Abstract
MicroRNAs (miRNAs) are important regulators of gene expression, and the dysregulation of miRNAs is a common feature of several diseases. More miRNAs are identified almost daily, revealing the complexity of these transcripts in eukaryotic cellular networks. The study of renal miRNAs, using genetically modified mice or by perturbing endogenous miRNA levels, has revealed the important biologic roles miRNAs have in the major cell lineages that compose the glomerulus. Here, we provide an overview of miRNA biogenesis and function in regulating key genes and cellular pathways in glomerular cells during development and homeostasis. Moreover, we focus on the emerging mechanisms through which miRNAs contribute to different diseases affecting the glomerulus, such as FSGS, IgA nephropathy, lupus nephritis, and diabetic nephropathy. In-depth knowledge of miRNA-based gene regulation has made it possible to unravel pathomechanisms, enabling the design of new therapeutic strategies for glomerular diseases for which available therapies are not fully efficacious.
Keywords: glomerular disease, miRNA, podocyte, mesangial cells, parietal epithelial cells
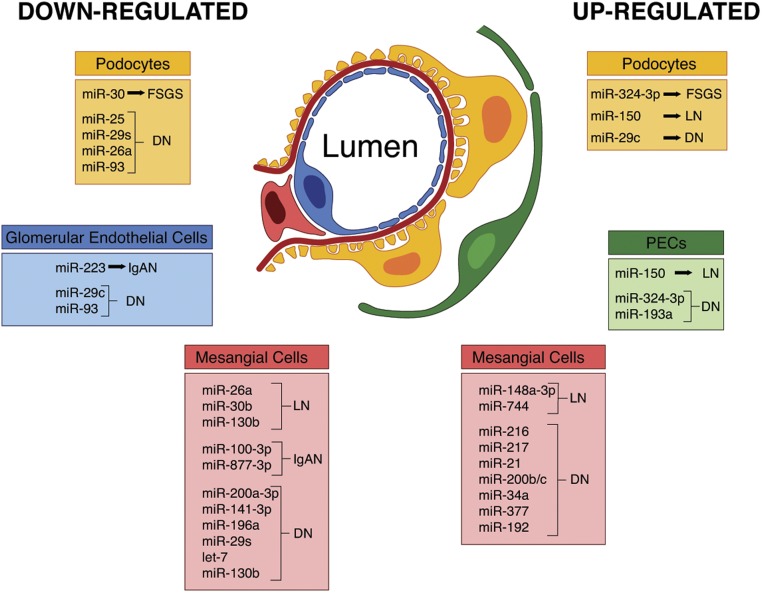
The kidneys fulfill several key tasks in the human body, from the removal of uremic waste products to the regulation of whole body ion homeostasis, acid-base balance, and BP. All of these functions depend on an adequate filtration rate. The filtering unit of the kidney is the glomerulus, a specialized bundle of capillaries composed of fenestrated endothelial cells surrounded by the glomerular (Bowman’s) capsule. The capillaries are wrapped by podocytes and the entire glomerular tuft is structurally supported by mesangial cells, which secrete growth factors and matrix proteins. Crosstalk between different glomerular cell types is required for the glomerular filtration barrier to be functional, and this crosstalk is tightly orchestrated by controlled gene expression and epigenetic mechanisms. Among epigenetic regulators, microRNAs (miRNAs) have emerged as endogenous molecules that negatively control target genes. miRNAs have been found to be dysregulated in glomerular cells, leading to cell-specific response and damage, and could therefore be exploited as novel targets for glomerular diseases (Figure 1).
Figure 1.
miRNA dysregulation in glomerular diseases. Changes in miRNAs in different populations of glomerular cells (podocytes, PECs, glomerular endothelial cells, and mesangial cells) occurring in FSGS, lupus nephritis (LN), IgA nephropathy (IgAN), and diabetic nephropathy (DN).
miRNA Biogenesis and Function
miRNAs are short (approximately 21 nucleotides) noncoding RNAs that function as guide molecules in RNA silencing by inducing mRNA degradation or blocking protein translation.1 Since the discovery of the first miRNA in Caenorhabditis elegans, the field has exploded, so much so that current estimates suggest that the human miRNAome is composed of >2500 miRNAs (http://www.mirbase.org).2 miRNA genes are individual or clustered and reside in intergenic regions and in the introns of noncoding or coding transcripts, although a minority are in exonic regions or embedded in long–noncoding RNAs (lncRNAs, ≥200 nt).3 miRNAs are transcribed by RNA polymerase II as long capped and polyadenylated hairpin transcripts (pri-miRNA) and their expression is regulated either by epigenetic mechanisms, such as DNA methylation and histone modifications,4 or transcription factors including p535,6 and ZEB1/2.7 Pri-miRNAs—thousands of base pairs in length—are cleaved by the nuclear RNase III Drosha, and release approximately 70-nucleotide stem-looped structures called precursor miRNAs (pre-miRNAs). Drosha processing can be accelerated by different proteins, including p538 and SMADs, the signal transducers of the TGF-β/bone morphogenetic protein,9 or slowed down, as occurs upon phosphorylated MeCP2 interacting with the Drosha cofactor DGCR8.10 Pre-miRNAs are then shuttled by the exportin-5-RanGTP complex to the cytoplasm, where they are cleaved by the RNAse type III endonuclease Dicer, liberating small duplexes of the mature miRNA strand (guide) and a complementary strand of similar length (passenger). It is notable that Dicer action can also be counteracted via interaction with the immune regulator MCPIP1.11 The duplex is finally loaded onto an Argonaute protein (AGO2), forming a pre-RNA–induced silencing complex (pre-RISC) which quickly removes the passenger strand to generate a mature RISC. The passenger strand is rapidly degraded after displacement from the duplex. The mature RISC is an effector complex where miRNAs function as guides by binding through their seed sequence (nucleotides 2–8) to miRNA binding sites usually located in the 3′-untranslated region of target mRNAs, whereas Argonaute proteins function as effectors by recruiting factors which induce translational repression, mRNA deadenylation, and mRNA decay.
The inhibitory activity of an endogenous miRNA is not a function of its absolute expression level in the cells, but depends on its loading into the RISC complex. The miRNAs’ association with RISC varies from cell to cell, due to the relative abundance of mRNA species and their complementarity.12 It is notable that a given miRNA can regulate several hundred transcripts belonging to different cellular pathways and networks. Because of this, miRNAs are able to switch between cellular programs and are therefore often considered master regulators of the genome.
miRNAs during Glomerular Development
Kidney development depends on reciprocal interaction between two tissues, the ureteric bud, which gives rise to the cells of the collecting duct, and the metanephric mesenchyme. The metanephric mesenchyme contains nephron progenitors that generate glomerular podocytes and renal tubular epithelia, stromal progenitors that differentiate into interstitial cells, mesangial cells, vascular smooth muscle cells and pericytes, and finally vascular progenitors which give rise to glomerular and vascular endothelia.13,14
miRNAs have been identified in the kidney at all embryonic stages and their sequential expression is crucial during organ development, as demonstrated in mammalian and nonmammalian models with spatial conditional deletion of specific miRNAs and the miRNA processing enzymes Drosha and Dicer. miRNAs are required during early kidney organogenesis as demonstrated by the crucial role played by the miR-30 family during pronephros development in Xenopous. miR-30s target the LIM-class homeobox factor Xlim/Lhx1, a key transcriptional regulator of kidney development.15 Moreover, miRNA absence in the early metanephric mesenchyme leads to severe renal dysgenesis due to loss of nephron progenitors.16,17 Similarly, during midgestation the depletion of miRNAs in nephron progenitors results in premature termination of nephrogenesis and nephron apoptosis,18 whereas the specific inactivation of the miR-17∼92 cluster leads to the defective proliferation of progenitor cells and reduced numbers of developing nephrons which predisposes mice to glomerular dysfunction and proteinuria.19
Finally, the deletion of Dicer-1 in Foxd1-derived stromal cells during kidney development resulted in hypoplastic kidneys with abnormal differentiation of the nephron tubules and vasculature, leading to kidney failure and perinatal mortality. Notably, the number of glomeruli in mutant kidneys was reduced and abnormalities, including cyst formation and dilated vessels, affected the glomeruli. Podocytes appeared to be immature, with cuboidal epithelial morphology, whereas mesangial cells failed to form in 20% of the glomeruli.20 The role of stromal miRNAs for normal nephrogenesis and glomerular mesangial maintenance was also confirmed in a concomitant study with similar results.21
miRNAs in Glomerular Visceral and Parietal Epithelial Cells
Podocytes are terminally differentiated epithelial cells that are located on the visceral side of the Bowman’s capsule. They develop primary and secondary foot processes that wrap around the capillary of the glomerulus.22 miRNAs are critical players in podocyte homeostasis, because targeted deletion of Dicer or Drosha in these cells, both in the developing as well as in the adult kidney, leads to proteinuria and glomerulosclerosis.23–26 Moreover, several miRNAs have been found to be dysregulated in podocyte injury, a pathologic mechanism causing glomerular injury and sclerosis.
