Abstract
Bombesin receptor subtype-3(BRS-3) is an orphan G-protein coupled receptor which is classified in the bombesin receptor(BnR) family with which it shares high homology. It is present widely in the central nervous system and peripheral tissues and primarily receptor-knockout studies suggest it is involved in metabolic-glucose-insulin homeostasis, feeding and other CNS behaviors, gastrointestinal motility and cancer growth. However, the role of BRS-3 physiologically or in pathologic disorders has been not well defined because the natural ligand is unknown. Until recently, no selective agonists/antagonists were available; however, recently synthetic high-affinity agonists, chiral-diazepines nonpeptide-analogs(3F, 9D, 9F, 9G) with low CNS penetrance, were described, but are not well-categorized pharmacologically or in different labarotory species. The present study attempted to characterize(affinity, potency, selectivity) of the chiral diazepine BRS-3 agonists in human and rodents(mice, rat). In human BRS-3 receptors, the relative affinities of the chiral diazepines was 9G > 9D > 9F > 3F; each was selective for BRS-3. For stimulating PLC activity, in hBRS-3 each of the four chiral diazepine analogs was fully efficacious and their relative potencies were: 9G (EC50: 9 nM) > 9D (EC50: 9.4 nM) > 9F (EC50: 39 nM) > 3F (EC50: 48 nM). None of the four chiral diazepine analogs activated r,m,hGRPR. The nonpeptide agonists showed marked differences from each other and a peptide agonist in receptor-coupling-stiochiometry and in affinities/potencies in different species. These results demonstrate that chiral diazepine analogs (9G, 9D, 9F, 3F) have high/affinity/ptoency for the BRS-3 receptor in human and rodent cells, but different coupling relationships and species differences from a peptide agonist.
Keywords: BRS-3, chiral diazepine, bombesin receptor, obesity, diabetes, nonpeptide agonist
1. Introduction
Bombesin receptor subtype-3 (BRS-3) is an orphan G-protein coupled orphan receptor present in the central nervous systems and peripheral tissues [10,13,22,30]. Because of its 51% and 47% amino acid homology with the two human Bombesin (Bn) receptors, gastrin-releasing peptide receptor (GRPR) and neuromedin B receptor (NMBR), it is classified in the bombesin receptor (BnR) family[10,22,43].
BRS-3 is receiving increased attention because studies, primarily using BRS-3 receptor knockout mice, suggest it is involved in insulin, glucose, lipid and metabolic homeostasis [13,17,40,52]; appetite and regulation of food intake [13,25,40], behavioral regulation [13,67,68]; gastrointestinal motility [22]; lung development and injury [55,59] and regulation of growth of normal and neoplastic tissues, as well as oncogene expression [13,24,37,57,59,65,66]. This has resulted in BRS-3 receiving considerable attention for a possible therapeutic role in treatment of obesity and/or diabetes mellitus [13,29]. However, the exact role of BRS-3 in physiological and pathologic disorders has not been well defined, because the natural ligand is unknown [22], and, in contrast to the other Bn receptors, until recently, no selective agonists/antagonists were available [13,19,22,29,43,62,64].
In contrast to other Bn receptors, BRS-3 has a low affinity for all natural occurring Bn-related peptides [13,34,48,49]. The only known high-affinity agonist for the hBRS-3 is the synthetic peptide agonist [Tyr 6, β-Ala11, Phe13, Nle14]Bn-(6–14), however this Bn analog also has high affinity for GRPR and NMBR in different species [22,34,41,50,61] and has low affinity for the rat and mouse BRS-3 (r,mBRS-3)[27]. Recent studies report that the nonpeptide MK-5046 is a potent and selective agonist in different species [18,44,54] and it also has potent effects on glucose/energy homeostasis [13,17,18,54]. However, in animal and human studies, MK-5046 in addition to having the desired effects on body weight, appetite and glucose/metabolic control, had cardiovascular and body temperature/thermogenesis side effects [13,17,26,44,54]. It was proposed that these side effects could be due to a CNS action of MK-5046, because it is CNS permeable and BRS-3 receptors are widely distributed in the CNS [17,21,51,69]. To investigate this possibility further, recently, chiral diazepine nonpeptide analogs with low CNS penetrance (3F, 9D, 9F, 9G) have been reported which are potent agonists for the human BRS-3 [35,36]. However, a full pharmacological characterization of their selectivity and affinities for human and rodent Bn receptors (mouse, rat) has not been done which is needed before detailed in vivo studies are performed. This characterization is particularly important with Bn receptor agonists, because previously studies demonstrate this class of receptors has considerable species variations with agonists, as well as antagonists [8,22,28,61,63,64] and because until recently, similar to many other gastrointestinal hormone/neurotransmitter G-protein-coupled receptors [12,20,38], no nonpeptide agonists existed and little is known of their pharmacology at these receptors. Therefore, in the present study, we have investigated the affinity and potency of these novel chiral diazepine analogs for in human, rat and mouse BnR’s.
2. Materials and methods
2.1. Materials
BALB 3T3 cells (mouse fibroblast) and AR42J cells (rat pancreatic acinar cells) were from American Type Culture Collection (ATCC), Rockville, MD; Dulbecco’s minimum essential medium (DMEM), phosphate-buffered saline (PBS), G418 sulfate, fetal bovine serum (FBS), penicillin, streptomycin and sodium pyruvate from Gibco Life Technology (Grand Island, NY); bacitracin, soybean trypsin inhibitor, 3-isobutyl-1-methylxanthine (IBMX), formic acid, ammonium formate, disodium tetraborate, and alumina were obtained from Sigma-Aldrich (St. Louis, MO); iodine-125 (100 mCi/ml) and myo-[2-3H]inositol were from Perkin Elmer Life Sciences (Boston, MA); 1,2,4,6-tetrachloro-3 -6 -diphenylglycouril (Iodo-Gen) from Pierce Chemical Co. (Rockford, IL); AG 1-X8 resin from BIO-RAD (Richmond, CA); human brain cDNA and mouse hypothalamic and brain cDNA library were from Zyagen (San Diego, CA). Standard protected amino acids and other synthetic reagents were obtained from Bachem Bioscience Inc. (King of Prussia, PA). MK-5046,(2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]-3-(4-[[1-(trifluoromethyl)cyclopropyl]methyl]-1H-imidazol-2-yl)propan-2-ol[54] was a gift from Merk, Sharp and Dohme (West Point, PA.); 9f, [(5R)-4-((3-[(6-methylpyridin-3-yl)oxy]phenyl)acetyl)-8-(trifluoromethyl)-2,3,4,5-tetrahydro-1H-pyrido[2,3-e][1,4]diazepin-5-yl]acetic acid [36]; 9g, [(5R)-4-([3-(2-methylpropoxy)phenyl]acetyl)-8-(trifluoromethyl)- 2,3,4,5-tetrahydro-1H-pyrido[2,3-e][1,4]diazepin-5-yl]acetic acid [36], 3F and 9D were gifts from Daiichi Sankyo Co. (Tokyo, Japan).
2.2. Stable transfections
hBRS-3, h,mGRPR and h,rNMBR stably transfected into BALB cells were made as described previously [2-5,48]. The rat BRS-3 receptor (rBRS-3) was obtained by PCR from a rat brain cDNA library from Zyagen (San Diego, CA) and the mouse BRS-3 receptor (mBRS-3) and NMBR (mNMBR) from a mouse hypothalamic and brain cDNA library Zyagen (San Diego, CA). The PCR product for r,mBRS-3 and mNMBR were inserted in pCMV6-Entry (OriGene, Rockville, MD) and they then subcloned into pcDNA3 at the EcoRI site. BALB cells were stably transfected with r,mBRS-3 or mNMBR as previous described [2–5,48]. Three days after transfection, cells were split in a ratio of 1:3, and the selection antibiotic G418 (Life Technologies, Grand Island, NY) was added to the regular growth medium at a concentration of 800 μg/ml. Single colonies were isolated 2 weeks later and expanded in growth medium containing G418 (300 μg/ml). Colonies containing mNMBR were selected by binding studies using 125I-[D-Tyr6, β-Ala11, Phe13, Nle14]Bn-(6–14). Colonies containing mBRS-3 or rBRS-3 were selected by assessing MK-5046-stimulated charge in [3H]Inositol phosphates (IP) performed as described below.
2.3. Cell culture
h,r,mBRS-3 stably transfected BALB cells; h,mGRPR stably transfected BALB cells; h,r,mNMBR stably transfected BALB cells were made grown in DMEM media supplemented with 10% FBS, 100 U/ml of penicillin, 100 μg g/ml of streptomycin and 300 μg/ml of G418 sulfate. AR42J cells containing native rGRPR [31,47] were grown in DMEM media supplemented with 10% FBS, 100 U/ml of penicillin and 100 μg/ml of streptomycin. All cells were incubated at 37°C in a 5% CO2 atmosphere.
2.4. Preparation of 125I-Labeled Peptides
125I-[D-Tyr6, β-Ala11, Phe13, Nle14]Bn-(6–14), with specific activity of 2200 Ci/mmol, was prepared by a modification of methods described elsewhere [33,34]. In brief, 0.8 μg of IODO-GEN (in 0.02 mg/ml chloroform) was transferred to a vial, dried under a stream of nitrogen, and washed with 100 μl of 0.5 M KH2PO4, pH 7.4. To the reaction vial 20 μl of 0.5 M KH2PO4, pH 7.4, 8 μg of peptide in 4 μl of water, and 2 mCi (20 μl) Na125I were added, mixed gently, and incubated at room temperature for 6 minutes. The incubation was stopped by the addition of 100 μl of distilled water. Radiolabeled peptide was separated using a Sep-Pak (Waters Associates, Milford, MA) and high-performance liquid chromatography as described previously elsewhere [34,50]. The radioligand was stored with 0.5% BSA at 20°C.
