Abstract
Two metrics, a rise in serum creatinine concentration and a decrease in urine output, are considered tantamount to the injury of the kidney tubule and the epithelial cells thereof (AKI). Yet neither criterion emphasizes the etiology or the pathogenetic heterogeneity of acute decreases in kidney excretory function. In fact, whether decreased excretory function due to contraction of the extracellular fluid volume (vAKI) or due to intrinsic kidney injury (iAKI) actually share pathogenesis and should be aggregated in the same diagnostic group remains an open question. To examine this possibility, we created mouse models of iAKI and vAKI that induced a similar increase in serum creatinine concentration. Using laser microdissection to isolate specific domains of the kidney, followed by RNA sequencing, we found that thousands of genes responded specifically to iAKI or to vAKI, but very few responded to both stimuli. In fact, the activated gene sets comprised different, functionally unrelated signal transduction pathways and were expressed in different regions of the kidney. Moreover, we identified distinctive gene expression patterns in human urine as potential biomarkers of either iAKI or vAKI, but not both. Hence, iAKI and vAKI are biologically unrelated, suggesting that molecular analysis should clarify our current definitions of acute changes in kidney excretory function.
Keywords: transcriptional profiling, acute kidney injury, volume depletion, renal ischemia, biomarkers
The critical function of the kidney, conserved from planaria1 to mammals,2,3 is to regulate the extracellular fluid volume (ECFV). When Na+ and water are scarce and ECFV decreases, the kidney’s excretory function also decreases, ensuring the conservation of Na+ and water and the maintenance of ECFV. However, the same homeostatic neuronal and hormonal pathways (e.g., sympathetic and angiotensin-aldosterone systems) that regulate effectors of volume retention (epithelial sodium channel, Na/KATPase, and osmolytes) are appropriated by diseases such as congestive heart failure and cirrhosis in the absence of volume depletion. Consequently, in patients with such diseases, the kidney’s excretory function decreases independently of the ECFV. Adding further complexity, mechanisms of injury that affect the cells of the nephron, such as severe ischemia, bacterial endotoxins, pancreatic enzymes, and nephrotoxic drugs may also decrease the kidney’s excretory function.
Poor or absent kidney excretory function due to extrarenal causes (i.e., ECFV depletion or diseases such as heart failure) has traditionally been labeled “prerenal” renal failure or “hemodynamic” renal failure, emphasizing the notion that cells of the nephron were likely uninjured, consequently distinguishing “prerenal” conditions from direct or “intrinsic” nephron injury. Although conceptually straightforward and of critical importance to guide therapeutic interventions, the separation of patients with acute decreases in kidney excretory functions into categories of “prerenal” or “intrinsic” kidney failure has bedeviled clinicians for decades.4,5 The discovery of biomarkers specific for intrinsic kidney injury6,7 has facilitated diagnosis, but the usefulness of these markers remains to be established in patients subject to different stressors that reduce excretory function.
Recent epidemiologic studies found that patients with acute decreases in kidney excretory function of similar magnitude, as determined by the concentration of circulating waste products (serum creatinine, sCr), had poor prognoses, regardless of the stimulus.8 Further, because acute increases in sCr of equal magnitude had equal clinical significance and presaged a similar clinical course,5 it followed that any acute decrease in kidney excretory function reflected some degree of kidney injury. In this view, even modest elevations in sCr due to nonrenal diseases are but an initial phase of a continuous pathogenesis resulting in kidney cell damage.9–11 In fact, even increases in sCr due to ECFV depletion have been proposed to represent a forme fruste of intrinsic kidney disease11–13 in that both might activate the same pathways of injury, but with different timing or with varying degrees of intensity. Although experimental data supporting this hypothesis is scant, it has gained widespread backing from several influential renal community associations such as the Acute Dialysis Quality Initiative (ADQI),14 the Acute Kidney Injury Network (AKIN),15 and the Kidney Disease Improving Global Outcomes (KDIGO).16 These groups all recommend that patients with acute decreases in kidney excretory function be diagnosed principally on the basis of the sCr level and urine output (RIFLE and AKIN), a concept encapsulated in the diagnostic acronym AKI. Although some guidelines suggest that ECFV depletion is an important “risk factor for AKI”16 and may influence sCr measurements,15 the biologic relationship between ECFV depletion and AKI remains indeterminate.
Although grouping all patients with acute rises in sCr into the single clinical entity of “AKI” has provided quantifiable data across many different clinical scenarios, it is at variance with the striking lack of correlation between the degree of sCr elevation and kidney pathology in biopsies of critical care and other patients,4,17,18 implying that there may be different cellular mechanisms that mediate decreases in kidney excretory function. To directly test this hypothesis, we examined the patterning of gene expression in the kidneys of mice subjected to either severe ECFV depletion (vAKI) or transient renal ischemia (iAKI). Because the elevation of sCr currently defines AKI stage, which is thought to reflect the intensity of kidney injury,14–16 we chose conditions to match sCr. In addition, because whole kidney analyses could render the genetic signatures of different nephron segments undetectable, we chose laser capture microdissection to assay different microanatomic kidney regions.
Our results indicate that equivalent acute decreases of kidney excretory function (e.g., rises in sCr) due to intrinsic kidney injury activate sharply different genetic programs than those activated by homeostatic responses to volume depletion, with very limited overlap. Further, we translated these data to humans where we found that the same genes were activated in clinically distinct groups of patients. Thus, the term “AKI” as based on acute increases in sCr is likely to identify patients with fundamentally different processes, and although it might be useful for epidemiologic and actuarial purposes, it obfuscates etiology and potentially confounds therapeutic decision-making.
Results
Patterning of the Genetic Responses to vAKI and iAKI
The concentration of sCr increased to the same extent (no significant differences) in the two models of AKI, the first due to volume depletion (vAKI; 1.9-fold increase in sCr; P<0.01) and the second due to transient kidney ischemia (iAKI; 1.5-fold increase in sCr; P<0.03; Supplemental Figure 1).
The isolation of microanatomic domains of the kidneys by laser capture was first validated in kidneys by both RNA sequencing and quantitative real-time PCR, which confirmed the appropriate enrichment of known nephron segment–specific markers (Supplemental Figure 2).
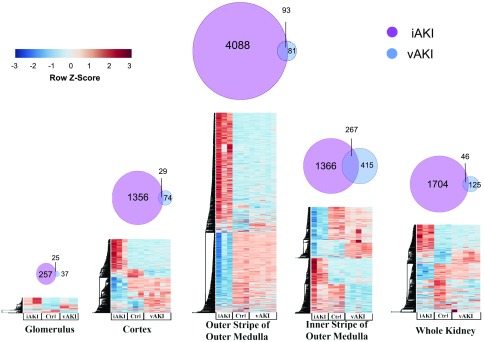
An unsupervised hierarchic clustering analysis (Supplemental Figure 3) revealed that transcriptional profiles were grouped by anatomic domains, and were separated according to the etiology of the rise in sCr. iAKI induced 12-fold more differentially expressed genes (DEGs) than vAKI (q-value<0.01). Remarkably, the majority of expressed genes were different and nonoverlapping between vAKI and iAKI. In addition, these genes had distinct expression patterns: iAKI DEGs localized predominately to the outer stripe of the outer medulla (OSOM), whereas vAKI DEGs localized predominately to the inner stripe of the outer medulla (ISOM; Figure 1). When we applied statistical cut offs, 92.3% (1158) of the genes upregulated in iAKI (>2-fold; P value <10−5) were not expressed in the vAKI model (Supplemental Table 1A). Similarly, 51.7% (103 genes) of the upregulated genes in vAKI (>2-fold; P value <10−5) were not expressed in the iAKI model (Supplemental Table 1B).