Because mature podocytes must withstand fluctuating pressures and potentially harmful molecules contained in the primary filtrate, they are unlikely to be static structures.22 This remodeling of the actin cytoskeleton plays a primary role in the structural adaptations made by podocytes to preserve their glomerular filtration properties. The podocyte cytoskeleton is finely regulated by a set of miRNAs that are expressed mainly in the adult kidney, such as miR-30, miR-132, miR-134, and miR-29a. miR-30 family members are highly expressed in human podocytes and, in addition to protecting them against apoptosis by directly targeting Notch1 and p53,27 they promote podocyte actin fiber stability by controlling calcium/calcineurin signaling through the inhibition of several components of this pathway (TRPC6, PP3CA, PP3CB, PPP3R1, and NFATC3).28,29 Dysregulation of calcium/calcineurin signaling leads to podocyte cytoskeletal damage, which is a key feature of various glomerular diseases, such as FSGS, characterized by the early onset of podocyte injury.28 It is notable that miR-30s are significantly downregulated in the podocytes of patients with FSGS and in the rat model of FSGS induced by the podocyte toxin puromycin aminonucleoside. Furthermore, fibrogenic factors, such as TGF-β, reduce miR-30 expression both in vivo as well as in cultured podocytes, possibly due to Smad2-dependent mechanisms.27,30 On the other hand, the renoprotective effect of glucocorticoids in glomerular disease has been shown to be associated with increased expression of miR-30s.27
A recent work underlines the role of miRNAs in regulating podocyte cytoskeletal dynamics. The study showed that brain-derived neurotrophic factor is able to repair podocyte damage both in vitro and in a mouse model of FSGS by inducing miR-132 and inhibiting miR-134 expression upon binding to its receptor on podocytes. Brain-derived neurotrophic factor induced modulation of miR-132 and miR-134 has been found to be essential for increasing actin polymerization, favoring foot process elongation that contrasts the cell flattening induced by proteinuric conditions.31
Adult podocytes also express miR-29 family members that possess potent antifibrotic and proapoptotic activities and have been found to be dysregulated in several diseases affecting both the kidney and the heart.32 An elegant study highlighted the renoprotective effect of miR-29a in diabetes-induced loss of podocyte integrity and renal homeostasis. Diabetic miR-29a transgenic mice had higher nephrin levels and podocyte viability and less glomerular fibrosis compared with diabetic wild-type mice. The overexpression of miR-29a attenuated nephrin ubiquitination and restored proper protein acetylation through the inhibition of histone deacetylase 4. These data demonstrate the importance of post-translational acetylation reactions in podocyte nephrin and suggest that increasing miR-29a action may protect against diabetic podocytopathy.33
The role of miRNAs has been ascertained in glomerular parietal epithelial cells (PECs) lining the Bowman’s capsule. PECs recently gained attention because they are a heterogeneous population of renal progenitor cells,34–36 as well as for their contribution to glomerular diseases.34 Robust miR-150 expression in PECs and podocytes of patients with lupus nephritis correlated positively with disease chronicity scores and the expression of profibrotic proteins. Increased miR-150 would foster the production of profibrotic molecules through the downregulation of its predicted target, SOCS1. The latter protein acts as a negative regulator of the JAK/STAT signaling pathway, which promotes the transcription of genes involved in cell proliferation, inflammation, and fibrosis.37,38 Similarly to miR-150, we identified miR-324–3p increased expression in PECs as well as podocytes in an FSGS rat model. Increased expression of miR-324–3p was associated with the downregulation of its target prolyl endopeptidase (Prep)—involved in the formation of the antifibrotic peptide Ac-SDKP—in fibrotic areas of the kidneys of diseased rats.39 hPECs isolated from naive Bowman’s capsules express significant levels of miR-193a, which works as a suppressor of podocyte differentiation by inhibiting the expression of the transcription factor Wilms tumor protein (WT1).40,41 WT1 is essential for the development and maintenance of podocytes and glomeruli by inducing the expression of podocalyxin, nephrin, and other genes governing podocyte architecture.42 The downregulation of miR-193a is associated with PEC transdifferentiation toward a podocyte phenotype, whereas its over-expression leads to their abnormal activation, a prerequisite for the formation of crescents in proliferative GN.41 In line with this, extracapillary lesions in humans and mice are mainly constituted by PECs, which over-express miR-193a. Moreover, isolated glomeruli from individuals with FSGS are characterized by increased expression of miR-193a, compared with normal kidneys or kidneys affected by other glomerular diseases.40 The involvement of miR-193a in the pathogenesis of FSGS was also supported by data from miR-193a knock-in mice, which develop FSGS with extensive podocyte foot process effacement.40 All of the above evidence, together with findings that the number of crescents was reduced by anti–miR-193a in the mouse model of nephrotoxic nephritis,41 concur to indicate that miR-193 is a promising therapeutic target for FSGS. The mouse model of nephrotoxic nephritis has been also instrumental for identifying the key role of miRNAs in orchestrating T cell–mediated crescentic GN. Mice with miRNA-deficient CD4+ T cells develop less severe GN upon toxin injection. Moreover, the kidneys of patients with ANCA-associated crescentic GN and mice with nephrotoxic nephritis are characterized by the upregulation of miR-155 that drives the Th17 immune response and tissue injury.43
miRNAs in Mesangial and Glomerular Endothelial cells
miRNAs are essential for mesangial cell integrity and function and are involved in their response to injury. Mesangial cells form the structural support of the capillary network and provide a counter force to changes in intraglomerular capillary pressure, contributing to the modulation of GFR. They respond to injury in different ways by producing reactive oxygen species, cytokines, chemokines, and excess matrix proteins, and undergoing apoptosis, abnormal proliferation, and migration.44
The deposition of immune complexes or Igs in the mesangium activates mesangial cells, causing their proliferation and excess matrix production, which lead to glomerular injury.44 The activation of mesangial cells is a typical hallmark of immune-mediated glomerular diseases, of which lupus nephritis and IgA nephropathy are the main examples. In patients and mice with lupus nephritis, mesangial expansion is mediated by the upregulation of miR-148a-3p through the post-transcriptional inhibition of PTEN (phosphatase and tensin homolog), the major negative regulator of PI3K/Akt signaling, with consequent Akt activation and cell proliferation.45 The activation of human mesangial cells after the deposition of immune complexes leads to the exuberant production of chemokines and cytokines, which contribute to cell proliferation and cause intense inflammation. In this context, the activation of the type I IFN signaling pathway in kidney tissues was recently shown to be key to the pathogenesis of lupus nephritis, because the expression level of IFN-inducible genes correlates with disease activity and severity.46 miR-744 acts as a feed-forward regulator of IFN signaling in human mesangial cells. Type I IFN promotes the transcription of proinflammatory genes through miR-744, which targets phosphatase PTP1B, leading to the activation of downstream signal components of the type I IFN pathway.47 Moreover, in mesangial cells, type I IFN, via the transcription factor IRF-1, induces the expression of human EGF 2 (HER-2), which switches off protective miRNAs including miR-26a and miR-30b, which directly regulate the cell cycle in mesangial cells.48 More recent studies provide another clue to the complex activation of the type I IFN pathway in the kidney, showing that this pathway might be negatively regulated by miR-130b. Downregulated miR-130b expression was observed in kidney tissues from patients and lupus-prone mice, which correlated negatively with abnormal activation of the IFN response. Interestingly, the administration of miR-130b agomir reduced the IFNα-accelerated progression of experimental lupus nephritis and ameliorated glomerular lesions.49