2.5. Binding studies
Binding studies with mBRS-3 and rBRS-3 could not be performed because they have low affinity for the universal Bn ligand 125I-[D-Tyr6, β-Ala11, Phe13, Nle14]Bn-(6–14), and no other suitable ligand is available [22,27]. hBRS-3/BALB (0.5 x 106 cells/ml), hGRPR/BALB (0.5 x 106 cells/ml), rGRPR/AR42J (1 x 106 cells/ml), mGRPR/BALB (0.5 x 106 cells/ml), hNMBR/BALB (0.05 x 106 cells/ml), rNMBR/BALB (0.2 x 106 cells/ml) and mNMBR/BALB (0.8 x 106 cells/ml) cells were incubated for 60 minutes at 21°C with 50 pM 125I-[D-Tyr6, β-Ala11, Phe13, Nle14]Bn-(6–14) in 300 μl of binding buffer as described previously elsewhere [14,34,38,61]. The standard binding buffer contained 24.5 mM HEPES, pH 7.4, 98 mM NaCl, 6 mM KCl, 2.5 mM KH2PO4, 5 mM sodium pyruvate, 5 mM sodium fumarate, 5 mM sodium glutamate, 2 mM glutamine, 11.5 mM glucose, 0.5 mM CaCl2, 1.0 mM MgCl2, 0.01% (w/v) soybean trypsin inhibitor, 0.2% (v/v) amino acid mixture, 0.2% (w/v) BSA, and 0.05% (w/v) bacitracin. After the incubation, 100 μl of each sample were removed and added to 300 μl of incubation buffer in 400-μl Microfuge tubes and centrifuged for 1 minute at 10,000g (Microfuge B; Beckman Coulter, Fullerton, CA) to separate the bound radioligand from unbound radioligand. The supernatant was aspirated, and the pelleted cells were rinsed twice with a washing buffer that contained 1% (w/v) BSA in PBS. The amount of radioactivity bound to the cells was measured in a Cobra II Gamma Counter (Packard Instruments, Meriden, CT). Binding was expressed as the percentage of the total radioactivity that was associated with the cell pellet. Nonsaturable binding was <10% of the total binding in all experiments. Each point was measured in duplicate, and each experiment was replicated at least 3 times. Calculation of affinity was performed by determining the IC50 using the curve-fitting program Prism GraphPad 4.0 (GraphPad Software, Inc., La Jolla, CA).
2.6. Measurement of [3H]Inositol Phosphates
[3H]Inositol phosphates (IP) were determined in the different cells as described previously [2,38,46,61]. In brief, h,r,mBRS-3 cells, h,mGRPR/BALB cells, AR42J cells containing native rGRPR and h,r,mNMBR/BALB cells (4.0 x 104 cells/ml) were subcultured into 24-well plates in regular propagation media and then were incubated for 24 hours at 37°C in a 5% CO2 atmosphere. The cells were then incubated with 3 μCi/ml of myo-[2-3H]inositol in growth media supplemented with 2% FBS for an additional 24 hours. After the incubation, the 24-well plates were washed by incubating for 30 minutes at 37°C with 1 ml/well of PBS (pH 7.0) containing 20 mM lithium chloride. The wash buffer was aspirated and replaced with 500 μl of IP assay buffer containing 135 mM sodium chloride, 20 mM HEPES (pH 7.4), 2 mM calcium chloride, 1.2 mM magnesium sulfate, 1 mM EGTA, 20 mM lithium chloride, 11.1 mM glucose, and 0.05% BSA (w/v), and was incubated without (control) or with different concentrations of the peptides studied. After 60 minutes of incubation at 37°C, the experiments were terminated by the addition of 1 ml of ice-cold 1% (v/v) hydrochloric acid in methanol. The total [3H]IP was isolated by anion-exchange chromatography as described previously [2,38,46]. Briefly, samples were loaded onto Dowex AG1-X8 anion-exchange resin columns, washed with 5 ml of distilled water to remove free [3H]IP and then washed with 2 ml of 5 mM disodium tetraborate/60 mM sodium formate solution to remove [3H]glycerophosphorylinositol. Finally, 2 ml of 1 mM ammonium formate/100 mM formic acid solution were added to the columns to elute the total [3H]IP. Each eluate was mixed with scintillation cocktail and measured for radioactivity in a scintillation counter.
2.7. Statistical Analysis
The results are the mean and S.E.M. from at least three separate experiments. IC50 and EC50 were calculated using the curve-fitting program Prism GraphPad 4.0.
3. Results
In this study, we compared the ability of four new chiral diazepines [35,36] with that of the naturally Bn-related peptides GRP and NMB, the universal Bn-receptor agonist peptide #1 [34,41] and the nonpeptide BRS-3 agonist MK-5046 [13,38,54] to interact and activate the three Bn-receptor subtypes –GRPR, NMBR and BRS-3– in human, rat and mouse.
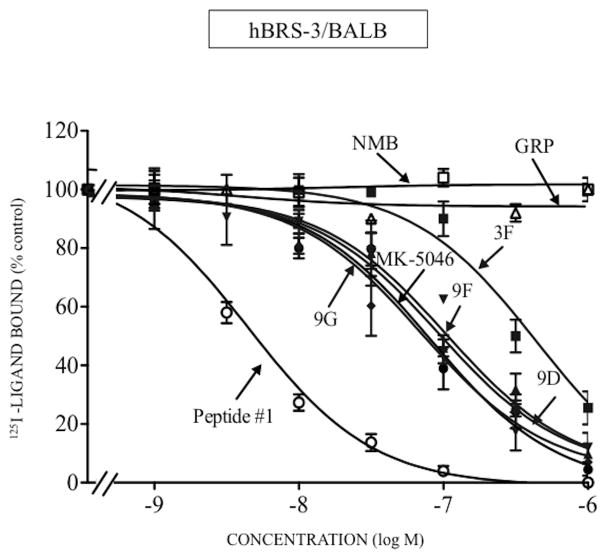
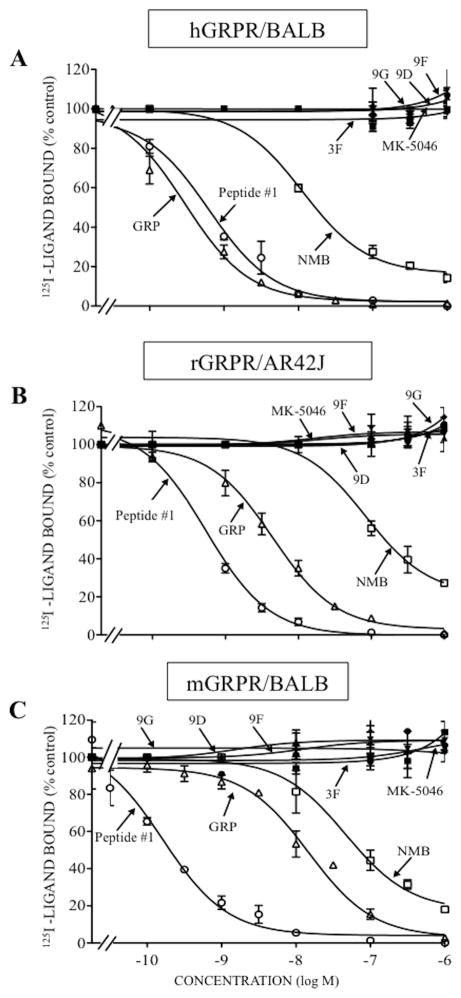
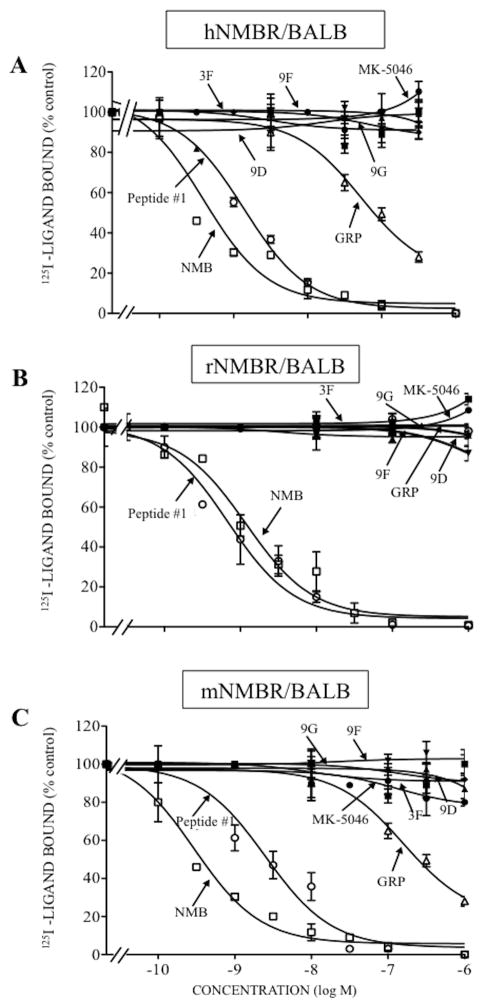
In hBRS-3/BALB cells, the affinity of chiral diazepine 9G was 1.5-fold greater than MK-5046 (9G; IC50: 70 nM and MK-5046; IC50: 102 nM, Table 1, Fig. 1). The remaining three chiral diazepines had a 1.7-13-fold lower affinity compared with 9G (chiral diazepines 9D, 9F and 3F; IC50: 121–909 nM, Table 1, Fig. 1). As described previously [13,32,34,61] for hBRS-3, GRP and NMB had very low affinity (IC50: >3000 nM, Table 1, Fig. 1); peptide #1 had a high affinity (IC50: 2.89 nM, Table 1, Fig. 1) and it was 24-fold higher than 9G (IC50: 70 nM, Table 1, Fig. 1). None of the four chiral diazepines interact with hGRPR or hNMBR at concentration up to 3,000 nM (Table 1, Fig. 2–3). In contrast, GRP and peptide #1 or NMB and peptide #1 interacted with high affinity with GRPR or NMBR, respectively (GRPR; GRP; IC50: 0.48 nM; peptide #1; IC50: 0.71 nM, Table 1, Fig. 2A and NMBR; NMB; IC50: 0.60 nM; peptide #1; IC50: 0.21 nM, Table 1, Fig. 3A). Because of a lack of a radioligand for rat and mouse BRS-3, the affinities of various ligands the could not be assessed by binding studies, however their potency for cell activation could be assesed.
