142.3±0.6 |
| K, mmol/L | 3.7±0.1 | 2.9±0.1a |
| Cl, mmol/L | 111.5±0.9 | 105.9±0.6b |
| Ca, mmol/L | 1.2±0.02 | 1.1±0.01 |
| TCO2, mmol/L | 19.7±0.7 | 26.4±1.0b |
| HCT, % | 36.2±0.4 | 39.0±0.5c |
| Hb, g/dl | 12.2±0.2 | 13.3±0.2c |
| BUN, mg/dl | 22.4±1.4 | 38.2±2.9b |
| Mg, mg/dl | 2.4±0.1 | 2.3±0.1 |
Values are mean±SEM. n=8 per group. TCO2, total carbon dioxide; HCT, hematocrit; Hb, hemoglobin.
P<0.05.
P<0.001 versus Kcnj10fl/fl mice.
P<0.01.
Basolateral DCT K Channels in Control and KS-kcnj10−/− Mice
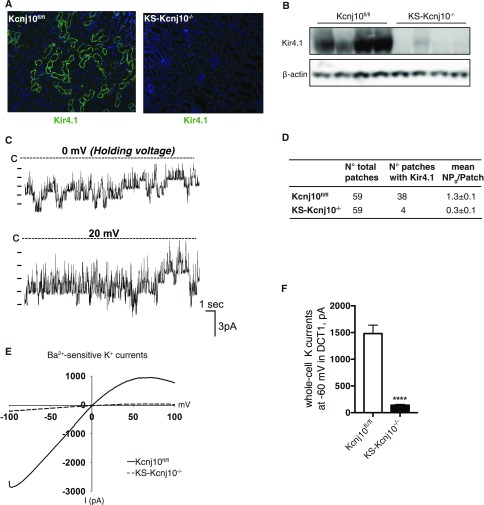
After 2 weeks of induced deletion with doxycycline, Kir4.1 was nearly absent, as assessed by both immunofluorescence and Western blot (Figure 2, A and B). In control (kcnj10fl/fl) mice, an inwardly-rectifying 40 pS K+ channel was highly expressed in the basolateral membrane of the DCT1 cells (the first segment of the DCT; Figure 2C), as reported previously.14,16 These 40 pS K+ channels were detected in the basolateral membrane of DCT1 cells from 38 patches in 59 experiments, using control mice. The mean channel activity (NPo) was 1.3±0.1 (n=10) (Figure 2D), a result similar to that observed in neonatal mice.14 Moreover, this 40 pS K+ channel was the only type of K+ channel detected in the basolateral membrane of both DCT1 and DCT2 segments (DCT2 is the more distal segment that also expresses ENaC). In contrast, 40 pS channels were detected in only four DCT1 patches from 59 experiments conducted using KS-kcnj10−/− mice. Channel activity, defined as NPo, was also much lower in these mice (0.3±0.1, n=10), providing strong evidence that Kir4.1 is the key component of the 40 pS K+ channel.
Figure 2.
K+ channel activity is reduced in KS-kcnj10−/− mice. (A) Representative immunostaining of Kir4.1 in kcnj10fl/fl and KS-kcnj10−/− mice. Original magnification, ×100. (B) Western analysis of Kir4.1 from kcnj10fl/fl and KS-kcnj10−/− mouse kidneys. Actin was the loading control. (C) Single channel recording of a 40 pS inwardly-rectifying K channel from the basolateral membrane of DCT1 in control mice. The experiments were performed in a cell-attached patch with 140 mM K in the pipette and 140 mM Na/5 mM K in the bath. The closed level is indicated by a dotted line and “C.” (D) The probability of finding the 40 pS K channel and channel activity (NPo) in DCT1 of kcnj10fl/fl and KS-kcnj10−/− mice. (E) Whole-cell recording showing Ba2+-sensitive K+ currents in DCT1 of kcnj10fl/fl (solid line) and KS-kcnj10−/− KO (dotted line) mice. The experiments were performed with a ramp protocol from −100 to 100 mV with symmetrical 140 mM KCl in the bath and in the pipette. (F) Bar graph summarizing the above results measured at −60 mV (n=5) is shown in the bottom panel. ***P<0.001 by t test.
The notion that Kir4.1 plays a predominant role in contributing to basolateral K+ conductance in the DCT is further confirmed by the experiments in which we used the whole-cell recording to measure K+ currents. Because there is no electrically detectable apical K+ channel in the DCT1,11 and because these cells are not coupled electrically, the whole-cell K+ current represents basolateral K+ channel activity, as documented previously.11,14 Figure 2E shows whole-cell currents from control and KS-kcnj10−/− mouse DCT1 cells with symmetrical 140 mM KCl in the bath and in the pipette. As summarized in Figure 2F, the mean whole K+ current from DCT1 at −60 mV was −1480±130 pA/cell (n=5) in cells from control mice, and −141±15 pA/cell (n=5) in KS-kcnj10−/− mice. Thus, we have successfully generated KS Kir4.1 knockout (KS-kcnj10−/−) mice that are viable for further characterization.