Mesangial cells are also strongly affected in IgA nephropathy, due to the massive deposition of aberrantly O-glycosilated polymeric IgA1. IgA1 O-glycosilation occurs in immune cells due to two enzymes, GALNT2 and C1GALT1, whose expression is controlled by let-7b and miR-148b, respectively.50,51 Of interest, miR-148b and let-7b were found upregulated in PBMCs of patients with IgA nephropathy.50–52 Several pieces of evidence indicate that IgA nephropathy is often aggravated by mucosal infection, with secretory IgA being the most important antibody in mucosal immunity. A recent study demonstrated that secretory IgA isolated from patients with IgA nephropathy induces mesangial cell proliferation and fosters the production of proinflammatory cytokines such as IL-8 and IL-1β, through the downregulation of miR-100–3p and miR-877–3p, respectively.53
It goes without saying that miRNAs also play a key role in glomerular endothelial cells, highly flattened and fenestrated cells. This unique feature allows them to selectively filter large volumes of plasma at a prodigious rate.54 Endocapillary hypercellularity contributes to renal injury in IgA nephropathy,55 a process in which miR-223 plays an active role.56 Patients with IgA nephropathy are characterized by low levels of miR-223, which induces glomerular endothelial cell activation by upregulating importin α4 and α5, which are responsible for NF-κB and STAT3-mediated cell proliferation and monocyte adhesion.56 Moreover, glomerular endothelial cell injury is a critical pathologic process at the early stage of diabetic nephropathy57 and it has been suggested that it is mediated by miR-29c58 and miR-93.59
miRNAs during Glomerular Obsolescence
Diabetic nephropathy has been instrumental for the identification of various miRNAs and their roles in different phases of damage leading to glomerular obsolescence. The development and progression of diabetic nephropathy involve complex interplay between metabolic, hemodynamic, growth, and inflammatory factors. Hyperglycemia is associated with the excessive production of reactive oxygen species generated by NADPH oxidase, together with the overactivation of the aldose reductase/polyol pathway. NADPH oxidase threshold activity is settled by miR-25 levels that directly silence NOX4, one of the major NADPH oxidase subunits.60 miR-25 expression is suppressed under hyperglycemic conditions leading to increased NADPH oxidase activity and ROS production.60 Moreover, aldose reductase favors oxidative stress by downregulating miR-200a-3p and miR-141–3p, which are able to suppress Keap1-Nrf2, TGF-β1/2, and Zeb1/2 signaling.61 Little is known about miRNA regulation of inflammation during diabetic nephropathy. During early diabetic nephropathy, miR-146a was upregulated in the kidneys and macrophages of diabetic mice.62 The induction of diabetes in miR-146a−/− mice resulted in greater proteinuria, higher renal macrophage infiltration, and more fibrosis than in wild-type diabetic mice. Furthermore, a lack of miR-146a was associated with the upregulation of proinflammatory and profibrotic genes and the expression of markers of inflammasome activation. This evidence pointed to miR-146a acting as a protective epigenetic regulator in diabetic nephropathy by tilting the balance toward anti-inflammatory signaling.62
Podocyte loss, mesangial hypertrophy, matrix accumulation, and basement membrane thickening are among the earliest pathologic features of diabetic nephropathy, with TGF-β playing a key role in their development.63 Podocyte loss, due to both detachment from the glomerular basement membrane and apoptosis, appears to be a prerequisite early event that leads to glomerular obsolescence. Podocyte apoptosis is controlled by miR-29c and miR-21. miR-29c expression is increased in db/db mice and activates Rho kinase by targeting Sprouty homolog 1, thus favoring fibronectin assembly and apoptosis.58 In contrast, there are data that suggest that miR-21 has an antiapoptotic role in podocytes because it ameliorates TGF-β and hyperglycemia-induced glomerular injury through the repression of proapoptotic signals.64
The hypertrophic response of mesangial cells results either from the activation of the PI3K/Akt pathway or the modulation of cell cycle–regulated proteins. Different miRNAs govern mesangial hypertrophy by serving as endogenous silencers of PTEN expression, as occurs for miR-216, miR-217,65,66 and miR-21,67–69 which are increased in diabetic mice glomeruli and in cultured mesangial cells exposed to TGF-β or high glucose. PI3K/Akt signaling is also controlled by miR-200b/c, which repress the PI3K inhibitor FOG2.70 Finally, under hyperglycemic conditions, miR-34a upregulation induces mesangial proliferation and glomerular hypertrophy by targeting Growth arrest specific 1 (GAS1),71 whereas miR-196a downregulation contributes to mesangial hypertrophy by switching on the expression of p27kip1, a cyclin-dependent kinase inhibitor, and therefore arresting mesangial cells in the G1 phase.72
Soon after hyperglycemia-induced mesangial hypertrophy, extracellular matrix proteins accumulate in the glomeruli. Excessive matrix production in glomerular cells is orchestrated by several miRNAs, the majority of which are induced by TGF-β. miR-26a, miR-93, and miR-29b/c are antifibrotic miRNAs whose expression is suppressed in podocytes in the presence of a diabetic milieu. miR-26a inhibition leads to increased levels of its target CTGF, a major downstream profibrotic factor of TGF-β. It is notable that miR-26a was found to be downregulated in patients with diabetic nephropathy,73 autoimmune lupus nephritis, and IgA nephropathy.74 miR-93 expression was found to be significantly reduced in the kidneys of patients with diabetic nephropathy and in experimental models of diabetes. It is associated with the upregulation of vascular endothelial growth factor A, together with its targets fibronectin and Col4a3 in db/db mice,59 and of Msk2 (mitogen and stress-activated kinase 2), a histone kinase that phosphorylates H3S10 (Histone H3 Ser10), which in turn mediates chromatin remodeling and gene activation.75 It is notable that miR-93 over-expression reverses high glucose–induced changes in chromatin structure and the global gene transcription of podocytes. The knock-down of Msk2 through the pharmacologic restoration of miR-93 or by a kidney-targeted shRNA system in diabetic mice results in significant improvements in biochemical and histologic features of diabetic nephropathy. Moreover, Msk2 expression is elevated in human patients with diabetic nephropathy in the face of reduced miR-93.75 Finally, miR-29s suppression results in the increased expression of type I, II, and IV collagens in rodent models of renal fibrosis.76 Increased matrix production in mesangial cells is induced by a fine balance between the upregulation of miR-192, miR-200b/c, miR-216a, and miR-377 and the downregulation of miR-29s and let-7 in experimental diabetes. Specifically, miR-19277,78 and miR-200b/c79 target the E-box repressors Zeb1/2, with the subsequent activation of Col1a2, Col4a1, miR-200b/c, miR-217, and miR-216a. The latter increases Col1a2 expression through a mechanism that favors the interaction of Tsc22 with the transcription factor E3 on the far upstream E-box region of Col1a2.80 High glucose, via TGF-β1, upregulates miR-377, which suppresses the expression of p21-activated kinase and superoxide dismutases leading to enhanced susceptibility to oxidant stress and the accumulation of fibronectin.81 In mesangial cells, TGF-β1 lowers the expression of miR-29s76 and let-7,82 antifibrotic miRNAs targeting different isoforms of collagen. Moreover, decreased miR-29a expression attenuates the Dickkopf-1/Wnt/β-catenin signaling, contributing to apoptosis and extracellular matrix deposition in STZ-treated mice.83 Notably, in mesangial cells TGF-β induces signaling loops that amplify and create a chronic state of profibrotic pathway activation, modulating the expression of miR-192, miR-200s, miR-21, and miR-130b.79,84,85