Figure 1.
Limited overlap of gene expression between iAKI and vAKI. Differential gene expression was most prominent in the OSOM in the iAKI model and in the ISOM in the vAKI model. The heatmaps portray only the significant DEGs (q-value<0.01). Gene expression was z-score–transformed on a per-gene basis and then hierarchically clustered.
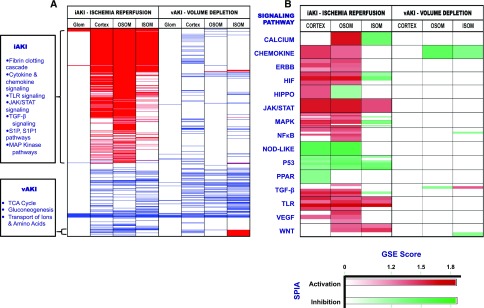
To examine whether the transcriptional profiles of the two types of AKI suggested distinct functional responses, we performed gene-set enrichment analyses (false discovery rate <25%).19 As shown in Figure 2A, this analysis indicated that iAKI yielded many more significantly induced gene sets than vAKI, including classic injury/repair (Hippo, ErbB, MAPK) and inflammatory (JAK/STAT, NOD-Like, NFκB, TLR, and Chemokine) pathways, probably reflecting the fact that kidney injury and repair overlap in time. In addition, the signaling pathway impact analysis20 (q=0.01), which provides directionality to gene interactions, identified Wnt and PPAR pathway activation in iAKI (Figure 2B). In marked contrast, none of these pathways were modulated by vAKI. Instead, vAKI induced metabolic (TCA, gluconeogenesis, oxidative phosphorylation, respiratory electron transport), transport (metal transport), and osmo-regulatory (sulfur amino acid, glycine-serine-threonine pathways) gene sets.21
Figure 2.
Different patterning of iAKI and vAKI pathways. (A) Functional analyses using gene set enrichment analysis (GSEA) against KEGG, Reactome, Biocarta, and PID Pathway databases. Each row (thin lines) demonstrates a pathway found in one or more of the queried databases. Significant pathway enrichment is represented in a binary manner (enrichment=red; de-enrichment=blue; unchanged=white; false discovery rate <25%). (B) Functional analysis using GSEA was supplemented with signaling pathway impact analysis leveraging the topologic information available from canonical signaling pathways. Each horizontal division (boxes) contains an aggregate of gene sets ascribable to a known signaling pathway analyzed by one or more of the queried databases (i.e., KEGG, Reactome, Biocarta, PID). Each shaded row within the division represents an individual gene set analyzed by a single database; most signaling pathways were analyzed by multiple gene sets and databases. Pathway activation (red) or inhibition (green) was determined by signaling pathway impact analysis; the depth of shading of red or green reflects the degree of GSEA enrichment or de-enrichment.
In sum, the patterning of gene expression (the number and location of DEGS) differed sharply in vAKI and iAKI. This indicated that cellular responses to the two stimuli were unrelated, as supported by histologic and TUNEL analyses of the kidneys (Supplemental Figure 1). Ischemic kidneys consistently demonstrated regions of coagulative necrosis at the outer edge of the OSOM, a region known to be most sensitive to ischemic damage.22 In contrast, vAKI kidneys had no detectable histologic cellular derangements, including apical megalin and actin in the proximal tubule (data not shown). Moreover, the patterning and number of apoptotic cells differed. Consistent with the histologic analysis, iAKI kidneys demonstrated focal clusters of TUNEL+ cells in the kidney cortex and OSOM (104 TUNEL+ per 630 µm2 kidney region), whereas vAKI kidneys had scant positive cells (1 TUNEL+ cell per 630 µm2 kidney region) and only scattered TUNEL+ cells in the papilla. Hence, two stimuli that induce equivalent reductions in kidney excretory function displayed markedly different genetic and pathologic responses.
Differential Expression of Specific Genes
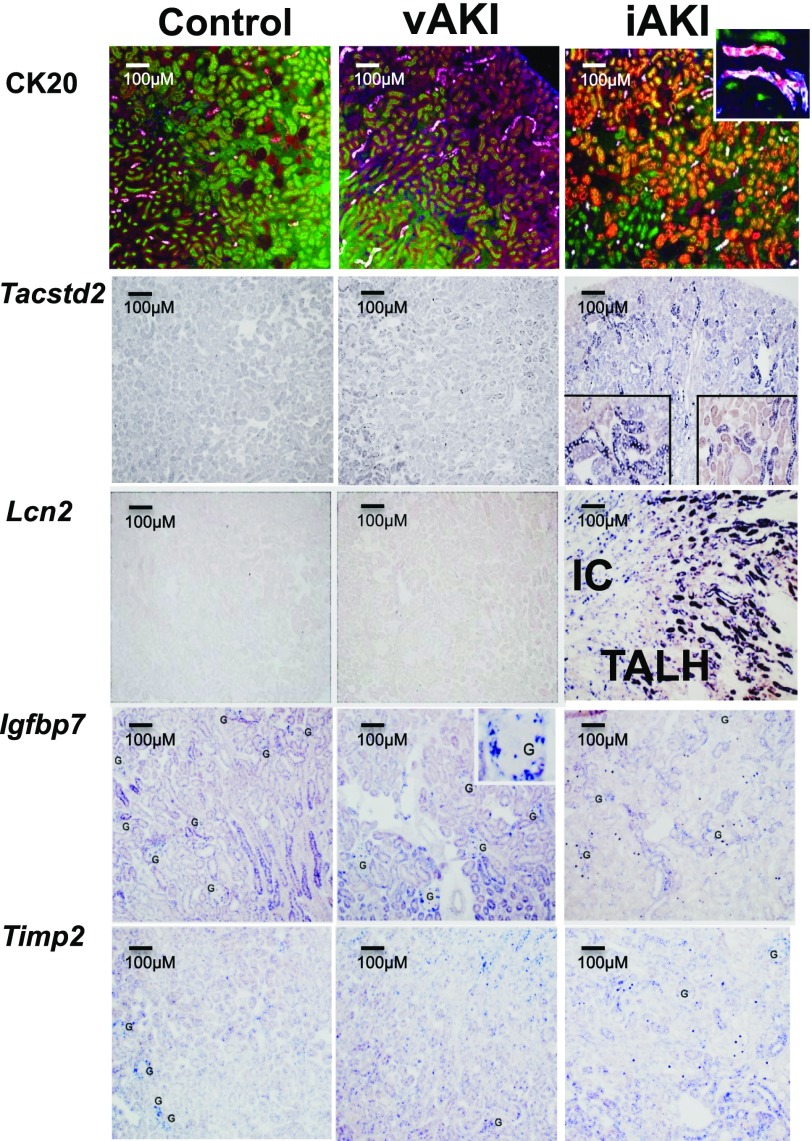
In iAKI kidneys, we confirmed the upregulation of genes known to be associated with intrinsic injury, specifically: Spp1 (OPN), Cxcl1 (GRO-α), and Lcn2 (NGAL) with P values <10−21; Clu (clusterin), Havcr (KIM-1), and Timp1 (TIMP1) with P values <10−10; and S100a8/9 (calprotectin) with P values <10−7 to <10−9 (Supplemental Table 2). They were upregulated on average approximately 180-fold. As an example of a well studied iAKI-specific gene, Lcn2 was intensely expressed (214-fold, P value <10−21) and like many other known iAKI genes, it localized to the OSOM and ISOM (Figure 3, Supplemental Table 2). More interestingly, we found nearly 1000 novel genes that were markedly upregulated in iAKI. Most localized in the OSOM and they were upregulated from 2- to 3067-fold (mean=29-fold). Particularly notable were Krt20 (CK20; 1643-fold, P<10−12), Tactstd2 (TROP2; 4.26-fold, P<10−9), and Gc (VDBP; 8.26-fold, P<10−6) (Figure 3, Supplemental Table 1A). Surprisingly, a separate group of genes previously suggested as iAKI biomarkers (β2M, Timp2, Netrin, Igfbp7, Tnfsf10, Hgf) were unchanged or even downregulated, on average 0.48-fold (see references, Gauer et al. and Mar et al.). Downregulation of the expression of these genes was specific for iAKI because these genes were not modulated by vAKI (Figure 1). Some of these genes were expressed by the glomerulus and by some tubules (Figure 3, Supplemental Table 2), rather than by the OSOM where iAKI changes in gene expression were generally found.