KS-Kcnj10−/− Mice Exhibit Hypokalemic Metabolic Alkalosis with Urine Na+ and K+ Wasting
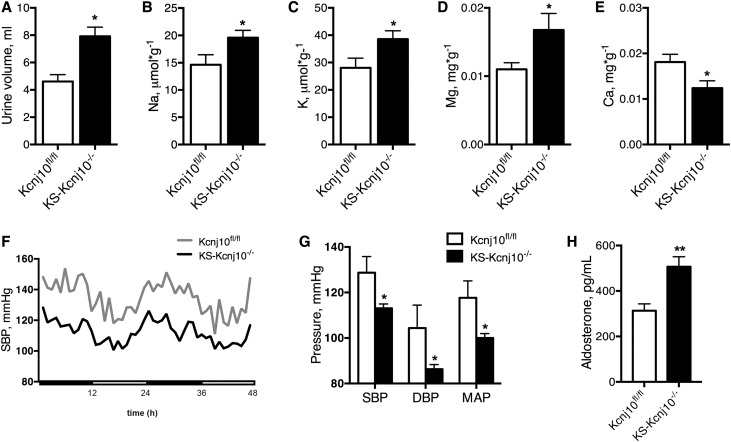
KS-Kcnj10−/− mice exhibited hypochloremic metabolic alkalosis, with hypokalemia (Table 1). The urine volume was higher in KS-Kcnj10−/− mice than in controls, with greater rates of urine K+ and Na+ excretion as well as hypocalciuria and hypermagnesuria (Figure 3, A–E). The hematocrit was also higher (Table 1). KS-Kcnj10−/− adult mice had lower BP (systolic BP [SBP] 113.1±1.9 mmHg) along with elevated plasma aldosterone level (507±44 pg/ml) compared with Kcnj10fl/fl mice (SBP 128.7±7.1 mmHg; aldosterone level 313±30 pg/ml) (Figure 3, F–H), further supporting an extracellular fluid volume–depleted phenotype.
Figure 3.
KS-Kcnj10−/− mice fed with standard diet exhibit Na+ and K+ wasting and hypotension. Twenty-four-hour urine collection reveals a significant increase in urine volume output (A), and urine excretion of sodium (B), potassium (C), and magnesium (D), whereas urine calcium excretion (E) decreased in KS-Kcnj10−/− compared with Kcnj10fl/fl mice. Bars are mean±SEM (n=8 in each group). *P<0.05 versus Kcnj10fl/fl. (F) SBP recorded for 48 hours (n=4 for Kcnj10 fl/fl and n=7 for KS-Kcnj10−/− mice). (G) Systolic, median, and diastolic BP average in 48 hours. (H) Plasma aldosterone levels (n=8 per group) increased in KS-Kcnj10−/− mice. Bars are mean±SEM. *P<0.05, ***P<0.001 versus Kcnj10fl/fl mice.
The DCT Basolateral Membrane is Depolarized in KS-kcnj10−/− Mice
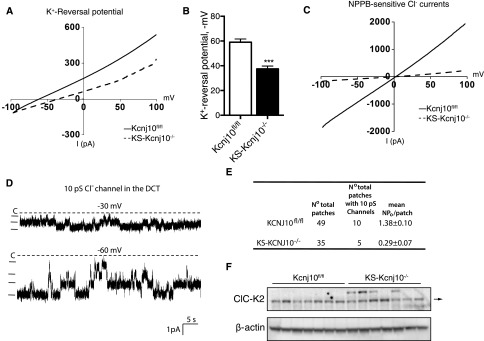
The basolateral K+ conductance participates in generating the cell membrane potential. Therefore, a decrease in the K+ conductance induced by the deletion of Kir4.1 is expected to reduce the negativity of DCT membrane potential. This possibility was examined by measuring the K+ reversal potential (an index of the cell membrane potential) with 140 mM NaCl and 5 mM KCl in the bath, and 140 mM KCl in the pipette. Figure 4A is a typical recording showing the currents measured in the DCT1 cells, which were clamped from −100 to 100 mV with a ramp protocol. The K reversal potential was −59±2.6 mV (n=5) in the DCT1 of WT mice, but was only −37.5±2.3 mV (n=4) in the KS-kcnj10−/− mice (Figure 4B). Chloride exits across the basolateral membrane of thick ascending limb and DCT cells, at least in part, via the chloride channel ClC-K2.17 Because the negative membrane potential is the main driving force for Cl− to exit the cell through the basolateral membrane, we expected that basolateral Cl channel activity would be altered in KS-kcnj10−/− mice. Figure 4C shows NPPB-sensitive Cl− currents in adult control and KS-kcnj10−/− mice. It is apparent that Cl− currents in the DCT1 of KS-kcnj10−/− mice (−98±12 pA/cell at −60 mV, n=5) were significantly lower than those of control mice (−1140±80 pA/cell at −60 mV, n=5). The probability of finding Cl channels was lower in KS-kcnj10−/− mice than in controls (Figure 4D), as was the channel activity, defined as NPo in the patch (Figure 4E). This decrease raised the possibility that chloride channel expression was altered by Kir4.1 deletion. To test this, we estimated ClC-K2 abundance by immunoblot. No difference was apparent (Figure 4F), a finding supported by immunofluorescent analysis (Supplemental Figure 2). This suggests that loss of Kir4.1 channels also inhibits NPPB-sensitive Cl− channel activity in the DCT1, through an allosteric mechanism (barium application had the same effect, data not shown).
Figure 4.
Deletion of Kir4.1 depolarized membrane and suppressed the basolateral chloride conductance in the DCT1. (A) Perforated whole-cell recording showing K reversal potential in the DCT1 cells of adult kcnj10fl/fl and KS-kcnj10−/− mice. (B) Bar graph summarizes the results of experiments in which K reversal potentials of the DCT were measured with the perforated whole-cell recording in kcnj10fl/fl (n=5) and KS-kcnj10−/− mice (n=4). For measurement of K+ reversal potential, the bath solution contains 140 mM NaCl and 5 mM KCl, whereas the pipette solution has 140 mM KCl. (C) NPPB (10 μM)-sensitive Cl− currents in the DCT1 of adult kcnj10fl/fl (solid line) and KS-kcnj10−/− (dotted line) mice. The measurements were carried out with whole-cell recording with symmetrical 140 mM KCl in the bath and pipette. (D) Single channel recording showing 10 pS Cl channel activity in the DCT of WT mice. The experiments were performed in a cell-attached patch and the holding potential was indicated at the top of each trace. The channel closed line is indicated by a dotted line and “C.” (E) Results of experiments in which the Cl channel activity was examined with single channel recording. (F) Immunoblot of ClC-K2 abundance. No differences were noted.