Recent work has demonstrated that diabetic conditions upregulate a megacluster of approximately 40 miRNAs (miR-379 megacluster) and their host lnc-MGC, an lncRNA that is regulated by an endoplasmic reticulum stress–related transcription factor (CHOP).3 miRNAs in the megacluster have several common target genes involved in protein synthesis and endoplasmic reticulum stress and their dysregulation leads to the hypertrophy and fibrosis typical of diabetic nephropathy. In diabetic mice, the inhibition of the cluster miRNAs by an antisense oligonucleotide targeting lnc-MGC results in reduced expression of profibrotic genes, reduced mesangial expansion, and decreased glomerular size in the kidneys. An increase in miR-379 megacluster expression was also observed in human mesangial cells treated with high glucose or TGF-β1. Moreover, most of the cluster miRNAs were expressed in the glomeruli of patients with diabetes, and the expression of some of their precursors increased with glomerular damage, suggesting relevance for human glomerular diseases.3
miRNAs as Potential Therapeutic Agents
Because of the role of miRNAs in regulating several pathways, miRNAs hold the promise of being able to yield a new class of therapeutics. Basically, miRNA-based therapies comprise two approaches: miRNA inhibition or replacement. Several intriguing methods have been developed to inhibit endogenous miRNAs that show a gain-of-function in diseased tissues, from miRNA sponges (target sequences able to capture miRNAs) to the masking approach (oligonucleotides complementary to the target mRNA binding site sequence), but the most widely adopted tool is antisense oligonucleotides (antagomir), designed against the mature sequence of miRNAs. Instead, the replacement of miRNAs is pursued by oligonucleotides, agomirs that “mimic” native miRNAs. Both antagomirs and agomirs need to be chemically modified (primarily with phosphorothioates or 2’O-methoxyethyl or locked nucleic acid backbones) to increase stability (otherwise they are degraded by serum nucleases in the bloodstream) and entrance into the cell through the negatively charged cell membrane.86 Intravenously injected antisense oligonucleotides yielded significant inhibition of related targets in both the livers and kidneys of mice87 and nonhuman primates.88
No matter what strategy is used, the major concerns of miRNA-based therapies include target delivery and safety. In the context of glomerular diseases, the target miRNA should be delivered specifically to the kidney in order to avoid any potential adverse effects in other tissues and organs, and should affect only one target (or targets acting in the same pathway) to avoid effects on unintended templates.
Of the miRNA-based clinical studies, only one relates to kidney disease. RG-012, an antagomir against miR-21, was recently developed to treat Alport nephropathy, a disease for which there is yet no effective therapy. Alport nephropathy is caused by defects in the genes encoding α3, α4, and α5 chains of type IV collagen, a key component of the glomerular basement membrane,89 resulting in the disruption of the glomerular filtration barrier, podocyte damage, and glomerular obsolescence. Preclinical studies proving the efficacy of anti–miR-21 were conducted in a mouse model of Alport nephropathy with a null mutation in the α3 chain of type IV collagen.90 Renal cells of knock-out mice respond to stress caused by defects in the glomerular basement membrane by increasing miR-21 expression that induces inflammatory and profibrotic signaling.90,91 Anti–miR-21–treated mice had better kidney function, with less glomerulosclerosis, interstitial fibrosis, tubular injury, and inflammation, and an increase in lifespan compared with vehicle-treated mice.
In December of 2015, a phase 1 clinical trial to evaluate the safety, tolerability, and pharmacokinetics of subcutaneous anti–miR-21, RG-012, in healthy volunteers was successfully completed. RG-012 was well tolerated and no serious adverse effects occurred. A phase 2 therapeutic intervention study of RG-012 in patients with Alport syndrome (NCT02855268) will start as soon as the ATHENA study provides data on the natural history of the disease (NCT02136862). Anti–miR-21 treatment developed for the cure of Alport nephropathy seems to be a very promising antifibrotic therapy that is translatable to other renal diseases, such as diabetic nephropathy and IgA nephropathy, where miR-21 has been shown to be actively involved in mediating kidney fibrosis.92,93 To date, anti–miR-21–based therapies have been successful at curing different CKD in experimental animal models.91,94,95 miR-21 knockdown ameliorates microalbuminuria and renal fibrosis in a model of type 2 diabetes,95 but a recent work has demonstrated miR-21 may have a potential protective role in glomerular injury, indicating that more studies are needed to fully understand the role of mir-21–targeting in diabetes.64
Cells have an endogenous machinery that can control miRNA expression, consisting of lncRNAs, which, besides hosting miRNAs, might act as miRNA sponges.96 An example is offered by lncRNA H19, which contains several binding sites for the miRNA let-7. The study of lncRNA involvement in glomerular disease is still in its infancy. The few available findings indicate that certain lncRNAs interfere with inflammatory/developmental processes related to diabetic nephropathy. There are data indicating that increased expression of lncRNA MALAT1 in endothelial cells exposed to high glucose, as well as in the kidneys of diabetic mice, is associated with robust expression of inflammatory genes.97 Moreover, H19 expression is reduced in the kidneys of E13 embryos carried by hyperglycemic mothers and E13 embryo explants exposed to high glucose. Such a decrease may be responsible for the perturbed epithelial/mesenchymal interactions in the developing kidney, leading to epigenetic changes during the prenatal period that could contribute to disease susceptibility in adult life.98
lncRNAs could also block the transcription of miRNAs, and more in general of genes, by recruiting the Polycomb repressive complex 2 (PRC2), an enzyme able to add methyl groups to histone proteins thus silencing transcription of the surrounding genes, to certain areas in the genome.96 Interestingly, a biotechnology company has recently developed an innovative RNA-targeted tool comprising short, single-stranded RNA oligonucleotides that interfere with the binding of the lncRNA to PRC2 protein leading to the transcription of specific genes (http://ranarx.com/our-science/).
The Role of miRNAs as Biomarkers
Scientists are motivated to look for novel biomarkers that provide earlier warning signs before the kidney is irreversibly damaged, more specific information on disease progression, and, ideally, response to treatment. Several studies have looked for circulating and urinary miRNAs as biomarkers in patients with glomerular diseases. Urine miRNAs are passively filtered from plasma or possibly shed from the urinary tract. They are stable in both plasma and urine thanks to the fact that they circulate either in association with Argonaute 2 complexes and lipoproteins or packaged into microvesicles, exosomes, and apoptotic bodies.99,100 Stability, together with the fact that urine can be collected noninvasively and repeatedly, and the fact that the miRNA profile changes according to the type of renal disease, makes urine miRNAs ideal candidates for glomerular diseases.101 Moreover, urine miRNAs are quantifiable by real-time quantitative PCR, microarrays, and next-generation sequencing. In order to monitor kidney diseases, urinary exosome miRNA content would be preferable to the assessment of urinary miRNAs. Exosomes are actively secreted from live renal cells, whereas the urinary miRNA population might also contain plasma miRNAs.
To date, several small clinical studies have found a correlation between the urinary expression of miRNAs and clinical and histologic parameters in diabetic nephropathy, IgA nephropathy, lupus nephritis, and FSGS (Table 1). To strengthen miRNA diagnostic power, future work in this area should focus on large-scale studies and should identify their causal relevance.