Figure 3.
Detection of novel biomarkers in mouse. Immunofluorescence (CK20) and in situ hybridization (Tacstd2, Lcn2) demonstrate specificity for iAKI. Note that CK20 (red) was expressed in proximal tubules (megalin=green) and in intercalated cells (IC) (inset: CK20=red; AQP2=white). Note that Tacstd2 was expressed in distal nephron segments. Lcn2 RNA was expressed by thick ascending limbs of Henle (TALH) as well as ICs of the collecting ducts in iAKI. Timp2 and Igfbp7 RNA are shown for comparison. Note the expression of these genes in scattered glomerular (G) and tubular cells.
In vAKI kidneys, upregulated genes were particularly localized to cortex and ISOM; e.g., Pappa2 (6.35-fold, P<10−8) and Stc1 (10.72-fold, P<10−15). They were upregulated from 2-fold to 128, with a mean of 10.8-fold (see Supplemental Table 1B). Because vAKI not only decreased kidney excretory function but also increased serum osmolality (see Supplemental Material), which is known to significantly alter kidney gene transcription,25 we also examined a model of vAKI in the absence of hyper-osmolarity (see Concise Methods). This model elevated sCr 1.5-fold and induced the same genes as the standard vAKI model; e.g., Pappa2 (38-fold, P<10−3), Stc1 (3-fold, P<10−3), Ddit4l (2.8-fold, P<10−2), Tuba4a (2-fold, P=0.05), and Impa1 (4.7-fold, P<10−2). In contrast, neither model of vAKI upregulated iAKI genes Lcn2 (NGAL) and Havcr (KIM1) (p=NS).
It has been proposed that vAKI is a forme fruste of iAKI and that vAKI can rapidly progress to iAKI.11–13 On the other hand, it may be that vAKI is an appropriate kidney homeostatic response to ECFV depletion, which does not cause tubular cell damage. In this case, correction of the ECFV depletion should quickly reverse the activated genetic program, whereas tubular damage would be expected to require a longer period of repair. To test this, we restored ad libitum water access to mice with vAKI (see Concise Methods) and found that differentially expressed vAKI genes normalized within 24 hours (Supplemental Figure 4). Hence, genes overexpressed in vAKI were sensitive to transient changes in ECFV.
Translational Application of iAKI and vAKI Genes
Because our results establish a clear-cut difference between iAKI and vAKI, we examined whether the data could be translated to humans. In clinical medicine, it may be initially difficult to determine whether an acute decrease in kidney excretory function is due to vAKI or iAKI. However, because the former rapidly responds to fluid resuscitation, but the latter generally has a longer time to resolution, the patient’s evolution facilitates the diagnosis. Hence, to first establish the prevalence of iAKI and vAKI in patients, we reviewed the creatinine kinetics in a cohort of patients from our previous emergency department studies.26,27 Remarkably, the majority of changes in sCr were very transient; e.g., analysis of >2000 emergency department patients26,27 showed that volume administration normalized sCr in 72% of patients within 48 hours of presentation. To determine whether transient changes in sCr are common, we developed an algorithm to examine the electronic health records of 3.8 million patients at New York Presbyterian-Columbia University Medical Center. We detected 61,726 events of “AKI,” defined as a deflection of sCr ≥0.3 mg/dl above baseline, but like in our emergency department studies, we again found that most sCr elevations were transient: 33% of events resolved within 1 day, 60% within 2 days, and 73% within 3 days of the initial rise in sCr. Hence, the evolution of sCr in the majority of patients entering the hospital indicates that vAKI is likely to be among the most common causes of acute decrease in kidney excretory function.4,8,28 Accordingly, although the major focus of clinical research in AKI concerns intrinsic forms of AKI, genes expressed in vAKI may be helpful in clinical practice as well.
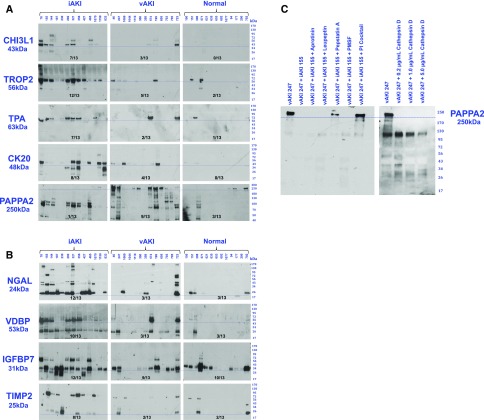
We first selected secreted gene products from the Secreted Protein Database29 or from the Max Planck Unified Proteome Database30 of human urine. This informatics pipeline identified 267 secreted iAKI (Supplemental Table 3A) and 30 secreted vAKI (Supplemental Table 3B) candidate urinary biomarkers. Of these, we tested 40 proteins that originated from different regions of the nephron using urine collected from iAKI or vAKI patients that were previously adjudicated by strict criteria26 (see Concise Methods). In agreement with our studies with mouse models, CHI3L1 (Chi3l1), TROP2 (Tacstd2), TPA (Plat), and CK20 (Krt20) (Figure 4A) were prominently expressed in the urine of patients who achieved the diagnosis of intrinsic kidney injury (i.e., iAKI) during their hospitalization. Further, these proteins were minimally or inconsistently expressed in patients diagnosed with volume-reversible vAKI. For comparison, we also assayed standard biomarkers of iAKI such as NGAL,6 VDBP,31 TIMP2/IGFBP732 (Figure 4B) which demonstrated a range of specificity for iAKI.