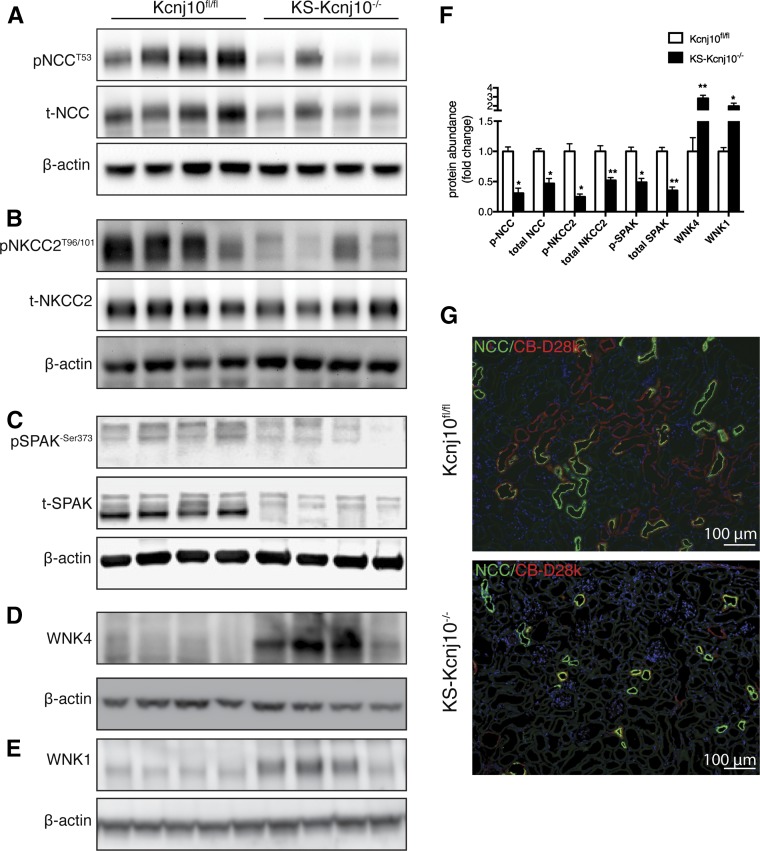
KS-kcnj10−/− Mice Exhibit Altered SPAK, NCC, NKCC2, and WNK4 Abundance
To determine whether KS-Kcnj10−/− mice have alterations in transporters and regulatory kinases along the DCT and TAL, we evaluated the abundance of WNK4, SPAK, and total and phosphorylated NCC and NKCC2 by Western blot. The abundance of WNK4, detected using a well validated antibody,18 was significantly greater in KS-Kcnj10−/− mice than in controls (Figure 5A), as was WNK1 (Figure 5B). SPAK is the predominant kinase that phosphorylates and activates NCC,19,20 so it was surprising that there appeared to be a “SPAK isoform switch”21,22 in KS-Kcnj10−/− mice (Figure 5C). Despite this “switch,” the abundance of phosphorylated SPAK, an indication of active SPAK, was lower in the KS-kcnj10−/− mice than in controls (Figure 5D).
Figure 5.
KS deletion of Kir4.1 decreased abundances of total and phosphorylated form of NCC, NKCC2, and modulated WNK-SPAK kinases. Representative immunoblot showing abundance of total and NCC phosphorylated at threonine 53 (A); abundance of total and NKCC2 phosphorylated at threonine 96/101 (B); abundance of total and SPAK phosphorylated at Serine 373 (C); total WNK4 abundance (D); WNK1 abundance (E). Protein abundance was normalized using β-actin. (F) Quantification of immunoblot results. Bars are mean±SEM. * P<0.001, **P<0.001 versus Kcnj10fl/fl mice. (G) Representative immunostaining for NCC on Kcnj10fl/fl and KS-Kcnj10−/− mouse kidneys. Original magnification, ×100.
As expected,14 phosphorylated and total NCC abundance was lower in KS-Kcnj10−/− mice than in controls (Figure 5E). The abundance of phosphorylated and total NKCC2 (Figure 5F) was also lower in KS-kcnj10−/− mice than in controls. These data are shown quantitatively in Figure 5G. Immunofluorescence confirmed that deletion of Kir4.1 reduced NCC abundance (Figure 5H), although this was not analyzed quantitatively.