Table 1.
miRNAs as biomarkers of glomerular diseases
| Disease | Body Fluid | Candidate miRNAs | Notes | Ref |
| FSGS | ||||
| Blood | ↑miR-186, ↑miR-192, ↑miR-205 | Correlated with proteinuria | 104, 105 | |
| Urine | ↑miR-196a, ↑miR-490, ↑miR-30a-5p | Correlated with disease activity | 102 | |
| Urine | ↓miR-1915, ↓miR-663, ↑miR-155 | 103 | ||
| Urine | ↑miR-200c | Correlated with eGFR | 106 | |
| LN | ||||
| Blood | ↓miR-342-3p, ↓miR-223, ↓miR-20a | Correlated with the onset of LN | 108 | |
| Blood | ↓miR-200b/c, ↓miR-429, ↓miR-205, ↓miR-192 | Correlated with eGFR | 110 | |
| PBMCs | ↑miR-371-5p, ↑miR-423-5p, ↓miR-1224-3p | Correlated with the onset of LN | 107 | |
| Urine | ↓miR-26a, ↓miR-30b | 48 | ||
| Urine | ↑miR-221, ↑miR-222 | Correlated with disease activity | 109 | |
| Urine | ↑miR-146a | Correlated with eGFR | 111 | |
| Urine | ↑miR-155 | Correlated with proteinuria | 111 | |
| IgAN | ||||
| Blood | ↑miR-148b, ↑let-7b | The combined miRNA levels have diagnostic accuracy | 52 | |
| CECs | ↓miR-223 | Correlated with proteinuria | 56 | |
| Urine | ↑miR-146a, ↑miR-155, ↓miR-3613–3p, ↓miR-4668–5p ↓miR-29b/c, ↓miR-200a/b, ↓miR-429 | Correlated with proteinuria | 112, 113, 114, 115 | |
| Urine | ↑miR-93 | Correlated with glomerular scarring | 114 | |
| Urine | ↑miR-17 | 101 | ||
| DN | ||||
| Blood | ↑let-7b-5p, ↑miR-21-5-p, ↓let-7c-5p, ↓miR-29a-3p | Correlated with risk of ESRD | 117 | |
| Urine | miRNA signature specific for each stage | 116 | ||
| Urine | ↓miR-15, ↓miR-192 | Correlated with proteinuria | 101, 106 | |
| Urine | ↑miR-126 | 121 | ||
| Urinary Ex | ↑miR-130a, ↑miR-145, ↓miR-155, ↓miR-424 | 118 | ||
| Urinary Ex | ↑miR-15b, ↑miR-34a, ↑miR-636 | Correlated with proteinuria | 120 | |
| Urinary EV | ↑miR-192 | Correlated with albuminuria | 119 |
Ref, references; ↑, increased; ↓, decreased; LN, lupus nephritis; IgAN, IgA nephropathy; DN, diabetic nephropathy; CEC, circulating endothelial cell; Ex, exosomes; EV, extracellular vescicles.
In conclusion, significant progress has been attained in our knowledge of how miRNA biology is essential for the dynamic control of gene expression in the kidney and is a crucial facet of glomerular cell function. It turns out that miRNAs are at the forefront of glomerular kidney disease, behaving either as the pathogenic agent or as a stress responder. Because miRNAs induce potentially reversible epigenetic changes, they could serve as potential therapeutic targets for glomerular diseases. An example of miRNA-based therapeutics is provided by the use of anti–miR-21 to cure Alport syndrome, which has recently successfully completed a phase 1 clinical trial. The most recent, promising therapeutic strategy for activating endogenous miRNA expression contemplates interfering lncRNA-mediated silencing of target miRNAs, although a lot of work should be done to achieve this. miRNAs have gained a great deal of attention in the field of the biomarker search for kidney diseases, with those derived from urinary exosomes likely providing a snapshot of the kidney’s status. A single miRNA is unlikely to offer diagnoses, instead a clear miRNA signature is needed for glomerular diseases on the basis of studies confirmed in appropriate cohorts of patients of different ethnicity and disease severity.
Disclosures
None.
Acknowledgments
We are deeply indebted to Professor Giuseppe Remuzzi for his critical appraisal and suggestions and to Dr. Susanna Tomasoni for helpful discussion. We are also grateful to Kerstin Mierke for English language editing.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ha M, Kim VN: Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15: 509–524, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Kozomara A, Griffiths-Jones S: miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 42: D68–D73, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato M, Wang M, Chen Z, Bhatt K, Oh HJ, Lanting L, Deshpande S, Jia Y, Lai JY, O’Connor CL, Wu Y, Hodgin JB, Nelson RG, Bitzer M, Natarajan R: An endoplasmic reticulum stress-regulated lncRNA hosting a microRNA megacluster induces early features of diabetic nephropathy. Nat Commun 7: 12864, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis-Dusenbery BN, Hata A: Mechanisms of control of microRNA biogenesis. J Biochem 148: 381–392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshpande SD, Putta S, Wang M, Lai JY, Bitzer M, Nelson RG, Lanting LL, Kato M, Natarajan R: Transforming growth factor-β-induced cross talk between p53 and a microRNA in the pathogenesis of diabetic nephropathy. Diabetes 62: 3151–3162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao B, Li H, Liu J, Han P, Zhang C, Bai H, Yuan X, Wang X, Li L, Ma H, Jin X, Chu Y: MicroRNA-23b targets ras GTPase-activating protein SH3 domain-binding protein 2 to alleviate fibrosis and albuminuria in diabetic nephropathy. J Am Soc Nephrol 27: 2597–2608, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krol J, Loedige I, Filipowicz W: The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11: 597–610, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki HI, Miyazono K: p53 actions on microRNA expression and maturation pathway. Methods Mol Biol 962: 165–181, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A: Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell 39: 373–384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng TL, Wang Z, Liao Q, Zhu Y, Zhou WH, Xu W, Qiu Z: MeCP2 suppresses nuclear microRNA processing and dendritic growth by regulating the DGCR8/Drosha complex. Dev Cell 28: 547–560, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki HI, Arase M, Matsuyama H, Choi YL, Ueno T, Mano H, Sugimoto K, Miyazono K: MCPIP1 ribonuclease antagonizes dicer and terminates microRNA biogenesis through precursor microRNA degradation. Mol Cell 44: 424–436, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Flores O, Kennedy EM, Skalsky RL, Cullen BR: Differential RISC association of endogenous human microRNAs predicts their inhibitory potential. Nucleic Acids Res 42: 4629–4639, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little MH, McMahon AP: Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb Perspect Biol 4: pii:a008300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Combes AN, Davies JA, Little MH: Cell-cell interactions driving kidney morphogenesis. Curr Top Dev Biol 112: 467–508, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Agrawal R, Tran U, Wessely O: The miR-30 miRNA family regulates Xenopus pronephros development and targets the transcription factor Xlim1/Lhx1. Development 136: 3927–3936, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu JY, Sims-Lucas S, Bushnell DS, Bodnar AJ, Kreidberg JA, Ho J: Dicer function is required in the metanephric mesenchyme for early kidney development. Am J Physiol Renal Physiol 306: F764–F772, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagalakshmi VK, Ren Q, Pugh MM, Valerius MT, McMahon AP, Yu J: Dicer regulates the development of nephrogenic and ureteric compartments in the mammalian kidney. Kidney Int 79: 317–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho J, Pandey P, Schatton T, Sims-Lucas S, Khalid M, Frank MH, Hartwig S, Kreidberg JA: The pro-apoptotic protein Bim is a microRNA target in kidney progenitors. J Am Soc Nephrol 22: 1053–1063, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrone AK, Stolz DB, Bastacky SI, Kostka D, Bodnar AJ, Ho J: MicroRNA-17~92 is required for nephrogenesis and renal function. J Am Soc Nephrol 25: 1440–1452, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa N, Xin C, Roach AM, Naiman N, Shankland SJ, Ligresti G, Ren S, Szak S, Gomez IG, Duffield JS: Dicer1 activity in the stromal compartment regulates nephron differentiation and vascular patterning during mammalian kidney