Figure 4.
Detection of novel biomarkers in human. (A) Secreted proteins (CHI3L1, TROP2, TPA, CK20) were elevated in most human iAKI urine but not in most vAKI urine. Conversely, secreted protein PAPPA2 was elevated in many vAKI urines, and some normal urines, but it is degraded in iAKI patients. Blue bars denote canonical molecular weights of the analyte. (B) Secreted proteins (NGAL, VDBP, TIMP2, IGFBP7) are shown for comparison and demonstrate different degrees of specificity of iAKI patients, as listed. Blue bars denote canonical molecular weights of the analyte. (C) Proteolysis of vAKI urinary PAPPA2 by iAKI urine was rescued by protease inhibitors, pepstatin A, or combinations of inhibitors. PAPPA2 was also degraded by cathepsin D.
Of the genes we detected in vAKI mouse kidneys, we found that full-length PAPPA2 (Figure 4A) was detected in the urine of patients with vAKI (9 of 13) and in the urine of some normal patients (3 of 13), but not in the urine of patients with iAKI. Interestingly, iAKI urine contained PAPPA2 immuno-reactive fragments rather than the full-length protein suggesting that iAKI urine contained proteases that cleave PAPPA2, and that the molecular weight of this biomarker in the setting of an elevated sCr might distinguish between iAKI and vAKI. To examine this, we mixed urine of vAKI patients and iAKI patients and found that PAPPA2 was degraded, particularly when urine pH was acidic (pH 5.5)33 (Figure 4C). A preliminary proteomic analysis of the iAKI urine samples identified proteases known to be active in tubular damage; e.g., cathepsin D,34 cathepsin B,35 aminopeptidase N,36 MMP-9,37 and MMP-8.38 Further, when cathepsin D was added to vAKI urine samples, PAPPA2 was digested, whereas the addition of pepstatin A to the urine coincubation preserved PAPPA2 immunoreactivity. These data show that full-length urinary PAPPA2 reports vAKI or normal urine, but degraded protein reports intrinsic kidney injury.
Discussion
The acute failure of the kidney to excrete salt, water, urea, creatinine, and other wastes results from a variety of processes including ECFV depletion, extrarenal diseases, and damage to cells of the nephron. It may be the case that all causes of defective renal excretion activate a common molecular pathway. On the other hand, if genetic readouts specific to different forms of defective renal excretion were evident at the time of patient presentation, then a “precision medicine” approach to AKI could be implemented, akin to the efforts currently underway in the study of CKD.
By isolating different domains of the kidney, we demonstrated that thousands of genes were unique to either iAKI or vAKI. Although these data were obtained with laser microdissection, which analyzes RNA from a variety of cells within the captured domain, they were in agreement with an emerging map of gene expression in specific kidney cells. For example, we adapted the method of Gay et al.39 to create a novel mouse to isolate newly-synthesized collecting duct RNA and found 86.3% of iAKI genes (>2-fold; P value <0.05) were not expressed in the vAKI model, and 95.9% of vAKI genes (>2-fold; P value <0.05) were not expressed in the iAKI model (T. Shen, unpublished data) consistent with laser capture microscopy. Our findings were also in agreement with transcriptomic analyses by Star and colleagues40 and with measurements of tubular cell energetics (which were preserved in vAKI but not in iAKI models).41 Hence, it is apparent that vAKI is not an attenuated form of iAKI; rather, each activated a distinct genetic program despite similar levels of sCr.
iAKI induced changes in inflammatory, epithelial growth, and cell repair genes. These include the Hippo signaling pathway,42,43 particularly Yap1, an antiapoptotic transcription cofactor which increased 1.7-fold (P<105), whereas its inactivator, Lats2,44 demonstrated a 0.65-fold decrease (P<104), implicating the Hippo pathway in epithelial regeneration.45 In addition, iAKI activated Wnt7a (80.5-fold, P<104), a gene which is essential for tubular repair and regeneration,46 but that can drive transformation to chronic damage when its expression is sustained.47,48 Other classic inflammatory and repair pathways, such as MAPK, JAK/STAT, NFκB, TLR, and chemokine, were also markedly activated in iAKI, in agreement with landmark studies.49–52 Yet, at the same sCr, none of the iAKI pathways were activated in vAKI. Perhaps the absence of injury in vAKI was due to protective mechanisms such as the renal protective factors known to be modulated in iAKI (e.g., prostaglandins, NO, HIF).4,53–57 As an example, PAPPA2 is a metalloproteinase secreted by the thick ascending limb of Henle, which targets the IGFBP system, permitting IGF-mediated cell survival and growth.58–60 However, PAPPA2 is degraded in iAKI through proteolysis, thereby removing a potential protective mechanism that is found in salt-sensitive volume stress.58 In sum, iAKI and vAKI models sharply diverged in clinical phenotype (Supplemental Figure 1) as well as in transcriptional (Figure 1, Supplemental Tables 1–3) and in specific pathways including cell survival and cell repair (Figure 2).
The stark distinctions between iAKI and vAKI raise the question of whether these entities represent continuous stages of injury that might ultimately converge. As our vAKI mice were subjected to severe and prolonged volume derangements and could not have survived further volume depletion, we argue that the volume stimulus was of sufficient severity to induce kidney injury, if capable. Hence, vAKI and iAKI phenotypes may be difficult to interconvert, given that the vAKI mice do not demonstrate iAKI histology, iAKI genes, or iAKI molecular pathways. In fact, the vAKI gene set surprisingly resembled the control gene set more closely than the iAKI gene set (see Figure 1, Supplemental Figure 3). Although there is no doubt that additional more prolonged (>3 days) or even more severe (acute shock) models of vAKI might cause iAKI (e.g., hemorrhagic or cardiogenic shock), our two models were consistent with human presentations of vAKI and iAKI.61–63 In this light, perhaps the conversion of vAKI to iAKI may generally require a “second hit” (a well known phenomenon in nephrotoxicity64–66 and sepsis67).