KS-Kcnj10−/− Mice Exhibit Altered Diuretic Responses
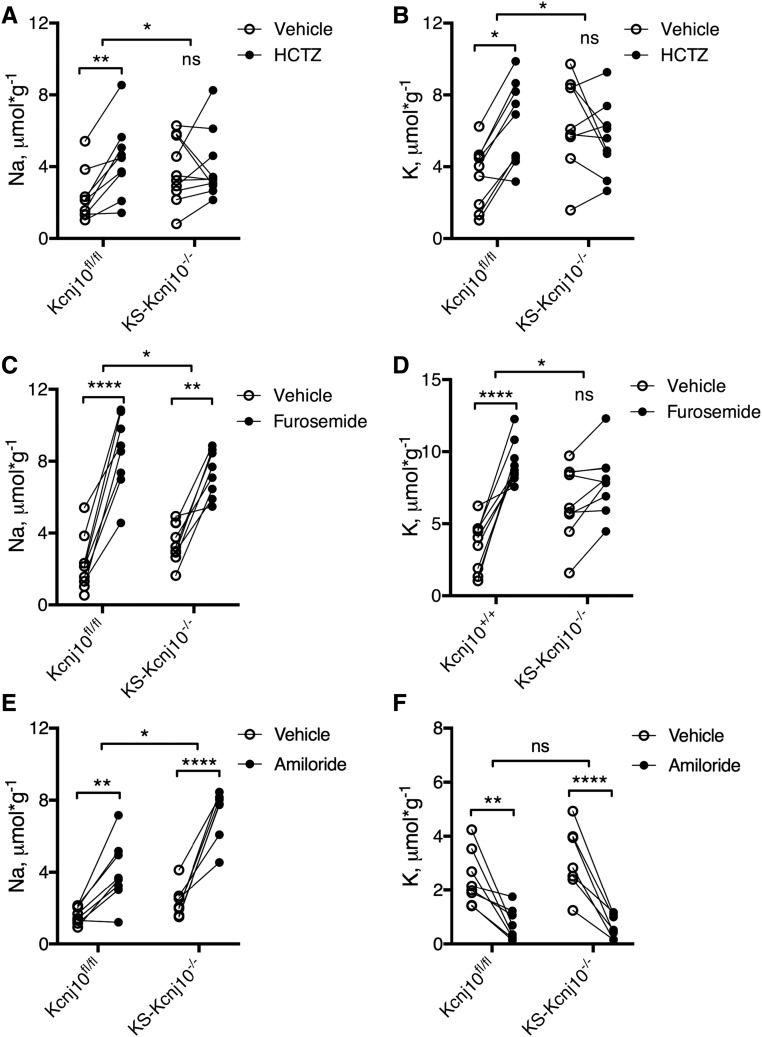
The studies above suggest that NCC activity in DCT is compromised in KS-kcnj10−/− mice; they also suggest that the activity of NKCC2 might be affected. To determine whether the observed changes had functional consequences, the Na+ and K+ excretory response to diuretics that act along the TAL, DCT, and connecting tubule/collecting duct were assessed. DCT function was evaluated by using hydrochlorothiazide (HCTZ), an inhibitor of NCC function. Figure 6, A and B show that HCTZ increased urine Na+ and K+ excretion in control mice, as expected (Na+: vehicle 2.3±0.5 and HCTZ 4.4±0.7 μmol*g−1, P<0.01; K+: vehicle 3.5±0.6 and HCTZ 6.4±0.8 μmol*g−1, P<0.05), whereas it did not in KS-Kcnj10−/− mice (Na+: vehicle 3.8±0.6 and HCTZ 4.0±0.6; K+: vehicle 6.5±0.8 and HCTZ 5.6±0.8). This indicates that thiazide-sensitive NaCl transport is essentially eliminated by kcnj10 deletion.
Figure 6.
KS-Kcnj10−/− adult mice show impaired response to HCTZ and furosemide. (A) Urine sodium and (B) potassium excretion before and after a single dose of HCTZ. (C) Urine sodium and (D) potassium excretion before and after a single dose of furosemide. (E) Urine sodium and (F) urine potassium after a single dose of amiloride (n=8–9). *P<0.05, **P<0.01, ****P<0.001 analyzed by two-way ANOVA.
We next investigated TAL function by evaluating the effect of furosemide, an inhibitor of NKCC2. Although furosemide induced an increase in urinary Na excretion in both kcnj10fl/fl (n=9) and KS-Kcnj10−/− (n=9) mice (Figure 6C), the response was slightly blunted in KS-kcnj10−/− mice (Kcnj10fl/fl: vehicle 2.3±0.5 and furosemide 8.5±0.7 μmol*g−1, P<0.001; KS-Kcnj10−/−: vehicle 3.8±0.6 and furosemide 7.3±0.5 μmol*g−1, P<0.001). Additionally, whereas furosemide increased in K+ excretion in control mice (Figure 6D), the effect in KS-kcnj10−/− mice was not significant; thus, the response differed in the two strains (P<0.05 by two-way ANOVA). Thus, deletion of kir4.1 from the kidney of adult mice appears to reduce TAL function modestly.
ENaC activity, estimated as the change in urinary Na+ excretion induced by amiloride, was greater in the KS-kcnj10−/− mice than in control (Figure 6E). Although the effect of amiloride on K+ excretion was not different (Figure 6F), amiloride did reduce K+ excretion to near zero in both control and KS-kcnj10−/− mice.
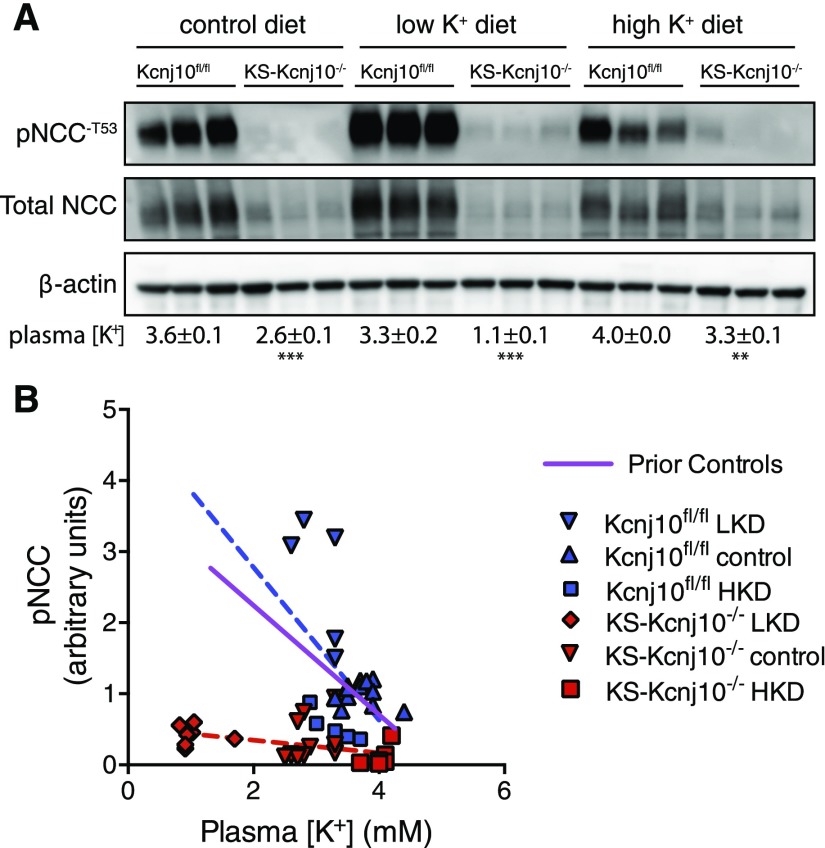
The Effect of Plasma [K+] on pNCC is Nearly Abrogated in KS-Kcnj10−/− Mice
To test whether Kir4.1 plays an essential role in the DCT’s ability to sense plasma [K+], mice were fed diets with low, high, and normal K+ content, and the relative abundance of pNCC was determined, as described.8 Low plasma [K+] did not increase pNCC abundance in KS-kcnj10−/− mice, as it did in control mice (Figure 7A). To examine this quantitatively, pNCC abundance was evaluated as a function of plasma [K+]. Figure 7B compares the previously published relationship between pNCC and plasma [K+]8 with that in kcnj10fl/fl mice, showing good agreement (blue and purple lines). This relationship was strikingly altered in KS-kcnj10−/− mice, where the slope was near to zero. The control and kcnj10−/− slopes are statistically different (P=0.01).