organogenesis. Kidney Int 87: 1125–1140, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phua YL, Chu JY, Marrone AK, Bodnar AJ, Sims-Lucas S, Ho J: Renal stromal miRNAs are required for normal nephrogenesis and glomerular mesangial survival. Physiol Rep 3: pii:e12537, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perico L, Conti S, Benigni A, Remuzzi G: Podocyte-actin dynamics in health and disease. Nat Rev Nephrol 12: 692–710, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Shi S, Yu L, Chiu C, Sun Y, Chen J, Khitrov G, Merkenschlager M, Holzman LB, Zhang W, Mundel P, Bottinger EP: Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol 19: 2159–2169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH: Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol 19: 2150–2158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho J, Ng KH, Rosen S, Dostal A, Gregory RI, Kreidberg JA: Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol 19: 2069–2075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhdanova O, Srivastava S, Di L, Li Z, Tchelebi L, Dworkin S, Johnstone DB, Zavadil J, Chong MM, Littman DR, Holzman LB, Barisoni L, Skolnik EY: The inducible deletion of Drosha and microRNAs in mature podocytes results in a collapsing glomerulopathy. Kidney Int 80: 719–730, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Zheng C, Fan Y, Zeng C, Chen Z, Qin W, Zhang C, Zhang W, Wang X, Zhu X, Zhang M, Zen K, Liu Z: Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J Am Soc Nephrol 25: 92–104, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Zheng C, Wang X, Yun S, Zhao Y, Liu L, Lu Y, Ye Y, Zhu X, Zhang C, Shi S, Liu Z: MicroRNA-30 family members regulate calcium/calcineurin signaling in podocytes. J Clin Invest 125: 4091–4106, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng R, Zhou L, Zhou Y, Zhao Y, Li Q, Ni D, Hu Y, Long Y, Liu J, Lyu Z, Mao Z, Yuan Y, Huang L, Zhao H, Li G, Zhou Q: MiR-30a inhibits the epithelial--mesenchymal transition of podocytes through downregulation of NFATc3. Int J Mol Sci 16: 24032–24047, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi S, Yu L, Zhang T, Qi H, Xavier S, Ju W, Bottinger E: Smad2-dependent downregulation of miR-30 is required for TGF-β-induced apoptosis in podocytes. PLoS One 8: e75572, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Armelloni S, Zennaro C, Wei C, Corbelli A, Ikehata M, Berra S, Giardino L, Mattinzoli D, Watanabe S, Agostoni C, Edefonti A, Reiser J, Messa P, Rastaldi MP: BDNF repairs podocyte damage by microRNA-mediated increase of actin polymerization. J Pathol 235: 731–744, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M: The miR-29 family: Genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics 44: 237–244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin CL, Lee PH, Hsu YC, Lei CC, Ko JY, Chuang PC, Huang YT, Wang SY, Wu SL, Chen YS, Chiang WC, Reiser J, Wang FS: MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J Am Soc Nephrol 25: 1698–1709, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, Ghezzi S, Remuzzi A, Remuzzi G: Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol 179: 628–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shankland SJ, Smeets B, Pippin JW, Moeller MJ: The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol 10: 158–173, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P: Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Zhou H, Hasni SA, Perez P, Tandon M, Jang SI, Zheng C, Kopp JB, Austin H 3rd, Balow JE, Alevizos I, Illei GG: miR-150 promotes renal fibrosis in lupus nephritis by downregulating SOCS1. J Am Soc Nephrol 24: 1073–1087, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomasoni S, Benigni A: Post-transcriptional gene regulation makes things clearer in renal fibrosis. J Am Soc Nephrol 24: 1026–1028, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Macconi D, Tomasoni S, Romagnani P, Trionfini P, Sangalli F, Mazzinghi B, Rizzo P, Lazzeri E, Abbate M, Remuzzi G, Benigni A: MicroRNA-324-3p promotes renal fibrosis and is a target of ACE inhibition. J Am Soc Nephrol 23: 1496–1505, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gebeshuber CA, Kornauth C, Dong L, Sierig R, Seibler J, Reiss M, Tauber S, Bilban M, Wang S, Kain R, Böhmig GA, Moeller MJ, Gröne HJ, Englert C, Martinez J, Kerjaschki D: Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med 19: 481–487, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Kietzmann L, Guhr SS, Meyer TN, Ni L, Sachs M, Panzer U, Stahl RA, Saleem MA, Kerjaschki D, Gebeshuber CA, Meyer-Schwesinger C: MicroRNA-193a regulates the transdifferentiation of human parietal epithelial cells toward a podocyte phenotype. J Am Soc Nephrol 26: 1389–1401, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hohenstein P, Pritchard-Jones K, Charlton J: The yin and yang of kidney development and Wilms’ tumors. Genes Dev 29: 467–482, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krebs CF, Kapffer S, Paust HJ, Schmidt T, Bennstein SB, Peters A, Stege G, Brix SR, Meyer-Schwesinger C, Müller RU, Turner JE, Steinmetz OM, Wolf G, Stahl RA, Panzer U: MicroRNA-155 drives TH17 immune response and tissue injury in experimental crescentic GN. J Am Soc Nephrol 24: 1955–1965, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abboud HE: Mesangial cell biology. Exp Cell Res 318: 979–985, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Qingjuan L, Xiaojuan F, Wei Z, Chao W, Pengpeng K, Hongbo L, Sanbing Z, Jun H, Min Y, Shuxia L: miR-148a-3p overexpression contributes to glomerular cell proliferation by targeting PTEN in lupus nephritis. Am J Physiol Cell Physiol 310: C470–C478, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Feng X, Wu H, Grossman JM, Hanvivadhanakul P, FitzGerald JD, Park GS, Dong X, Chen W, Kim MH, Weng HH, Furst DE, Gorn A, McMahon M, Taylor M, Brahn E, Hahn BH, Tsao BP: Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum 54: 2951–2962, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Han X, Tang Y, Wu Y, Qu B, Shen N: miR-744 enhances type I interferon signaling pathway by targeting PTP1B in primary human renal mesangial cells. Sci Rep 5: 12987, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costa-Reis P, Russo PA, Zhang Z, Colonna L, Maurer K, Gallucci S, Schulz SW, Kiani AN, Petri M, Sullivan KE: The role of microRNAs and human epidermal growth factor receptor 2 in proliferative lupus nephritis. Arthritis Rheumatol 67: 2415–2426, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han X, Wang Y, Zhang X, Qin Y, Qu B, Wu L, Ma J, Zhou Z, Qian J, Dai M, Tang Y, Chan EK, Harley JB, Zhou S, Shen N: MicroRNA-130b ameliorates murine lupus nephritis through targeting the type I interferon pathway on renal mesangial cells. Arthritis Rheumatol 68: 2232–2243, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Serino G, Sallustio F, Cox SN, Pesce F, Schena FP: Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol 23: 814–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serino G, Sallustio F, Curci C, Cox SN, Pesce F, De Palma G, Schena FP: Role of let-7b in the regulation of N-acetylgalactosaminyltransferase 2 in IgA nephropathy. Nephrol Dial Transplant 30: 1132–1139, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Serino G, Pesce F, Sallustio F, De Palma G, Cox SN, Curci C, Zaza G, Lai KN, Leung JC, Tang SC, Papagianni A, Stangou M, Goumenos D, Gerolymos M, Takahashi K, Yuzawa Y, Maruyama S, Imai E, Schena FP: In a retrospective international study, circulating miR-148b and let-7b were found to be serum markers for detecting primary IgA nephropathy. Kidney Int 89: 683–692, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Liang Y, Zhao G, Tang L, Zhang J, Li T, Liu Z: MiR-100-3p and miR-877-3p regulate overproduction of IL-8 and IL-1β in mesangial cells activated by secretory IgA from IgA nephropathy patients. Exp Cell Res 347: 312–321, 2016 [DOI] [PubMed] [Google Scholar]