The distinct patterning of vAKI and iAKI genes may have a number of clinical applications. First, because vAKI genes rapidly reversed with volume resuscitation, the proteins encoded by these genes may serve as novel biomarkers of volume disorders, perhaps useful to guide rehydration (or cardiotonic) therapy and limit volume overload. Second, simple ratios of iAKI and vAKI genes might confirm the presence of tubular damage and clarify the meaning of an elevated sCr. Lastly, the distinct patterning of genes may provide an explanation for the dissociation of iAKI biomarkers from sCr at lower levels of RIFLE, AKIN, and KDIGO scores. Not only do the limitations of sCr reduce its capacity to judge the performance of an iAKI biomarker,68,69 but vAKI-induced elevated sCr should be compared with vAKI-induced genes rather than iAKI-induced genes. Pooling molecularly and spatially distinct programs on the basis of sCr reduces the utility of iAKI biomarkers, because many of these proteins are not even expressed when sCr rises due to vAKI.26,70,71 For example, Lcn2 (siderocalin-NGAL) was rapidly stimulated by injurious stimuli,27,72–76 but it was poorly responsive to even prolonged volume derangements27,70,72 (including cirrhosis71,76 and diuretics72) despite elevation of sCr in all of these cases. Consequently, we suggest that elevated sCr even at the same stage can reflect very different clinical, cellular, and molecular responses, but simultaneous measurement of both iAKI and vAKI markers could provide discriminatory power.
In sum, the kidney’s response to environmental challenges is fine-tuned, producing different genetic readouts in different cells in different parts of the nephron. A transient elevation of sCr is the most prevalent renal abnormality in clinical medicine and if these rapid fluctuations are volume-sensitive elevations (as suggested by our emergency department series26), new tools specific to vAKI (in addition to those for iAKI) could provide quick prospective diagnoses and treatment plans for a broad range of patients.
Concise Methods
Clinical Samples
Patient samples and data collection were approved by the Institutional Review Board of Columbia University with written informed consent of the patients. Emergency room urine samples were selected at random from our multicenter prospective cohort study,26,27 using our published criteria for iAKI, vAKI, and control including clinical history, time to resolution of elevated sCr (vAKI<72 hours; iAKI≥7 days), and rapid responses to volume challenges, all aimed at identifying only “gold standard” patients (Supplemental Material).
Analysis of Large Clinical Dataset
We examined the electronic health records of 3.8 million patients at New York Presbyterian-Columbia University Data Warehouse. Of these patients, 569,519 had at least one creatinine available. We defined baseline creatinine during each of their hospitalizations if at least three sCr were available (83,601 patients) and demonstrated stable sCr (change≤0.2 mg/dl; median number of sCr measurements defining baseline per hospitalization was four). The baseline creatinine was defined as the mean of the longest sequence of stable creatinine values. An AKI event was defined as a deflection from baseline of ≥0.3 mg/dl evident in 61,726 events in 27,464 patients. We defined the time to resolution as the number of hours between the peak creatinine value during the event and the time of the first drop ≥0.3 mg/dl from this peak.
Mouse Husbandry
Female wild-type C57Bl/6 mice, aged 10–12 weeks (Jackson Labs, Bar Harbor, ME), were used according to protocols approved by the Columbia Institutional Animal Care and Use Committee.
Renal Volume Depletion Model
We tested different durations of water deprivation72 which reduced food intake (6.2±0.3 versus 1.2±0.4 g on day 3; P<0.001), until we found a significant rise in sCr at 72 hours. Consequently, water was withheld from mice for 72 hours. Body weight and food intake were measured daily. Food intake was determined by weighing chow pellets and spillage. Kidneys were harvested and blood was collected at 72 hours or mice were rehydrated for an additional 24 hours. The furosemide model utilized a single dose of 50 mg/kg followed by briefer water and food deprivation (48 hours).77,78
Renal Ischemia Reperfusion Injury Model
To compare the vAKI model with acute renal vascular ischemia, we evaluated a range of ischemic doses until we matched the elevated sCr in the volume depletion model. We found that brief bilateral renal artery ischemia raised sCr at the 24 hours point, similar to the volume depletion model. Consequently, mice were anesthetized with isoflurane and placed on a warming table to maintain a rectal temperature of 37°C. Left and right renal pedicles were clamped using microvascular clamps (Fine Science Tools, Foster City, CA) for 10 minutes. After the clamps were removed, reperfusion of the kidneys was visually confirmed. The kidneys and blood were harvested at 24 hours.
Clinical Measurements
sCR, sodium, and blood urea nitrogen were measured using Creatinine and EC8+ cartridges read by an i-STAT Handheld (Abbott Point of Care, Princeton, NJ).
Immunohistochemistry
Kidneys were fixed (4% PFA/0.1M PB at 4°C overnight), transferred to 30% sucrose/0.1M PB (4°C overnight), and embedded in O.C.T. Compound (Tissue-Tek). Frozen sections of 20 µm were used for immunofluorescence staining with rabbit anti-CK20 (1:200; ab118574; Abcam), goat anti-AQP2 (1:400; sc-9880; Santa Cruz Biotechnology), and Fluorescein-labeled Lotus Tetragonolobus Lectin (1:200; FL-1321; Vector Laboratories). Fluorescent secondary antibodies, Alexa Fluor 594-AffiniPure F(ab′)2 Fragment Donkey Anti-Rabbit IgG and Alexa Fluor 647-AffiniPure F(ab′)2 Fragment Donkey Anti-Goat IgG (1:1000; Jackson Immunoresearch Laboratories), were used for CK20 and AQP2 identification, respectively. All slides were costained with 4′,6-diamidino-2-phenylindole to identify nuclei.
Laser Capture Microdissection
Kidneys were embedded in O.C.T. Compound and immediately snap frozen in dry ice and kept at −80°C until time of sectioning. Sections of 8–10 µm (20 µm for glomeruli) were collected on nuclease-free glass slides covered with a thin membrane (Zeiss Microscopy, Thornwood, NY), fixed in 70% ethanol for 30 seconds, stained with 1% cresyl violet acetate solution, and dehydrated in 70% and 100% ethanol followed by air-drying for 30 minutes. Regions of interest were identified morphologically and 15–20 cross sections (for cortex, OSOM; ISOM) or approximately 1500 cross sections (glomerulus) were microdissected (PALM MicroBeam; Zeiss Microscopy, Thornwood, NY). We collected RNA from vAKI (n=5), iAKI (n=3), and control (n=3) (50 independent samples in total). Domain-specific RNA was validated by RT-qPCR (Supplemental Figure 2; Supplemental Table 4).
RNA Extraction and RNA Sequencing
Total RNA was isolated using Ambion RNAqueous Micro Kit (Life Technologies, Carlsbad, CA). RNA concentration and integrity for each sample were assessed on RNA 6000 Chips using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Poly-A pull-down was used to enrich mRNAs (200 ng to 1 μg per sample, sample RIN was >8.0) and then libraries were prepared using single-end 100 bp reads for each sample with Illumina TruSeq RNA prep kits (Illumina, San Diego, CA). Libraries were sequenced using Illumina HiSeq2000 at Columbia Genome Center. Batch effects were analyzed using PC analysis (Supplemental Figure 5, Supplemental Material).