Figure 7.
NCC abundance is dissociated from plasma [K+] in KS-kcnj10−/− mice. (A) Western blot of NCC and pNCC abundances in control kcnj10fl/fl and KS-kcnj10−/− mice. Values below blot are plasma [K+] for the designated groups±SEM. (B) Scatter plot of the relation between plasma [K+] and relative pNCC abundance in mice. The purple regression line is from data previously published;8 this serves as an historical control. Results from control mice (kcnj10fl/fl) are shown in blue (pNCC=[−1.1× plasma [K+]]+4.9), with the diets as indicated and superimposed prior results. Results shown in red are from KS-kcnj10−/− mice (pNCC=[−0.09 plasma [K+]]+0.53). The slopes of the regression lines are significantly different, F=7.3, P=0.01.
Discussion
Mammals tolerate variations in potassium intake because they adjust urinary excretion appropriately. Although total body K+ abundance is important for physiologic processes, plasma [K+] typically determines K+ toxicity. When plasma [K+] deviates from normal, serious adverse consequences, such as cardiac arrhythmias and death, can result.23,24
It has been recognized recently that the DCT plays an essential and nonredundant role in K+ homeostasis. We suggested that DCT cells sense plasma [K+] through effects on membrane voltage and intracellular chloride.3 On the basis of its conductive properties and its localization, the channel involved likely comprises heteromers of kir4.1 with kir5.1.16 The current results show that this channel is required for DCT cells to sense plasma [K+] and modulate NaCl entry. Such modulation is required to address cellular constraints and to adjust systemic K+ balance. The current results, therefore, provide new insight into an adrenal/renal axis that maintains K+ excretion within normal bounds.3
Recent results have indicated that the DCT and NCC play previously unrecognized, but essential, roles in adjusting urinary potassium excretion. Deficiency of NCC, as occurs in GS, causes profound renal K+ wasting, and hypokalemia, essential criteria for the diagnosis of GS.25 Conversely, activation of NCC, as occurs in FHHt,26,27 causes potassium retention and hyperkalemia, findings required for its diagnosis.28 The present results indicate that DCT segments in mature mice, in which kcnj10 is deleted, no longer sense low plasma [K+] or activate NCC; they therefore develop unrelenting K+ wasting and hypokalemia. Hypokalemia is typically a potent stimulus to NCC,3,4,7 so the failure to activate NCC in KS-kcnj10−/− mice provides strong evidence for the centrality of Kir4.1 in this process. Attempts to activate NCC, using low K+ chow in the KS-kcnj10−/− mice, not only were ineffective, but led to potentially fatal levels of hypokalemia (Figure 7A).
It has long been known that kidneys play a dominant role in [K+] balance, except in the short term, by responding to plasma [K+] and aldosterone, as well as to circadian and perhaps gut factors.29 Potassium excretion derives predominantly from [K+] secreted along the aldosterone-sensitive distal nephron, a segment comprising the connecting tubule and collecting duct, and perhaps including a portion of the DCT.30 Along this segment, sodium reabsorption via ENaC at the apical membrane generates a transepithelial voltage, oriented with the lumen negative relative to the basolateral side. This voltage drives K+ secretion through ROMK and BK.31 Potassium secretion along this segment is stimulated by aldosterone, a hormone secreted by adrenal zona glomerulosa cells.
As noted, however, the DCT and NCC also play essential roles in [K+] homeostasis.3,4,32–35 These roles must be indirect, however, as NCC itself does not transport [K+] and the proximal portion of the DCT secretes very little or no K+.36 Despite this, NCC abundance and activity are exquisitely sensitive to plasma [K+], which can override the effects of aldosterone and extracellular fluid volume.3,4,37 We suggested that NCC is regulated by the WNK/SPAK signaling pathway in response to changes in plasma [K+], through effects on cell voltage and intracellular chloride.3,14 Here, we establish that the 40 pS K+ channel, comprising Kir4.1 and likely Kir5.1, is absolutely required for DCT cells to respond to plasma [K+] and maintain normal K+ homeostasis.
Treatment with doxycycline in adult kcnj10fl/fl mice led to nearly complete renal deletion of Kir4.1, as detected by Western blot, immunofluorescence, and patch clamp, but was otherwise tolerated well. Importantly, whole-cell K+ currents were almost absent in DCT from kcnj10−/− mice, demonstrating that, unlike in TAL and collecting duct,38,39 Kir4.1 is essential for K+ to exit DCT cells. Thus, the reversal potential was strikingly depolarized in DCT1 cells from KS-kcnj10−/− mice, confirming the central role of Kir4.1 in setting the membrane potential of these cells. Interestingly, Cl− currents were also lower in KS-kcnj10−/− mice than in controls, suggesting that basolateral K+ and Cl− conductances are regulated pari passu.