- 54.Obeidat M, Obeidat M, Ballermann BJ: Glomerular endothelium: A porous sieve and formidable barrier. Exp Cell Res 318: 964–972, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts IS, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, Rollino C, Amore A, Praga M, Feriozzi S, Segoloni G, Pani A, Cancarini G, Durlik M, Moggia E, Mazzucco G, Giannakakis C, Honsova E, Sundelin BB, Di Palma AM, Ferrario F, Gutierrez E, Asunis AM, Barratt J, Tardanico R, Perkowska-Ptasinska A; VALIGA study of the ERA-EDTA Immunonephrology Working Group : Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 86: 828–836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao H, Chen H, Zhu X, Zhang M, Yao G, Yu Y, Qin W, Zeng C, Zen K, Liu Z: MiR-223 downregulation promotes glomerular endothelial cell activation by upregulating importin alpha4 and alpha5 in IgA nephropathy. Kidney Int 85: 624–635, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Cheng H, Harris RC: Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc Hematol Disord Drug Targets 14: 22–33, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long J, Wang Y, Wang W, Chang BH, Danesh FR: MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem 286: 11837–11848, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long J, Wang Y, Wang W, Chang BH, Danesh FR: Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem 285: 23457–23465, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu Y, Zhang Y, Wang Z, Wang L, Wei X, Zhang B, Wen Z, Fang H, Pang Q, Yi F: Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am J Nephrol 32: 581–589, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Wei J, Zhang Y, Luo Y, Wang Z, Bi S, Song D, Dai Y, Wang T, Qiu L, Wen L, Yuan L, Yang JY: Aldose reductase regulates miR-200a-3p/141-3p to coordinate Keap1-Nrf2, Tgfβ1/2, and Zeb1/2 signaling in renal mesangial cells and the renal cortex of diabetic mice. Free Radic Biol Med 67: 91–102, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Bhatt K, Lanting LL, Jia Y, Yadav S, Reddy MA, Magilnick N, Boldin M, Natarajan R: Anti-inflammatory role of microRNA-146a in the pathogenesis of diabetic nephropathy. J Am Soc Nephrol 27: 2277–2288, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.López-Hernández FJ, López-Novoa JM: Role of TGF-β in chronic kidney disease: An integration of tubular, glomerular and vascular effects. Cell Tissue Res 347: 141–154, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Lai JY, Luo J, O’Connor C, Jing X, Nair V, Ju W, Randolph A, Ben-Dov IZ, Matar RN, Briskin D, Zavadil J, Nelson RG, Tuschl T, Brosius FC 3rd, Kretzler M, Bitzer M: MicroRNA-21 in glomerular injury. J Am Soc Nephrol 26: 805–816, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R: TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11: 881–889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahimainathan L, Das F, Venkatesan B, Choudhury GG: Mesangial cell hypertrophy by high glucose is mediated by downregulation of the tumor suppressor PTEN. Diabetes 55: 2115–2125, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Denby L, Ramdas V, McBride MW, Wang J, Robinson H, McClure J, Crawford W, Lu R, Hillyard DZ, Khanin R, Agami R, Dominiczak AF, Sharpe CC, Baker AH: miR-21 and miR-214 are consistently modulated during renal injury in rodent models. Am J Pathol 179: 661–672, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Z, Peng H, Chen J, Chen X, Han F, Xu X, He X, Yan N: MicroRNA-21 protects from mesangial cell proliferation induced by diabetic nephropathy in db/db mice. FEBS Lett 583: 2009–2014, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Lu X, Fan Q, Xu L, Li L, Yue Y, Xu Y, Su Y, Zhang D, Wang L: Ursolic acid attenuates diabetic mesangial cell injury through the up-regulation of autophagy via miRNA-21/PTEN/Akt/mTOR suppression. PLoS One 10: e0117400, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park JT, Kato M, Yuan H, Castro N, Lanting L, Wang M, Natarajan R: FOG2 protein down-regulation by transforming growth factor-β1-induced microRNA-200b/c leads to Akt kinase activation and glomerular mesangial hypertrophy related to diabetic nephropathy. J Biol Chem 288: 22469–22480, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L, He S, Guo S, Xie W, Xin R, Yu H, Yang F, Qiu J, Zhang D, Zhou S, Zhang K: Down-regulation of miR-34a alleviates mesangial proliferation in vitro and glomerular hypertrophy in early diabetic nephropathy mice by targeting GAS1. J Diabetes Complications 28: 259–264, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Shen E, Wang Y, Jiang Z, Gui D, Cheng D, Chen T, Wang N: MiR-196a regulates high glucose-induced mesangial cell hypertrophy by targeting p27kip1. J Lab Autom 20: 491–499, 2015 [DOI] [PubMed] [Google Scholar]
- 73.Koga K, Yokoi H, Mori K, Kasahara M, Kuwabara T, Imamaki H, Ishii A, Mori KP, Kato Y, Ohno S, Toda N, Saleem MA, Sugawara A, Nakao K, Yanagita M, Mukoyama M: MicroRNA-26a inhibits TGF-β-induced extracellular matrix protein expression in podocytes by targeting CTGF and is downregulated in diabetic nephropathy. Diabetologia 58: 2169–2180, 2015 [DOI] [PubMed] [Google Scholar]
- 74.Ichii O, Otsuka-Kanazawa S, Horino T, Kimura J, Nakamura T, Matsumoto M, Toi M, Kon Y: Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS One 9: e110383, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Badal SS, Wang Y, Long J, Corcoran DL, Chang BH, Truong LD, Kanwar YS, Overbeek PA, Danesh FR: miR-93 regulates Msk2-mediated chromatin remodelling in diabetic nephropathy. Nat Commun 7: 12076, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang B, Komers R, Carew R, Winbanks CE, Xu B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K, Gregorevic P, Cooper ME, Kantharidis P: Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J Am Soc Nephrol 23: 252–265, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R: MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA 104: 3432–3437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R: Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol 23: 458–469, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kato M, Arce L, Wang M, Putta S, Lanting L, Natarajan R: A microRNA circuit mediates transforming growth factor-β1 autoregulation in renal glomerular mesangial cells. Kidney Int 80: 358–368, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kato M, Wang L, Putta S, Wang M, Yuan H, Sun G, Lanting L, Todorov I, Rossi JJ, Natarajan R: Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-beta-induced collagen expression in kidney cells. J Biol Chem 285: 34004–34015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X, Quigg RJ: MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J 22: 4126–4135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park JT, Kato M, Lanting L, Castro N, Nam BY, Wang M, Kang SW, Natarajan R: Repression of let-7 by transforming growth factor-β1-induced Lin28 upregulates collagen expression in glomerular mesangial cells under diabetic conditions. Am J Physiol Renal Physiol 307: F1390–F1403, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin CL, Wang JY, Ko JY, Huang YT, Kuo YH, Wang FS: Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J Am Soc Nephrol 21: 124–135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trionfini P, Benigni A, Remuzzi G: MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 11: 23–33, 2015 [DOI] [PubMed] [Google Scholar]