Data Analysis
Illumina RTA was used to perform base calling, and CASAVA (version 1.8.2) was used for converting base call files (.BCL) to FASTQ format and for performing sequence adaptor trimming. Reads were then mapped to the mouse reference genome (mm9) using Tophat79 (version 2.0.4) allowing four mismatches (–read-mismatches=4) and a maximum of ten multiple hits (–max-multihits=10). The relative expression was calculated using cufflinks80 (version 2.0.2) with default settings. Gene expression levels were normalized by library size and gene length into FPKMs81 and log2 transformed. Counts tables were generated with HTSeq (http://www-huber.embl.de/users/anders/HTSeq) version 0.6.1. Transcripts with zero counts across all samples were removed and mathematical artifacts (e.g., negative infinites) were replaced with “NA.” Statistical analysis was performed in R version 3.1.0 and additional Bioconductor packages were part of release 2.14.
Data Availability
The FASTQ dataset can be accessed from NCBI’s GEO Accession GSE81741 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE81741).
Generation of Heatmaps
Genes that were differentially expressed in iAKI versus control and vAKI versus control were included. The expression data shown is the variance-stabilized data generated using the DESeq package from Bioconductor according to the DESeq vignette, (http://bioconductor.org/packages/release/bioc/html/DESeq.html, http://bioconductor.org/packages/release/bioc/vignettes/DESeq/inst/doc/DESeq.pdf). Hierarchic clustering used Pearson correlation distance plus single linkage. The variance-stabilized expression values were visualized with heatmap.2 (gplots package, http://cran.r-project.org/web/packages/gplots/index.html).
Identification of Genes and Pathways
DEGs were identified using edgeR package82 version 3.6. We used the Benjamini & Hochberg83 procedure for controlling false discovery rate of the multiple tests and accepted as significant a q-value<0.01. Pathway enrichment analysis was performed using the commercially available version of signaling pathway impact analysis, PathwayGuide (Advaita Corporation http://www.advaitabio.com/)20 against KEGG84 and Reactome,85 whereas gene-set enrichment analyses19 were performed against MSigDB canonical pathways from the curated gene sets (C2) v4.0.
Identification of Biomarkers
Candidate biomarkers were filtered according to the Max Plank Unified Proteome (1542 proteins assessed March 20, 2014), Secreted ProteinDB,29 or by prediction (signal peptide and without a transmembrane domain—according to Ensembl!).86,87 We identified the proteins that were exclusive to condition, demonstrated ≥2-fold change compared with control, and were expressed with an FPKM>1.
Protein Identification by Nano-Liquid Chromatography Coupled to Tandem Mass Spectrometry Analysis
Urinary protein preparations were resolved briefly by SDS-PAGE, stained with Coomassie Blue, and separated protein bands excised for in situ trypsin digestion of polypeptides.88 Peptides were eluted with 3 µl of 40% acetonitrile containing 0.1% formic acid and diluted to 20 µl with 0.1% formic acid for nano-liquid chromatography coupled to tandem mass spectrometry as described.89 If not analyzed immediately, the peptide pools are stable when kept in organic buffer (40% acetonitrile/0.1%formic acid) and stored at −80°C.
Post–Liquid Chromatography-tandem Mass Spectrometry Analysis
Database searches (parameters in Supplemental Material) were carried out using Mascot version 2.5.090 with the human segment of Uniprot protein database (20,210 sequences; European Bioinformatics Institute, Swiss Institute of Bioinformatics, and Protein Information Resource).
Western Blot
Urine (8.3 μl) was loaded on 4%–15% SDS-polyacrylamide gel (Bio-Rad Laboratories), blotted using nitrocellulose (GE Healthcare, Pittsburgh, PA), and proteins detected using anti-human antibodies: polyclonal human CHI3L1 (1:1000; AF2599; R&D Systems), polyclonal human TROP2 (1:1000; AF650; R&D Systems), monoclonal human TPA (PLAT) (1:1000; ab157469; Abcam), monoclonal human CK20 (1:1000; ab118574; Abcam), polyclonal human PAPPA2 (1:1000; AF1668; R&D Systems), monoclonal human NGAL (1:1,000; BPD-HYB-211–01–02; Enzo Lifesciences), monoclonal human VDBP (1:1000; MAB3778; R&D), polyclonal human IGFBP7 (1:1000; AF1334; R&D Systems), and polyclonal human TIMP2 (1:1000; AF971; R&D Systems).
Probe Synthesis for In Situ Hybridization
Mouse kidney mRNA was reverse transcribed using SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen), and target genes were amplified using the following primers: Timp2, forward: 5′-gatcagagccaaagcagtgag-3′ and T7 embedded reverse: 5′-ggattaccTAATACGACTCACTATAGGGttctctgtgacccagtccatc-3′; IGFBP7, forward: 5′-ctctcctcttcctcctcttcg-3′ and T7 embedded reverse: 5′-ggattaccTAATACGACTCACTATAGGG tgacctcacagctcaagaaca-3′; Ngal, forward: 5′-aaaaacagaaggcagctttacg-3′ and T7 embedded reverse: 5′-ggattaccTAATACGACTCACTATAGGGaaagatggagtggcagacaga-3′; Trop2, forward: 5′-GCAATGGGCTCACAGGTATT-3′, and T7-embedded reverse: 5′-GGCCAGTGAATTGTAATACGACTCACTATAGGGAGGCGGTTTGTATTTGCCCGACTTCC-3′. The PCR products were used as templates for in vitro transcription. Probes were synthesized by T7 RNA polymerase (Roche) and digoxigenin-labeled RNAs were subsequently purified by PureLink RNA Mini Kit (Life Tech).
In Situ Hybridization for Frozen Sections
Kidneys fixed in 4% PFA were sectioned (8 μm), air-dried for 1–3 hours, then refixed in 4% PFA for 10 minutes and treated with proteinase K (1 μg/ml), acetylated and prehybridized, and hybridizations were at 68°C–72°C overnight in 50% formamide, 5× SSC, 5× Denhardts, 250 μg/ml baker’s yeast RNA (Sigma), and 500 μg/ml herring sperm DNA (Sigma). Washes were at 72°C in 5× SSC for 5–10 minutes, then at 72°C in 0.2× SSC for 1 hour. Sections were stained overnight with anti-digoxigenin antibody (1:5000 dilution; Boehringer-Ingelheim, Mannheim, Germany) and alkaline phosphatase activity detected with BCIP, NBT (Boehringer-Ingelheim), and 0.25 mg/ml levamisole. Sections were dehydrated and mounted in Permount (Fisher Scientific).
Disclosures
Columbia University has issued patents and patent applications for the use of biomarkers.
Supplementary Material
Acknowledgments
J.B. was supported by R21 DK091729, R01DK073462, R01DK092684, and by a March of Dimes Research Grant. K.X. was supported by F31 DK105799.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016090974/-/DCSupplemental.