TABLE 1.
Affinities of different ligands for human, rat and mouse BRS-3, GRP, and NMB receptors in various cells.
| IC50 (nM) | |||||||
|---|---|---|---|---|---|---|---|
| Human | Rat | Mouse | |||||
| BRS-3 | GRPR | NMBR | GRPR | NMBR | GRPR | NMBR | |
| Peptides | hBRS-3/BALB | hGRPR/BALB | hNMBR/BALB | rGRPR/AR42J | rNMBR/BALB | mGRPR/BALB | mNMBR/BALB |
| Peptide #1 | 2.89 ± 0.70 | 0.71 ± 0.03 | 0.21 ± 0.01 | 0.03 ± 0.00 | 0.53 ± 0.08 | 0.42 ± 0.04 | 5.76 ± 1.02 |
| MK-5046 | 102 ± 2 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 |
| 3 F | 909 ± 2 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 |
| 9 D | 121 ± 1 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 |
| 9 F | 495 ± 1 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 |
| 9 G | 70 ± 1 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 |
| GRP | >3,000 | 0.48 ± 0.05 | 65 ± 17 | 0.41 ± 0.00 | 440 ± 70 | 2.70 ± 0.20 | 206 ± 13 |
| NMB | >3,000 | 31.5 ± 2.4 | 0.60 ± 0.02 | 80 ± 5 | 1.21 ± 0.15 | 170 ± 5 | 0.60 ± 0.02 |
The indicated cell type was incubated with 50 pM 125I-[D-Tyr6, β-Ala11, Phe13, Nle14]Bn-(6–14) for 60 minutes at 21°C and binding was determined as described in Materials and Methods. The IC50 was the concentration causing half-maximal inhibition of the saturable binding, calculated using a nonlinear regression curve-fitting program (Prism). In each experiment each value was determined in duplicate, and values given are means and S.E.M. from at least three separate experiments. Abbreviations: BALB 3T3, mouse fibroblast cells; BRS-3, bombesin receptor subtype 3; GRPR, gastrin-related peptide receptor; NMBR, neuromedin B receptor; h, human; r, rat; m, mouse; 3F, 9D, 9F, 9G, nonpeptide chiral diazepine analogs; peptide #1, [D-Tyr6, β-Ala11, Phe13, Nle14]Bn-(6–14); MK-5046, (2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]-3-(4-[[1- (trifluoromethyl)cyclopropyl]methyl]-1H-imidazol-2-yl)propan-2-ol; IC50, half- maximal inhibitory concentration; h,r,mBRS-3 stably transfected into BALB cells; h,mGRPR stably transfected into BALB cells; h,r,mNMBR stably transfected into BALB cells and AR42J cells containing native rGRPR. Binding studies could not be performed with rat or mouse BRS-3 because no radioligand is available. Data are calculated from dose-inhibition curves shown in Fig. 1–3.
Figure 1.
Comparison of the ability of four chiral diazepine analogs (3F, 9D, 9F, 9G); a nonselective Bn analog agonist (Peptide #1); a nonpeptide agonist MK-5046; GRP and NMB to inhibit binding to cells containing human BRS-3. The peptides were incubated with 50 pM 125I- [D-Tyr6, β-Ala11, Phe13, Nle14]Bn-(6–14) for 60 minutes at 21°C in 300 μl of binding buffer with hBRS-3/BALB cells (0.5 x 106 cells/ml), and the saturable binding was determined as described in Materials and Methods. The results are expressed as the percentage of saturable binding without unlabeled peptide added (percentage control). The results are the mean and S.E.M. from at least three separate experiments and in each experiment the data points were determined in duplicated. Abbreviations: See in table 1 legend.
Figure 2.
Comparison of the ability of four chiral diazepine analogs (3F, 9D, 9F, 9G); a nonselective Bn analog agonist (Peptide #1); a nonpeptide agonist MK-5046; GRP and NMB to inhibit binding to cells containing human, rat or mouse GRPR. The peptides were incubated with 50 pM 125I- [D-Tyr6, β-Ala11, Phe13, Nle14]Bn-(6–14) for 60 minutes at 21°C in 300 μl of binding buffer with hGRPR/BALB cells (0.5 x 106 cells/ml) (A), rGRPR/AR42J cells (1 x 106 cells/ml) (B) or mGRPR/BALB cells (0.5 x 106 cells/ml) (C), and the saturable binding was determined as described under Materials and Methods. The results are expressed as the percentage of saturable binding without unlabeled peptide added (percentage control). The results are the mean and S.E.M. from at least three separate experiments and in each experiment the data points were determined in duplicated. Abbreviations: See in table 1 legend.
Figure 3.
Comparison of the ability of four chiral diazepine analogs (3F, 9D, 9F, 9G); a nonselective Bn analog agonist (Peptide #1); a nonpeptide agonist MK-5046; GRP and NMB to inhibit binding of 125I- labeled peptide in cells containing human, rat or mouse NMBR. The peptides were incubated with 50 pM 125I- [D-Tyr6, β-Ala11, Phe13, Nle14]Bn-(6–14) for 60 minutes at 21°C in 300 μl of binding buffer with hNMBR/BALB cells (0.05 x 106 cells/ml) (A), rNMBR/BALB cells (0.2 x 106 cells/ml) (B) or mNMBR/BALB cells (0.8 x 106 cells/ml) (c), and the saturable binding was determined as described under Materials and Methods. The results are expressed as the percentage of saturable binding without unlabeled peptide added (percentage control). The results are the mean and S.E.M. from at least three separate experiments and in each experiment the data points were determined in duplicated. Abbreviations: See in table 1 legend.
In binding studies with rGRPR/AR42J cells and rNMBR/BALB cells, the four chiral diazapines and MK-5046 did not interact with rGRPR or rNMBR, even at concentrations of 3,000 nM (Table 1, Fig. 2B, 3B). However, peptide #1 showed the highest affinity for rGRPR or rNMBR (IC50: 0.03–0.53 nM, Table 1, Fig. 2B, 3B). GRP showed a high affinity for rGRPR (IC50: 0.41 nM, Table 1, Fig. 2B) and it was >1,000-fold greater than GRP for rNMBR (IC50: 440 nM, Table 1, Fig. 3B). Similarly, NMB had a high affinity for rNMBR (IC50: 1.21 nM, Table 1, Fig. 3B) and 66-fold lower in affinity for rGRPR (IC50: 80 nM, Table 1, Fig. 2B).
In binding studies with mGRPR/BALB and mNMBR/BALB cells, the four chiral diazapines and MK-5046 did not interact with mGRPR or mNMBR at concentrations up to 3,000 nM (Table 1, Fig. 2C, 3C). In contrast, peptide #1 had a high affinity for mGRPR and mNMBR (IC50: 0.42 and 5.76 nM, Table 1, Fig. 2C, 3C); and GRP and NMB showed a high affinity for mGRPR and mNMBR, respectively (IC50: 0.60 and 5.76 nM, Table 1, Fig. 2C, 3C). GRP had a low affinity for mNMBR and NMB had low affinity for mGRPR (IC50: 206 and 170 nM, Table 1, Fig. 2C, 3C).
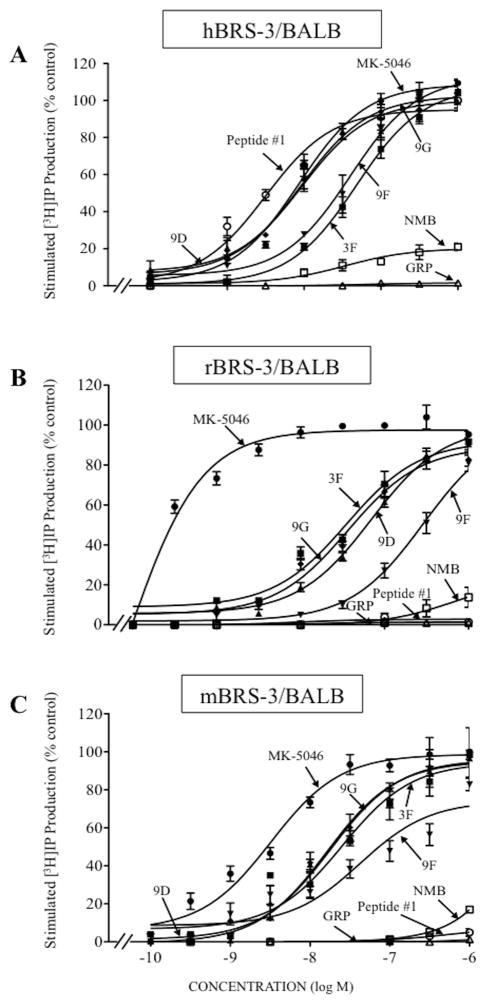
To assess the potencies of the various BRS-3 selective agonists, we utilized the fact that each of the Bn-receptor subtypes is coupled to PLC activation [15,22,43], and assessed their abilities to stimulate [3H]IP generation in each of the BRS-3, GRPR, and NMBR containing cells. We comapred the results with the natural ligands (GRP, NMB), the universal Bn-receptor agonist peptide #1, the nonpeptide BRS-3 agonist MK-5046 and the four chiral diazepines (3F, 9D, 9F, 9G) (Fig. 4–6).