- 85.Castro NE, Kato M, Park JT, Natarajan R: Transforming growth factor β1 (TGF-β1) enhances expression of profibrotic genes through a novel signaling cascade and microRNAs in renal mesangial cells. J Biol Chem 289: 29001–29013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simonson B, Das S: MicroRNA therapeutics: The next magic bullet? Mini Rev Med Chem 15: 467–474, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geary RS, Norris D, Yu R, Bennett CF: Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 87: 46–51, 2015 [DOI] [PubMed] [Google Scholar]
- 88.Straarup EM, Fisker N, Hedtjärn M, Lindholm MW, Rosenbohm C, Aarup V, Hansen HF, Ørum H, Hansen JB, Koch T: Short locked nucleic acid antisense oligonucleotides potently reduce apolipoprotein B mRNA and serum cholesterol in mice and non-human primates. Nucleic Acids Res 38: 7100–7111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kruegel J, Rubel D, Gross O: Alport syndrome--insights from basic and clinical research. Nat Rev Nephrol 9: 170–178, 2013 [DOI] [PubMed] [Google Scholar]
- 90.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, Xia TH, Liu X, Borza DB, Grafals M, Shankland SJ, Himmelfarb J, Portilla D, Liu S, Chau BN, Duffield JS: Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest 125: 141–156, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS: MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 4: 121ra18, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McClelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, Gregorevic P, Kantharidis P, Cooper ME: miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond) 129: 1237–1249, 2015 [DOI] [PubMed] [Google Scholar]
- 93.Hennino MF, Buob D, Van der Hauwaert C, Gnemmi V, Jomaa Z, Pottier N, Savary G, Drumez E, Noël C, Cauffiez C, Glowacki F: miR-21-5p renal expression is associated with fibrosis and renal survival in patients with IgA nephropathy. Sci Rep 6: 27209, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong X, Chung AC, Chen HY, Meng XM, Lan HY: Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol 22: 1668–1681, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhong X, Chung AC, Chen HY, Dong Y, Meng XM, Li R, Yang W, Hou FF, Lan HY: miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia 56: 663–674, 2013 [DOI] [PubMed] [Google Scholar]
- 96.Lorenzen JM, Thum T: Long noncoding RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol 12: 360–373, 2016 [DOI] [PubMed] [Google Scholar]
- 97.Puthanveetil P, Chen S, Feng B, Gautam A, Chakrabarti S: Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J Cell Mol Med 19: 1418–1425, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanwar YS, Pan X, Lin S, Kumar A, Wada J, Haas CS, Liau G, Lomasney JW: Imprinted mesodermal specific transcript (MEST) and H19 genes in renal development and diabetes. Kidney Int 63: 1658–1670, 2003 [DOI] [PubMed] [Google Scholar]
- 99.Lorenzen JM, Thum T: Circulating and urinary microRNAs in kidney disease. Clin J Am Soc Nephrol 7: 1528–1533, 2012 [DOI] [PubMed] [Google Scholar]
- 100.Papadopoulos T, Belliere J, Bascands JL, Neau E, Klein J, Schanstra JP: miRNAs in urine: A mirror image of kidney disease? Expert Rev Mol Diagn 15: 361–374, 2015 [DOI] [PubMed] [Google Scholar]
- 101.Szeto CC, Ching-Ha KB, Ka-Bik L, Mac-Moune LF, Cheung-Lung CP, Gang W, Kai-Ming C, Kam-Tao LP: Micro-RNA expression in the urinary sediment of patients with chronic kidney diseases. Dis Markers 33: 137–144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang W, Zhang C, Chen H, Li L, Tu Y, Liu C, Shi S, Zen K, Liu Z: Evaluation of microRNAs miR-196a, miR-30a-5P, and miR-490 as biomarkers of disease activity among patients with FSGS. Clin J Am Soc Nephrol 9: 1545–1552, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramezani A, Devaney JM, Cohen S, Wing MR, Scott R, Knoblach S, Singhal R, Howard L, Kopp JB, Raj DS: Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: A pilot study. Eur J Clin Invest 45: 394–404, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang C, Zhang W, Chen HM, Liu C, Wu J, Shi S, Liu ZH: Plasma microRNA-186 and proteinuria in focal segmental glomerulosclerosis. Am J Kidney Dis 65: 223–232, 2015 [DOI] [PubMed] [Google Scholar]
- 105.Cai X, Xia Z, Zhang C, Luo Y, Gao Y, Fan Z, Liu M, Zhang Y: Serum microRNAs levels in primary focal segmental glomerulosclerosis. Pediatr Nephrol 28: 1797–1801, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang G, Kwan BC, Lai FM, Chow KM, Li PK, Szeto CC: Urinary sediment miRNA levels in adult nephrotic syndrome. Clin Chim Acta 418: 5–11, 2013 [DOI] [PubMed] [Google Scholar]
- 107.Te JL, Dozmorov IM, Guthridge JM, Nguyen KL, Cavett JW, Kelly JA, Bruner GR, Harley JB, Ojwang JO: Identification of unique microRNA signature associated with lupus nephritis. PLoS One 5: e10344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carlsen AL, Schetter AJ, Nielsen CT, Lood C, Knudsen S, Voss A, Harris CC, Hellmark T, Segelmark M, Jacobsen S, Bengtsson AA, Heegaard NH: Circulating microRNA expression profiles associated with systemic lupus erythematosus. Arthritis Rheum 65: 1324–1334, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guan J, Wang G, Tam LS, Kwan BC, Li EK, Chow KM, Li PK, Szeto CC: Urinary sediment ICAM-1 level in lupus nephritis. Lupus 21: 1190–1195, 2012 [DOI] [PubMed] [Google Scholar]
- 110.Wang G, Tam LS, Li EK, Kwan BC, Chow KM, Luk CC, Li PK, Szeto CC: Serum and urinary free microRNA level in patients with systemic lupus erythematosus. Lupus 20: 493–500, 2011 [DOI] [PubMed] [Google Scholar]
- 111.Wang G, Tam LS, Kwan BC, Li EK, Chow KM, Luk CC, Li PK, Szeto CC: Expression of miR-146a and miR-155 in the urinary sediment of systemic lupus erythematosus. Clin Rheumatol 31: 435–440, 2012 [DOI] [PubMed] [Google Scholar]
- 112.Wang G, Kwan BC, Lai FM, Chow KM, Li PK, Szeto CC: Elevated levels of miR-146a and miR-155 in kidney biopsy and urine from patients with IgA nephropathy. Dis Markers 30: 171–179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang N, Bu R, Duan Z, Zhang X, Chen P, Li Z, Wu J, Cai G, Chen X: Profiling and initial validation of urinary microRNAs as biomarkers in IgA nephropathy. PeerJ 3: e990, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang G, Kwan BC, Lai FM, Chow KM, Li PK, Szeto CC: Urinary miR-21, miR-29, and miR-93: Novel biomarkers of fibrosis. Am J Nephrol 36: 412–418, 2012 [DOI] [PubMed] [Google Scholar]
- 115.Wang G, Kwan BC, Lai FM, Chow KM, Kam-Tao Li P, Szeto CC: Expression of microRNAs in the urinary sediment of patients with IgA nephropathy. Dis Markers 28: 79–86, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Argyropoulos C, Wang K, McClarty S, Huang D, Bernardo J, Ellis D, Orchard T, Galas D, Johnson J: Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS One 8: e54662, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pezzolesi MG, Satake E, McDonnell KP, Major M, Smiles AM, Krolewski AS: Circulating TGF-β1-regulated miRNAs and the risk of rapid progression to ESRD in type 1 diabetes. Diabetes 64: 3285–3293, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barutta F, Tricarico M, Corbelli A, Annaratone L, Pinach S, Grimaldi S, Bruno G, Cimino D, Taverna D, Deregibus MC, Rastaldi MP, Perin PC, Gruden G: Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One 8: e73798, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jia Y, Guan M, Zheng Z, Zhang Q, Tang C, Xu W, Xiao Z, Wang L, Xue Y: miRNAs in urine extracellular vesicles as predictors of early-stage diabetic nephropathy. J Diabetes Res 2016: 1–10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eissa S, Matboli M, Aboushahba R, Bekhet MM, Soliman Y: Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J Diabetes Complications 30: 1585–1592, 2016 [DOI] [PubMed] [Google Scholar]
- 121.Liu Y, Gao G, Yang C, Zhou K, Shen B, Liang H, Jiang X: Stability of miR-126 in urine and its potential as a biomarker for renal endothelial injury with diabetic nephropathy. Int J Endocrinol 2014: 393109, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]