Coupling of ion transport across apical and basolateral membranes has long perplexed investigators.1,40,41 The DCT, however, presents a unique challenge in this regard because the apical membrane lacks any significant K+ (or Na+) conductance,11 so the signal to the apical membrane is not electrical. The present results, coupled with prior studies,3,8,19,42,43 suggest that one role of the WNK/SPAK pathway, at least in DCT1, is to couple apical and basolateral transport. Dawson emphasized40 that such coupling can serve both as a systemic control process (modulating systemic K+ balance) and a local homeostatic one (the need to maintain cell composition and volume). It is not surprising, therefore, that DCT cells of an individual with EAST/SeSAME syndrome were found to be substantially atrophic.12 Conversely, treatment with furosemide, leading to hypokalemia and extracellular fluid volume depletion, is associated with DCT cell hypertrophy.44
The abundance of NCC, and its functional activity, were substantially lower in KS-kcnj10−/− mice than in controls, consistent with predictions from humans with EAST/SeSAME syndrome,12,13 and from constitutive neonatal kcnj10−/− mice.14 As SPAK is the predominant kinase that phosphorylates and activates NCC,21,22,45 it seemed likely that SPAK activity in the KS-kcnj10−/−mice would be lower. This was confirmed, as phosphorylated SPAK abundance was lower in these mice, although it appeared to have undergone an “isoform switch,” a change typically associated with SPAK activation. Surprisingly, given the low pNCC abundance and low NCC activity, WNK1 and WNK4 abundances were strikingly higher in the KS-kcnj10−/− mice. In vivo, WNK4 abundance often parallels phosphorylated NCC,46 as in WNK4−/− mice,47 and in mouse models of FHHt.26,27 A possible explanation for the fact that DCT cells from mice that lack Kir4.1 have low phosphorylated SPAK and NCC, despite high WNK4 abundance, is that high intracellular [Cl−] has turned WNK4 from its active state to an inactive one.8 Alternatively, the sites of WNK1, WNK4, and SPAK expression may have been altered, a possibility currently under investigation.
The phenotype of KS-kcnj10−/− mice contrasts strikingly with that of mice lacking Kir5.1 (kcnj16),48 despite the fact that Kir4.1 and Kir5.1 form heteromeric channels. Although kcnj16−/− mice have hypokalemia, they have acidosis rather than alkalosis, and hypercalciuria, rather than hypocalciuria; further, the activity of NCC is increased, not decreased.48 This likely results from the appearance of a 23 pS K+ channel in the kcnj16−/− mice, which preserves the K+ conductance, and may be homomeric Ki4.1.48
Surprisingly, kcnj10−/− mice exhibited moderate salt wasting even on normal salt intake; they also had BP that was lower than controls. These phenotypic effects differ from those of NCC deletion, at least in mice.49 The fact that there is more apparent salt wasting in the KS-Kcnj10−/− mice than in slc12a3−/− mice may involve the decreased activity of NKCC2 along the thick ascending limb in addition to disruption of the DCT, as the effect of furosemide, a diuretic acting along the loop segment, was reduced in the KS-kcnj10−/− mice. These observations are in contrast to observations in mice with constitutive kcnj10 deletion, studied in the neonatal period, pointing to the importance of the new model. In those mice, kcnj10 disruption, and loss of the 40 pS basolateral conductance, appeared to be compensated along the TAL. Further studies will be required to resolve these differences.
The current results indicate that pNCC abundance is nearly independent of plasma [K+] in KS-kcnj10−/− mice, in contrast to control mice, where [K+] appears to be the dominant modulator (see Figure 7B). Coupled with the observation that conductive K+ movement across the basolateral membrane of DCT cells is nearly absent in KS-kcnj10−/− mice (see Figure 2F), the present results provide strong support for a model in which Kir4.1/Kir5.1 channels sense basolateral (extracellular fluid) [K+] in the DCT, thereby permitting this nephron segment to act as a systemic K+ gatekeeper. The results help to explain the near identical presentations of GS and EAST syndrome tubulopathies, and illuminate how the kidney can control Na+ and K+ excretion independently. Manipulation of this DCT control system is an attractive target for drug development.
Concise Methods
Generation of Inducible KS-Kcnj10 Knockout Mice
All animal studies were approved by the Animal Care and Use Committee of Oregon Health and Science University IS00918, A858 and the Institutional Animal Care and Use Committee at New York Medical College #82–2-1114.
Kcnj10 deletion was carried out in 8-week-old mice homozygous for floxed Kcnj10 gene and hemizygous for Pax8-rtTA/LC-1 transgene by providing doxycycline (5 mg/ml, 5% sucrose) in the drinking water for 2 weeks. This was followed by at least 2 additional weeks without doxycycline treatment, before performing experiments. Littermate mice of the same age and genetic background drinking 5% sucrose were used as controls (Kcnj10fl/fl). Complete procedure and genotyping are described in the Supplemental Material.
Preparation of the DCT
Mice were euthanized by cervical dislocation and the abdomen was opened to expose the left kidney. We perfused the left kidney with 2 ml L-15 medium (Life Technologies) containing type 2 collagenase (250 unit/ml) and then removed the collagenase-perfused kidney. The renal cortex was separated and further cut into small pieces for additional incubation in collagenase-containing L-15 media for 30–50 minutes at 37°C. The tissue was then washed three times with fresh L-15 medium and transferred to an ice-cold chamber for dissection. The method for identifying DCT1/DCT2 was described previously.11 The isolated DCT tubules were placed on a small cover glass coated with poly-lysine, and the cover glass was placed on a chamber mounted on an inverted microscope.
Patch-Clamp Experiments
Patch-clamp techniques were used to study the basolateral membrane of both early DCT (DCT1) and late DCT (DCT2). A Narishige electrode puller (Narishige, Japan) was used to manufacture the patch-clamp pipettes from borosilicate glass (1.7-mm OD). The resistance of pipettes was 5 MΩ (for single channel recording) or 2 MΩ (for whole-cell recording) when filled with solution containing (in mM) 140 KCl, 1.8 MgCl2, and 10 HEPES (pH 7.4). For the measurement of K+ reversal potential and single channel recording, the tubule was superfused with a bath solution containing 140 mM NaCl, 5 mM KCl, 1.8 mM MgCl2, 1.8 mM CaCl2, and 10 mM HEPES (pH 7.4).