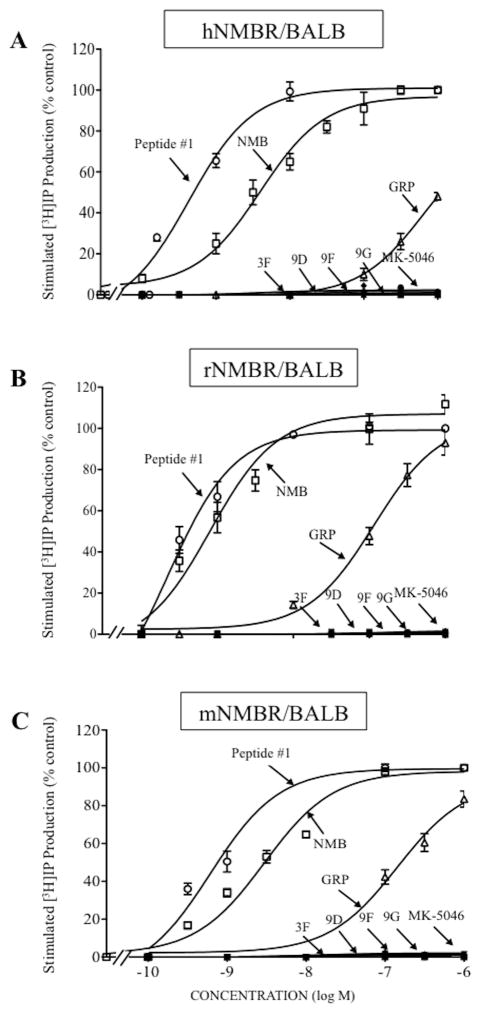
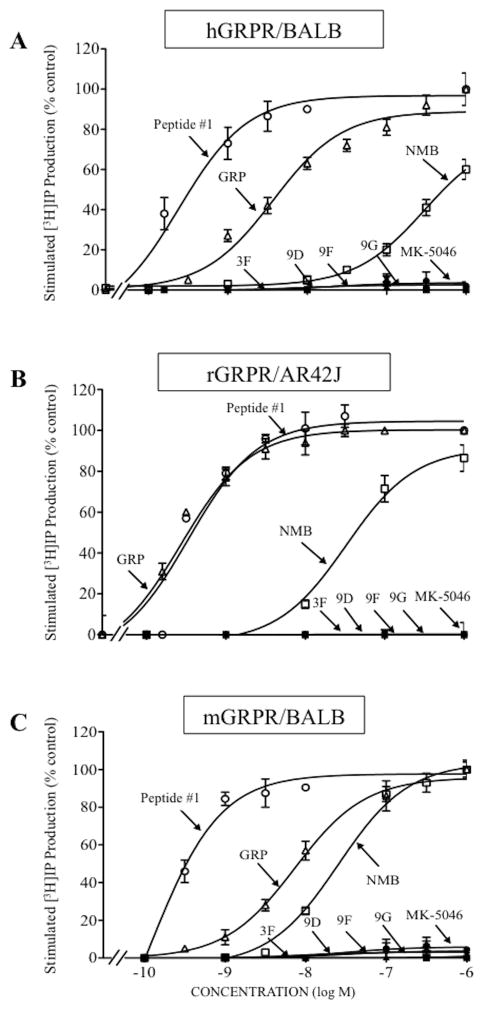
Figure 4.
Ability of four chiral diazepine analogs (3F, 9D, 9F, 9G); a nonselective Bn analog agonist (Peptide #1); a nonpeptide agonist MK-5046; GRP and NMB to stimulate [3H]IP generation in cells containing human, rat or mouse BRS-3: (A) hBRS-3/BALB, (B) rBRS-3/BALB and (C) mBRS-3/BALB cells. [3H]IP was determined after a 60 minutes incubation at 37°C after loading the cells for 48 hours with 3 μCi/ml myo-[2-3H]inositol as described in Materials and Methods. The results are the mean and S.E.M. from at least three separate experiments and in each experiment the data points were determined in duplicated. With hBRS-3/BALB cells, the maximal stimulated [3H]IP value by 1 μM MK-5046 was 70,268 ± 4,912 dpm, and the control value was 18,251 ± 1,632 dpm (n=13). With rBRS-3/BALB cells, the maximal stimulation of a 1 μM concentration of MK-5046 was 21,812 ± 1,721 dpm, and the control value was 3,749 ± 315 dpm (n=7). With mBRS-3/BALB cells, the maximal stimulation of a 1 μM concentration of MK-5046 was 62,992 ± 9,298 dpm, and the control value was 16,160 ± 2,396 dpm (n=3). Abbreviations: See in table 1 and 2 legends.
Figure 6.
Comparison of abilities of four chiral diazepine analogs (3F, 9D, 9F, 9G); a nonselective Bn analog agonist (Peptide #1); a nonpeptide agonist MK-5046; GRP and NMB to stimulate [3H]IP generation in cells containing human, rat or mouse NMBR: (A) hNMBR/BALB, (B) rNMBR/BALB and (C) mNMBR/BALB cells. The experimental conditions were the same as described in Fig. 4 legend. The results are the mean and S.E.M. from at least three experiments, and in each experiment the data points were determined in duplicate. The results are expressed as the percentage of stimulation causing by maximal effective concentration of peptide #1 (1 μM). The control and the maximal stimulated [3H]IP with peptide #1 (1 μM): hNMBR/BALB cells, 2,046 ± 171 and 56,260 ± 916 dpm, respectively (n=8); rNMBR/BALB cells, 2,501 ± 519 and 69,250 ± 13,261 dpm, respectively (n=6) and mNMBR/BALB cells, 3,692 ± 413 and 125,207 ± 4,108 dpm, respectively (n=3). Abbreviations: See in table 1 and 2 legends.
In human, rat and mouse BRS-3 containing cells, each of the four chiral diazepines, as well as MK-5046, were full efficacious for activating PLC causing a 3.8-, 5.8- and 3.9-fold increase, respectively (Table 2, Fig. 4). In human BRS-3/BALB cells, of the four chiral diazepines, 9G and 9D were the most potent (IC50: 9.2 and 9.4 nM, Table 2, Fig. 4A); which was equal in potency to MK-5046 (EC50: 8.70 nM, Table 2, Fig. 4A); with 9F and 3F having, respectively a 4.3- and 5.2-fold lower potency than 9G or 9D (3F; EC50: 47.7 nM, and 9F; EC50: 39.6 nM, Table 2, Fig. 4A). For rat and mouse BRS-3, the relative and absolute potencies for the chiral agonists, MK-5046 and peptide #1 differed from that seen in hBRS-3/BALB cells. Specifically, in rBRS-3/BALB cells, the chiral diazepines 9G and 3F were equally potent (3F; EC50: 34.6 nM and 9G; EC50: 37.9 nM, Table 2, Fig. 4B), but were 165-fold less potent than MK-5046 (EC50: 0.23 nM, Table 2, Fig. 4B); whereas 9D and 9F had 2- and 7.8-fold lower potency for rBRS-3/BALB (9D; EC50: 70.4 nM and 9F; EC50: 295 nM, Table 2, Fig. 4B). In mBRS-3/BALB cells, of the four chiral agonists, 9D and 9G were the most potent (9D; EC50: 18.4 nM and 9G; EC50: 20.1 nM, Table 2, Fig. 4C), but were 3.5–3.8-fold less potent than MK-5046 (EC50: 5.24 nM, Table 2, Fig. 4C). In contrast, the chiral diazepines 3F and 9F, compared to 9D or 9G, showed a 1.5- and 4.3-fold lower for mBRS-3/BALB cells (3F; EC50: 31 nM and 9F; EC50: 86.8 nM, Table 2, Fig. 4C). In hBRS-3/BALB cells, GRP or NMB, and in r,mBRS-3/BALB cells, GRP or NMB, did not interact with BRS-3 at concentrations up to 3,000 nM (Table 2, Fig. 4A–C). Similarly, peptide #1 did not activate r,mBRS-3 at concentrations up to 3,000 nM (Table 2, Fig. 4B, 4C).
TABLE 2.