References
- 1.McKanna JA: Fine structure of the protonephridial system in Planaria. I. Flame cells. Z Zellforsch Mikrosk Anat (Vienna, Austria 1948) 92: 509–523, 1968. Available at http://www.ncbi.nlm.nih.gov/pubmed/4894087. Accessed May 23, 2016 [DOI] [PubMed]
- 2.Laverty G, Skadhauge E: Adaptive strategies for post-renal handling of urine in birds. Comp Biochem Physiol A Mol Integr Physiol 149: 246–254, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Van Sant MJ, Oufiero CE, Muñoz-Garcia A, Hammond KA, Williams JB: A phylogenetic approach to total evaporative water loss in mammals. Physiol Biochem Zool 85: 526–532, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Parikh CR, Coca SG: Acute kidney injury: Defining prerenal azotemia in clinical practice and research. Nat Rev Nephrol 6: 641–642, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Bellomo R, Bagshaw S, Langenberg C, Ronco C: Pre-renal azotemia: A flawed paradigm in critically ill septic patients? Contrib Nephrol 156: 1–9, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Uchino S, Bellomo R, Bagshaw SM, Goldsmith D: Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant 25: 1833–1839, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Basile DP, Anderson MD, Sutton TA: Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton TA, Fisher CJ, Molitoris BA: Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Macedo E, Mehta RL: Prerenal failure: From old concepts to new paradigms. Curr Opin Crit Care 15: 467–473, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goren O, Matot I: Perioperative acute kidney injury. Br J Anaesth 115: ii3–ii14, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup : Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network : Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 17.Labban B, Arora N, Restaino S, Markowitz G, Valeri A, Radhakrishnan J: The role of kidney biopsy in heart transplant candidates with kidney disease. Transplantation 89: 887–893, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Bergler-Klein J, Pirich C, Laufer G, Grimm M, Regele H, Mayer G, Oberbauer R: The long-term effect of simultaneous heart and kidney transplantation on native renal function. Transplantation 71: 1597–1600, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP: Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarca AL, Draghici S, Khatri P, Hassan SS, Mittal P, Kim J-S, Kim CJ, Kusanovic JP, Romero R: A novel signaling pathway impact analysis. Bioinformatics 25: 75–82, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck F-X, Neuhofer W: Response of renal medullary cells to osmotic stress. Contrib Nephrol 148: 21–34, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Heyman SN, Evans RG, Rosen S, Rosenberger C: Cellular adaptive changes in AKI: Mitigating renal hypoxic injury. Nephrol Dial Transplant 27: 1721–1728, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Gauer S, Sichler O, Obermüller N, Holzmann Y, Kiss E, Sobkowiak E, Pfeilschifter J, Geiger H, Mühl H, Hauser IA: IL-18 is expressed in the intercalated cell of human kidney. Kidney Int 72: 1081–1087, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Mar D, Gharib SA, Zager RA, Johnson A, Denisenko O, Bomsztyk K: Heterogeneity of epigenetic changes at ischemia/reperfusion- and endotoxin-induced acute kidney injury genes. Kidney Int 88: 734–744, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brocker C, Thompson DC, Vasiliou V: The role of hyperosmotic stress in inflammation and disease. Biomol Concepts 3: 345–364, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, Elger A, Maarouf O, Sola-Del Valle DA, O’Rourke M, Sherman E, Lee P, Geara A, Imus P, Guddati A, Polland A, Rahman W, Elitok S, Malik N, Giglio J, El-Sayegh S, Devarajan P, Hebbar S, Saggi SJ, Hahn B, Kettritz R, Luft FC, Barasch J: Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: A multicenter prospective cohort study. J Am Coll Cardiol 59: 246–255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J: Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 148: 810–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coca SG, King JT Jr, Rosenthal RA, Perkal MF, Parikh CR: The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int 78: 926–933, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Zhang Y, Yin Y, Gao G, Li S, Jiang Y, Gu X, Luo J: SPD--a web-based secreted protein database. Nucleic Acids Res 33: D169–D173, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhang Y, Adachi J, Olsen JV, Shi R, de Souza G, Pasini E, Foster LJ, Macek B, Zougman A, Kumar C, Wisniewski JR, Jun W, Mann M: MAPU: Max-Planck Unified database of organellar, cellular, tissue and body fluid proteomes. Nucleic Acids Res 35: D771–D779, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vicente-Vicente L, Ferreira L, González-Buitrago JM, López-Hernández FJ, López-Novoa JM, Morales AI: Increased urinary excretion of albumin, hemopexin, transferrin and VDBP correlates with chronic sensitization to gentamicin nephrotoxicity in rats. Toxicology 304: 83–91, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EAJ, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmelé T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent J-L, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA: Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17: R25, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banay-Schwartz M, Bracco F, Dahl D, Deguzman T, Turk V, Lajtha A: The pH dependence of breakdown of various purified brain proteins by cathepsin D preparations. Neurochem Int 7: 607–614, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Aregger F, Uehlinger DE, Witowski J, Brunisholz RA, Hunziker P, Frey FJ, Jörres A: Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney Int 85: 909–919, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Svara T, Pogacnik M, Juntes P: Distribution and amount of cathepsin B in gentamicin-induced acute kidney injury in rats. Pol J Vet Sci 13: 75–82, 2010 [PubMed] [Google Scholar]
- 36.Bagshaw SM, Langenberg C, Haase M, Wan L, May CN, Bellomo R: Urinary biomarkers in septic acute kidney injury. Intensive Care Med 33: 1285–1296, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Bengatta S, Arnould C, Letavernier E, Monge M, de Préneuf HM, Werb Z, Ronco P, Lelongt B: MMP9 and SCF protect from apoptosis in acute kidney injury. J Am Soc Nephrol 20: 787–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL: Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol 9: 654–662, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gay L, Miller MR, Ventura PB, Devasthali V, Vue Z, Thompson HL, Temple S, Zong H, Cleary MD, Stankunas K, Doe CQ: Mouse TU tagging: A chemical/genetic intersectional method for purifying cell type-specific nascent RNA. Genes Dev 27: 98–115, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuen PST, Jo S-K, Holly MK, Hu X, Star RA: Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiol Genomics 25: 375–386, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zager RA: Alterations of intravascular volume: Influence on renal susceptibility to ischemic injury. J Lab Clin Med 108: 60–69, 1986 [PubMed] [Google Scholar]
- 42.Zhao B, Li L, Lei Q, Guan K-L: The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev 24: 862–874, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao B, Tumaneng K, Guan K-L: The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 13: 877–883, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao Y, Chun A, Cheung K, Rashidi B, Yang X: Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem 283: 5496–5509, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Li P-X, Wu J, Gao Y-J, Yin M-X, Lin Y, Yang M, Chen D-P, Sun H-P, Liu Z-B, Gu X-C, Huang H-L, Fu L-L, Hu H-M, He L-L, Wu W-Q, Fei Z-L, Ji H-B, Zhang L, Mei C-L: Involvement of the Hippo pathway in regeneration and fibrogenesis after ischaemic acute kidney injury: YAP is the key effector. Clin Sci (Lond) 130: 349–363, 2016. [DOI] [PMC free article] [PubMed]
- 46.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y: Sustained Activation of Wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 2015. [DOI] [PMC free article] [PubMed]