Single Channel Recording
Single K channel currents were recorded with an Axon200B amplifier (Axon), low-pass filtered at 1 KHz, and digitized by an Axon interface (Digidata 1332) with sampling rate of 4 KHz. For the calculation of channel numbers, we selected a channel recording at least 10 minutes long. We determined the channel open probability (Po) from the channel number (N) and NPo (a product of channel number and open probability), which was calculated from data samples of 60 seconds’ duration in the steady state. NPo was determined using the following equation:
where ti is the fractional open time spent at each of the observed current levels. The channel conductance was determined by measuring the current amplitudes over several voltages.
Whole-Cell Recording
An Axon 200A amplifier was used for the measurement of K+ reversal potential, Ba2+-sensitive K currents, and NPPB-sensitive Cl currents. For measuring K+ reversal potential, the tip of the pipette was filled with pipette solution containing (in mM) 140 KCl, 1.8 MgCl2, 1 EGTA, and 5 HEPES (pH 7.4). The pipette was then back-filled with the pipette solution containing amphotericin B (20 μg/0.1 ml). The bath solution was the same as the one we used to perform the single channel recordings. For the measurement of whole-cell Ba2+-sensitive K current and NPPB-sensitive Cl− current, the bath solution contained (in mM) 140 KCl, 1.8 MgCl2, 1.8 CaCl2, and 10 HEPES (pH=7.4). After forming a high resistance seal (>2 GΩ), the membrane capacitance was monitored until the whole-cell patch configuration was formed. The currents were low-pass filtered at 1 KHz, digitized by an Axon interface with 4 KHz sampling rate (1440A; DigiData). Data were analyzed using the pClamp software system 9.0 (Axon).
Urine and Serum/Plasma Analysis
KS-Kcnj10 and Kcnj10fl/fl mice were housed in individual metabolic cages and fed with gel control diet (adjusted to 0.49% NaCl, 0.8% KCl). Urine was collected for 24 hours under saturated mineral oil. Urine sodium and potassium concentrations were measured using a dual-channel flame photometer (Cole-Parmer Instrument, Vernon Hills, IL). Urine calcium and magnesium were measured by using colorimetric reagent sets (cat C7503–120 and HM929–120, respectively; Pointe Scientific INC, Canton, MI).
Blood Chemistry
Blood was obtained through terminal cardiac puncture and analyzed using an i-STAT Portable Clinical Analyzer (Abbott Pointe, Princeton, NJ). Plasma aldosterone levels were measured by ELISA kit (cat IB79134; IBL-America, Minneapolis, MN).
BP
SBP, diastolic BP, and median artery pressure were measured by radiotelemetry probes (model TA11PA-C10; Data Science International) implanted into the left carotid artery. BP was recorded for 48 hours after 10 days of surgery recovery.
Immunoblotting
Whole kidney protein extract was separated on 4%–12% (wt/vol) Bis-Tris gel (Novex; ThermoFisher Scientific) and transferred overnight to PVDF membrane. The membranes were incubated overnight at 4°C with primary (antibodies and specific conditions are described in the Supplemental Material). Protein bands were visualized by chemiluminescence (ECL plus; Amersham Bioscience) and images captured using PXi gel imaging system (Syngene). Densitometry analysis was performed using ImageJ (https://imagej.nih.gov/ij/).
Immunofluorescence
Kidneys were fixed by retrograde abdominal aortic perfusion of 3% paraformaldehyde and frozen in OCT. Five micrometer sections were washed in 1× PBS and permeabilized with 0.5% Triton ×100 for 30 minutes, followed by 1 hour of blockade in 5% nonfat milk or BSA in PBS. Primary antibody in 5% nonfat milk or BSA was incubated for 1 hour. Sections were incubated for 1 hour with 1:800 Cy3 (Invitrogen, Carlsbad, CA) or FITC-conjugated antibody (Zymed Laboratories, San Francisco, CA).
Statistical Analyses
Data were analyzed using Prism v6 (GraphPad software Inc., La Jolla, CA). Comparisons between two groups were performed using parametric t test. Comparisons between more than two groups were performed using one-way ANOVA followed by Tukey post hoc test. P values <0.05 were considered statistically significant. Data are presented as the mean±SEM.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Institutes of Health (DK051496 to D.H.E. and C.L.Y., DK098141 to J.A.M., and DK54983 to W.-H.W) and the Department of Veterans Affairs (1I0BX002228-01A1 to D.H.E.).
Portions of this work were presented at Experimental Biology, April 6, 2016, San Diego, CA.
D.H.E. and W.-H.W. conceived of the study with C.A.C., X.-T.S., J.A.M., and C.-L.Y. C.A.C. and X.-T.S. performed the experiments, with assistance from M.X.W., A.S.T., and D.-H.L. C.A.C., X-T.S., D.H.E., and W.-H.W. wrote the manuscript. All authors approved the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016090935/-/DCSupplemental.