Ability of different ligands to activate phospholipase C and stimulate [3H]IP production in BRS-3, GRP, and NMB receptor containing cells.
| EC50 (nM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Human | Rat | Mouse | |||||||
| BRS-3 | GRPR | NMBR | BRS-3 | GRPR | NMBR | BRS-3 | GRPR | NMBR | |
| Peptides | hBRS-3/BALB | hGRPR/BALB | hNMBR/BALB | rBRS-3/BALB | rGRPR/AR42J | rNMBR/BALB | mBRS-3/BALB | mGRPR/BALB | mNMBR/BALB |
| Peptide #1 | 3.92 ± 0.26 | 0.57 ± 0.08 | 0.71 ± 0.04 | >3,000 | 0.12 ± 0.01 | 0.46 ± 0.06 | >3,000 | 0.58 ± 0.04 | 0.91 ± 0.09 |
| MK-5046 | 8.70 ± 0.47 | >3,000 | >3,000 | 0.23 ± 0.08 | >3,000 | >3,000 | 5.24 ± 0.36 | >3,000 | >3,000 |
| 3 F | 47.7 ± 0.7 | >3,000 | >3,000 | 34.55 ± 0.56 | >3,000 | >3,000 | 31.08 ± 1.02 | >3,000 | >3,000 |
| 9 D | 9.40 ± 0.46 | >3,000 | >3,000 | 70.40 ± 0.37 | >3,000 | >3,000 | 18.4 ± 0.5 | >3,000 | >3,000 |
| 9 F | 39.6 ± 1.0 | >3,000 | >3,000 | 295 ± 0.5 | >3,000 | >3,000 | 86.8 ± 0.8 | >3,000 | >3,000 |
| 9 G | 9.20 ± 0.39 | >3,000 | >3,000 | 37.9 ± 0.8 | >3,000 | >3,000 | 20.1 ± 0.9 | >3,000 | >3,000 |
| GRP | >3,000 | 4.79 ± 0.19 | 1,300 ± 170 | >3,000 | 0.30 ± 0.10 | 125 ± 10 | >3,000 | 1.00 ± 0.20 | 499 ± 32 |
| NMB | >3,000 | 530 ± 23 | 2.52 ± 0.10 | >3,000 | 17.01 ± 1.90 | 1.59 ± 0.16 | >3,000 | 23.0 ± 8.0 | 4.73 ± 0.21 |
Cells were incubated with 3 μCi/ml myo-[2-3H]inositol for 24 hours, then washed and incubated with unlabeled ligand for 60 minutes at 37°C. [3H]-IP production was determined as described in Materials and Methods. The EC50 for each ligand was the concentration causing half-maximal increase in [3H]-IP production with the maximal response seen with MK-5046 or peptide #1 at 1 μM. Data were calculated from the experiments shown in Fig. 4–6. In each experiment each value was determined in duplicate, and values given are means and S.E.M. from at least three separate experiments. Abbreviations: EC50, concentration causing half-maximal stimulation; for others see, Table 1 legend.
In h,mGRPR/BALB cells, rGRPR/AR42J cells and h,r,mNMBR/BALB cells, none of the four chiral diazepines or MK-5046 activated GRPR or NMBR at concentrations up to 3,000 nM (Table 2, Fig. 5A–C, 6A–C). However, peptide #1 had a high potency for activating GRPR and NMBR in human, rat or mouse (EC50: 0.12–0.58 nM, Table 2, Fig. 5A–C, 6A–C); which was 8.4-, 0.3- and 0.2-fold greater than GRP, respectively (EC50: 4.79, 0.30 and 1 nM, Table 2, Fig. 5A–C, 6A–C). Similarly, peptide #1 showed a 3.4-, 3.5- and 5.2-fold greater potency than NMB for human, rat or mouse NMBR (EC50: 2.5, 1.6, 4.7 nM, Table 2, Fig. 5A–C, 6A–6C). In contrast, peptide #1 had a >250-fold higher potency than GRP for h,r,mNMBR (EC50: 1,300, 125 and 499 nM, Table 2, Fig. 5A–C, 6A–C) and 40-930-fold greater potency than NMB for h,r,mGRPR (EC50: 530, 17 and 23 nM, Table 2, Fig. 5A–C, 6A–C).
Figure 5.
Comparison of abilities of four chiral diazepine analogs (3F, 9D, 9F, 9G); a nonselective Bn analog agonist (Peptide #1); a nonpeptide agonist MK-5046; GRP and NMB to stimulate [3H]IP generation in cells containing human, rat or mouse GRPR: (A) hGRPR/BALB, (B) rGRPR/AR42J and (C) mGRPR/BALB cells. The experimental conditions were the same as described in Fig. 4 legend. The results are the mean and S.E.M. from at least three experiments, and in each experiment the data points were determined in duplicate. The results are expressed as the percentage of stimulation causing by maximal effective concentration of peptide #1 (1 μM). The control and the maximal stimulated [3H]IP with peptide #1 (1 μM): hGRPR/BALB cells, 2,567 ± 211 and 20,974 ± 2,520 dpm, respectively (n=6). rGRPR/AR42J cells, 577 ± 6 and 9,887 ± 465 dpm, respectively (n=3). mGRPR/BALB cells, 1,490 ± 113 and 12,527 ± 2087 dpm, respectively (n=7). Abbreviations: See in table 1 and 2 legends.
Comparison of affinities for occupying the hBRS-3 receptor determined from binding studies (IC50) and its potencies for activating hBRS-3 (EC50) in [3H]IP demonstrated the different chiral diazepines, peptide #1 and MK-5046 had markedly different degrees of receptor sparseness. Specifically, for the four chiral agonists, 9G had the lowest IC50/EC50 ratio of 7.6; whereas with 9D, 9F, 3F and the IC50/EC50 ratios were 12.5, 12.8 and 19, respectively. With MK-5046 the IC50/EC50 ratio was 11.7, whereas with peptide #1 the IC50/EC50 ratio was 0.74, demonstrating no receptor sparseness (Table 3).
TABLE 3.
Comparison of affinities/potencies of different ligands for human BRS-3/BALB cells.
| IC50 (nM) | EC50 (nM) | IC50/ EC50 | |
|---|---|---|---|
| Peptides | hBRS-3/BALB | hBRS-3/BALB | hBRS-3/BALB |
| Peptide #1 | 2.89 ± 0.70 | 3.92 ± 0.26 | 0.74 |
| MK-5046 | 102 ± 2 | 8.70 ± 0.47 | 11.72 |
| 3 F | 909 ± 2 | 47.7 ± 0.7 | 19.06 |
| 9 D | 121 ± 1 | 9.40 ± 0.46 | 12.87 |
| 9 F | 495 ± 1 | 39.6 ± 1.0 | 12.50 |
| 9 G | 70 ± 1 | 9.20 ± 0.39 | 7.61 |
4. Discussion
The propose of this study was to assess the abilities of four new nonpeptide chiral diazepines with low CNS penetrance [35,36] to interact with and activate Bn receptors. This study was performed because of the increasing interest in BRS-3 both in physiological and pathological conditions, as well as in it therapeutic potential in diseases such as obesity/diabetes [13,17,18,29,30,36,40,43,44]. Until recently, little was known of the role of BRS-3 in physiological, or pathological conditions, because it is an orphan receptor with an unknown ligand, ever though it is included in the Bn receptor family, because of its high homology to other receptors in this family [10,13,22]. The report that BRS-3 knockout animals develop, hypertension, diabetes and obesity, resulted in increased interest in this receptor [13,40]. This resulted in different pharmacological companies developing, nonpeptide agonists and antagonists [11,13,17,18,35,36,38,44,54], aimed at exploring BRS-3’s role in physiology/pathophysiology and possible role in treatment of obesity/diabetes [13,17,23,29,30,44]. Initial human and animal studies with the benzodiazepine analogue, MK-5046, showed promise with weight loss, stimulation of insulin release and correction of hyperglycemia/diabetes [13,18,54]. However, an area of prominent concern was that MK-5046, caused cardiovascular side-effects in both human and mice [18,26,44], which could possibly be due to a CNS effect of MK-5046, because it is a CNS penetrance compound and BRS-3 receptors are widely present in the CNS [13,17,69]. To circumvent this possibility, peripherally acting BRS-3 agonists which are nonpeptide chiral diazepine analogs were recently reported [35,36]. However, little is known of the pharmacology of these new nonpeptide chiral diazepines, including their affinities for the different human BnRs, their receptor selectivity, specifically in comparison to the widely used BRS-3 agonist MK-5046, and their potency and efficacy for activating the various BnR’s. Because these compounds will be widely used to investigate the physiological/pathological role of BRS-3 and because they are the first peripheral-acting BRS-3 potentially, selective agonists described, the assessment of their pharmacology is important. This is particularly important for BnRs because numerous studies have reported with this class of receptors that various peptide agonists and antagonists show marked species difference in affinity, potency and efficacy, such that in different species a given analogue can function as an antagonist, agonist or partial agonist [14,22,28,61,63,64]. Furthermore, little information exists for nonpeptide agonists of Bn receptors, similar to case for many other G-protein-coupled receptors (GPCRs) for gastrointestinal (GI) hormones/neurotransmitters. This has occurred because for many GPCR for GI hormones/neurotransmitters such as those for VIP, secretin, substance P, PACAP or gastrin, as well as for the BnR receptors, NMBR and GRPR, no nonpeptide agonists have been described. With other GI hormone/neurotransmitter GPCRs receptors such as for CCK, BRS-3, neurotensin and motilin, only recently have nonpeptide agonists been described[6,58,60], with the result that their pharmacology is largely unknown, including whether they also demonstrated species variability for affinity, potency and efficacy.
Furthermore, the pharmacology, as well as the ability of given Bn receptor ligand to function as agonist/antagonist, various markedly in different species. Because of this, in the present study a comparison for the ability of the different chiral diazepines to activate BnRs of mice, rat and human was performed.