- 48.Edeling M, Ragi G, Huang S, Pavenstädt H, Susztak K: Developmental signalling pathways in renal fibrosis: The roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol 2016. [DOI] [PMC free article] [PubMed]
- 49.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuk A, Bonventre JV: Acute kidney injury. Annu Rev Med 67: 293–307, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park KM, Chen A, Bonventre JV: Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem 276: 11870–11876, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Markó L, Vigolo E, Hinze C, Park J-K, Roël G, Balogh A, Choi M, Wübken A, Cording J, Blasig IE, Luft FC, Scheidereit C, Schmidt-Ott KM, Schmidt-Ullrich R, Müller DN: Tubular epithelial NF-κB Activity Regulates Ischemic AKI. J Am Soc Nephrol 27: 2658–2669, 2016. [DOI] [PMC free article] [PubMed]
- 53.Arany I, Megyesi JK, Reusch JEB, Safirstein RL: CREB mediates ERK-induced survival of mouse renal tubular cells after oxidant stress. Kidney Int 68: 1573–1582, 2005 [DOI] [PubMed] [Google Scholar]
- 54.di Mari JF, Davis R, Safirstein RL: MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol 277: F195–F203, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Goldfarb M, Abassi Z, Rosen S, Shina A, Brezis M, Heyman SN: Compensated heart failure predisposes to outer medullary tubular injury: Studies in rats. Kidney Int 60: 607–613, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Gobé G, Willgoss D, Hogg N, Schoch E, Endre Z: Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int 56: 1299–1304, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Bonventre JV: Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14[Suppl 1]: S55–S61, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Cowley AW Jr, Yang C, Kumar V, Lazar J, Jacob H, Geurts AM, Liu P, Dayton A, Kurth T, Liang M: Pappa2 is linked to salt-sensitive hypertension in Dahl S rats. Physiol Genomics 48: 62–72, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christians JK, Bath AK, Amiri N: Pappa2 deletion alters IGFBPs but has little effect on glucose disposal or adiposity. Growth Horm IGF Res 25: 232–239, 2015 [DOI] [PubMed] [Google Scholar]
- 60.Yan X, Baxter RC, Firth SM: Involvement of pregnancy-associated plasma protein-A2 in insulin-like growth factor (IGF) binding protein-5 proteolysis during pregnancy: A potential mechanism for increasing IGF bioavailability. J Clin Endocrinol Metab 95: 1412–1420, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Harden LM, Kent S, Pittman QJ, Roth J: Fever and sickness behavior: Friend or foe? Brain Behav Immun 50: 322–333, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Eccles R: Understanding the symptoms of the common cold and influenza. Lancet Infect Dis 5: 718–725, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Exton MS: Infection-induced anorexia: Active host defence strategy. Appetite 29: 369–383, 1997 [DOI] [PubMed] [Google Scholar]
- 64.Ghane Shahrbaf F, Assadi F: Drug-induced renal disorders. J Renal Inj Prev 4: 57–60, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldfarb S, McCullough PA, McDermott J, Gay SB: Contrast-induced acute kidney injury: Specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin Proc 84: 170–179, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perazella MA: Onco-nephrology: Renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol 7: 1713–1721, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin J Am Soc Nephrol 2: 431–439, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Waikar SS, Betensky RA, Emerson SC, Bonventre JV: Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol 23: 13–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siew ED, Ware LB, Ikizler TA: Biological markers of acute kidney injury. J Am Soc Neph 22: 810–820, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, Endre ZH: Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int 81: 1254–1262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR; TRIBE-AKI Consortium : Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 60: 622–632, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paragas N, Qiu A, Zhang Q, Samstein B, Deng S-X, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin C-S, Barasch J: The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 17: 216–222, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Basu RK, Wong HR, Krawczeski CD, Wheeler DS, Manning PB, Chawla LS, Devarajan P, Goldstein SL: Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 64: 2753–2762, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling C-R, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR: The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: A multicenter pooled analysis of prospective studies. J Am Coll Cardiol 57: 1752–1761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT: Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 105: 485–491, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Verna EC, Brown RS, Farrand E, Pichardo EM, Forster CS, Sola-Del Valle DA, Adkins SH, Sise ME, Oliver JA, Radhakrishnan J, Barasch JM, Nickolas TL: Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci 57: 2362–2370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dennen P, Altmann C, Kaufman J, Klein CL, Andres-Hernando A, Ahuja NH, Edelstein CL, Cadnapaphornchai MA, Keniston A, Faubel S: Urine interleukin-6 is an early biomarker of acute kidney injury in children undergoing cardiac surgery. Crit Care 14: R181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee C-T, Chen H-C, Lai L-W, Yong K-C, Lien Y-HH: Effects of furosemide on renal calcium handling. Am J Physiol Renal Physiol 293: F1231–F1237, 2007 [DOI] [PubMed] [Google Scholar]
- 79.Trapnell C, Pachter L, Salzberg SL: TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L: Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28: 511–515, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B: Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5: 621–628, 2008 [DOI] [PubMed] [Google Scholar]
- 82.Robinson TJ, Dinan MA, Dewhirst M, Garcia-Blanco MA, Pearson JL: SplicerAV: A tool for mining microarray expression data for changes in RNA processing. BMC Bioinformatics 11: 108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995 [Google Scholar]
- 84.Kanehisa M, Goto S: KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28: 27–30, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Croft D, Mundo AF, Haw R, Milacic M, Weiser J, Wu G, Caudy M, Garapati P, Gillespie M, Kamdar MR, Jassal B, Jupe S, Matthews L, May B, Palatnik S, Rothfels K, Shamovsky V, Song H, Williams M, Birney E, Hermjakob H, Stein L, D’Eustachio P: The Reactome pathway knowledgebase. Nucleic Acids Res 42: D472–D477, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fitzgerald S, Gil L, Girón CG, Gordon L, Hourlier T, Hunt S, Johnson N, Juettemann T, Kähäri AK, Keenan S, Kulesha E, Martin FJ, Maurel T, McLaren WM, Murphy DN, Nag R, Overduin B, Pignatelli M, Pritchard B, Pritchard E, Riat HS, Ruffier M, Sheppard D, Taylor K, Thormann A, Trevanion SJ, Vullo A, Wilder SP, Wilson M, Zadissa A, Aken BL, Birney E, Cunningham F, Harrow J, Herrero J, Hubbard TJP, Kinsella R, Muffato M, Parker A, Spudich G, Yates A, Zerbino DR, Searle SMJ: Ensembl 2014. Nucleic Acids Res 42: D749–D755, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.The UniProt Consortium : Activities at the Universal Protein Resource (UniProt): Nucleic Acids Res 42: D191–D198, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sebastiaan Winkler G, Lacomis L, Philip J, Erdjument-Bromage H, Svejstrup JQ, Tempst P: Isolation and mass spectrometry of transcription factor complexes. Methods 26: 260–269, 2002 [DOI] [PubMed] [Google Scholar]
- 89.Beverly LJ, Lockwood WW, Shah PP, Erdjument-Bromage H, Varmus H: Ubiquitination, localization, and stability of an anti-apoptotic BCL2-like protein, BCL2L10/BCLb, are regulated by Ubiquilin1. Proc Natl Acad Sci USA 109: E119–E126, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS: Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The FASTQ dataset can be accessed from NCBI’s GEO Accession GSE81741 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE81741).