References
- 1.Diamond JM: Transcellular cross-talk between epithelial cell membranes. Nature 300: 683–685, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC: Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ: K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl- cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA: Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrier R, Boscardin E, Malsure S, Sergi C, Maillard MP, Loffing J, Loffing-Cueni D, Sorensen MV, Koesters R, Rossier BC, Frateschi S, Hummler E: Severe salt-losing syndrome and hyperkalemia induced by adult nephron-specific knockout of the epithelial sodium channel alpha-subunit. J Am Soc Nephrol 27: 2309–2318, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitzthum H, Seniuk A, Schulte LH, Müller ML, Hetz H, Ehmke H: Functional coupling of renal K+ and Na+ handling causes high blood pressure in Na+ replete mice. J Physiol 592: 1139–1157, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang G, Ellison DH: Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ: Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7: ra41, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M: Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Zhang C, Wang L, Thomas S, Wang K, Lin DH, Rinehart J, Wang WH: Src family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10 protein. J Biol Chem 288: 26135–26146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R: Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP: Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A 106: 5842–5847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Wang L, Zhang J-H, Su X-T, Lin D-H, Scholl UI, Giebisch GH, Lifton RP, Wang W-H: KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci U S A 111:11864-11869, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandulik S, Schmidt K, Bockenhauer D, Zdebik AA, Humberg E, Kleta R, Warth R, Reichold M: The salt-wasting phenotype of EAST syndrome, a disease with multifaceted symptoms linked to the KCNJ10 K+ channel. Pflugers Arch 461: 423–435, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J: An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: Similarities with Kir4-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer LG, Frindt G: Cl- channels of the distal nephron. Am J Physiol Renal Physiol 291: F1157–F1168, 2006 [DOI] [PubMed] [Google Scholar]
- 18.McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, West M, Borschewski A, Himmerkus N, Bleich M, Bachmann S, Mutig K, Argaiz ER, Gamba G, Singer JD, Ellison DH: Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest 124: 4723–4736, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferdaus MZ, Barber KW, López-Cayuqueo KI, Terker AS, Argaiz ER, Gassaway BM, Chambrey R, Gamba G, Rinehart J, McCormick JA: SPAK and OSR1 play essential roles in potassium homeostasis through actions on the distal convoluted tubule. J Physiol 594: 4945–4966, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazua-Valenti S, Gamba G: Revisiting the NaCl cotransporter regulation by with no-lysine kinases. Am J Physiol Cell Physiol 308: C779–C791, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang C-L, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH: A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA: SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC: The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 169: 1156–1162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nordrehaug JE, Johannessen KA, von der Lippe G: Serum potassium concentration as a risk factor of ventricular arrhythmias early in acute myocardial infarction. Circulation 71: 645–649, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Matsunoshita N, Nozu K, Shono A, Nozu Y, Fu XJ, Morisada N, Kamiyoshi N, Ohtsubo H, Ninchoji T, Minamikawa S, Yamamura T, Nakanishi K, Yoshikawa N, Shima Y, Kaito H, Iijima K: Differential diagnosis of bartter syndrome, gitelman syndrome, and pseudo-bartter/gitelman syndrome based on clinical characteristics. Genet Med 18: 180–188, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S: Molecular pathogenesis of pseudohypoaldosteronism type II: Generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP: Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Pathare G, Hoenderop JG, Bindels RJ, San-Cristobal P: A molecular update on pseudohypoaldosteronism type II. Am J Physiol Renal Physiol 305: F1513–F1520, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Gumz ML, Rabinowitz L, Wingo CS: An integrated view of potassium homeostasis. N Engl J Med 373: 60–72, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC, Loffing J: In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: Differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol 299: F1473–F1485, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Sansom SC, Welling PA: Two channels for one job. Kidney Int 72: 529–530, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Czogalla J, Vohra T, Penton D, Kirschmann M, Craigie E, Loffing J: The mineralocorticoid receptor (MR) regulates ENaC but not NCC in mice with random MR deletion. Pflugers Arch 468: 849–858, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Canonica J, Sergi C, Maillard M, Klusonova P, Odermatt A, Koesters R, Loffing-Cueni D, Loffing J, Rossier B, Frateschi S, Hummler E: Adult nephron-specific MR-deficient mice develop a severe renal PHA-1 phenotype. Pflugers Arch 468: 895–908, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J: Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol 26: 425–438, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoorn EJ, Loffing J, Ellison DH: An integrated view of potassium homeostasis. N Engl J Med 373: 1786, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Velázquez H, Ellison DH, Wright FS: Chloride-dependent potassium secretion in early and late renal distal tubules. Am J Physiol 253: F555–F562, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, Park HJ, McCormick JA, Yang CL, Ellison DH: Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su XT, Zhang C, Wang L, Gu R, Lin DH, Wang WH: Disruption of KCNJ10 (Kir4.1) stimulates the expression of ENaC in the collecting duct. Am J Physiol Renal Physiol 310: F985–F993, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Wang L, Su XT, Lin DH, Wang WH: KCNJ10 (Kir4.1) is expressed in the basolateral membrane of the cortical thick ascending limb. Am J Physiol Renal Physiol 308: F1288–F1296, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawson DC, Richards NW: Basolateral K conductance: Role in regulation of NaCl absorption and secretion. Am J Physiol 259: C181–C195, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Feraille E, Dizin E: Coordinated control of ENaC and Na+,K+-ATPase in renal collecting duct. J Am Soc Nephrol 27: 2554–2563, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welling PA, Chang YP, Delpire E, Wade JB: Multigene kinase network, kidney transport, and salt in essential hypertension. Kidney Int 77: 1063–1069, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chávez-Canales M, Zhang C, Soukaseum C, Moreno E, Pacheco-Alvarez D, Vidal-Petiot E, Castañeda-Bueno M, Vázquez N, Rojas-Vega L, Meermeier NP, Rogers S, Jeunemaitre X, Yang C-L, Ellison DH, Gamba G, Hadchouel J: WNK-SPAK-NCC cascade revisited: WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4. Hypertension 64: 1047–1053, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellison DH, Velázquez H, Wright FS: Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest 83: 113–126, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH: SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi D, Mori T, Nomura N, Khan MZ, Araki Y, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S: WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep 34: 195–205, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G: Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U S A 106: 4384–4389, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paulais M, Bloch-Faure M, Picard N, Jacques T, Ramakrishnan SK, Keck M, Sohet F, Eladari D, Houillier P, Lourdel S, Teulon J, Tucker SJ: Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome. Proc Natl Acad Sci U S A 108: 10361–10366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE: Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+-Cl- cotransporter of the distal convoluted tubule. J Biol Chem 273: 29150–29155, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.