A new number of our results support the conclusion that each of four new chiral diazepines and nonpeptide MK-5046 are highly selective and fully efficacious agonists for the BRS-3 receptor in human, rat and mouse cells. First, in hBRS-3 containing cells, the two chiral diazepines (9D and 9G) had a similar affinity for the hBRS-3 receptor (69.69–121.1 nM) to that seen with MK-5046 (102 nM). Second, each of the four new chiral diazepines were selective for hBRS-3 and did not bind to either GRPR or NMBR in human, rat or mouse at concentrations as high as 3,000 nM. In contrast, the agonist, peptide #1, as was reported previously [32–34,41,61], showed a high affinity for all human, rat or mouse Bn receptors (IC50: 0.03–5.76 nM), except rat, mouse BRS-3. Third, our results demonstrated that the new four chiral diazepines are fully efficacious BRS-3 agonists for activating phospholipase C in cells containing BRS-3 from human, rat or mouse; that they were selective BRS-3 in each of these cells, not activating activate GRPR or NMBR in human, rat or mouse cells, even at concetrations up to 3,000 nM.
Previous studies report wide variation in the affinity of peptide agonists and peptide antagonists for GRPR and NMBR in different species [8,9,14,22,28,34,61,63,64], as well as their potency/efficiency for activating GRPR or NMBR and functioning as an agonist, partial agonist or antagonist in different species [8,9,22,63,64]. Much less information is available for the BRS-3 receptor became the endogenous ligand is unknown [13,30]. Furthermore, only recently has nonpeptide agonists been described for BRS-3, similar to numerous other gastrointestinal hormone/neurotransmitter GPCRs [12], so there is very little information of whether this variability in potency of a given receptor in different species extends also to a nonpeptide agonist, and exists for BRS-3. This study for the first time demonstrates that the nonpeptide BRS-3 receptor agonists examined in the present study (MK-5046, 3F, 9D, 9F and 9G) demonstrate wide variation in their potencies for receptor activation between human, rat and mouse BRS-3, but not in they efficacy for activating the receptor and stimulating phospholipase C activation. This conclusion is supported by a number of findings. First, MK-5046 in rBRS-3 cell was 22-fold and 38-fold more potent than in mBRS-3 or hBRS-3, whereas the chiral diazepine 3F was approximately equally potent in the BRS-3 receptors of all these species and the related chiral diazepines 9G, 9F were more potent in hBRS-3 over r,mBRS-3. Second, the relative potencies of the different nonpeptide agonists varied markedly between the different species. For example, MK-5046, 9D and 9G were approximately equally potent at activating hBRS-3; whereas for rBRS-3, 9F and 9G were 8-fold and 165-fold less potent than MK-5046 and with mouse BRS-3, and they were 5-fold and 4-fold less potent. Third, in h,r,mBRS-3 cells, each of the the nonpeptide demonstrated similar efficacy in activating phospholipase C, when sufficiently high correlations were used, demonstrate none were partial agonists. These results are consistent with studies of nonpeptide agonists for serotonin 2A [7], histamine (H1, H2, H3, H4) [56], endothelium [45] and Kinin-B2 receptors, which are report to demonstrate species variation in their affinities/potencies. These results suggested that if physiological/pathological functions of BRS-3 are explored in different laboratory animals, it will be important to consider potency of the selected agonists in the studied species.
Recently with peptide and nonpeptide agonists as well as antagonists, marked different in receptor coupling efficiency for various G-protein coupled receptors have been reported [1,6,16,38,39,42,53,61]. Until recently, no nonpeptide agonists existed for BnRs, so that it was unclear whether a similar relationship frequently occurred with these receptors for nonpeptide agonists. Our results support the conclusion that it frequently occurs with BRS-3 receptors in human cells. This conclusion was supported by the fact that with the peptide agonist (peptide #1), the IC50/ EC50 ratio, a measure of receptor spareness, was almost unity, showing no receptor sparseness. In contrast, with the nonpeptide BRS-3 agonists the coupling ratio varied from 7.61 to 19.06 demonstrating receptor sparseness in each case, as well as demonstrating this variation occurred even with closely chemically related agonists such as the four chiral diazepines (IC50/ EC50: 7.61–19.06). These results demonstrated the receptor coupling efficiency both of peptide agonists and nonpeptide agonists can vary considerably for a given receptor of the BnR family. This result is, in contrast, to the findings recently reported with a nonpeptide agonist’s ability to activate and occupy CCK [53], vasopressin V2 [39], or bradykinin B2R [1] receptors. With each of these receptors there was no receptor spareness for the nonpeptide agonist and it resembles the ability of a peptide agonist in its receptor occupation-coupling stoichiometry. This marked variation in receptor coupling activation is important to consider in the use of the BRS-3 compounds, because it can lead to different conclusions on their selectivity for a given BnR [38].
Highlights.
Recently reported nonpeptide-chiral-diazepine BRS-3 agonists are characterized pharmacologically.
The chiral-dizapeines have high affinity and selectivity for human, rat, mouse hBRS-3.
Each analog has high potency, selectivity and functions as full agonist at human, rat, mouse BRS-3.
Compared to peptide agonists, the chiral diazepines had variable receptor spareness in human BRS-3
The chiral diazepines nonpeptides analogs had variable potencies in BRS-3 from different species
Acknowledgments
This work was partially supported by intramural funds of the NIDDK, NIH.
Abbreviations
- AR42J cells
rat pancreatic acinar cells
- BALB 3T3
mouse embryonic fibroblast cells
- Bn
Bombesin
- BnR
Bombesin receptors
- BRS-3
Bombesin receptor subtype-3
- BSA
bovine serum albumin fraction V; chiral diazepine analogs (3F, 9G, 9F, 9G)
- CNS
central nervous system
- DMEM
Dulbecco’s minimum essential medium
- h
human
- EC50
concentration causing half-stimulation
- FBS
fetal bovine serum
- GPCR
G-protein-coupled receptor
- GRP
gastrin-releasing peptide
- GRPR
gastrin-releasing peptide receptor
- IC50
half maximal inhibitor concentration
- IBMX
3-isobutyl-1-methylxanthine
- IP
inositol phosphate
- m
mouse
- MK-5046
nonpeptide BRS3 agonist
- NMB
neuromedin B
- NMBR
neuromedin B receptor
- peptide #1
[D-Tyr6, β-Ala11, Phe13, Nle14]Bn-(6–14)
- PBS
phosphate buffered saline
- PLC
phospholipase C
- r
rat
Footnotes
Conflicts of Interest
The authors have no conflicts of interest
References
- 1.Aramori I, Zenkoh J, Morikawa N, Asano M, Hatori C, Sawai H, et al. Nonpeptide mimic of bradykinin with long-acting properties at the bradykinin B2 receptor. Mol Pharmacol. 1997;52:16–20. doi: 10.1124/mol.52.1.16. [DOI] [PubMed] [Google Scholar]
- 2.Benya RV, Fathi Z, Battey JF, Jensen RT. Serines and threonines in the gastrin-releasing peptide receptor carboxyl terminus mediate internalization. J Biol Chem. 1993;268:20285–90. [PubMed] [Google Scholar]
- 3.Benya RV, Fathi Z, Pradhan T, Battey JF, Kusui T, Jensen RT. Gastrin-releasing peptide receptor-induced internalization, down-regulation, desensitization and growth: Possible role of cAMP. Mol Pharmacol. 1994;46(2):235–45. [PubMed] [Google Scholar]
- 4.Benya RV, Kusui T, Pradhan TK, Battey JF, Jensen RT. Expression and characterization of cloned human bombesin receptors. Mol Pharmacol. 1995;47:10–20. [PubMed] [Google Scholar]
- 5.Benya RV, Wada E, Battey JF, Fathi Z, Wang LH, Mantey SA, et al. Neuromedin B receptors retain functional expression when transfected into BALB 3T3 fibroblasts: analysis of binding, kinetics, stoichiometry, modulation by guanine nucleotide-binding proteins, and signal transduction and comparison with natively expressed receptors. Mol Pharmacol. 1992;42(6):1058–68. [PubMed] [Google Scholar]
- 6.Bignon E, Bachy A, Boigegrain R, Brodin R, Cottineau M, Gully D, et al. SR146131: a new potent, orally active, and selective nonpeptide cholecystokinin subtype 1 receptor agonist. I. In vitro studies. J Pharmacol Exp Ther. 1999;289:742–51. [PubMed] [Google Scholar]
- 7.Canal CE, Cordova-Sintjago T, Liu Y, Kim MS, Morgan D, Booth RG. Molecular pharmacology and ligand docking studies reveal a single amino acid difference between mouse and human serotonin 5-HT2A receptors that impacts behavioral translation of novel 4-phenyl-2-dimethylaminotetralin ligands. J Pharmacol Exp Ther. 2013;347:705–16. doi: 10.1124/jpet.113.208637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coy DH, Wang LH, Jiang NZ, Jensen RT. Short chain bombesin pseudopeptides which are potent and more general bombesin receptor antagonists. Eur J Pharmacol. 1990;190(1/2):31–8. doi: 10.1016/0014-2999(90)94109-b. [DOI] [PubMed] [Google Scholar]
- 9.Falconieri Erspamer G, Severini C, Erspamer V, Melchiorri P, Delle Fave G, Nakajima T. Parallel bioassay of 27 bombesin-like peptides on 9 smooth muscle preparations, structure-activity relationships and bombesin receptor subtypes. Regul Pept. 1988;21:1–11. doi: 10.1016/0167-0115(88)90085-7. [DOI] [PubMed] [Google Scholar]
- 10.Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, et al. BRS-3: novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J Biol Chem. 1993;268(8):5979–84. [PubMed] [Google Scholar]
- 11.Feng Y, Guan XM, Li J, Metzger JM, Zhu Y, Juhl K, et al. Bombesin Receptor Subtype-3 (BRS-3) Regulates Glucose-Stimulated Insulin Secretion in Pancreatic Islets across Multiple Species. Endocrinology. 2011;152:4106–15. doi: 10.1210/en.2011-1440. [DOI] [PubMed] [Google Scholar]
- 12.Freidinger RM. Toward peptide receptor ligand drugs: progress on nonpeptides. Prog Drug Res. 1993;40:33–98. doi: 10.1007/978-3-0348-7147-1_4. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales N, Moreno P, Jensen RT. Bombesin receptor -subtype 3 as a potential target for obesity and diabetes. Curr Opin Therapeutic Targets. 2015 doi: 10.1517/14728222.2015.1056154. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez N, Mantey SA, Pradhan TK, Sancho V, Moody TW, Coy DH, et al. Characterization of putative GRP- and NMB-receptor antagonist’s interaction with human receptors. Peptides. 2009;30:1473–86. doi: 10.1016/j.peptides.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez N, Moody TW, Igarashi H, Ito T, Jensen RT. Bombesin-related peptides and their receptors: recent advances in their role in physiology and disease states. Curr Opin Endocrinol Diabetes Obes. 2008;15:58–64. doi: 10.1097/MED.0b013e3282f3709b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gouldson P, Legoux P, Carillon C, Delpech B, Le Fur G, Ferrara P, et al. Contrasting roles of leu(356) in the human CCK(1) receptor for antagonist SR 27897 and agonist SR 146131 binding. Eur J Pharmacol. 1999;383:339–46. doi: 10.1016/s0014-2999(99)00612-3. [DOI] [PubMed] [Google Scholar]
- 17.Guan XM, Chen H, Dobbelaar PH, Dong Y, Fong TM, Gagen K, et al. Regulation of Energy Homeostasis by Bombesin Receptor Subtype-3: Selective Receptor Agonists for the Treatment of Obesity. Cell Metab. 2010;11:101–12. doi: 10.1016/j.cmet.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Guan XM, Metzger JM, Yang L, Raustad KA, Wang SP, Spann SK, et al. Antiobesity effect of MK-5046, a novel bombesin receptor subtype-3 agonist. J Pharmacol Exp Ther. 2011;336:356–64. doi: 10.1124/jpet.110.174763. [DOI] [PubMed] [Google Scholar]
- 19.Heinz-Erian P, Coy DH, Tamura M, Jones SW, Gardner JD, Jensen RT. [D-Phe12]bombesin analogues: a new class of bombesin receptor antagonists. Am J Physiol. 1987;252:G439–G442. doi: 10.1152/ajpgi.1987.252.3.G439. [DOI] [PubMed] [Google Scholar]
- 20.Hoyer D, Bartfai T. Neuropeptides and neuropeptide receptors: drug targets, and peptide and non-peptide ligands: a tribute to Prof. Dieter Seebach Chem Biodivers. 2012;9:2367–87. doi: 10.1002/cbdv.201200288. [DOI] [PubMed] [Google Scholar]
- 21.Jennings CA, Harrison DC, Maycox PR, Crook B, Smart D, Hervieu GJ. The distribution of the orphan bombesin receptor subtype-3 in the rat CNS. Neuroscience. 2003;120:309–24. doi: 10.1016/s0306-4522(03)00260-4. [DOI] [PubMed] [Google Scholar]
- 22.Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LVIII. Mammalian Bombesin Receptors: Nomenclature, distribution, pharmacology, signaling and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen RT, Delle Fave G. Promising advances in the treatment of malignant pancreatic endocrine tumors. N Engl J Med. 2011;364:564–5. doi: 10.1056/NEJMe1013903. [DOI] [PubMed] [Google Scholar]
- 24.Jensen RT, Moody TW. Bombesin-Related Peptides. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Amsterdam: Elsevier; 2013. pp. 1188–96. [Google Scholar]
- 25.Ladenheim EE, Hamilton NL, Behles RR, Bi S, Hampton LL, Battey JF, et al. Factors contributing to obesity in bombesin receptor subtype-3-deficient mice. Endocrinology. 2008;149:971–8. doi: 10.1210/en.2007-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lateef DM, breu-Vieira G, Xiao C, Reitman ML. Regulation of body temperature and brown adipose tissue thermogenesis by bombesin receptor subtype-3. Am J Physiol Endocrinol Metab. 2014;306:E681–E687. doi: 10.1152/ajpendo.00615.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Lao ZJ, Zhang J, Schaeffer MT, Jiang MM, Guan XM, et al. Molecular basis of the pharmacological difference between rat and human bombesin receptor subtype-3 (BRS-3) Biochemistry (Mosc) 2002;41:8954–60. doi: 10.1021/bi0202777. [DOI] [PubMed] [Google Scholar]
- 28.Maina T, Nock BA, Zhang H, Nikolopoulou A, Waser B, Reubi JC, et al. Species differences of bombesin analog interactions with GRP-R define the choice of animal models in the development of GRP-R-targeting drugs. J Nucl Med. 2005;46:823–30. [PubMed] [Google Scholar]
- 29.Majumdar ID, Weber HC. Appetite-modifying effects of bombesin receptor subtype-3 agonists. Handb Exp Pharmacol. 2012:405–32. doi: 10.1007/978-3-642-24716-3_19. [DOI] [PubMed] [Google Scholar]
- 30.Majumdar ID, Weber HC. Biology and pharmacology of bombesin receptor subtype-3. Curr Opin Endocrinol Diabetes Obes. 2012;19:3–7. doi: 10.1097/MED.0b013e32834ec77d. [DOI] [PubMed] [Google Scholar]
- 31.Mantey S, Frucht H, Coy DH, Jensen RT. Characterization of bombesin receptors using a novel, potent, radiolabeled antagonist that distinguishes bombesin receptor subtypes. Mol Pharmacol. 1993;45:762–74. [PubMed] [Google Scholar]
- 32.Mantey SA, Coy DH, Pradhan TK, Igarashi H, Rizo IM, Shen L, et al. Rational design of a peptide agonist that interacts selectively with the orphan receptor, bombesin receptor subtype 3. J Biol Chem. 2001;276:9219–29. doi: 10.1074/jbc.M008737200. [DOI] [PubMed] [Google Scholar]
- 33.Mantey SA, Gonzalez N, Schumann M, Pradhan TK, Shen L, Coy DH, et al. Identification of bombesin receptor subtype-specific ligands: effect of N-methyl scanning, truncation, substitution, and evaluation of putative reported selective ligands. J Pharmacol Exp Ther. 2006;319:980–9. doi: 10.1124/jpet.106.107011. [DOI] [PubMed] [Google Scholar]
- 34.Mantey SA, Weber HC, Sainz E, Akeson M, Ryan RR, Pradhan TK, et al. Discovery of a high affinity radioligand for the human orphan receptor, bombesin receptor subtype 3, which demonstrates it has a unique pharmacology compared to other mammalian bombesin receptors. J Biol Chem. 1997;272(41):26062–71. doi: 10.1074/jbc.272.41.26062. [DOI] [PubMed] [Google Scholar]
- 35.Matsufuji T, Shimada K, Kobayashi S, Ichikawa M, Kawamura A, Fujimoto T, et al. Synthesis and biological evaluation of novel chiral diazepine derivatives as bombesin receptor subtype-3 (BRS-3) agonists incorporating an antedrug approach. Bioorg Med Chem. 2015;23:89–104. doi: 10.1016/j.bmc.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Matsufuji T, Shimada K, Kobayashi S, Kawamura A, Fujimoto T, Arita T, et al. Discovery of novel chiral diazepines as bombesin receptor subtype-3 (BRS-3) agonists with low brain penetration. Bioorg Med Chem Lett. 2014;24:750–5. doi: 10.1016/j.bmcl.2013.12.106. [DOI] [PubMed] [Google Scholar]
- 37.Moody TW, Sancho V, Di Florio A, Nuche-Berenguer B, Mantey S, Jensen RT. Bombesin receptor subtype-3 agonists stimulate the growth of lung cancer cells and increase EGF receptor tyrosine phosphorylation. Peptides. 2011;32:1677–84. doi: 10.1016/j.peptides.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno P, Mantey SA, Nuche-Berenguer B, Reitman ML, Gonzalez N, Coy DH, et al. Comparative pharmacology of bombesin receptor subtype-3, nonpeptide agonist MK-5046, a universal peptide agonist, and peptide antagonist Bantag-1 for human bombesin receptors. J Pharmacol Exp Ther. 2013;347:100–16. doi: 10.1124/jpet.113.206896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura S, Yamamura Y, Itoh S, Hirano T, Tsujimae K, Aoyama M, et al. Characterization of a novel nonpeptide vasopressin V(2)-agonist, OPC-51803, in cells transfected human vasopressin receptor subtypes. Br J Pharmacol. 2000;129:1700–6. doi: 10.1038/sj.bjp.0703221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohki-Hamazaki H, Watase K, Yamamoto K, Ogura H, Yamano M, Yamada K, et al. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature. 1997;390(6656):165–9. doi: 10.1038/36568. [DOI] [PubMed] [Google Scholar]
- 41.Pradhan TK, Katsuno T, Taylor JE, Kim SH, Ryan RR, Mantey SA, et al. Identification of a unique ligand which has high affinity for all four bombesin receptor subtypes. Eur J Pharmacol. 1998;343:275–87. doi: 10.1016/s0014-2999(97)01527-6. [DOI] [PubMed] [Google Scholar]
- 42.Ramos-Alvarez I, Mantey SA, Nakamura T, Nuche-Berenguer B, Moreno P, Moody TW, et al. A structure-function study of PACAP using conformationally restricted analogs: Identification of PAC1 receptor-selective PACAP agonists. Peptides. 2015;66:26–42. doi: 10.1016/j.peptides.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramos-Alvarez I, Moreno P, Mantey SA, Nakamura T, Nuche-Berenguer B, Moody TW, et al. Insights into bombesin receptors and ligands: highlighting recent advances. Peptides. 2015 doi: 10.1016/j.peptides.2015.04.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reitman ML, Dishy V, Moreau A, Denney WS, Liu C, Kraft WK, et al. Pharmacokinetics and pharmacodynamics of MK-5046, a bombesin receptor subtype-3 (BRS-3) agonist, in healthy patients. J Clin Pharmacol. 2012;52:1306–16. doi: 10.1177/0091270011419854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds EE, Hwang O, Flynn MA, Welch KM, Cody WL, Steinbaugh B, et al. Pharmacological differences between rat and human endothelin B receptors. Biochem Biophys Res Commun. 1995;209:506–12. doi: 10.1006/bbrc.1995.1530. [DOI] [PubMed] [Google Scholar]
- 46.Rowley WH, Sato S, Huang SC, Collado-Escobar DM, Beaven MA, Wang LH, et al. Cholecystokinin-induced formation of inositol phosphates in pancreatic acini. Am J Physiol. 1990;259:G655–G665. doi: 10.1152/ajpgi.1990.259.4.G655. [DOI] [PubMed] [Google Scholar]
- 47.Ryan RR, Katsuno T, Mantey SA, Pradhan TP, Weber HC, Battey JF, et al. Comparative pharmacology of a nonpeptoid neuromedin B antagonist PD 168368. J Pharmacol Exp Ther. 1999;290:1202–11. [PubMed] [Google Scholar]
- 48.Ryan RR, Weber HC, Hou W, Sainz E, Mantey SA, Battey JF, et al. Ability of various bombesin receptor agonists and antagonists to alter intracellular signaling of the human orphan receptor BRS-3. J Biol Chem. 1998;273:13613–24. doi: 10.1074/jbc.273.22.13613. [DOI] [PubMed] [Google Scholar]
- 49.Ryan RR, Weber HC, Mantey SA, Hou W, Hilburger ME, Pradhan TK, et al. Pharmacology and intracellular signaling mechanisms of the native human orphan receptor BRS-3 in lung cancer cells. J Pharmacol Exp Ther. 1998;287:366–80. [PubMed] [Google Scholar]
- 50.Sancho V, Moody TW, Mantey SA, Di Florio A, Uehara H, Coy DH, et al. Pharmacology of putative selective hBRS-3 receptor agonists for human bombesin receptors (BnR): Affinities, potencies and selectivity in multiple native and BnR transfected cells. Peptides. 2010;31:1569–78. doi: 10.1016/j.peptides.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sano H, Feighner SD, Hreniuk DL, Iwaasa H, Sailer AW, Pan J, et al. Characterization of the bombesin-like peptide receptor family in primates. Genomics. 2004;84:139–46. doi: 10.1016/j.ygeno.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Sayegh AI. The role of bombesin and bombesin-related peptides in the short-term control of food intake. Prog Mol Biol Transl Sci. 2013;114:343–70. doi: 10.1016/B978-0-12-386933-3.00010-8. [DOI] [PubMed] [Google Scholar]
- 53.Schaeffer P, Nestor AL, Prabonnaud V, Bachy A, Laplace MC, Keane PE, et al. Characterisation of the effects of SR146131, a novel non-peptide CCK(1) receptor agonist, on IMR-32 human neuroblastoma cells. Eur J Pharmacol. 2000;397:303–10. doi: 10.1016/s0014-2999(00)00274-0. [DOI] [PubMed] [Google Scholar]
- 54.Sebhat IK, Franklin C, Lo MC, Chen D, Jewell JP, Miller R, et al. Discovery of MK-5046, a potent, selective bombesin recptor subtype-3 agonist for the treatment of obesity. ACS Medicinal Chemistry Letters. 2011;2:43–7. doi: 10.1021/ml100196d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shan L, Emanuel RL, Dewald D, Torday JS, Asokanathan N, Wada K, et al. Bombesin-like peptide receptor gene expression, regulation, and function in fetal murine lung. Am J Physiol (Lung Cell Mol Physiol ) 2004;286:L165–L173. doi: 10.1152/ajplung.00436.2002. [DOI] [PubMed] [Google Scholar]
- 56.Strasser A, Wittmann HJ, Buschauer A, Schneider EH, Seifert R. Species-dependent activities of G-protein-coupled receptor ligands: lessons from histamine receptor orthologs. Trends Pharmacol Sci. 2013;34:13–32. doi: 10.1016/j.tips.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Subramaniam M, Sugiyama K, Coy DH, Kong Y, Miller YE, Weller PF, et al. Bombesin-like peptides and mast cell responses: relevance to bronchopulmonary dysplasia? Am J Respir Crit Care Med. 2003;168:601–11. doi: 10.1164/rccm.200212-1434OC. [DOI] [PubMed] [Google Scholar]
- 58.Takanashi H, Yogo K, Ozaki K, Koga H, Itoh Z, Omura S. In vitro pharmacological characterization of mitemcinal (GM-611), the first acid-resistant non-peptide motilin receptor agonist, in smooth muscle of rabbit small intestine. Pharmacology. 2007;79:137–48. doi: 10.1159/000098129. [DOI] [PubMed] [Google Scholar]
- 59.Tan YR, Qi MM, Qin XQ, Xiang Y, Li X, Wang Y, et al. Wound repair and proliferation of bronchial epithelial cells enhanced by bombesin receptor subtype 3 activation. Peptides. 2006;27:1852–8. doi: 10.1016/j.peptides.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 60.Thomas JB, Giddings AM, Olepu S, Wiethe RW, Harris DL, Narayanan S, et al. Identification of N-{[6-chloro-4-(2,6-dimethoxyphenyl)quinazolin-2-yl]carbonyl}-l-leucine (NTRC-808), a novel nonpeptide chemotype selective for the neurotensin receptor type 2. Bioorg Med Chem Lett. 2015;25:292–6. doi: 10.1016/j.bmcl.2014.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uehara H, Gonzalez N, Sancho V, Mantey SA, Nuche-Berenguer B, Pradhan T, et al. Pharmacology and selectivity of various natural and synthetic bombesin related peptide agonists for human and rat bombesin receptors differs. Peptides. 2011;32:1685–99. doi: 10.1016/j.peptides.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Schrenck T, Wang LH, Coy DH, Villanueva ML, Mantey S, Jensen RT. Potent bombesin receptor antagonists distinguish receptor subtypes. Am J Physiol. 1990;259:G468–G473. doi: 10.1152/ajpgi.1990.259.3.G468. [DOI] [PubMed] [Google Scholar]
- 63.Wang LH, Coy DH, Taylor JE, Jiang NY, Kim SH, Moreau JP, et al. Desmethionine alkylamide bombesin analogues: a new class of bombesin receptor antagonists with a potent antisecretory activity in pancreatic acini and antimitotic activity in Swiss 3T3 cells. Biochemistry (Mosc) 1990;29(3):616–22. doi: 10.1021/bi00455a004. [DOI] [PubMed] [Google Scholar]
- 64.Wang LH, Coy DH, Taylor JE, Jiang NY, Moreau JP, Huang SC, et al. Des-Met carboxyl-terminally modified analogues of bombesin function as potent bombesin receptor antagonists, partial agonists, or agonists. J Biol Chem. 1990;265(26):15695–703. [PubMed] [Google Scholar]
- 65.Wang Y, Zhang M, Tan Y, Xiang Y, Liu H, Qu F, et al. BRS-3 activation transforms the effect of human bronchial epithelial cells from PGE2 mediated inhibition to TGF-beta1 dependent promotion on proliferation and collagen synthesis of lung fibroblasts. Cell Biol Int. 2007;31:1495–500. doi: 10.1016/j.cellbi.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 66.Weber HC, Walters J, Leyton J, Casibang M, Purdom S, Jensen RT, et al. A bombesin receptor subtype-3 peptide increases nuclear oncogene expression in a MEK-1 dependent manner in human lung cancer cells. Europ J Pharmacol. 2001;412:13–20. doi: 10.1016/s0014-2999(00)00941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamada K, Santo-Yamada Y, Wada E, Wada K. Role of bombesin (BN)-like peptides/receptors in emotional behavior by comparison of three strains of BN-like peptide receptor knockout mice. Mol Psychiatry. 2002;7:113–7. 6. doi: 10.1038/sj.mp.4000974. [DOI] [PubMed] [Google Scholar]
- 68.Yamada K, Wada E, Wada K. Bombesin-like peptides: studies on food intake and social behaviour with receptor knock-out mice. Ann Med. 2000;32:519–29. doi: 10.3109/07853890008998831. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Parks GS, Wang Z, Wang L, Lew M, Civelli O. Anatomical characterization of bombesin receptor subtype-3 mRNA expression in the rodent central nervous system. J Comp Neurol. 2013;521:1020–39. doi: 10.1002/cne.23216. [DOI] [PubMed] [Google Scholar]